Notch Signal Mediates the Cross-Interaction between M2 Muscarinic Acetylcholine Receptor and Neuregulin/ErbB Pathway: Effects on Schwann Cell Proliferation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statements for Experiments Involving the Use of Animals
2.2. Schwann Cell Cultures
2.3. Drug Treatments
2.4. Cell Viability
2.5. Flow Cytometry Analysis
2.6. RT-PCR and qRT-PCR Analysis
- Nrg1 Forward 5′-CCATCACTCCACGACTGTC-3′
- Reverse 5′-GTGCCTGCTGTTCTCTACC-3′
- Nrg1/I Forward 5′-TCATCTTCGGCGAGATGTCTG-3′
- Reverse 5′-CTCCTGGCTTTCATTTCTTTCA-3′
- erbB2 Forward 5′-CGAGTGTCAGCCTCAAAACA-3′
- Reverse 5′-CTCATCCGGGTACTTCCAGA-3′
- erbB3 Forward 5′-CTGTTTAGGCCAAGCAGAGG-3′
- Reverse 5′-GACTTTGTTTGCCTTCTCGC-3′
- Gapdh Forward 5′-GTGCCAGCCTCGTCTCATAG-3′
- Reverse 5′-TGATGGCAACAATGTCCACT-3′
2.7. Protein Extraction and Western Blot
2.8. Immunocytochemistry
2.9. Cell Infection with Adenovirus Expressing Notch-NICD
2.10. Data Analysis
3. Results
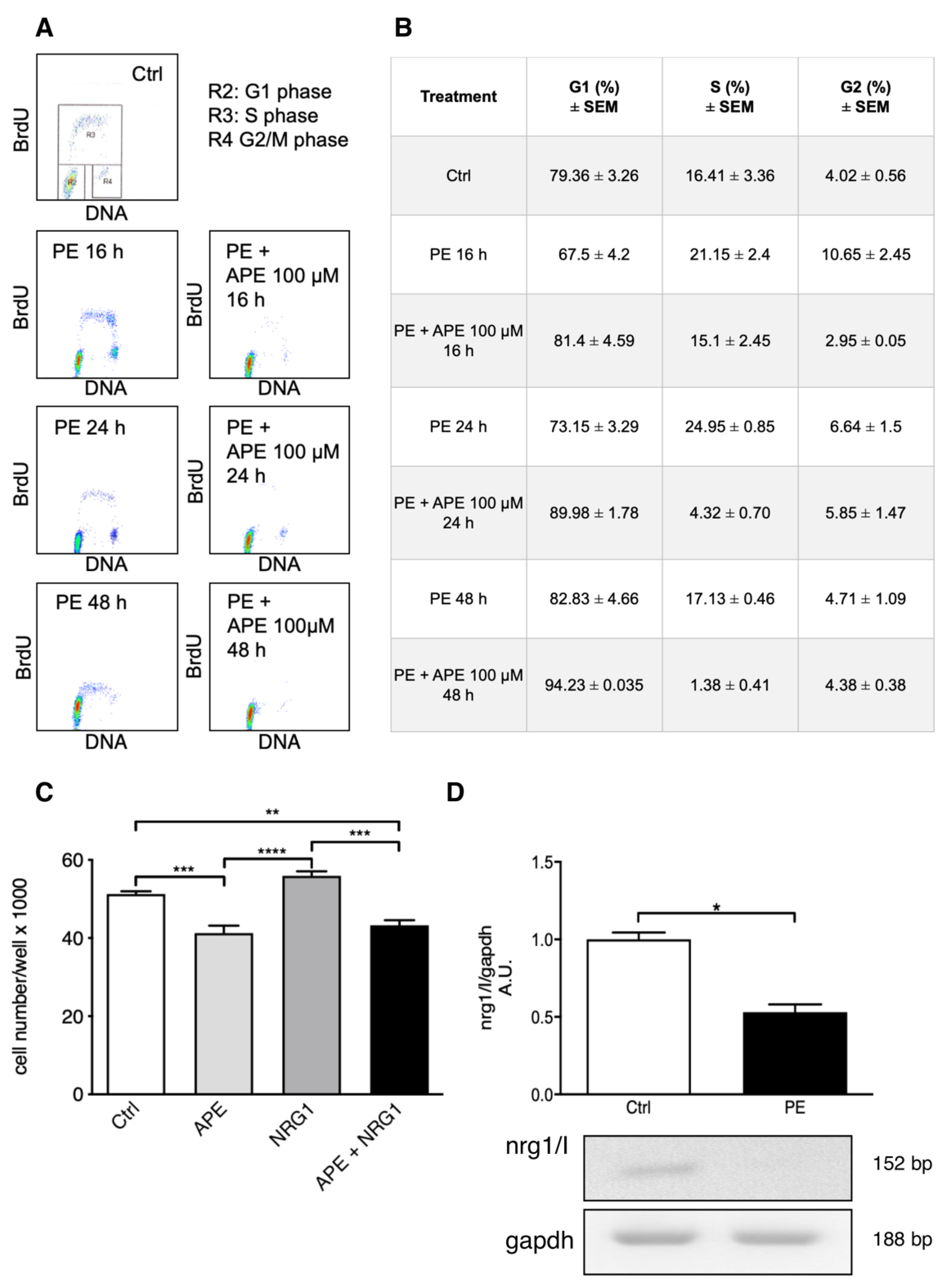
3.1. M2 Receptor Stimulation Counteracts SC Proliferation Mediated by PE/NRG1
3.2. M2 Receptor Activation Downregulates NRG1 Expression
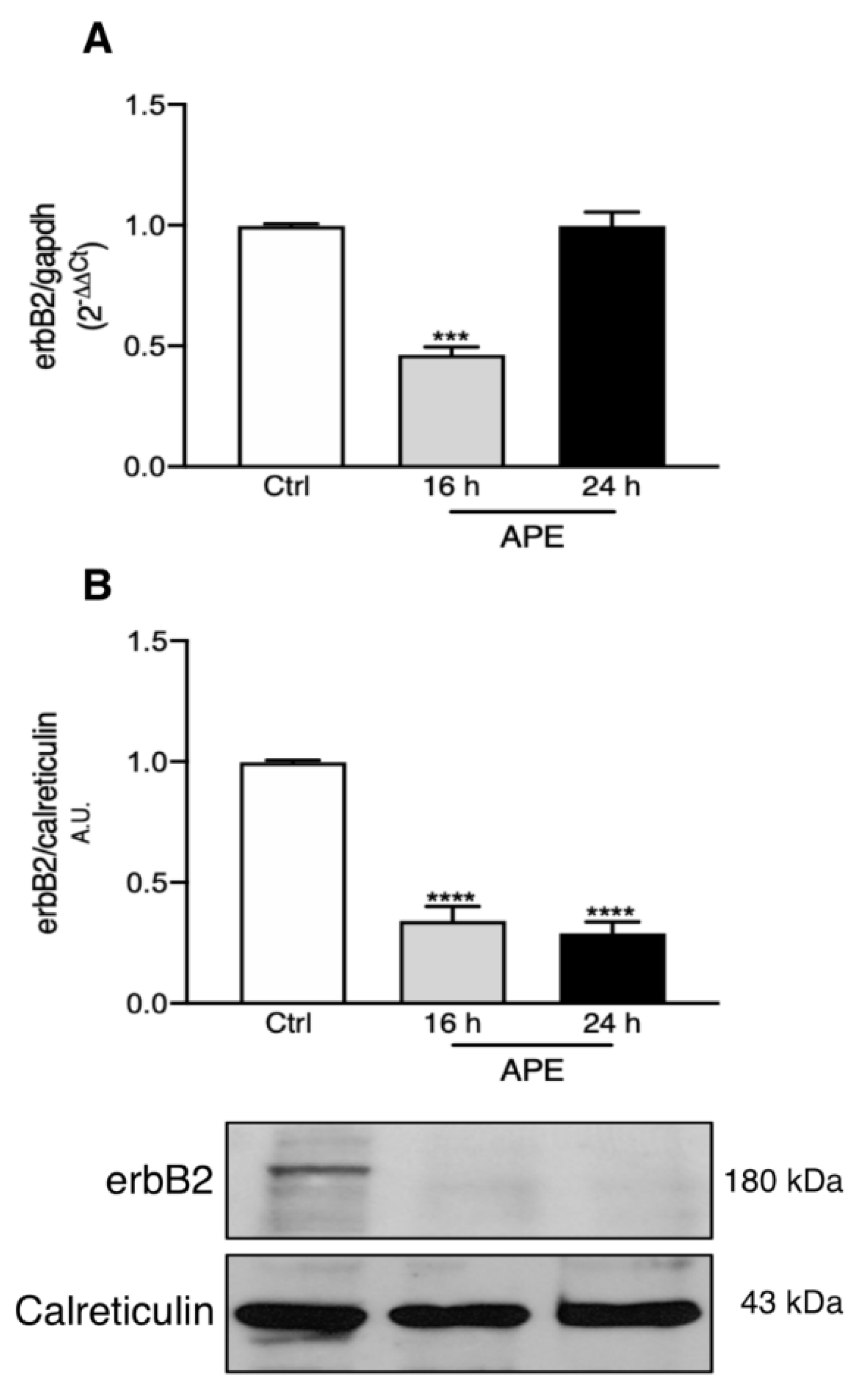
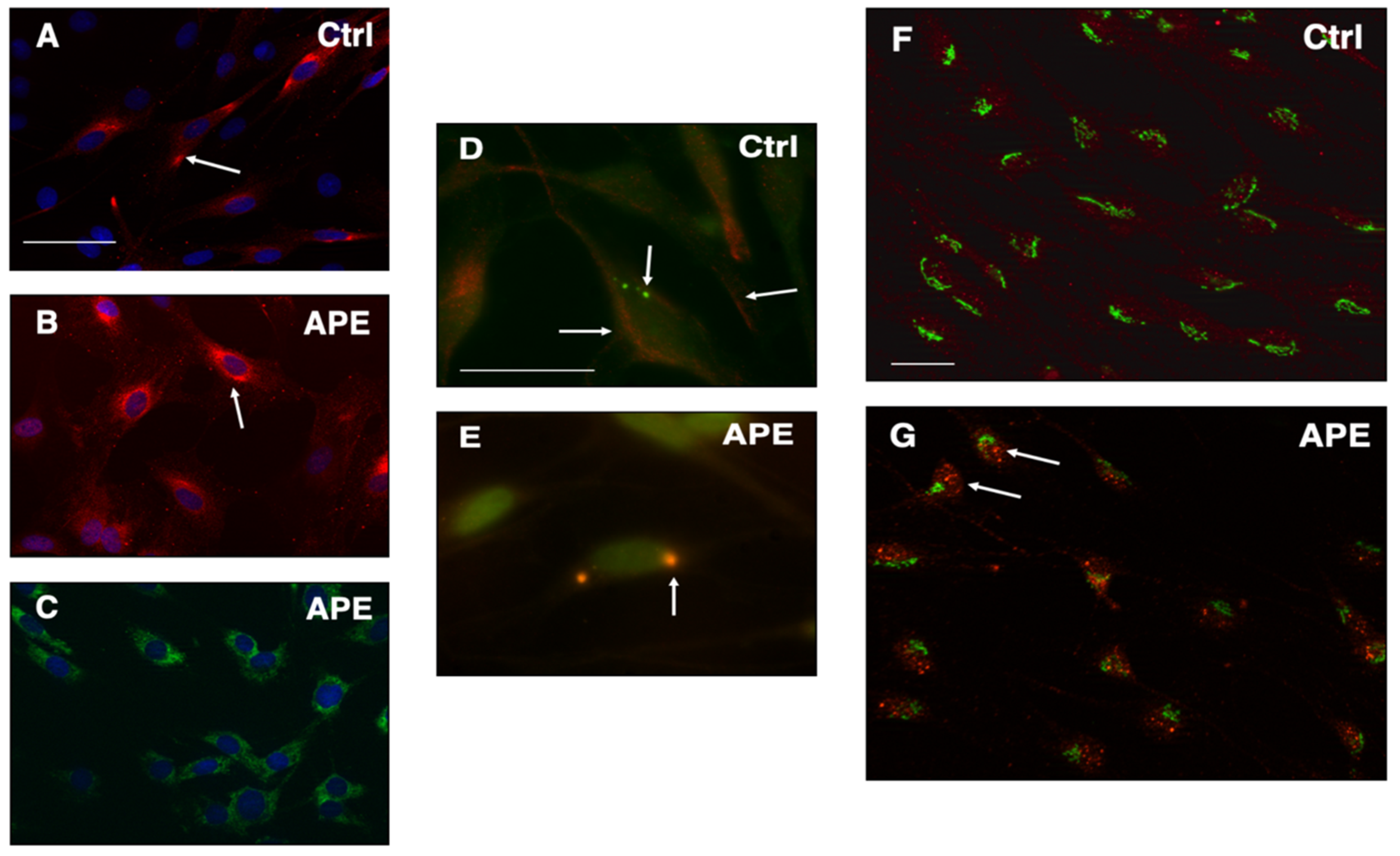
3.3. M2 Stimulation Alters erbB2 Receptor Expression and Distribution
3.4. M2 Receptor Modulates erbB2 Expression via Notch-1 Pathway
3.5. M2 Receptor Activation Negatively Controls the Expression of erbB3 Receptor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Verkhratsky, A.; Butt, A. Glial Physiology and Pathophysiology; John Wiley & Sons, Ltd.: Chichester, UK, 2013; ISBN 9781118402061. [Google Scholar]
- Wang, F.; Yuan, T.; Pereira, A.; Verkhratsky, A.; Huang, J.H. Glial Cells and Synaptic Plasticity. Neural Plast. 2016, 2016, 5042902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, B.; Fields, R.D. Response of Schwann Cells to Action Potentials in Development. Science 2000, 287, 2267–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taveggia, C. Schwann cells–axon interaction in myelination. Curr. Opin. Neurobiol. 2016, 39, 24–29. [Google Scholar] [CrossRef]
- Falls, D.L. Neuregulins: Functions, forms, and signaling strategies. Exp. Cell Res. 2003, 284, 14–30. [Google Scholar] [CrossRef]
- Newbern, J.; Birchmeier, C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin. Cell Dev. Biol. 2010, 21, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, A. Neuregulin-1 Type I: A Hidden Power Within Schwann Cells for Triggering Peripheral Nerve Remyelination. Sci. Signal. 2013, 6, jc1. [Google Scholar] [CrossRef] [PubMed]
- Taveggia, C.; Zanazzi, G.; Petrylak, A.; Yano, H.; Rosenbluth, J.; Einheber, S.; Xu, X.; Esper, R.M.; Loeb, J.A.; Shrager, P.; et al. Neuregulin-1 Type III Determines the Ensheathment Fate of Axons. Neuron 2005, 47, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Velardez, M.O.; Warot, X.; Yu, Z.-X.; Miller, S.J.; Cros, D.; Corfas, G. Neuregulin 1-erbB Signaling Is Necessary for Normal Myelination and Sensory Function. J. Neurosci. 2006, 26, 3079–3086. [Google Scholar] [CrossRef]
- Stassart, R.M.; Nawaz, S.; Humml, C.; Velanac, V.; Radyuschkin, K.; Goebbels, S.; Nave, A. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 2009, 59, 581–595. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.; Rigo, J.-M.; Rocher, V.; Belachew, S.; Malgrange, B.; Rogister, B.; Leprince, P.; Moonen, G. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001, 305, 187–202. [Google Scholar] [CrossRef]
- Tata, A.M.; Cursi, S.; Biagioni, S.; Augusti-Tocco, G. Cholinergic modulation of neurofilament expression and neurite outgrowth in chick sensory neurons. J. Neurosci. Res. 2003, 73, 227–234. [Google Scholar] [CrossRef]
- Bernardini, N.; Tomassy, G.S.; Tata, A.M.; Augusti-Tocco, G.; Biagioni, S. Detection of basal and potassium-evoked acetylcholine release from embryonic DRG explants. J. Neurochem. 2004, 88, 1533–1539. [Google Scholar] [CrossRef]
- Magnaghi, V.; Procacci, P.; Tata, A.M. Chapter 15 Novel Pharmacological Approaches to Schwann Cells as Neuroprotective Agents for Peripheral Nerve Regeneration, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 87, ISBN 0074-7742. [Google Scholar]
- Coronas, V.; Durand, M.; Chabot, J.; Jourdan, F.; Quirion, R. Acetylcholine induces neuritic outgrowth in rat primary olfactory bulb cultures. Neuroscience 2000, 98, 213–219. [Google Scholar] [CrossRef]
- Loreti, S.; Vilaró, M.T.; Visentin, S.; Rees, H.; Levey, A.I.; Tata, A.M. Rat Schwann cells express M1–M4 muscarinic receptor subtypes. J. Neurosci. Res. 2006, 84, 97–105. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Bernardo, A.; Magnaghi, V.; Minghetti, L.; Tata, A.M. Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev. Neurobiol. 2011, 72, 713–728. [Google Scholar] [CrossRef]
- Ragheb, F.; Molina-Holgado, E.; Cui, Q.-L.; Khorchid, A.; Liu, H.-N.; LaRocca, J.N.; Almazan, G. Pharmacological and functional characterization of muscarinic receptor subtypes in developing oligodendrocytes. J. Neurochem. 2001, 77, 1396–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Zee, E.A.; de Jong, G.I.; Strosberg, A.D.; Luiten, P.G.M. Muscarinic acetylcholine receptor-expression in astrocytes in the cortex of young and aged rats. Glia 1993, 8, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Loreti, S.; Ricordy, R.; De Stefano, M.E.; Augusti-Tocco, G.; Tata, A.M. Acetylcholine inhibits cell cycle progression in rat Schwann cells by activation of the M2 receptor subtype. Neuron Glia Biol. 2007, 3, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Piovesana, R.; Faroni, A.; Taggi, M.; Matera, A.; Soligo, M.; Canipari, R.; Manni, L.; Reid, A.J.; Tata, A.M. Muscarinic receptors modulate Nerve Growth Factor production in rat Schwann-like adipose-derived stem cells and in Schwann cells. Sci. Rep. 2020, 10, 7159. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Piovesana, R.; Faroni, A.; Tata, A.M. Schwann-like adipose-derived stem cells as a promising therapeutic tool for peripheral nerve regeneration: Effects of cholinergic stimulation. Neural Regen. Res. 2021, 16, 1218–1220. [Google Scholar] [CrossRef]
- Piovesana, R.; Faroni, A.; Tata, A.M.; Reid, A.J. Functional Characterization of Muscarinic Receptors in Human Schwann Cells. Int. J. Mol. Sci. 2020, 21, 6666. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.B.; Stroobant, P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J. Cell Biol. 1990, 110, 1353–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piovesana, R.; Melfi, S.; Fiore, M.; Magnaghi, V.; Tata, A.M. M2 muscarinic receptor activation inhibits cell proliferation and migration of rat adipose-mesenchymal stem cells. J. Cell. Physiol. 2018, 233, 5348–5360. [Google Scholar] [CrossRef] [Green Version]
- Alessandrini, F.; Cristofaro, I.; Di Bari, M.; Zasso, J.; Conti, L.; Tata, A.M. The activation of M2 muscarinic receptor inhibits cell growth and survival in human glioblastoma cancer stem cells. Int. Immunopharmacol. 2015, 29, 105–109. [Google Scholar] [CrossRef]
- Pacini, L.; De Falco, E.; Di Bari, M.; Coccia, A.; Siciliano, C.; Ponti, D.; Pastore, A.L.; Petrozza, V.; Carbone, A.; Tata, A.M.; et al. M2muscarinic receptors inhibit cell proliferation and migration in urothelial bladder cancer cells. Cancer Biol. Ther. 2014, 15, 1489–1498. [Google Scholar] [CrossRef] [Green Version]
- Piovesana, R.; Faroni, A.; Magnaghi, V.; Reid, A.J.; Tata, A.M. M2 receptors activation modulates cell growth, migration and differentiation of rat Schwann-like adipose-derived stem cells. Cell Death Discov. 2019, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Uggenti, C.; De Stefano, M.E.; Costantino, M.; Loreti, S.; Pisano, A.; Avallone, B.; Talora, C.; Magnaghi, V.; Tata, A.M. M2 muscarinic receptor activation regulates schwann cell differentiation and myelin organization. Dev. Neurobiol. 2013, 74, 676–691. [Google Scholar] [CrossRef]
- Rangarajan, A.; Talora, C.; Okuyama, R.; Nicolas, M.; Mammucari, C.; Oh, H.; Aster, J.C.; Krishna, S.; Metzger, D.; Chambon, P.; et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001, 20, 3427–3436. [Google Scholar] [CrossRef] [Green Version]
- Birchmeier, C.; Bennett, D.L. Neuregulin/ErbB Signaling in Developmental Myelin Formation and Nerve Repair. Curr. Top. Dev. Biol. 2016, 116, 45–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodhoo, A.; Alonso, M.B.D.; Droggiti, A.; Turmaine, M.; D’Antonio, M.; Parkinson, D.B.; Wilton, D.K.; Al-Shawi, R.; Simons, P.; Shen, J.; et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat. Neurosci. 2009, 12, 839–847. [Google Scholar] [CrossRef] [Green Version]
- Fields, R.D. Release of neurotransmitters from glia. Neuron Glia Biol. 2010, 6, 137–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, R.D.; Dutta, D.J.; Belgrad, J.; Robnett, M. Cholinergic signaling in myelination. Glia 2017, 65, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, V.; Mozzetta, C.; Biagioni, S.; Tocco, G.A.; Tata, A. The mechanisms and possible sites of acetylcholine release during chick primary sensory neuron differentiation. Life Sci. 2012, 91, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Boerboom, A.; Dion, V.; Chariot, A.; Franzen, R. Molecular Mechanisms Involved in Schwann Cell Plasticity. Front. Mol. Neurosci. 2017, 10, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stassart, R.M.; Fledrich, R.; Velanac, V.; Brinkmann, B.G.; Schwab, M.H.; Meijer, D.; Sereda, M.W.; Nave, K.-A. A role for Schwann cell–derived neuregulin-1 in remyelination. Nat. Neurosci. 2012, 16, 48–54. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Control of Schwann cell myelination. F1000 Biol. Rep. 2010, 2, 19. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piovesana, R.; Pisano, A.; Loreti, S.; Ricordy, R.; Talora, C.; Tata, A.M. Notch Signal Mediates the Cross-Interaction between M2 Muscarinic Acetylcholine Receptor and Neuregulin/ErbB Pathway: Effects on Schwann Cell Proliferation. Biomolecules 2022, 12, 239. https://doi.org/10.3390/biom12020239
Piovesana R, Pisano A, Loreti S, Ricordy R, Talora C, Tata AM. Notch Signal Mediates the Cross-Interaction between M2 Muscarinic Acetylcholine Receptor and Neuregulin/ErbB Pathway: Effects on Schwann Cell Proliferation. Biomolecules. 2022; 12(2):239. https://doi.org/10.3390/biom12020239
Chicago/Turabian StylePiovesana, Roberta, Annalinda Pisano, Simona Loreti, Ruggero Ricordy, Claudio Talora, and Ada Maria Tata. 2022. "Notch Signal Mediates the Cross-Interaction between M2 Muscarinic Acetylcholine Receptor and Neuregulin/ErbB Pathway: Effects on Schwann Cell Proliferation" Biomolecules 12, no. 2: 239. https://doi.org/10.3390/biom12020239
APA StylePiovesana, R., Pisano, A., Loreti, S., Ricordy, R., Talora, C., & Tata, A. M. (2022). Notch Signal Mediates the Cross-Interaction between M2 Muscarinic Acetylcholine Receptor and Neuregulin/ErbB Pathway: Effects on Schwann Cell Proliferation. Biomolecules, 12(2), 239. https://doi.org/10.3390/biom12020239






