CCN2 Binds to Tubular Epithelial Cells in the Kidney
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model of CCN2-Induced Renal Damage
2.2. CCN2 Fluorescence Cy5 Labeling and siRNA FAM Labeling
- Antisense (5′-3′): AUAUUCGUAGCAUUUAUGGag;
- Sense (5′-3′): CCAUAAAUGCUACGAAUAUtt.
2.3. Confocal Microscopy Analysis of CCN2(IV) Binding In Vivo
2.4. Kidney Activation of EGFR Signaling Pathway
2.5. Cell Cultures
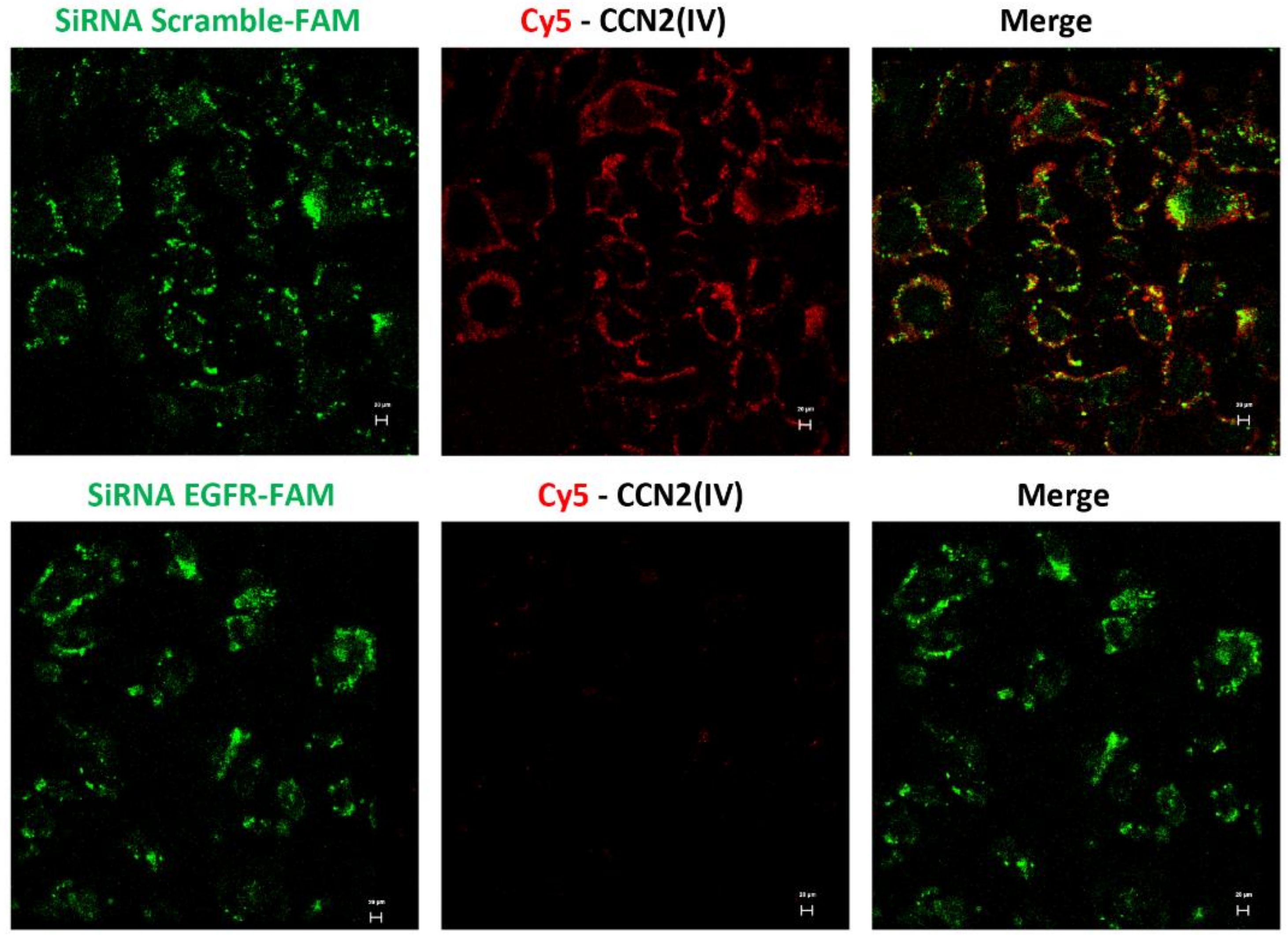
2.6. In Vitro Cy5-CCN2(IV) Binding to EGFR
2.7. Live-Cell Confocal Microscopy
3. Results
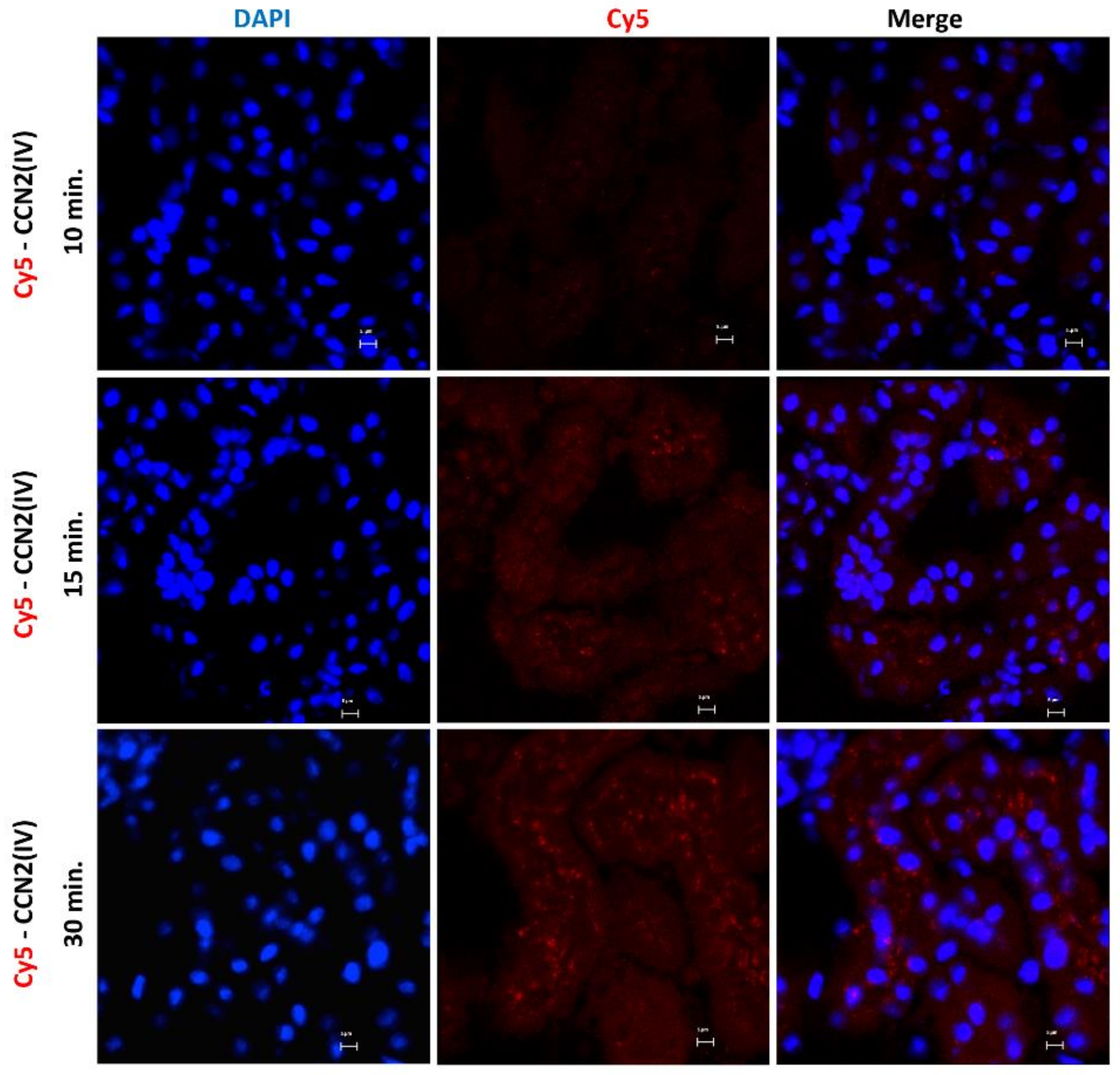
3.1. CCN2 Binds to Tubular Epithelial Cells, but Not to Glomerular Cells, in a Model of Cy5-CCN2(IV) Intra-Renal Injection
3.2. CCN2 Binds to Tubular Epithelial Cells, but Not to Glomerular Cells, in a Model of Systemic Administration of Cy5-CCN2(IV) into Mice
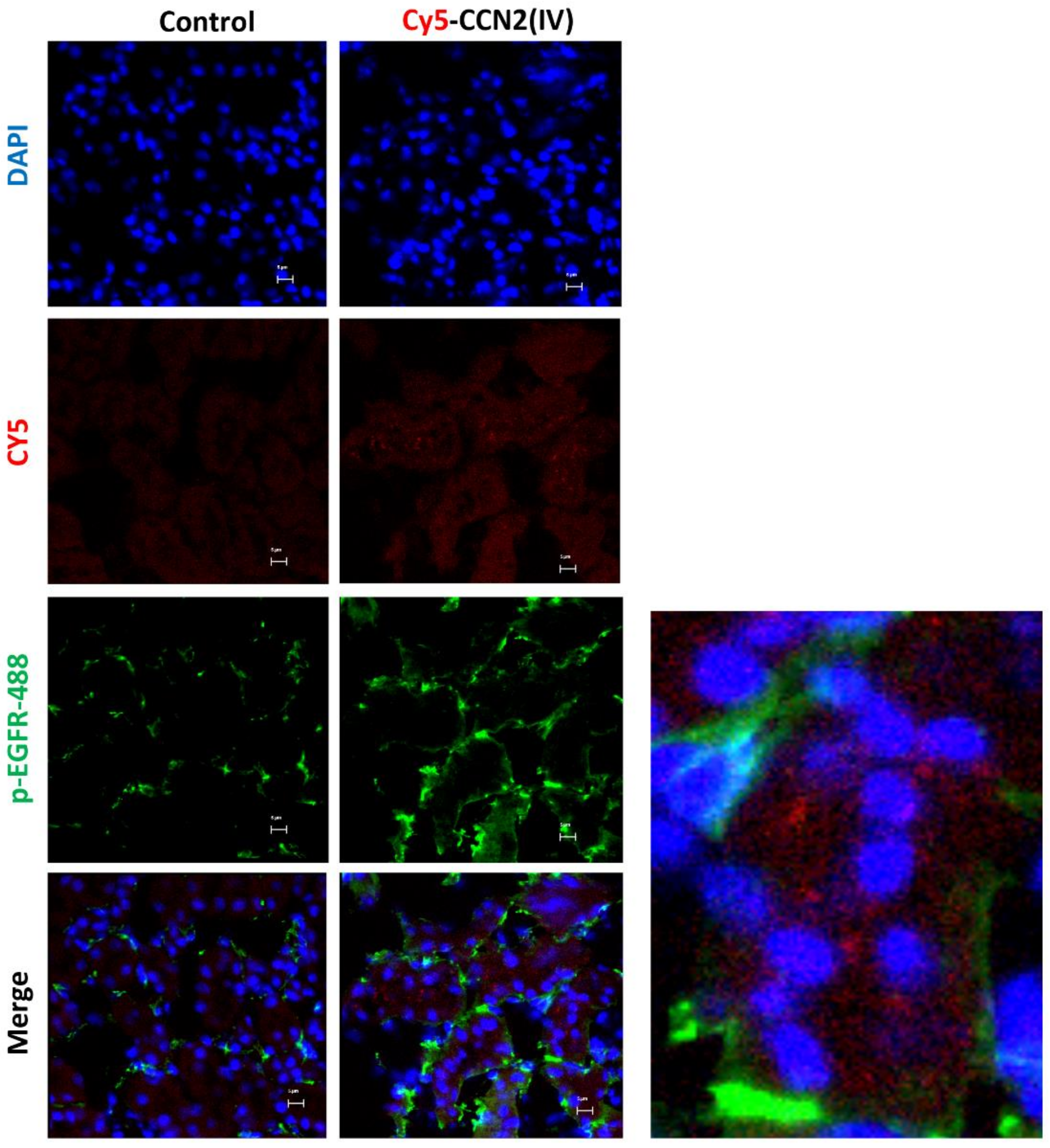
3.3. CCN2 Binding Is Linked to EGFR Pathway Activation in the Kidney
3.4. CCN2 Binding Is Linked to EGFR Pathway Activation in the Kidney
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takigawa, M. An early history of CCN2/CTGF research: The road to CCN2 via hcs24, ctgf, ecogenin, and regenerin. J. Cell Commun. Signal. 2018, 12, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Bedore, J.; Leask, A.; Séguin, C.A. Targeting the extracellular matrix: Matricellular proteins regulate cell-extracellular matrix communication within distinct niches of the intervertebral disc. Matrix Biol. 2014, 37, 124–130. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Campillo, S.; Rodrigues-Diez, R.R.; Tejera-Muñoz, A.; Marquez-Exposito, L.; Goldschmeding, R.; Rodríguez-Puyol, D.; Calleros, L.; Ruiz-Ortega, M. Interplay between extracellular matrix components and cellular and molecular mechanisms in kidney fibrosis. Clin. Sci. 2021, 135, 1999–2029. [Google Scholar] [CrossRef]
- Feng, D.; Ngov, C.; Henley, N.; Boufaied, N.; Gerarduzzi, C. Characterization of matricellular protein expression signatures in mechanistically diverse mouse models of kidney injury. Sci. Rep. 2019, 9, 16736. [Google Scholar] [CrossRef] [PubMed]
- Perbal, B. CCN proteins: Multifunctional signalling regulators. Lancet 2004, 363, 62–64. [Google Scholar] [CrossRef]
- de Winter, P.; Leoni, P.; Abraham, D. Connective tissue growth factor: Structure-function relationships of a mosaic, multifunctional protein. Growth Factors 2008, 26, 80–91. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Rodrigues-Diez, R.; Morgado-Pascual, J.L.; Valentijn, F.; Valdivielso, J.M.; Goldschmeding, R.; Ruiz-Ortega, M. Role of epidermal growth factor receptor (EGFR) and its ligands in kidney inflammation and damage. Mediat. Inflamm. 2018, 2018, 8739473. [Google Scholar] [CrossRef]
- Markiewicz, M.; Nakerakanti, S.S.; Kapanadze, B.; Ghatnekar, A.; Trojanowska, M. Connective tissue growth factor (CTGF/CCN2) mediates angiogenic effect of S1P in human dermal microvascular endothelial cells. Microcirculation 2011, 18, 1–11. [Google Scholar] [CrossRef]
- Rodrigues-Díez, R.; Rodrigues-Díez, R.R.; Rayego-Mateos, S.; Suarez-Alvarez, B.; Lavoz, C.; Stark Aroeira, L.; Sánchez-López, E.; Orejudo, M.; Alique, M.; Lopez-Larrea, C.; et al. The C-terminal module IV of connective tissue growth factor is a novel immune modulator of the Th17 response. Lab. Investig. 2013, 93, 812–824. [Google Scholar] [CrossRef]
- Jun, J.-I.; Lau, L.F. CCN2 induces cellular senescence in fibroblasts. J. Cell Commun. Signal. 2017, 11, 15–23. [Google Scholar] [CrossRef]
- Valentijn, F.A.; Knoppert, S.N.; Pissas, G.; Rodrigues-Diez, R.R.; Marquez-Exposito, L.; Broekhuizen, R.; Mokry, M.; Kester, L.A.; Falke, L.L.; Goldschmeding, R.; et al. CCN2 Aggravates the immediate oxidative stress-DNA damage response following renal ischemia-reperfusion injury. Antioxidants 2021, 10, 2020. [Google Scholar] [CrossRef]
- Liu, B.-C.; Zhang, J.-D.; Zhang, X.-L.; Wu, G.-Q.; Li, M.-X. Role of connective tissue growth factor (CTGF) module 4 in regulating epithelial mesenchymal transition (EMT) in HK-2 cells. Clin. Chim. Acta 2006, 373, 144–150. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chen, Z.; Martin, M.; Zhang, J.; Sangwung, P.; Woo, B.; Tremoulet, A.H.; Shimizu, C.; Jain, M.K.; Burns, J.C.; et al. miR-483 targeting of CTGF suppresses endothelial-to-mesenchymal transition: Therapeutic implications in kawasaki disease. Circ. Res. 2017, 120, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Rayego, S.; Rodrigues-Díez, R.; Rodriguez, J.S.; Rodrigues-Díez, R.; Rodríguez-Vita, J.; Carvajal, G.; Aroeira, L.S.; Selgas, R.; Mezzano, S.A.; et al. CTGF promotes inflammatory cell infiltration of the renal interstitium by activating NF-kappaB. J. Am. Soc. Nephrol. 2009, 20, 1513–1526. [Google Scholar] [CrossRef]
- Sakai, N.; Nakamura, M.; Lipson, K.E.; Miyake, T.; Kamikawa, Y.; Sagara, A.; Shinozaki, Y.; Kitajima, S.; Toyama, T.; Hara, A.; et al. Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis. Sci. Rep. 2017, 7, 5392. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Abraham, D.J. All in the CCN family: Essential matricellular signaling modulators emerge from the bunker. J. Cell Sci. 2006, 119, 4803–4810. [Google Scholar] [CrossRef]
- Chaqour, B. Caught between a “Rho” and a hard place: Are CCN1/CYR61 and CCN2/CTGF the arbiters of microvascular stiffness? J. Cell Commun. Signal. 2020, 14, 21–29. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Rodrigues Díez, R.; Rodríguez Vita, J.; Rayego Mateos, S.; Rodrigues Díez, R.R.; Rodríguez García, E.; Lavoz Barria, C.; Mezzano, S.; Egido, J.; Ortiz, A.; et al. Connective tissue growth factor (CTGF): A key factor in the onset and progression of kidney damage. Nefrologia 2009, 29, 382–391. [Google Scholar] [CrossRef]
- Phanish, M.K.; Winn, S.K.; Dockrell, M.E.C. Connective tissue growth factor-(CTGF, CCN2)--a marker, mediator and therapeutic target for renal fibrosis. Nephron. Exp. Nephrol. 2010, 114, e83–e92. [Google Scholar] [CrossRef]
- Ivkovic, S.; Yoon, B.S.; Popoff, S.N.; Safadi, F.F.; Libuda, D.E.; Stephenson, R.C.; Daluiski, A.; Lyons, K.M. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 2003, 130, 2779–2791. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Rodrigues-Diez, R.R.; Rodrigues-Diez, R.; Falke, L.L.; Mezzano, S.; Ortiz, A.; Egido, J.; Goldschmeding, R.; Ruiz-Ortega, M. Connective tissue growth factor induces renal fibrosis via epidermal growth factor receptor activation. J. Pathol. 2018, 244, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Díez Raul, R.; Tejera-Muñoz, A.; Esteban, V.; Steffensen Lasse, B.; Rodrigues-Díez, R.; Orejudo, M.; Rayego-Mateos, S.; Falke Lucas, L.; Cannata-Ortiz, P.; Ortiz, A.; et al. CCN2 (Cellular communication network factor 2) deletion alters vascular integrity and function predisposing to aneurysm formation. Hypertension 2021. [Google Scholar] [CrossRef]
- Gupta, S.; Clarkson, M.R.; Duggan, J.; Brady, H.R. Connective tissue growth factor: Potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int. 2000, 58, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Riser, B.L.; Denichilo, M.; Cortes, P.; Baker, C.; Grondin, J.M.; Yee, J.; Narins, R.G. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J. Am. Soc. Nephrol. 2000, 11, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, M.; Ruiz-Ortega, M.; Esteban, V.; Lorenzo, O.; Mezzano, S.; Plaza, J.J.; Egido, J. Angiotensin II increases connective tissue growth factor in the kidney. Am. J. Pathol. 2003, 163, 1937–1947. [Google Scholar] [CrossRef][Green Version]
- Toda, N.; Mukoyama, M.; Yanagita, M.; Yokoi, H. CTGF in kidney fibrosis and glomerulonephritis. Inflamm. Regen. 2018, 38, 14. [Google Scholar] [CrossRef] [PubMed]
- Shi-Wen, X.; Leask, A.; Abraham, D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008, 19, 133–144. [Google Scholar] [CrossRef]
- Slagman, M.C.J.; Nguyen, T.Q.; Waanders, F.; Vogt, L.; Hemmelder, M.H.; Laverman, G.D.; Goldschmeding, R.; Navis, G. Effects of antiproteinuric intervention on elevated connective tissue growth factor (CTGF/CCN-2) plasma and urine levels in nondiabetic nephropathy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1845–1850. [Google Scholar] [CrossRef]
- Tam, F.W.K.; Riser, B.L.; Meeran, K.; Rambow, J.; Pusey, C.D.; Frankel, A.H. Urinary monocyte chemoattractant protein-1 (MCP-1) and connective tissue growth factor (CCN2) as prognostic markers for progression of diabetic nephropathy. Cytokine 2009, 47, 37–42. [Google Scholar] [CrossRef]
- Guha, M.; Xu, Z.-G.; Tung, D.; Lanting, L.; Natarajan, R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 2007, 21, 3355–3368. [Google Scholar] [CrossRef]
- Okada, H.; Kikuta, T.; Kobayashi, T.; Inoue, T.; Kanno, Y.; Takigawa, M.; Sugaya, T.; Kopp, J.B.; Suzuki, H. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J. Am. Soc. Nephrol. 2005, 16, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Mukoyama, M.; Nagae, T.; Mori, K.; Suganami, T.; Sawai, K.; Yoshioka, T.; Koshikawa, M.; Nishida, T.; Takigawa, M.; et al. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2004, 15, 1430–1440. [Google Scholar] [CrossRef]
- Lau, L.F. Cell surface receptors for CCN proteins. J. Cell Commun. Signal. 2016, 10, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Rodrigues-Díez, R.; Morgado-Pascual, J.L.; Rodrigues Díez, R.R.; Mas, S.; Lavoz, C.; Alique, M.; Pato, J.; Keri, G.; Ortiz, A.; et al. Connective tissue growth factor is a new ligand of epidermal growth factor receptor. J. Mol. Cell Biol. 2013, 5, 323–335. [Google Scholar] [CrossRef]
- Wahab, N.A.; Weston, B.S.; Mason, R.M. Connective tissue growth factor CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J. Am. Soc. Nephrol. 2005, 16, 340–351. [Google Scholar] [CrossRef]
- Sibilia, M.; Kroismayr, R.; Lichtenberger, B.M.; Natarajan, A.; Hecking, M.; Holcmann, M. The epidermal growth factor receptor: From development to tumorigenesis. Differentiation 2007, 75, 770–787. [Google Scholar] [CrossRef] [PubMed]
- Bronte, G.; Terrasi, M.; Rizzo, S.; Sivestris, N.; Ficorella, C.; Cajozzo, M.; Di Gaudio, F.; Gulotta, G.; Siragusa, S.; Gebbia, N.; et al. EGFR genomic alterations in cancer: Prognostic and predictive values. Front. Biosci. 2011, 3, 879–887. [Google Scholar] [CrossRef]
- Martinez-Useros, J.; Garcia-Foncillas, J. The challenge of blocking a wider family members of EGFR against head and neck squamous cell carcinomas. Oral Oncol. 2015, 51, 423–430. [Google Scholar] [CrossRef]
- Zeng, F.; Singh, A.B.; Harris, R.C. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp. Cell Res. 2009, 315, 602–610. [Google Scholar] [CrossRef]
- Lautrette, A.; Li, S.; Alili, R.; Sunnarborg, S.W.; Burtin, M.; Lee, D.C.; Friedlander, G.; Terzi, F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: A new therapeutic approach. Nat. Med. 2005, 11, 867–874. [Google Scholar] [CrossRef]
- Terzi, F.; Burtin, M.; Hekmati, M.; Federici, P.; Grimber, G.; Briand, P.; Friedlander, G. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J. Clin. Investig. 2000, 106, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Flamant, M.; Bollée, G.; Hénique, C.; Tharaux, P.-L. Epidermal growth factor: A new therapeutic target in glomerular disease. Nephrol. Dial. Transplant. 2012, 27, 1297–1304. [Google Scholar] [CrossRef]
- Tang, J.; Liu, N.; Zhuang, S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int. 2013, 83, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Melderis, S.; Hagenstein, J.; Warkotsch, M.T.; Dang, J.; Herrnstadt, G.R.; Niehus, C.B.; Neumann, K.; Panzer, U.; Berasain, C.; Avila, M.A.; et al. Amphiregulin aggravates glomerulonephritis via recruitment and activation of myeloid cells. J. Am. Soc. Nephrol. 2020, 31, 1996–2012. [Google Scholar] [CrossRef]
- Rodrigues-Diez, R.R.; Garcia-Redondo, A.B.; Orejudo, M.; Rodrigues-Diez, R.; Briones, A.M.; Bosch-Panadero, E.; Kery, G.; Pato, J.; Ortiz, A.; Salaices, M.; et al. The C-terminal module IV of connective tissue growth factor, through EGFR/Nox1 signaling, activates the NF-κB pathway and proinflammatory factors in vascular smooth muscle cells. Antioxid. Redox Signal. 2015, 22, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.W.; Moeckel, G.W.; Lien, Y.H. Kidney-targeted liposome-mediated gene transfer in mice. Gene Ther. 1997, 4, 426–431. [Google Scholar] [CrossRef][Green Version]
- Lavoz, C.; Alique, M.; Rodrigues-Diez, R.; Pato, J.; Keri, G.; Mezzano, S.; Egido, J.; Ruiz-Ortega, M. Gremlin regulates renal inflammation via the vascular endothelial growth factor receptor 2 pathway. J. Pathol. 2015, 236, 407–420. [Google Scholar] [CrossRef]
- Sweeney, C.; Carraway, K.L. Ligand discrimination by ErbB receptors: Differential signaling through differential phosphorylation site usage. Oncogene 2000, 19, 5568–5573. [Google Scholar] [CrossRef]
- Crean, J.K.G.; Finlay, D.; Murphy, M.; Moss, C.; Godson, C.; Martin, F.; Brady, H.R. The role of p42/44 MAPK and protein kinase B in connective tissue growth factor induced extracellular matrix protein production, cell migration, and actin cytoskeletal rearrangement in human mesangial cells. J. Biol. Chem. 2002, 277, 44187–44194. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Rodriguez-Vita, J.; Cartier, C.; Rupérez, M.; Esteban, V.; Carvajal, G.; Rodrígues-Díez, R.; Plaza, J.J.; Egido, J.; Ruiz-Ortega, M. Inhibitory effect of interleukin-1beta on angiotensin II-induced connective tissue growth factor and type IV collagen production in cultured mesangial cells. Am. J. Physiol. Renal Physiol. 2008, 294, F149–F160. [Google Scholar] [CrossRef]
- Hu, X.; Wang, H.; Liu, J.; Fang, X.; Tao, K.; Wang, Y.; Li, N.; Shi, J.; Wang, Y.; Ji, P.; et al. The role of ERK and JNK signaling in connective tissue growth factor induced extracellular matrix protein production and scar formation. Arch. Dermatol. Res. 2013, 305, 433–445. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Meng, X.; Zhu, Z.; Yang, X.; Deng, A. Role of connective tissue growth factor in renal tubular epithelial-myofibroblast transdifferentiation and extracellular matrix accumulation in vitro. Life Sci. 2004, 75, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.S.; Paredes, A.; Dube, P.; Styer, E. Connective tissue growth factor expression in the rat remnant kidney model and association with tubular epithelial cells undergoing transdifferentiation. Vet. Pathol. 2000, 37, 328–335. [Google Scholar] [CrossRef]
- Rodrigues-Díez, R.; Carvajal-González, G.; Sánchez-López, E.; Rodríguez-Vita, J.; Rodrigues Díez, R.; Selgas, R.; Ortiz, A.; Egido, J.; Mezzano, S.; Ruiz-Ortega, M. Pharmacological modulation of epithelial mesenchymal transition caused by angiotensin II. Role of ROCK and MAPK pathways. Pharm. Res. 2008, 25, 2447–2461. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-C.; Liu, B.-C.; Zhang, X.-L.; Li, M.-X.; Zhang, J.-D. Role of ERK1/2 and PI3-K in the regulation of CTGF-induced ILK expression in HK-2 cells. Clin. Chim. Acta. 2007, 382, 89–94. [Google Scholar] [CrossRef]
- Ramazani, Y.; Knops, N.; Elmonem, M.A.; Nguyen, T.Q.; Arcolino, F.O.; van den Heuvel, L.; Levtchenko, E.; Kuypers, D.; Goldschmeding, R. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol. 2018, 68–69, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Kaasbøll, O.J.; Gadicherla, A.K.; Wang, J.-H.; Monsen, V.T.; Hagelin, E.M.V.; Dong, M.-Q.; Attramadal, H. Connective tissue growth factor (CCN2) is a matricellular preproprotein controlled by proteolytic activation. J. Biol. Chem. 2018, 293, 17953–17970. [Google Scholar] [CrossRef]
- Dean, R.A.; Butler, G.S.; Hamma-Kourbali, Y.; Delbé, J.; Brigstock, D.R.; Courty, J.; Overall, C.M. Identification of candidate angiogenic inhibitors processed by matrix metalloproteinase 2 (MMP-2) in cell-based proteomic screens: Disruption of vascular endothelial growth factor (VEGF)/heparin affin regulatory peptide (pleiotrophin) and VEGF/Connective. Mol. Cell. Biol. 2007, 27, 8454–8465. [Google Scholar] [CrossRef]
- Gerritsen, K.G.; Abrahams, A.C.; Peters, H.P.; Nguyen, T.Q.; Koeners, M.P.; den Hoedt, C.H.; Dendooven, A.; van den Dorpel, M.A.; Blankestijn, P.J.; Wetzels, J.F.; et al. Effect of GFR on plasma N-terminal connective tissue growth factor (CTGF) concentrations. Am. J. Kidney Dis. 2012, 59, 619–627. [Google Scholar] [CrossRef]
- Riser, B.L.; Cortes, P.; DeNichilo, M.; Deshmukh, P.V.; Chahal, P.S.; Mohammed, A.K.; Yee, J.; Kahkonen, D. Urinary CCN2 (CTGF) as a possible predictor of diabetic nephropathy: Preliminary report. Kidney Int. 2003, 64, 451–458. [Google Scholar] [CrossRef]
- Li, W.; Cui, M.; Wei, Y.; Kong, X.; Tang, L.; Xu, D. Inhibition of the expression of TGF-β1 and CTGF in human mesangial cells by exendin-4, a glucagon-like peptide-1 receptor agonist. Cell. Physiol. Biochem. 2012, 30, 749–757. [Google Scholar] [CrossRef]
- Mason, R.M. Fell-Muir lecture: Connective tissue growth factor (CCN2)—A pernicious and pleiotropic player in the development of kidney fibrosis. Int. J. Exp. Pathol. 2013, 94, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.G.; Schwartz, S.; Williams, M.E.; Arauz-Pacheco, C.; Bolton, W.K.; Lee, T.; Li, D.; Neff, T.B.; Urquilla, P.R.; Sewell, K.L. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin. J. Am. Soc. Nephrol. 2010, 5, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Higgins, B.; Kolinsky, K.; Smith, M.; Beck, G.; Rashed, M.; Adames, V.; Linn, M.; Wheeldon, E.; Gand, L.; Birnboeck, H.; et al. Antitumor activity of erlotinib (OSI-774, Tarceva) alone or in combination in human non-small cell lung cancer tumor xenograft models. Anti-Cancer Drugs 2004, 15, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Jonker, D.J.; O’Callaghan, C.J.; Karapetis, C.S.; Zalcberg, J.R.; Tu, D.; Au, H.-J.; Berry, S.R.; Krahn, M.; Price, T.; Simes, R.J.; et al. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 2007, 357, 2040–2048. [Google Scholar] [CrossRef]
- Li, D.; Ambrogio, L.; Shimamura, T.; Kubo, S.; Takahashi, M.; Chirieac, L.R.; Padera, R.F.; Shapiro, G.I.; Baum, A.; Himmelsbach, F.; et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008, 27, 4702–4711. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, M.; He, P.; Hidalgo, M.; Baker, S.D. Differential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymes. Clin. Cancer Res. 2007, 13, 3731–3737. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Thongprasert, S.; Yang, C.-H.; Chu, D.-T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef]
- Pirker, R.; Pereira, J.R.; Szczesna, A.; von Pawel, J.; Krzakowski, M.; Ramlau, R.; Vynnychenko, I.; Park, K.; Yu, C.-T.; Ganul, V.; et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): An open-label randomised phase III trial. Lancet 2009, 373, 1525–1531. [Google Scholar] [CrossRef]
- Ramos, A.M.; Fernández-Fernández, B.; Pérez-Gómez, M.V.; Carriazo Julio, S.M.; Sanchez-Niño, M.D.; Sanz, A.; Ruiz-Ortega, M.; Ortiz, A. Design and optimization strategies for the development of new drugs that treat chronic kidney disease. Expert Opin. Drug Discov. 2020, 15, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Thomson, S.C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Takagi, S.; Nitta, K.; Kitada, M.; Srivastava, S.P.; Takagaki, Y.; Kanasaki, K.; Koya, D. Renal protective effects of empagliflozin via inhibition of EMT and aberrant glycolysis in proximal tubules. JCI Insight 2020, 5, e129034. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayego-Mateos, S.; Morgado-Pascual, J.L.; Lavoz, C.; Rodrigues-Díez, R.R.; Márquez-Expósito, L.; Tejera-Muñoz, A.; Tejedor-Santamaría, L.; Rubio-Soto, I.; Marchant, V.; Ruiz-Ortega, M. CCN2 Binds to Tubular Epithelial Cells in the Kidney. Biomolecules 2022, 12, 252. https://doi.org/10.3390/biom12020252
Rayego-Mateos S, Morgado-Pascual JL, Lavoz C, Rodrigues-Díez RR, Márquez-Expósito L, Tejera-Muñoz A, Tejedor-Santamaría L, Rubio-Soto I, Marchant V, Ruiz-Ortega M. CCN2 Binds to Tubular Epithelial Cells in the Kidney. Biomolecules. 2022; 12(2):252. https://doi.org/10.3390/biom12020252
Chicago/Turabian StyleRayego-Mateos, Sandra, José Luis Morgado-Pascual, Carolina Lavoz, Raúl R. Rodrigues-Díez, Laura Márquez-Expósito, Antonio Tejera-Muñoz, Lucía Tejedor-Santamaría, Irene Rubio-Soto, Vanessa Marchant, and Marta Ruiz-Ortega. 2022. "CCN2 Binds to Tubular Epithelial Cells in the Kidney" Biomolecules 12, no. 2: 252. https://doi.org/10.3390/biom12020252
APA StyleRayego-Mateos, S., Morgado-Pascual, J. L., Lavoz, C., Rodrigues-Díez, R. R., Márquez-Expósito, L., Tejera-Muñoz, A., Tejedor-Santamaría, L., Rubio-Soto, I., Marchant, V., & Ruiz-Ortega, M. (2022). CCN2 Binds to Tubular Epithelial Cells in the Kidney. Biomolecules, 12(2), 252. https://doi.org/10.3390/biom12020252







