Protonation State of an Important Histidine from High Resolution Structures of Lytic Polysaccharide Monooxygenases
Abstract
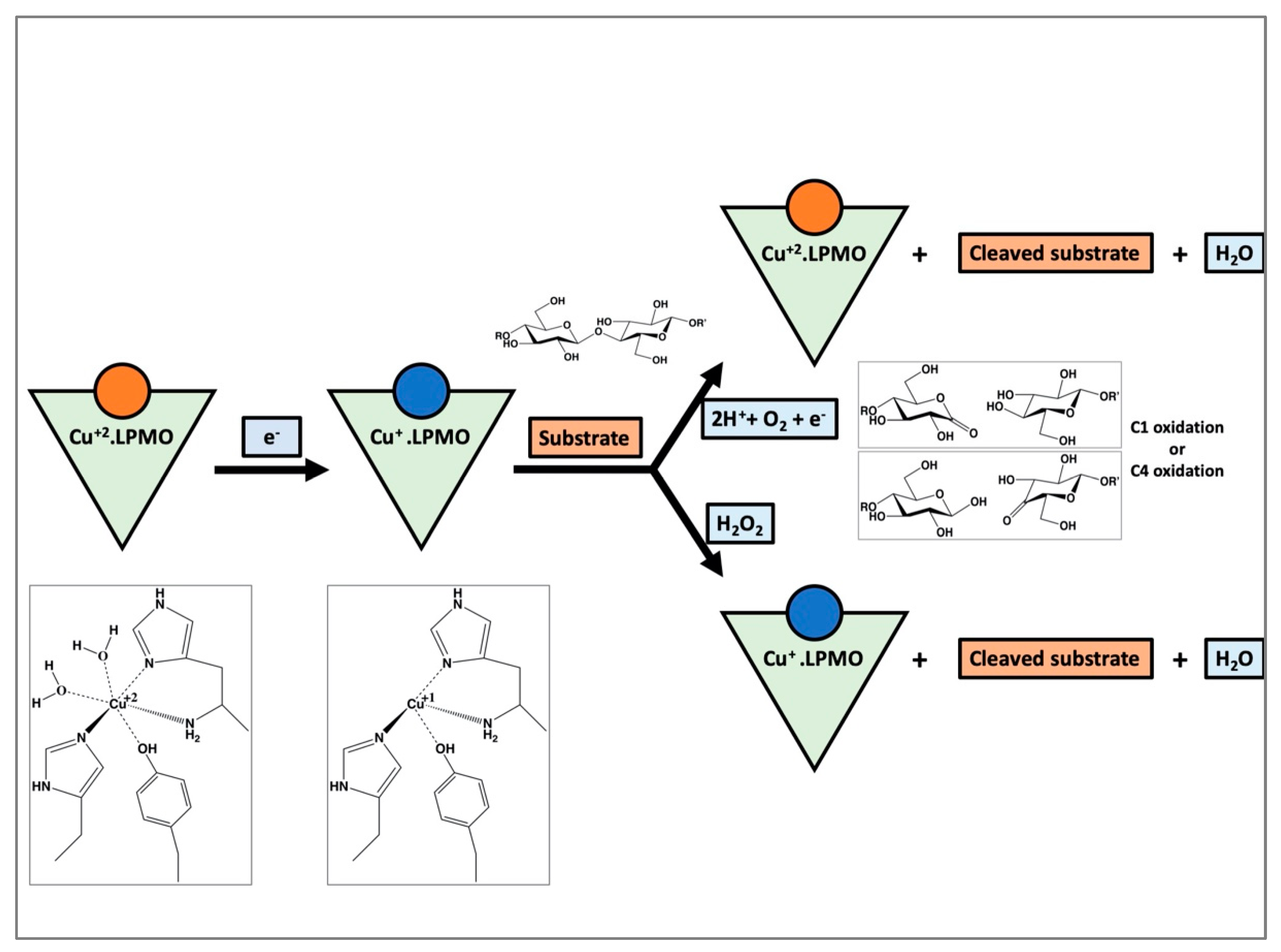
:1. Introduction
2. Materials and Methods
2.1. Crystallization
2.2. X-ray Data Collection and Structure Determination
2.3. Re-Refinement of Other Structures from PDB
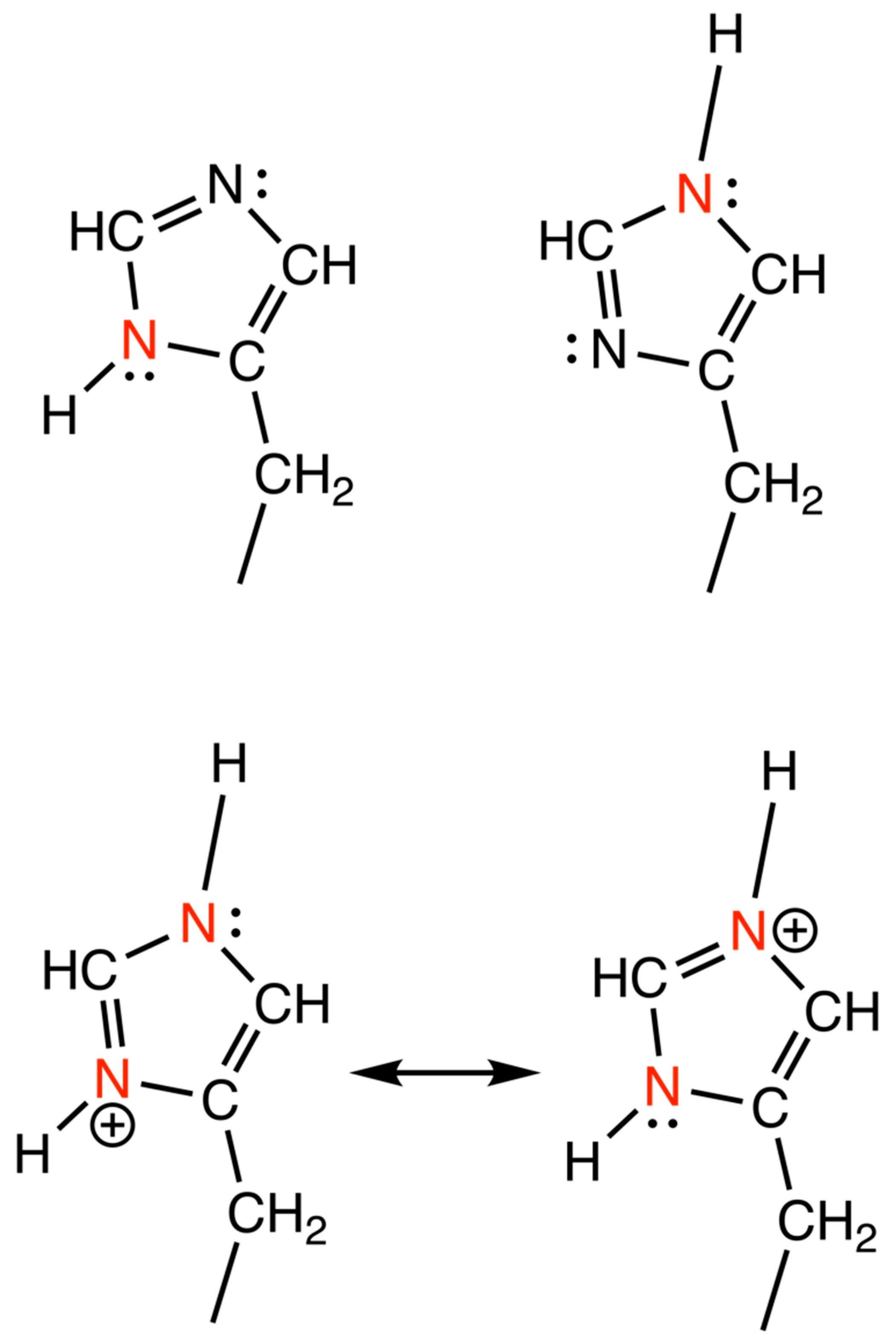
2.4. Determination of His Protonation
2.5. Refinement to Investigate Protonation
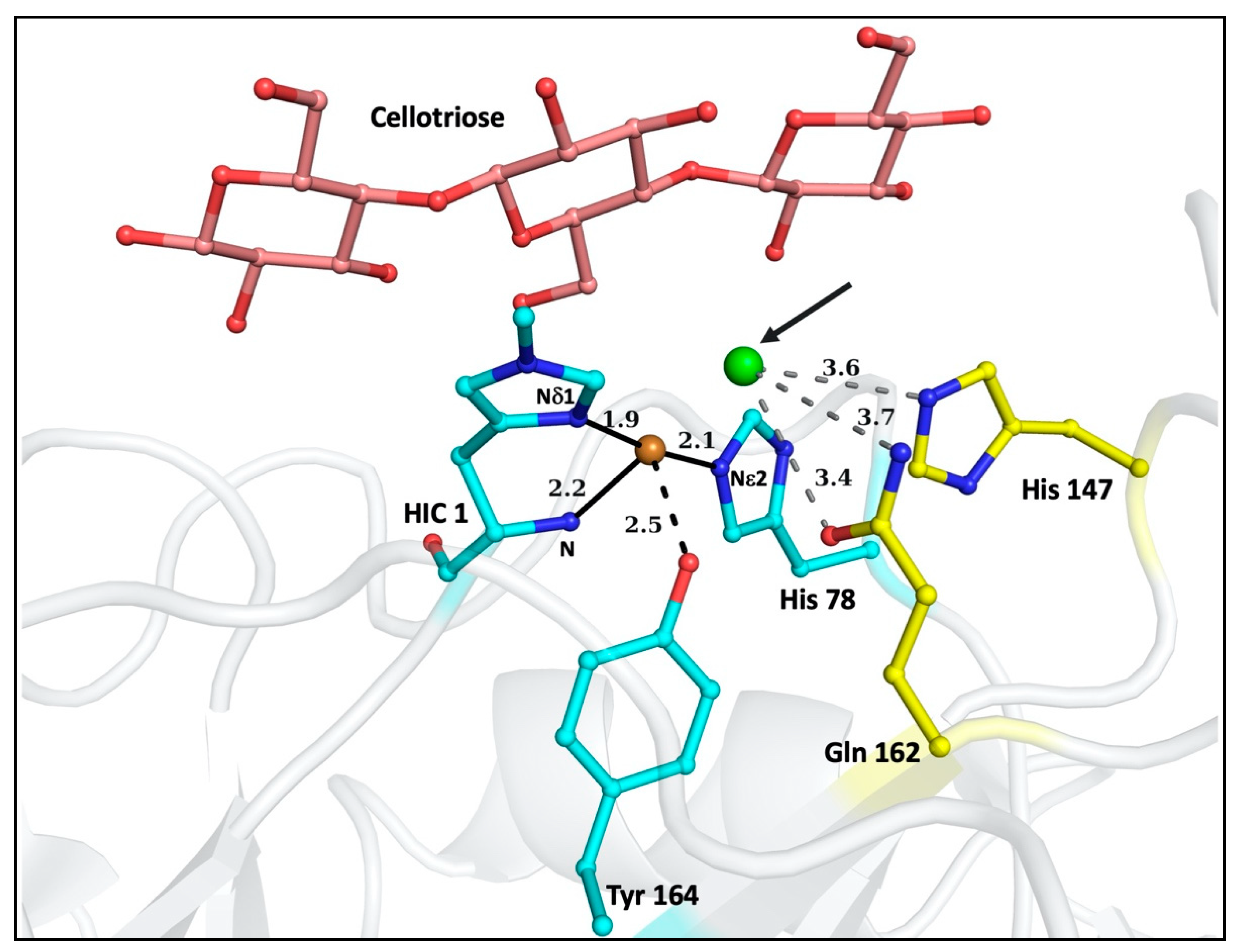
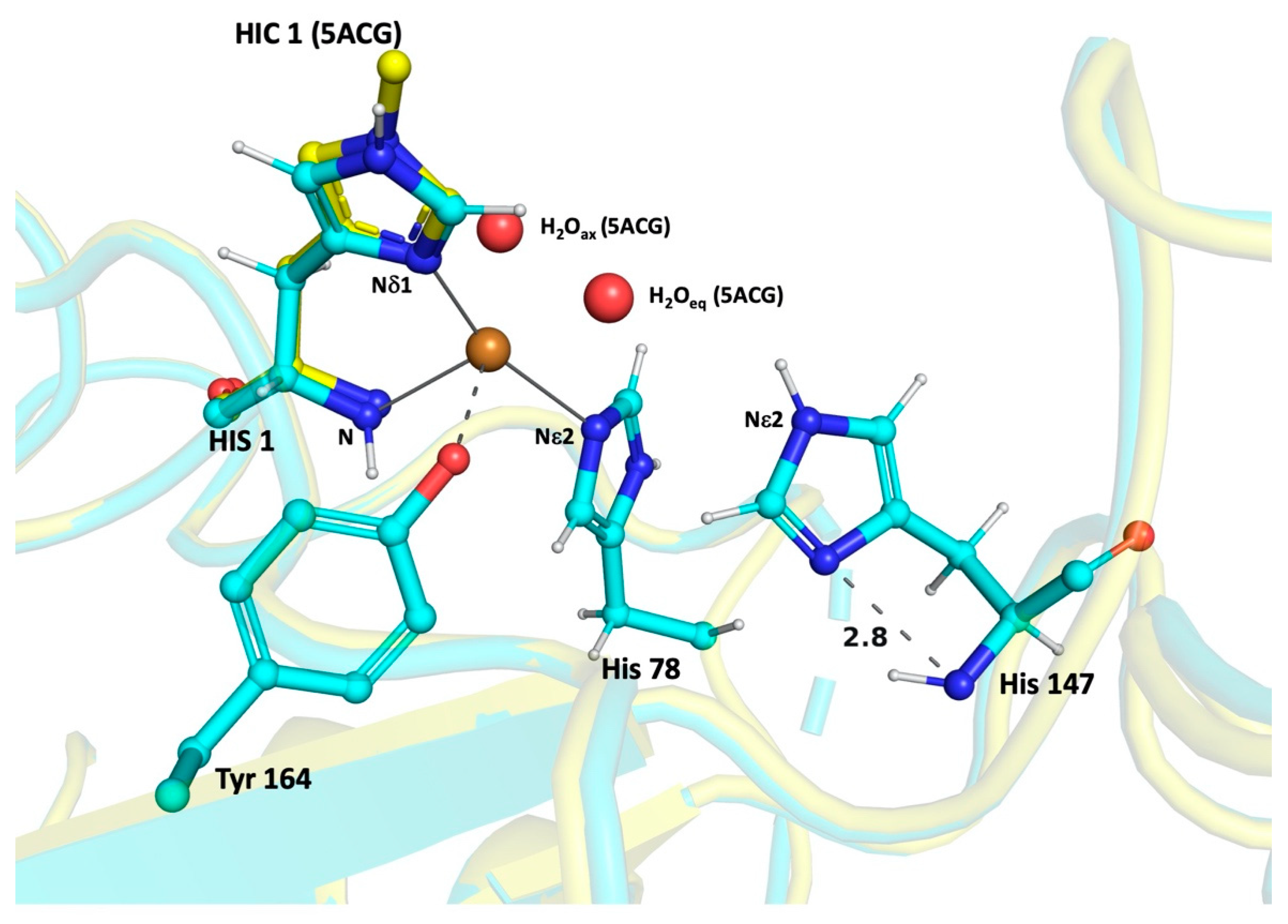
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horn, S.; Vaaje-Kolstad, G.; Westereng, B.; Eijsink, V.G. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 2012, 5, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Leggio, L.; Simmons, T.J.; Poulsen, J.-C.N.; Frandsen, K.E.H.; Hemsworth, G.R.; Stringer, M.A.; von Freiesleben, P.; Tovborg, M.; Johansen, K.S.; De Maria, L.; et al. Structure and boosting activity of a starch-degrading lytic polysaccharide monooxygenase. Nat. Commun. 2015, 6, 5961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.V.; Welner, D.; McFarland, K.C.; Re, E.; Navarro Poulsen, J.-C.; Brown, K.; Salbo, R.; Ding, H.; Vlasenko, E.; Merino, S.; et al. Stimulation of Lignocellulosic Biomass Hydrolysis by Proteins of Glycoside Hydrolase Family 61: Structure and Function of a Large, Enigmatic Family. Biochemistry 2010, 49, 3305–3316. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Várnai, A.; Johansen, K.S.; Eijsink, V.G.H.; Horn, S.J. Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions. Biotechnol. Biofuels 2015, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Johansen, K.S. Discovery and industrial applications of lytic polysaccharide mono-oxygenases. Biochem. Soc. Trans. 2016, 44, 143–149. [Google Scholar] [CrossRef]
- Shah, F.; Nicolás, C.; Bentzer, J.; Ellström, M.; Smits, M.; Rineau, F.; Canbäck, B.; Floudas, D.; Carleer, R.; Lackner, G.; et al. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors. New Phytol. 2016, 209, 1705–1719. [Google Scholar] [CrossRef]
- Blanco-Ulate, B.; Morales-Cruz, A.; Amrine, K.C.H.; Labavitch, J.M.; Powell, A.L.T.; Cantu, D. Genome-wide transcriptional profiling of Botrytis cinerea genes targeting plant cell walls during infections of different hosts. Front. Plant Sci. 2014, 5, 435. [Google Scholar] [CrossRef] [Green Version]
- Askarian, F.; Uchiyama, S.; Masson, H.; Sørensen, H.V.; Golten, O.; Bunæs, A.C.; Mekasha, S.; Røhr, Å.K.; Kommedal, E.; Ludviksen, J.A.; et al. The lytic polysaccharide monooxygenase CbpD promotes Pseudomonas aeruginosa virulence in systemic infection. Nat. Commun. 2021, 12, 1230. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandrup, T.; Frandsen, K.E.H.; Johansen, K.S.; Berrin, J.-G.; Lo Leggio, L. Recent insights into lytic polysaccharide monooxygenases (LPMOs). Biochem. Soc. Trans. 2018, 46, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Vu, V.V.; Ngo, S.T. Copper active site in polysaccharide monooxygenases. Coord. Chem. Rev. 2018, 368, 134–157. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Davies, G.J.; Walton, P.H.; Rovira, C. Activation of O2 and H2O2 by Lytic Polysaccharide Monooxygenases. ACS Catal. 2020, 10, 12760–12769. [Google Scholar] [CrossRef]
- Eijsink, V.G.H.; Petrovic, D.; Forsberg, Z.; Mekasha, S.; Røhr, Å.K.; Várnai, A.; Bissaro, B.; Vaaje-Kolstad, G. On the functional characterization of lytic polysaccharide monooxygenases (LPMOs). Biotechnol. Biofuels 2019, 12, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, R.J.; Sweeney, M.D.; Lo Leggio, L.; Otten, H.; Poulsen, J.-C.N.; Johansen, K.S.; Krogh, K.B.R.M.; Jorgensen, C.I.; Tovborg, M.; Anthonsen, A.; et al. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci. USA 2011, 108, 15079–15084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbadin, F.; Urresti, S.; Henrissat, B.; Avrova, A.O.; Welsh, L.R.J.; Lindley, P.J.; Csukai, M.; Squires, J.N.; Walton, P.H.; Davies, G.J.; et al. Secreted pectin monooxygenases drive plant infection by pathogenic oomycetes. Science 2021, 373, 774–779. [Google Scholar] [CrossRef]
- Frandsen, K.E.H.; Simmons, T.J.; Dupree, P.; Poulsen, J.-C.N.; Hemsworth, G.R.; Ciano, L.; Johnston, E.M.; Tovborg, M.; Johansen, K.S.; von Freiesleben, P.; et al. The molecular basis of polysaccharide cleavage by lytic polysaccharide monooxygenases. Nat. Chem. Biol. 2016, 12, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Frandsen, K.E.H.; Lo Leggio, L. Lytic polysaccharide monooxygenases: A crystallographer’s view on a new class of biomass-degrading enzymes. IUCrJ 2016, 3, 448–467. [Google Scholar] [CrossRef] [Green Version]
- Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sørlie, M.; Eijsink, V.G.H. An Oxidative Enzyme Boosting the Enzymatic Conversion of Recalcitrant Polysaccharides. Science 2010, 330, 219–222. [Google Scholar] [CrossRef]
- Langston, J.A.; Shaghasi, T.; Abbate, E.; Xu, F.; Vlasenko, E.; Sweeney, M.D. Oxidoreductive Cellulose Depolymerization by the Enzymes Cellobiose Dehydrogenase and Glycoside Hydrolase 61. Appl. Environ. Microbiol. 2011, 77, 7007–7015. [Google Scholar] [CrossRef] [Green Version]
- Fushinobu, S. A new face for biomass breakdown. Nat. Chem. Biol. 2014, 10, 88–89. [Google Scholar] [CrossRef]
- Westereng, B.; Cannella, D.; Wittrup Agger, J.; Jørgensen, H.; Larsen Andersen, M.; Eijsink, V.G.H.; Felby, C. Enzymatic cellulose oxidation is linked to lignin by long-range electron transfer. Sci. Rep. 2015, 5, 18561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kracher, D.; Andlar, M.; Furtmüller, P.G.; Ludwig, R. Active-site copper reduction promotes substrate binding of fungal lytic polysaccharide monooxygenase and reduces stability. J. Biol. Chem. 2018, 293, 1676–1687. [Google Scholar] [CrossRef] [Green Version]
- Hangasky, J.A.; Iavarone, A.T.; Marletta, M.A. Reactivity of O2 versus H2O2 with polysaccharide monooxygenases. Proc. Natl. Acad. Sci. USA 2018, 115, 4915–4920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissaro, B.; Røhr, Å.K.; Müller, G.; Chylenski, P.; Skaugen, M.; Forsberg, Z.; Horn, S.J.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat. Chem. Biol. 2017, 13, 1123–1128. [Google Scholar] [CrossRef]
- Hedegård, E.D.; Ryde, U. Molecular mechanism of lytic polysaccharide monooxygenases. Chem. Sci. 2018, 9, 3866–3880. [Google Scholar] [CrossRef] [Green Version]
- Brander, S.; Tokin, R.; Ipsen, J.Ø.; Jensen, P.E.; Hernández-Rollán, C.; Nørholm, M.H.H.; Lo Leggio, L.; Dupree, P.; Johansen, K.S. Scission of Glucosidic Bonds by a Lentinus similis Lytic Polysaccharide Monooxygenases Is Strictly Dependent on H2O2 while the Oxidation of Saccharide Products Depends on O2. ACS Catal. 2021, 11, 13848–13859. [Google Scholar] [CrossRef]
- Shook, R.L.; Borovik, A.S. Role of the secondary coordination sphere in metal-mediated dioxygen activation. Inorg. Chem. 2010, 49, 3646–3660. [Google Scholar] [CrossRef] [Green Version]
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and Functional Aspects of Metal Sites in Biology. Chem. Rev. 1996, 96, 2239–2314. [Google Scholar] [CrossRef] [PubMed]
- Span, E.A.; Suess, D.L.M.; Deller, M.C.; Britt, R.D.; Marletta, M.A. The Role of the Secondary Coordination Sphere in a Fungal Polysaccharide Monooxygenase. ACS Chem. Biol. 2017, 12, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- O’Dell, W.B.; Agarwal, P.K.; Meilleur, F. Oxygen Activation at the Active Site of a Fungal Lytic Polysaccharide Monooxygenase. Angew. Chem. Int. Ed. 2017, 56, 767–770. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Walton, P.H.; Rovira, C. Molecular Mechanisms of Oxygen Activation and Hydrogen Peroxide Formation in Lytic Polysaccharide Monooxygenases. ACS Catal. 2019, 9, 4958–4969. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Beckham, G.T.; Larsson, A.M.; Ishida, T.; Kim, S.; Payne, C.M.; Himmel, M.E.; Crowley, M.F.; Horn, S.J.; Westereng, B.; et al. Crystal Structure and Computational Characterization of the Lytic Polysaccharide Monooxygenase GH61D from the Basidiomycota Fungus Phanerochaete chrysosporium. J. Biol. Chem. 2013, 288, 12828–12839. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Beeson, W.T.; Phillips, C.M.; Marletta, M.A.; Cate, J.H.D. Structural Basis for Substrate Targeting and Catalysis by Fungal Polysaccharide Monooxygenases. Structure 2012, 20, 1051–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frericks Schmidt, H.L.; Shah, G.J.; Sperling, L.J.; Rienstra, C.M. NMR Determination of Protein p K a Values in the Solid State. J. Phys. Chem. Lett. 2010, 1, 1623–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, A.L.; Kay, L.E. Measurement of histidine pKa values and tautomer populations in invisible protein states. Proc. Natl. Acad. Sci. USA 2014, 111, E1705–E1712. [Google Scholar] [CrossRef] [Green Version]
- Wallerstein, J.; Weininger, U.; Khan, M.A.I.; Linse, S.; Akke, M. Site-Specific Protonation Kinetics of Acidic Side Chains in Proteins Determined by pH-Dependent Carboxyl 13 C NMR Relaxation. J. Am. Chem. Soc. 2015, 137, 3093–3101. [Google Scholar] [CrossRef] [PubMed]
- Kneller, D.W.; Phillips, G.; Weiss, K.L.; Pant, S.; Zhang, Q.; O’Neill, H.M.; Coates, L.; Kovalevsky, A. Protonation states in SARS-CoV-2 main protease mapped by neutron crystallography. bioRxiv 2020. bioRxiv: 2020.09.22.308668. [Google Scholar] [CrossRef]
- Wan, Q.; Parks, J.M.; Hanson, B.L.; Fisher, S.Z.; Ostermann, A.; Schrader, T.E.; Graham, D.E.; Coates, L.; Langan, P.; Kovalevsky, A. Direct determination of protonation states and visualization of hydrogen bonding in a glycoside hydrolase with neutron crystallography. Proc. Natl. Acad. Sci. USA 2015, 112, 12384–12389. [Google Scholar] [CrossRef] [Green Version]
- Zaccai, N.R.; Coquelle, N. Opportunities and challenges in neutron crystallography. EPJ Web Conf. 2020, 236, 02001. [Google Scholar] [CrossRef]
- Aachmann, F.L.; Sorlie, M.; Skjak-Braek, G.; Eijsink, V.G.H.; Vaaje-Kolstad, G. NMR structure of a lytic polysaccharide monooxygenase provides insight into copper binding, protein dynamics, and substrate interactions. Proc. Natl. Acad. Sci. USA 2012, 109, 18779–18784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madland, E.; Forsberg, Z.; Wang, Y.; Lindorff-Larsen, K.; Niebisch, A.; Modregger, J.; Eijsink, V.G.; Aachmann, F.L.; Courtade, G. NMR structures and functional roles of two related chitin-binding domains of a lytic polysaccharide monooxygenase from Cellvibrio japonicus. BioRxiv 2021. [Google Scholar] [CrossRef]
- Malinska, M.; Dauter, M.; Kowiel, M.; Jaskolski, M.; Dauter, Z. Protonation and geometry of histidine rings. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 1444–1454. [Google Scholar] [CrossRef]
- Forsberg, Z.; Bissaro, B.; Gullesen, J.; Dalhus, B.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Structural determinants of bacterial lytic polysaccharide monooxygenase functionality. J. Biol. Chem. 2018, 293, 1397–1412. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Rollán, C.; Falkenberg, K.B.; Rennig, M.; Bertelsen, A.B.; Ipsen, J.Ø.; Brander, S.; Daley, D.O.; Johansen, K.S.; Nørholm, M.H.H. LyGo: A Platform for Rapid Screening of Lytic Polysaccharide Monooxygenase Production. ACS Synth. Biol. 2021, 10, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Blossom, B.M.; Russo, D.A.; van Oort, B.; Croce, R.; Jensen, P.E.; Felby, C.; Bjerrum, M.J. Thermal unfolding and refolding of a lytic polysaccharide monooxygenase from Thermoascus aurantiacus. RSC Adv. 2019, 9, 29734–29742. [Google Scholar] [CrossRef] [Green Version]
- Incardona, M.-F.; Bourenkov, G.P.; Levik, K.; Pieritz, R.A.; Popov, A.N.; Svensson, O. EDNA: A framework for plugin-based applications applied to X-ray experiment online data analysis. J. Synchrotron Radiat. 2009, 16, 872–879. [Google Scholar] [CrossRef]
- Murshudov, G.N.; Vagin, A.A.; Dodson, E.J. Refinement of Macromolecular Structures by the Maximum-Likelihood Method. Acta. Crystallogr. Sect. D Biol. Crystallogr. 1997, 53, 240–255. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.W.; McCoy, A.; et al. Overview of the CCP 4 suite and current developments. Acta. Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta. Crystallogr. D. Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vagin, A.; Teplyakov, A. MOLREP: An Automated Program for Molecular Replacement. J. Appl. Crystallogr. 1997, 30, 1022–1025. [Google Scholar] [CrossRef]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B.; de Bakker, P.I.W.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Cα geometry: ϕ, ψ and Cβ deviation. Proteins Struct. Funct. Bioinforma. 2003, 50, 437–450. [Google Scholar] [CrossRef]
- Schrodinger, L.L.C. The PyMOL Molecular Graphics System, Version 2.0.1 2018. Available online: https://pymol.org (accessed on 17 January 2022).
- Hansson, H.; Karkehabadi, S.; Mikkelsen, N.; Douglas, N.R.; Kim, S.; Lam, A.; Kaper, T.; Kelemen, B.; Meier, K.K.; Jones, S.M.; et al. High-resolution structure of a lytic polysaccharide monooxygenase from Hypocrea jecorina reveals a predicted linker as an integral part of the catalytic domain. J. Biol. Chem. 2017, 292, 19099–19109. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.-C.; Kracher, D.; Gandini, R.; Sygmund, C.; Kittl, R.; Haltrich, D.; Hällberg, B.M.; Ludwig, R.; Divne, C. Structural basis for cellobiose dehydrogenase action during oxidative cellulose degradation. Nat. Commun. 2015, 6, 7542. [Google Scholar] [CrossRef] [Green Version]
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles; Wiley: Hoboken, NJ, USA, 2003; ISBN 9783527307203. [Google Scholar]
- Søndergaard, C.R.; Olsson, M.H.M.; Rostkowski, M.; Jensen, J.H. Improved Treatment of Ligands and Coupling Effects in Empirical Calculation and Rationalization of p K a Values. J. Chem. Theory Comput. 2011, 7, 2284–2295. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical p K a Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Arnold, E.; Himmel, D.M. International Tables for Crystallography, Vol. F; M.G. Kluwer Academic Publishers: New York, NY, USA, 2001; ISBN 978-0-470-66078-2. [Google Scholar]
- Ursby, T.; Åhnberg, K.; Appio, R.; Aurelius, O.; Barczyk, A.; Bartalesi, A.; Bjelčić, M.; Bolmsten, F.; Cerenius, Y.; Doak, R.B.; et al. BioMAX—The first macromolecular crystallography beamline at MAX IV Laboratory. J. Synchrotron Rad. 2020, 27, 1415–1429. [Google Scholar] [CrossRef]
| LsAA9A_Ec_1 | LsAA9A_Ec_2 | TaAA9A | |
|---|---|---|---|
| Beamline | BioMAX, MAXIV, Lund | BioMAX, MAXIV, Lund | BioMAX, MAXIV, Lund |
| Wavelength [Å] | 0.9762 | 0.9762 | 0.9538 Å |
| Space group | P41 | P41 | P21 |
| No. of mols/asymmetric unit | 1 | 1 | 2 |
| Cell parameters | |||
| (a, b, c) [Å] | 48.47, 48.47, 109.72 | 48.66, 48.66, 109.59 | 37.65, 89.05, 70.47 |
| (α,β,γ) [º] | 90.0 | 90.0 | 90.0, 103.3, 90.0 |
| Resolution [Å] | 48.5–1.09 (1.15–1.09) * | 48.7–1.09 (1.16–1.09) | 68.6–1.06 (1.12–1.06) |
| Completeness [%] | 97.2 (84.6) | 91.3 (59.7) | 84.2 (40.4) |
| Rmeas [%] | 5.3 (36.1) | 5.9 (113.4) | 5.4 (43.4) |
| I/σ(I) | 7.21 (3.37) | 19.3 (1.5) | 18.6 (3.6) |
| CC1/2 [%] | 99.8 (88.6) | 99.9 (73.1) | 99.9 (89.4) |
| Unique reflections | 102,068 (15,109) | 96,541 (10,733) | 171,807 (12,525) |
| Observed reflections | 1,163,180 (71,657) | 1,157,797 (63,079) | 1,126,749 (54,423) |
| Redundancy | 11.4 (4.7) | 12.0 (5.9) | 6.6 (4.3) |
| No. mol./ASU | 1 | 1 | 2 |
| RWork [%] | 11.23 | 12.35 | 11.73 |
| RFree [%] | 12.75 | 13.93 | 14.00 |
| RMSD | |||
| Bond lengths [Å] | 0.0191 | 0.0196 | 0.0193 |
| Bond Angles [°] | 2.1238 | 2.0322 | 2.3041 |
| Ramachandran Statistics (%) | |||
| Favored | 94.8 | 94.8 | 98.4 |
| Allowed | 5.2 | 5.2 | 1.1 |
| Outlier | 0.0 | 0.0 | 0.4 |
| Crystallization Condition | No. of Mols/Asymmetric Unit | Resolution (Å) | Restrained Refinement | Histidine-Unrestrained Refinement | |||
|---|---|---|---|---|---|---|---|
| Final RWork/RFree [%] | Final RWork/RFree [%] | Final RMSD Bond lengths [Å] | Final RMSD Bond Angles [°] | ||||
| LsAA9A_Ec_1 | 0.1 M citric acid pH 3.5 and 3.0 M NaCl | 1 | 1.09 | 11.04/12.36 | 11.39/12.82 | 0.0131 | 1.8818 |
| LsAA9A_Ec_2 | 0.1 M citric acid pH 3.5 and 3.0 M NaCl | 1 | 1.09 | 12.16/13.79 | 12.62/13.93 | 0.0170 | 1.9397 |
| TaAA9A | 0.2 M MgCl2, 0.1 M HEPES pH 7.5, 22% polyacrylic acid | 2 | 1.06 | 11.94/14.17 | 11.80/12.70 | 0.0121 | 2.0481 |
| 5O2X | 1.6 M ammonium sulfate, 0.1 M citric acid 4.0 | 1 | 0.95 | 11.29/12.53 | 11.39/12.58 | 0.0139 | 1.9586 |
| 4EIR | 20% PEG 3350, pH 6.7 | 2 | 1.10 | 12.07/13.52 | 11.20/12.73 | 0.0147 | 1.8145 |
| 5OPF | 0.04 M potassium phosphate monobasic, 16% w/v PEG8000, 20% v/v glycerol | 1 | 1.08 | 11.49/13.86 | 11.79/14.02 | 0.0124 | 1.7270 |
| 4QI8 | 0.2M ammonium nitrate, 20% (w/v) PEG 3350, pH 7.0 | 2 | 1.10 | 11.98/14.60 | 12.08/14.62 | 0.0161 | 1.8468 |
| Nδ1-Cε1 (X1) (Å) | Cε1-Nε2 (X2) (Å) | -Nδ1- (X3) (deg) | -Nε2- (X4) deg) | his1 | his2 | Protonated Group | Predicted pKa | |
|---|---|---|---|---|---|---|---|---|
| LsAA9A_Ec_1 | ||||||||
| 1 | 1.37 | 1.33 | 105.04 | 108.92 | 2.3922 | −0.9368 | Nε2 | 5.65 |
| 78 | 1.34 | 1.31 | 107.76 | 106.57 | −0.3255 | −0.2142 | Nδ1 | 3.70 |
| 147 | 1.35 | 1.37 | 107.88 | 110.02 | 2.7804 | 0.9156 | Nδ1 + Nε2 | 5.07 |
| LsAA9A_Ec_2 | ||||||||
| 1 | 1.36 | 1.33 | 105.33 | 107.53 | 1.5237 | −1.3366 | Nε2 | 5.53 |
| 78 | 1.33 | 1.37 | 110.10 | 106.26 | −0.751 | 0.6228 | Nδ1 | 3.49 |
| 147 | 1.34 | 1.43 | 107.53 | 107.20 | 2.1689 | −0.808 | Nε2 | 4.95 |
| TaAA9A_A chain | ||||||||
| 86 | 1.39 | 1.33 | 105.37 | 105.03 | −1.5224 | −2.429 | Nδ1 | 2.78 |
| 164 | 1.32 | 1.33 | 105.30 | 109.14 | 4.2605 | −0.5908 | Nε2 | 4.80 |
| TaAA9A_B chain | ||||||||
| 86 | 1.40 | 1.31 | 104.16 | 106.83 | −0.0649 | −2.2506 | Nδ1 | 2.83 |
| 164 | 1.31 | 1.33 | 106.09 | 107.37 | 2.7832 | −0.8702 | Nε2 | 4.82 |
| 5O2X | ||||||||
| 86 | 1.36 | 1.30 | 105.81 | 103.08 | −2.6326 | −2.7544 | Nδ1 | 2.45 |
| 163 | 1.34 | 1.34 | 105.72 | 107.36 | 2.0476 | −1.2072 | Nε2 | 4.63 |
| 4EIR_A chain | ||||||||
| 84 | 1.35 | 1.30 | 107.34 | 106.85 | −0.3731 | −0.2926 | Nδ1 | 2.21 |
| 157 | 1.30 | 1.32 | 106.39 | 108.31 | 3.5234 | −0.225 | Nε2 | 4.68 |
| 4EIR_B chain | ||||||||
| 84 | 1.33 | 1.32 | 108.83 | 105.27 | −1.4691 | −0.2002 | Nδ1 | 2.24 |
| 157 | 1.31 | 1.33 | 106.17 | 107.73 | 3.0056 | −0.6742 | Nε2 | 4.67 |
| 5OPF | ||||||||
| 37 | 1.33 | 1.32 | 105.02 | 103.23 | −0.5811 | −3.1906 | Nδ1 | 3.44 |
| 144 | 1.32 | 1.30 | 106.03 | 103.60 | −0.8842 | −2.3264 | Nδ1 | 2.18 |
| 216 | 1.30 | 1.34 | 103.87 | 104.49 | 2.5444 | −3.3622 | Nε2 | 4.83 |
| 4QI8_A chain | ||||||||
| 1 | 1.36 | 1.28 | 105.25 | 110.2 | 2.8256 | 0.044 | Nδ1 + Nε2 | 4.29 |
| 72 | 1.34 | 1.30 | 107.14 | 104.41 | −1.7260 | −1.4078 | Nδ1 | 2.19 |
| 146 | 1.33 | 1.36 | 105.18 | 105.13 | 1.3833 | −2.5462 | Nε2 | 4.75 |
| 4QI8_B chain | ||||||||
| 1 | 1.37 | 1.32 | 106.58 | 107.07 | −0.1551 | −0.7906 | Nδ1 | 4.23 |
| 72 | 1.36 | 1.32 | 107.96 | 105.84 | −1.5996 | −0.5128 | Nδ1 | 2.11 |
| 146 | 1.32 | 1.32 | 105.02 | 106.52 | 2.2928 | −1.7872 | Nε2 | 4.64 |
| Histidine Residue Number in This Study | Baverage (Å2) from Restrained Refinement | Baverage (Å2) from His-Only Unrestrained Refinement |
|---|---|---|
| LsAA9A_Ec_1 Overall_protein-chain | 14.1 | 14.1 |
| 1 | 12.7 | 12.8 |
| 78 | 13.1 | 13.1 |
| 147 | 14.1 | 14.2 |
| LsAA9A_Ec_2 Overall_protein-chain | 17.5 | 17.7 |
| 1 | 15.7 | 15.8 |
| 78 | 15.8 | 15.9 |
| 147 | 17.5 | 17.7 |
| TaAA9A Overall_protein-chainA | 12.5 | 12.3 |
| 86_chainA | 9.4 | 9.4 |
| 164_chainA | 10.0 | 10.0 |
| Overall_protein-chainB | 12.9 | 12.8 |
| 86_chainB | 9.8 | 9.7 |
| 164_chainB | 10.7 | 10.7 |
| 5O2X Overall_protein | 7.1 | 7.0 |
| 86 | 5.8 | 5.7 |
| 163 | 6.8 | 6.8 |
| 4EIR Overall_protein-chainA | 17.8 | 16.9 |
| 84_chainA | 12.7 | 12.6 |
| 157_chainA | 14.1 | 14.0 |
| Overall_protein-chainB | 13.1 | 12.8 |
| 84_chainB | 10.8 | 10.7 |
| 157_chainB | 11.4 | 11.4 |
| 5OPF Overall_protein | 9.4 | 9.3 |
| 37 | 8.0 | 8.0 |
| 144 | 7.2 | 7.1 |
| 216 | 7.8 | 7.8 |
| 4QI8 Overall_protein-chainA | 17.8 | 16.9 |
| 1_chainA | 10.7 | 10.6 |
| 72_chainA | 27.1 | 26.5 |
| 146_chainA | 13.5 | 13.4 |
| Overall_protein-chainB | 13.0 | 12.8 |
| 1_chainB | 10.2 | 10.2 |
| 72_chainB | 28.4 | 27.3 |
| 146_chainB | 12.4 | 12.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, S.; Muderspach, S.J.; Tandrup, T.; Frandsen, K.E.H.; Singh, R.K.; Ipsen, J.Ø.; Hernández-Rollán, C.; Nørholm, M.H.H.; Bjerrum, M.J.; Johansen, K.S.; et al. Protonation State of an Important Histidine from High Resolution Structures of Lytic Polysaccharide Monooxygenases. Biomolecules 2022, 12, 194. https://doi.org/10.3390/biom12020194
Banerjee S, Muderspach SJ, Tandrup T, Frandsen KEH, Singh RK, Ipsen JØ, Hernández-Rollán C, Nørholm MHH, Bjerrum MJ, Johansen KS, et al. Protonation State of an Important Histidine from High Resolution Structures of Lytic Polysaccharide Monooxygenases. Biomolecules. 2022; 12(2):194. https://doi.org/10.3390/biom12020194
Chicago/Turabian StyleBanerjee, Sanchari, Sebastian J. Muderspach, Tobias Tandrup, Kristian Erik Høpfner Frandsen, Raushan K. Singh, Johan Ørskov Ipsen, Cristina Hernández-Rollán, Morten H. H. Nørholm, Morten J. Bjerrum, Katja Salomon Johansen, and et al. 2022. "Protonation State of an Important Histidine from High Resolution Structures of Lytic Polysaccharide Monooxygenases" Biomolecules 12, no. 2: 194. https://doi.org/10.3390/biom12020194
APA StyleBanerjee, S., Muderspach, S. J., Tandrup, T., Frandsen, K. E. H., Singh, R. K., Ipsen, J. Ø., Hernández-Rollán, C., Nørholm, M. H. H., Bjerrum, M. J., Johansen, K. S., & Lo Leggio, L. (2022). Protonation State of an Important Histidine from High Resolution Structures of Lytic Polysaccharide Monooxygenases. Biomolecules, 12(2), 194. https://doi.org/10.3390/biom12020194








