Linking Cerebrovascular Dysfunction to Age-Related Hearing Loss and Alzheimer’s Disease—Are Systemic Approaches for Diagnosis and Therapy Required?
Abstract
1. Introduction: Alzheimer’s Disease—Etiology and Pathophysiology
1.1. AD Pathophysiology
1.2. Noteworthy, Adding to This, Other Non-Aβ-Based Hypotheses Exist to Explain the Pathogenesis of AD
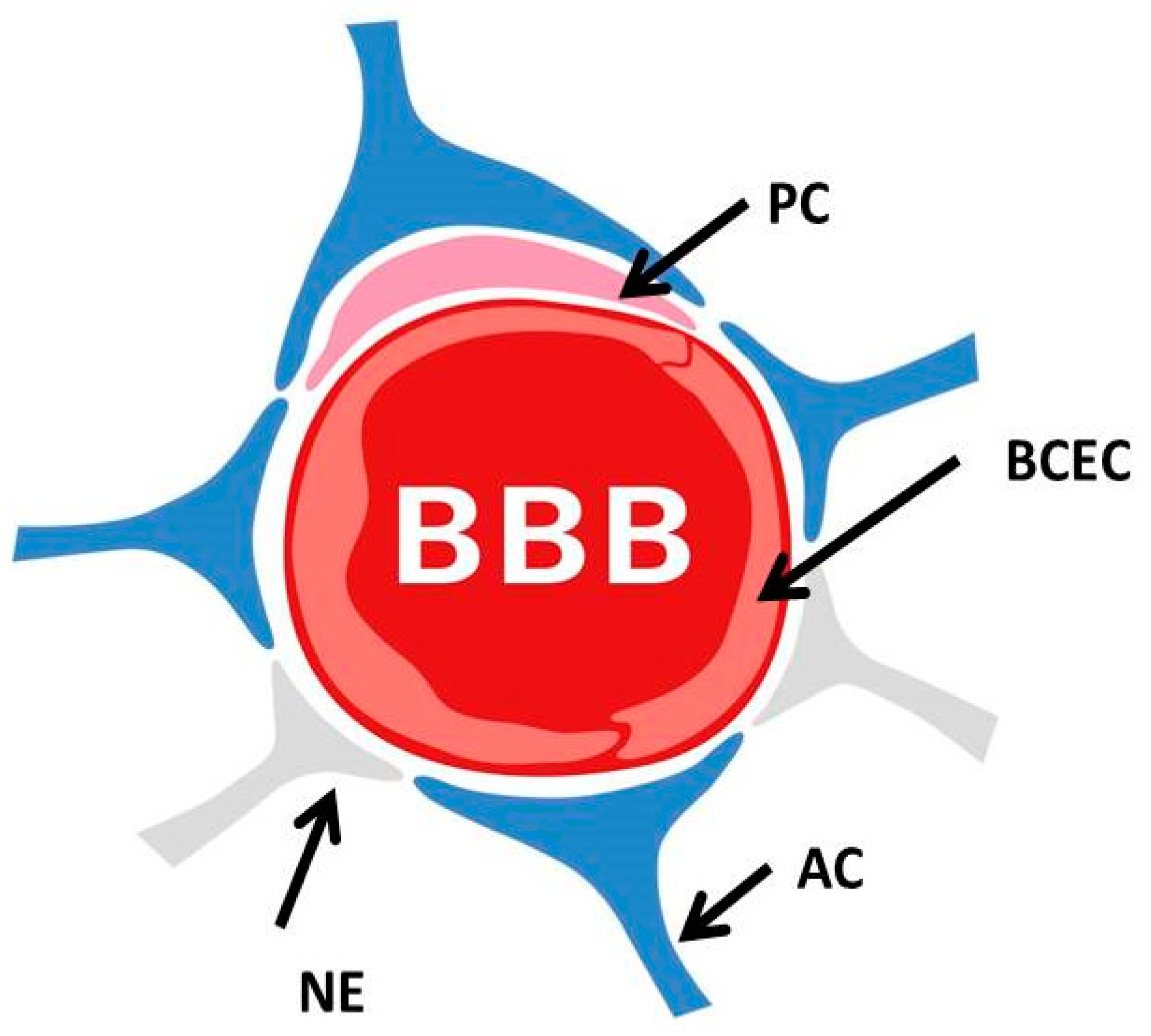
2. The Blood–Brain Barrier and Its Dysfunction in the Genesis of AD
3. Blood–Brain Barrier Dysfunction in the Pathogenesis of Alzheimer’s Disease—Available Knowledge
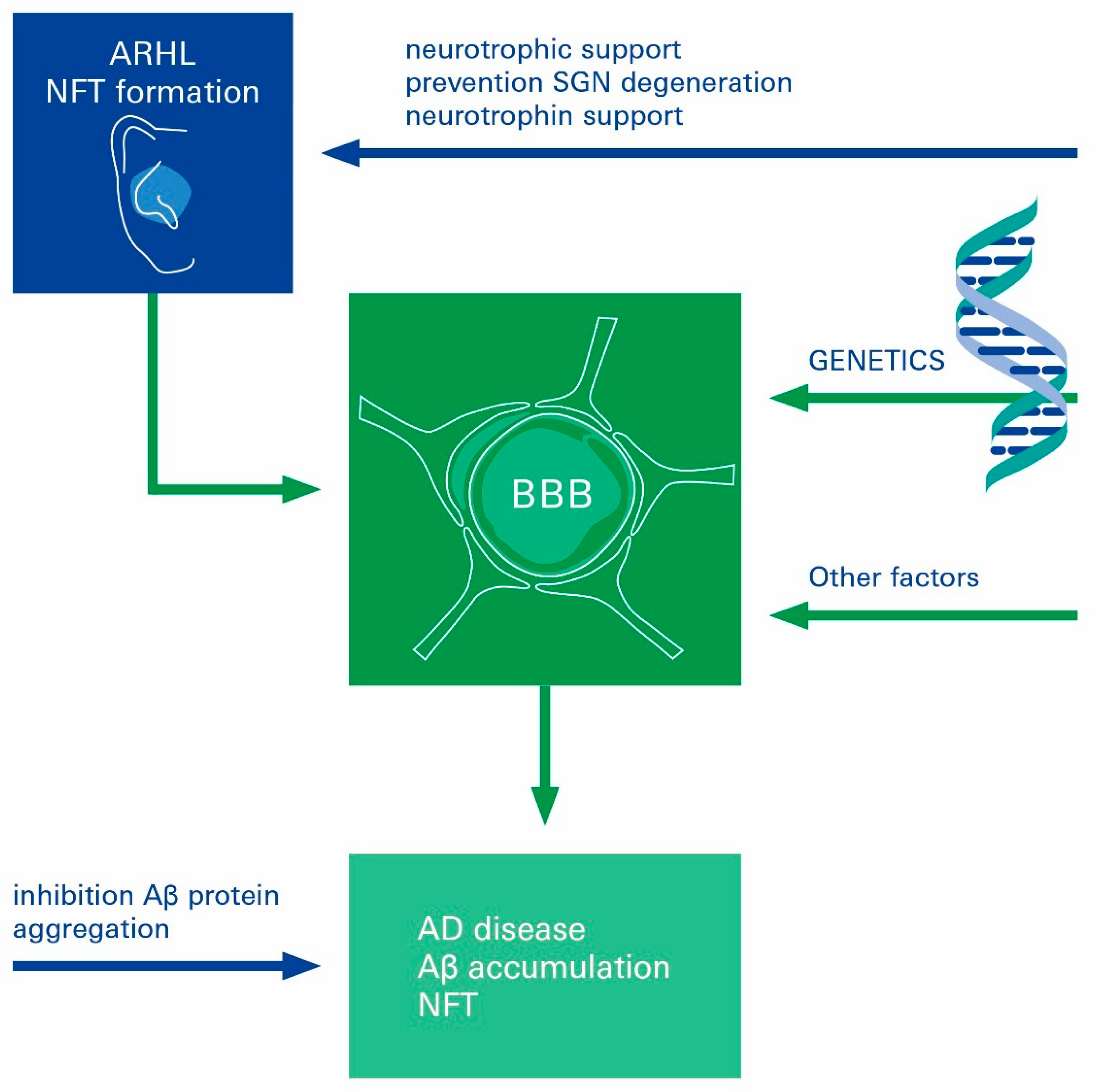
4. Age-Related Hearing Loss in the Genesis of AD
4.1. Functional Contribution of Age-Related Decline in BLB Integrity on ARHL
4.2. The Age-Related Decline of Spiral Ganglion Neurons—Could the Treatment of ARHL Improve Cognitive Outcomes or Prevent AD Development?
5. Neurotrophic Factors at the Interface of AD Pathophysiology and Neural ARHL
6. Current and Novel Treatment Modalities
7. Novel Inhibitors of Amyloid Fibril Formation in AD


8. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sagare, A.P.; Bell, R.D.; Zlokovic, B.V. Neurovascular dysfunction and faulty amyloid beta-peptide clearance in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a011452. [Google Scholar] [CrossRef]
- Swanberg, M.M.; Tractenberg, R.E.; Mohs, R.; Thal, L.J.; Cummings, J.L. Executive Dysfunction in Alzheimer Disease. Arch. Neurol. 2004, 61, 556–560. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2020, 15, 321–387. [Google Scholar]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Cummings, J.L. Alzheimer’s disease. N. Engl. J. Med. 2004, 351, 56–67. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- De la Torre, J.C. The vascular hypothesis of Alzheimer’s disease: Bench to bedside and beyond. Neuro-Degener. Dis. 2010, 7, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, V.T. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: Implications for early detection and therapy. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005, 28, 202–208. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2020, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Przybyłowska, M.; Dzierzbicka, K.; Kowalski, S.; Chmielewska, K.; Inkielewicz-Stepniak, I. Therapeutic Potential of Multifunctional Derivatives of Cholinesterase Inhibitors. Curr. Neuropharmacol. 2021, 19, 1323–1344. [Google Scholar] [CrossRef]
- Kametani, F.; Hasegawa, M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef]
- Shen, Y.; Hu, H.; Fan, C.; Wang, Q.; Zou, T.; Ye, B.; Xiang, M. Sensorineural hearing loss may lead to dementia-related pathological changes in hippocampal neurons. Neurobiol. Dis. 2021, 156, 105408. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.-Y.; Shen, C.-T.; Wang, L.-F.; Chien, C.-Y. Association of sudden sensorineural hearing loss with dementia: A nationwide cohort study. BMC Neurol. 2021, 21, 88. [Google Scholar] [CrossRef]
- Edwards, G.A.; Gamez, N., Jr.; Escobedo, G.; Calderon, O.; Moreno-Gonzalez, I. Modifiable Risk Factors for Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 146. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Golub, J.S.; Sharma, R.K.; Rippon, B.Q.; Brickman, A.M.; Luchsinger, J.A. The Association Between Early Age-Related Hearing Loss and Brain beta-Amyloid. Laryngoscope 2021, 131, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Jellinger, K.A. The overlap between vascular disease and Alzheimer’s disease—Lessons from pathology. BMC Med. 2014, 12, 633–638. [Google Scholar] [CrossRef]
- Miklossy, J. Cerebral hypoperfusion induces cortical watershed microinfarcts which may further aggravate cognitive decline in Alzheimer’s disease. Neurol. Res. 2003, 25, 605–610. [Google Scholar] [CrossRef]
- Llano, D.A.; Kwok, S.S.; Devanarayan, V.; (Adni), T.A.D.N.I. Reported Hearing Loss in Alzheimer’s Disease Is Associated with Loss of Brainstem and Cerebellar Volume. Front. Hum. Neurosci. 2021, 15, 739754. [Google Scholar] [CrossRef] [PubMed]
- Bu, N.; Churilov, L.; Khlif, M.S.; Lemmens, R.; Wouters, A.; Fiebach, J.B.; Chamorro, A.; Ringelstein, E.B.; Norrving, B.; Laage, R.; et al. Early Brain Volume Changes After Stroke: Subgroup Analysis From the AXIS-2 Trial. Front. Neurol. 2022, 12, 747343. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Moroni, F.; Magnoni, M.; Rocca, M.A.; Messina, R.; Anzalone, N.; De Filippis, C.; Scotti, I.; Besana, F.; Spagnolo, P.; et al. Extent and characteristics of carotid plaques and brain parenchymal loss in asymptomatic patients with no indication for revascularization. IJC Heart Vasc. 2020, 30, 100619. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. From blood–brain barrier to blood–brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Abbott, N.J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef]
- Shityakov, S.; Pastorin, G.; Foerster, C.; Salvador, E. Blood–brain barrier transport studies, aggregation, and molecular dynamics simulation of multiwalled carbon nanotube functionalized with fluorescein isothiocyanate. Int. J. Nanomed. 2015, 10, 1703–1713. [Google Scholar] [CrossRef]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef]
- Engelhardt, B.; Ransohoff, R.M. Capture, crawl, cross: The T cell code to breach the blood-brain barriers. Trends Immunol. 2012, 33, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Salvador, E.; Burek, M.; Förster, C.Y. Tight Junctions and the Tumor Microenvironment. Curr. Pathobiol. Rep. 2016, 4, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Blecharz, K.; Kahles, T.; Schwarz, T.; Kraft, P.; Göbel, K.; Meuth, S.G.; Burek, M.; Thum, T.; Stoll, G.; et al. Glucocorticoid Insensitivity at the Hypoxic Blood–Brain Barrier Can Be Reversed by Inhibition of the Proteasome. Stroke 2011, 42, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Kanekiyo, T. Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1965. [Google Scholar] [CrossRef] [PubMed]
- Hartz, A.M.; Bauer, B.; Soldner, E.L.; Wolf, A.; Boy, S.; Backhaus, R.; Mihaljevic, I.; Bogdahn, U.; Klünemann, H.H.; Schuierer, G.; et al. Amyloid-beta contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke 2012, 43, 514–523. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2013, 33, 1500–1513. [Google Scholar] [CrossRef]
- Grammas, P.; Samany, P.G.; Thirumangalakudi, L. Thrombin and inflammatory proteins are elevated in Alzheimer’s disease microvessels: Implications for disease pathogenesis. J. Alzheimer’s Dis. JAD 2006, 9, 51–58. [Google Scholar] [CrossRef]
- Goos, J.D.; Kester, M.I.; Barkhof, F.; Klein, M.; Blankenstein, M.A.; Scheltens, P.; van der Flier, W.M. Patients with Alzheimer disease with multiple microbleeds: Relation with cerebrospinal fluid biomarkers and cognition. Stroke 2009, 40, 3455–3460. [Google Scholar] [CrossRef]
- Lanfranconi, S.; Franco, G.; Borellini, L.; Denaro, F.; Basilico, P.; Parati, E.; Micieli, G.; Bersano, A. Genetics of cerebral hemorrhage and microbleeds. Panminerva Med. 2013, 55, 11–28. [Google Scholar]
- van Assema, D.M.; Lubberink, M.; Rizzu, P.; van Swieten, J.C.; Schuit, R.C.; Eriksson, J.; Scheltens, P.; Koepp, M.; Lammertsma, A.A.; van Berckel, B.N. Blood-brain barrier P-glycoprotein function in healthy subjects and Alzheimer’s disease patients: Effect of polymorphisms in the ABCB1 gene. EJNMMI Res. 2012, 2, 57. [Google Scholar] [CrossRef]
- Wolf, A.; Bauer, B.; Hartz, A.M.S. ABC Transporters and the Alzheimer’s Disease Enigma. Front. Psychiatry 2012, 3, 54. [Google Scholar] [CrossRef]
- Carrano, A.; Hoozemans, J.J.; van der Vies, S.M.; Rozemuller, A.J.; van Horssen, J.; de Vries, H.E. Amyloid Beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid. Redox. Signal 2011, 15, 1167–1178. [Google Scholar] [CrossRef]
- Brun, A.; Englund, E. A white matter disorder in dementia of the Alzheimer type: A pathoanatomical study. Ann. Neurol. 1986, 19, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.N.; Lee, L.; Saxena, R.; Bemis, K.G.; Wang, M.; Theodorakis, J.L.; Vuppalanchi, R.; Alloosh, M.; Sturek, M.; Chalasani, N. Serum proteomic analysis of diet-induced steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Am. J. Physiol. Liver Physiol. 2010, 298, G746–G754. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Wu, Z.; Sagare, A.; Davis, J.; Du Yan, S.; Hamm, K.; Xu, F.; Parisi, M.; LaRue, B.; Hu, H.W.; et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 2004, 43, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Atwood, C.S.; Martins, R.N.; Smith, M.A.; Perry, G. Senile plaque composition and posttranslational modification of amyloid-beta peptide and associated proteins. Peptides 2002, 23, 1343–1350. [Google Scholar] [CrossRef]
- Kumar-Singh, S.; Pirici, D.; McGowan, E.; Serneels, S.; Ceuterick, C.; Hardy, J.; Duff, K.; Dickson, D.; Van Broeckhoven, C. Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am. J. Pathol. 2005, 167, 527–543. [Google Scholar] [CrossRef]
- Hellberg, S.; Silvola, J.M.; Liljenbäck, H.; Kiugel, M.; Eskola, O.; Hakovirta, H.; Hörkkö, S.; Morisson-Iveson, V.; Hirani, E.; Saukko, P.; et al. Amyloid-Targeting PET Tracer [18F]Flutemetamol Accumulates in Atherosclerotic Plaques. Molecules 2019, 24, 1072. [Google Scholar] [CrossRef]
- Bell, R.D.; Zlokovic, B.V. Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009, 118, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Takuma, K.; Fang, F.; Zhang, W.; Yan, S.; Fukuzaki, E.; Du, H.; Sosunov, A.; McKhann, G.; Funatsu, Y.; Nakamichi, N.; et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc. Natl. Acad. Sci. USA 2009, 106, 20021–20026. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Luehmann, M.; Coomaraswamy, J.; Bolmont, T.; Kaeser, S.; Schaefer, C.; Kilger, E.; Neuenschwander, A.; Abramowski, D.; Frey, P.; Jaton, A.L.; et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science 2006, 313, 1781–1784. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Luehmann, M.; Spires-Jones, T.L.; Prada, C.; Garcia-Alloza, M.; De Calignon, A.; Rozkalne, A.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Bacskai, B.J.; Hyman, B.T. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature 2008, 451, 720–724. [Google Scholar] [CrossRef]
- Eisele, Y.S.; Obermüller, U.; Heilbronner, G.; Baumann, F.; Kaeser, S.A.; Wolburg, H.; Walker, L.C.; Staufenbiel, M.; Heikenwalder, M.; Jucker, M. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science 2010, 330, 980–982. [Google Scholar] [CrossRef]
- Prusiner, S.B. Human prion diseases and neurodegeneration. Curr. Top. Microbiol. Immunol. 1996, 207, 1–17. [Google Scholar]
- Zlokovic, B.V. New therapeutic targets in the neurovascular pathway in Alzheimer’s disease. Neurother. J. Am. Soc. Exp. 2008, 5, 409–414. [Google Scholar] [CrossRef]
- Jellinger, K. Cerebellar involvement in progressive supranuclear palsy. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 1104–1105. [Google Scholar] [CrossRef]
- Roberts, R.O.; Geda, Y.E.; Knopman, D.S.; Cha, R.H.; Boeve, B.F.; Ivnik, R.J.; Pankratz, V.S.; Tangalos, E.G.; Petersen, R.C. Metabolic syndrome, inflammation, and nonamnestic mild cognitive impairment in older persons: A population-based study. Alzheimer Dis. Assoc. Disord. 2010, 24, 11–18. [Google Scholar] [CrossRef]
- Iadecola, C.; Davisson, R.L. Hypertension and Cerebrovascular Dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Gustafson, D.R.; Barrett-Connor, E.; Haan, M.N.; Gunderson, E.P.; Yaffe, K. Central obesity and increased risk of dementia more than three decades later. Neurology 2008, 71, 1057–1064. [Google Scholar] [CrossRef]
- Jellinger, K.A. Prevalence and Impact of Cerebrovascular Lesions in Alzheimer and Lewy Body Diseases. Neurodegener. Dis. 2010, 7, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Ruitenberg, A.; den Heijer, T.; Bakker, S.L.; van Swieten, J.C.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Cerebral hypoperfusion and clinical onset of dementia: The Rotterdam Study. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2005, 57, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, S.E.; Prins, N.D.; Heijer, T.D.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Silent Brain Infarcts and the Risk of Dementia and Cognitive Decline. N. Engl. J. Med. 2003, 348, 1215–1222. [Google Scholar] [CrossRef]
- Snowdon, D.A.; Greiner, L.H.; Mortimer, J.A.; Riley, K.P.; Greiner, P.A.; Markesbery, W.R. Brain infarction and the clinical expression of Alzheimer disease: The Nun Study. JAMA 1997, 277, 813–817. [Google Scholar] [CrossRef]
- Di Marco, L.Y.; Venneri, A.; Farkas, E.; Evans, P.C.; Marzo, A.; Frangi, A.F. Vascular dysfunction in the pathogenesis of Alzheimer’s disease—A review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol. Dis. 2015, 82, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.G.; Eckert, G.P.; Igbavboa, U.; Müller, W.E. Amyloid beta-protein interactions with membranes and cholesterol: Causes or casualties of Alzheimer’s disease. Biochim. Biophys. Acta 2003, 1610, 281–290. [Google Scholar] [CrossRef]
- Wendell, C.R.; Waldstein, S.R.; Ferrucci, L.; O’Brien, R.J.; Strait, J.B.; Zonderman, A.B. Carotid Atherosclerosis and Prospective Risk of Dementia. Stroke 2012, 43, 3319–3324. [Google Scholar] [CrossRef]
- Dempsey, R.J.; Vemuganti, R.; Varghese, T.; Hermann, B.P. A review of carotid atherosclerosis and vascular cognitive decline: A new understanding of the keys to symptomology. Neurosurgery 2010, 67, 484–493. [Google Scholar] [CrossRef]
- A Dar, T.; A Sheikh, I.; A Ganie, S.; Ali, R.; R Singh, L.; Hua Gan, S.; A Kamal, M.; A Zargar, M. Molecular linkages between diabetes and Alzheimer’s disease: Current scenario and future prospects. CNS Neurol. Disord. Drug Targets 2014, 13, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.; Stolk, R.P.; van Harskamp, F.; Pols, H.A.P.; Hofman, A.; Breteler, M.M.B. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999, 53, 1937. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Bierhaus, A.; Schwaninger, M. Reactive Oxygen Species in Diabetes-induced Vascular Damage, Stroke, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2009, 16, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Nguyen, J.C.D.; Killcross, A.S.; Jenkins, T.A. Obesity and cognitive decline: Role of inflammation and vascular changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef]
- Petrovitch, H.; White, L.; Izmirilian, G.; Ross, G.; Havlik, R.; Markesbery, W.; Nelson, J.; Davis, D.; Hardman, J.; Foley, D.; et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: The HAAS☆. Neurobiol. Aging 2000, 21, 57–62. [Google Scholar] [CrossRef]
- Tzourio, C. Hypertension, cognitive decline, and dementia: An epidemiological perspective. Dialogues Clin. Neurosci. 2007, 9, 61–70. [Google Scholar] [CrossRef]
- Chakraborty, A.; de Wit, N.M.; van der Flier, W.M.; de Vries, H.E. The blood brain barrier in Alzheimer’s disease. Vasc. Pharmacol. 2017, 89, 12–18. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Khan, S.M. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med. Hypotheses 2004, 63, 8–20. [Google Scholar] [CrossRef]
- Diaz-Ruiz, C.; Wang, J.; Ksiezak-Reding, H.; Ho, L.; Qian, X.; Humala, N.; Thomas, S.; Martinez-Martin, P.; Pasinetti, G.M. Role of Hypertension in Aggravating Abeta Neuropathology of AD Type and Tau-Mediated Motor Impairment. Cardiovasc. Psychiatry Neurol. 2009, 2009, 107286. [Google Scholar] [CrossRef]
- Touyz, R.M. Oxidative stress and vascular damage in hypertension. Curr. Hypertens. Rep. 2000, 2, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Nadhimi, Y.; Llano, D.A. Does hearing loss lead to dementia? A review of the literature. Heart Res. 2020, 402, 108038. [Google Scholar] [CrossRef] [PubMed]
- Sinha, U.K.; Hollen, K.M.; Rodriguez, R.; Miller, C.A. Auditory system degeneration in Alzheimer’s disease. Neurology 1993, 43, 779–785. [Google Scholar] [CrossRef]
- Gates, G.A.; Mills, J.H. Presbycusis. Lancet 2005, 366, 1111–1120. [Google Scholar] [CrossRef]
- Losada, A.; Márquez-González, M.; Romero-Moreno, R.; Mausbach, B.T.; López, J.; Fernández-Fernández, V.; Nogales-González, C. Cognitive–behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for dementia family caregivers with significant depressive symptoms: Results of a randomized clinical trial. J. Consult. Clin. Psychol. 2015, 83, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, D.G.; Kelly, M.E.; Kelley, G.A.; Brennan, S.; Lawlor, B.A. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: A systematic review and meta-analysis. JAMA Otolaryngol.-Head Neck Surg. 2018, 144, 115–126. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Sardone, R.; Battista, P.; Piccininni, M.; Dibello, V.; La Montagna, M.; Stallone, R.; Venezia, P.; Liguori, A.; et al. Sensorial frailty: Age-related hearing loss and the risk of cognitive impairment and dementia in later life. Ther. Adv. Chronic Dis. 2018, 10, 2040622318811000. [Google Scholar] [CrossRef]
- Nagai, M.; Scheper, V.; Lenarz, T.; Förster, C.Y. The insular cortex as a vestibular area in relation to autonomic function. Clin. Auton. Res. 2020, 31, 179–185. [Google Scholar] [CrossRef]
- Bowl, M.R.; Dawson, S.J. Age-Related Hearing Loss. Cold Spring Harb. Perspect. Med. 2019, 9, a033217. [Google Scholar] [CrossRef]
- Fagan, A.M.; Xiong, C.; Jasielec, M.S.; Bateman, R.J.; Goate, A.M.; Benzinger, T.L.; Ghetti, B.; Martins, R.N.; Masters, C.L.; Mayeux, R.; et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet. Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Mann, D.M.A.; Yates, P.O. Lipoprotein Pigments—Their Relationship To Ageing In The Human Nervous System. I. The lipofuscin content of nerve cells. Brain J. Neurol. 1974, 97, 481–488. [Google Scholar] [CrossRef]
- Moreno-García, A.; Kun, A.; Calero, O.; Medina, M.; Calero, M. An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration. Front. Neurosci. 2018, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Förster, C.Y.; Scheper, V.; Lenarz, T. Hearing loss and strial microvascular pathology-towards unravelling the functional contribution of the blood-labyrinth barrier. Otorhinolaryngol. Neck Surg. 2019, 4–7. [Google Scholar] [CrossRef]
- Nyberg, S.; Abbott, N.J.; Shi, X.; Steyger, P.S.; Dabdoub, A. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Sci. Transl. Med. 2019, 11, eaao0935. [Google Scholar] [CrossRef]
- Heeringa, A.N.; Köppl, C. The aging cochlea: Towards unraveling the functional contributions of strial dysfunction and synaptopathy. Heart Res. 2019, 376, 111–124. [Google Scholar] [CrossRef]
- Kovacs, B.; Lumayag, S.; Cowan, C.; Xu, S. microRNAs in Early Diabetic Retinopathy in Streptozotocin-Induced Diabetic Rats. Investig. Opthalmology Vis. Sci. 2011, 52, 4402–4409. [Google Scholar] [CrossRef]
- Cheung, N.; Wong, T.Y. Diabetic retinopathy and systemic vascular complications. Prog. Retin. Eye Res. 2008, 27, 161–176. [Google Scholar] [CrossRef]
- Ruiz, M.A.; Chakrabarti, S. MicroRNAs: The Underlying Mediators of Pathogenetic Processes in Vascular Complications of Diabetes. Can. J. Diabetes 2013, 37, 339–344. [Google Scholar] [CrossRef]
- Wu, P.; Liberman, L.; Bennett, K.; de Gruttola, V.; O’Malley, J.; Liberman, M. Primary Neural Degeneration in the Human Cochlea: Evidence for Hidden Hearing Loss in the Aging Ear. Neuroscience 2018, 407, 8–20. [Google Scholar] [CrossRef]
- Schulze, J.; Staecker, H.; Wedekind, D.; Lenarz, T.; Warnecke, A. Expression pattern of brain-derived neurotrophic factor and its associated receptors: Implications for exogenous neurotrophin application. Heart Res. 2020, 413, 108098. [Google Scholar] [CrossRef]
- Keithley, E.M.; Croskrey, K.L. Spiral ganglion cell endings in the cochlear nucleus of young and old rats. Heart Res. 1990, 49, 169–177. [Google Scholar] [CrossRef]
- Madden, C.; Rutter, M.; Hilbert, L.; Greinwald Jr, J.H.; Choo, D.I. Clinical and audiological features in auditory neuropathy. Arch. Otolaryngol.-Head Neck Surg. 2002, 128, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Schuknecht, H.F. The Pathology of Several Disorders of the Inner Ear Which Cause Vertigo. South. Med. J. 1964, 57, 1161–1167. [Google Scholar] [CrossRef]
- Ohlemiller, K.K. Age-related hearing loss: The status of Schuknecht’s typology. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 439–443. [Google Scholar] [CrossRef]
- Konerding, W.; Janssen, H.; Hubka, P.; Tornøe, J.; Mistrik, P.; Wahlberg, L.; Lenarz, T.; Kral, A.; Scheper, V. Encapsulated cell device approach for combined electrical stimulation and neurotrophic treatment of the deaf cochlea. Heart Res. 2017, 350, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Schwieger, J.; Warnecke, A.; Lenarz, T.; Esser, K.-H.; Scheper, V. Neuronal Survival, Morphology and Outgrowth of Spiral Ganglion Neurons Using a Defined Growth Factor Combination. PLoS ONE 2015, 10, e0133680. [Google Scholar] [CrossRef][Green Version]
- Scheper, V.; Paasche, G.; Miller, J.M.; Warnecke, A.; Berkingali, N.; Lenarz, T.; Stöver, T. Effects of delayed treatment with combined GDNF and continuous electrical stimulation on spiral ganglion cell survival in deafened guinea pigs. J. Neurosci. Res. 2008, 87, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.; Webster, D.B. Spiral ganglion neuron loss following organ of corti loss: A quantitative study. Brain Res. 1981, 212, 17–30. [Google Scholar] [CrossRef]
- Bao, J.; Ohlemiller, K.K. Age-related loss of spiral ganglion neurons. Heart Res. 2010, 264, 93–97. [Google Scholar] [CrossRef]
- Ernfors, P.; Kucera, J.; Lee, K.F.; Loring, J.; Jaenisch, R. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. Int. J. Dev. Biol. 1995, 39, 799–807. [Google Scholar] [PubMed]
- Shepherd, R.K.; Hardie, N.A. Deafness-Induced Changes in the Auditory Pathway: Implications for Cochlear Implants. Audiol. Neurotol. 2001, 6, 305–318. [Google Scholar] [CrossRef]
- Butler, B.E.; Lomber, S.G. Functional and structural changes throughout the auditory system following congenital and early-onset deafness: Implications for hearing restoration. Front. Syst. Neurosci. 2013, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Kühne, D.; Scheper, V.; Kral, A. Congenital deafness affects deep layers in primary and secondary auditory cortex. J. Comp. Neurol. 2017, 525, 3110–3125. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, P.A.; Hubka, P.; Tillein, J.; Vinck, M.; Kral, A. Deafness Weakens Interareal Couplings in the Auditory Cortex. Front. Neurosci. 2021, 14, 625721. [Google Scholar] [CrossRef] [PubMed]
- Tarawneh, R. Biomarkers: Our Path Towards a Cure for Alzheimer Disease. Biomark. Insights 2020, 15, 1177271920976367. [Google Scholar] [CrossRef]
- Amieva, H.; Ouvrard, C. Does Treating Hearing Loss in Older Adults Improve Cognitive Outcomes? A Review. J. Clin. Med. 2020, 9, 805. [Google Scholar] [CrossRef]
- Lenarz, T. Cochlear implant—State of the art. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2017, 16, 4. [Google Scholar] [CrossRef]
- Roehm, P.C.; Hansen, M.R. Strategies to preserve or regenerate spiral ganglion neurons. Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 294–300. [Google Scholar] [CrossRef]
- Sampaio, T.B.; Savall, A.S.; Gutierrez, M.E.Z.; Pinton, S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: Implications for pathogenesis and therapy. Neural Regen. Res. 2017, 12, 549–557. [Google Scholar]
- Altschuler, R.A.; Cho, Y.; Ylikoski, J.; Pirvola, U.; Magal, E.; Miller, J.M. Rescue and Regrowth of Sensory Nerves Following Deafferentation by Neurotrophic Factors. Ann. N. Y. Acad. Sci. 1999, 884, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Mufson, E.J.; Weingartner, J.A.; Skau, K.A.; Crutcher, K.A. Nerve growth factor in Alzheimer’s disease: Increased levels throughout the brain coupled with declines in nucleus basalis. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 6213–6221. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015, 6, 331–341. [Google Scholar]
- Gao, L.; Ge, R.; Xie, G.; Yao, D.; Li, P.; Wang, O.; Ma, X.; Han, F. Hearing Improvement in A/J Mice via the Mouse Nerve Growth Factor. Clin. Exp. Otorhinolaryngol. 2017, 10, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Rüttiger, L.; Panford-Walsh, R.; Schimmang, T.; Tan, J.; Zimmermann, U.; Rohbock, K.; Köpschall, I.; Limberger, A.; Müller, M.; Fraenzer, J.-T.; et al. BDNF mRNA expression and protein localization are changed in age-related hearing loss. Neurobiol. Aging 2007, 28, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Yurek, D.M.; Fletcher-Turner, A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001, 891, 228–235. [Google Scholar] [CrossRef]
- Ibáñez, C.F.; Andressoo, J.-O. Biology of GDNF and its receptors—Relevance for disorders of the central nervous system. Neurobiol. Dis. 2017, 97, 80–89. [Google Scholar] [CrossRef]
- Bothwell, M. Recent advances in understanding neurotrophin signaling. F1000Research 2016, 5, 1885. [Google Scholar] [CrossRef]
- Leake, P.A.; Akil, O.; Lang, H. Neurotrophin gene therapy to promote survival of spiral ganglion neurons after deafness. Heart Res. 2020, 394, 107955. [Google Scholar] [CrossRef]
- Cordell, C.B.; Borson, S.; Boustani, M.; Chodosh, J.; Reuben, D.; Verghese, J.; Thies, W.; Fried, L.B.; Medicare Detection of Cognitive Impairment Workgroup. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2013, 9, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association; Thies, W.; Bleiler, L. 2013 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2013, 9, 208–245. [Google Scholar] [CrossRef]
- Lee, S.J.C.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an understanding of amyloid-beta oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, M.; Czapski, G.A.; Wójtowicz, S.; Wieczorek, I.; Wencel, P.L.; Strosznajder, R.P.; Jaber, V.; Lukiw, W.J.; Strosznajder, J.B. Alterations of Transcription of Genes Coding Anti-oxidative and Mitochondria-Related Proteins in Amyloid beta Toxicity: Relevance to Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 1374–1388. [Google Scholar] [CrossRef]
- Nakagami, Y.; Nishimura, S.; Murasugi, T.; Kaneko, I.; Meguro, M.; Marumoto, S.; Kogen, H.; Koyama, K.; Oda, T. A novel beta-sheet breaker, RS-0406, reverses amyloid beta-induced cytotoxicity and impairment of long-term potentiation in vitro. Br. J. Pharmacol. 2002, 137, 676–682. [Google Scholar] [CrossRef]
- Mehrazma, B.; Robinson, M.; Opare, S.K.A.; Petoyan, A.; Lou, J.; Hane, F.T.; Rauk, A.; Leonenko, Z. Pseudo-peptide amyloid-beta blocking inhibitors: Molecular dynamics and single molecule force spectroscopy study. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Kumar, M.G.; Gopi, H.N.; Paknikar, K.M. Inhibition of beta-Amyloid Aggregation through a Designed beta-Hairpin Peptide. Langmuir 2018, 34, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, S.; Cha, M. Neuroprotective effect of single-wall carbon nanotubes with built-in peroxidase-like activity against beta-amyloid-induced neurotoxicity. Medchemcomm 2017, 8, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Sohajda, T.; Puskás, I.; Roewer, N.; Förster, C.; Broscheit, J.A. Ionization states, cellular toxicity and molecular modeling studies of midazolam complexed with trimethyl-beta-cyclodextrin. Molecules 2014, 19, 16861–16876. [Google Scholar] [CrossRef]
- Shityakov, S.; Broscheit, J.; Förster, C. α-Cyclodextrin dimer complexes of dopamine and levodopa derivatives to assess drug delivery to the central nervous system: ADME and molecular docking studies. Int. J. Nanomed. 2012, 7, 3211–3219. [Google Scholar] [CrossRef]
- Yao, J.; Ho, D.; Calingasan, N.Y.; Pipalia, N.H.; Lin, M.T.; Beal, M.F. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J. Exp. Med. 2012, 209, 2501–2513. [Google Scholar] [CrossRef]
- Ştefănescu, R.; Stanciu, G.D.; Luca, A.; Caba, I.C.; Tamba, B.I.; Mihai, C.T. Contributions of Mass Spectrometry to the Identification of Low Molecular Weight Molecules Able to Reduce the Toxicity of Amyloid-beta Peptide to Cell Cultures and Transgenic Mouse Models of Alzheimer’s Disease. Molecules 2019, 24, 1167. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Shityakov, S.; Hayashi, K.; Störk, S.; Scheper, V.; Lenarz, T.; Förster, C.Y. Conspicuous Link between Ear, Brain and Heart-Could Neurotrophin-Treatment of Age-Related Hearing Loss Help Prevent Alzheimer’s Disease and Associated Amyloid Cardiomyopathy? Biomolecules 2021, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Salmas, R.E.; Salvador, E.; Roewer, N.; Broscheit, J.; Förster, C. Evaluation of the potential toxicity of unmodified and modified cyclodextrins on murine blood-brain barrier endothelial cells. J. Toxicol. Sci. 2016, 41, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Skorb, E.V.; Förster, C.Y.; Dandekar, T. Scaffold Searching of FDA and EMA-Approved Drugs Identifies Lead Candidates for Drug Repurposing in Alzheimer’s Disease. Front. Chem. 2021, 9, 736509. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Förster, C.Y.; Shityakov, S.; Scheper, V.; Lenarz, T. Linking Cerebrovascular Dysfunction to Age-Related Hearing Loss and Alzheimer’s Disease—Are Systemic Approaches for Diagnosis and Therapy Required? Biomolecules 2022, 12, 1717. https://doi.org/10.3390/biom12111717
Förster CY, Shityakov S, Scheper V, Lenarz T. Linking Cerebrovascular Dysfunction to Age-Related Hearing Loss and Alzheimer’s Disease—Are Systemic Approaches for Diagnosis and Therapy Required? Biomolecules. 2022; 12(11):1717. https://doi.org/10.3390/biom12111717
Chicago/Turabian StyleFörster, Carola Y., Sergey Shityakov, Verena Scheper, and Thomas Lenarz. 2022. "Linking Cerebrovascular Dysfunction to Age-Related Hearing Loss and Alzheimer’s Disease—Are Systemic Approaches for Diagnosis and Therapy Required?" Biomolecules 12, no. 11: 1717. https://doi.org/10.3390/biom12111717
APA StyleFörster, C. Y., Shityakov, S., Scheper, V., & Lenarz, T. (2022). Linking Cerebrovascular Dysfunction to Age-Related Hearing Loss and Alzheimer’s Disease—Are Systemic Approaches for Diagnosis and Therapy Required? Biomolecules, 12(11), 1717. https://doi.org/10.3390/biom12111717








