Potential Application of Recombinant Snake Prothrombin Activator Ecarin in Blood Diagnostics
Abstract
:1. Introduction
2. Methods
2.1. Cloning, Expression and Purification of Recombinant Ecarin (rEcarin)
2.2. Western Blot
2.3. Analytical SEC Coupled with MALS of Purified rEcarin
2.4. S-2238 One Stage MTP Ecarin Assay
2.5. Clotting Assay of Whole Blood Samples in Both Commercial Blood Collection and rEcarin-Containing Tubes
2.6. Thromboelastography (TEG) of Citrated Whole Blood
2.7. Statistical Analysis
2.8. Human Research Ethics
3. Results
3.1. Recombinant Ecarin (rEcarin) Expression, Purification and Identification
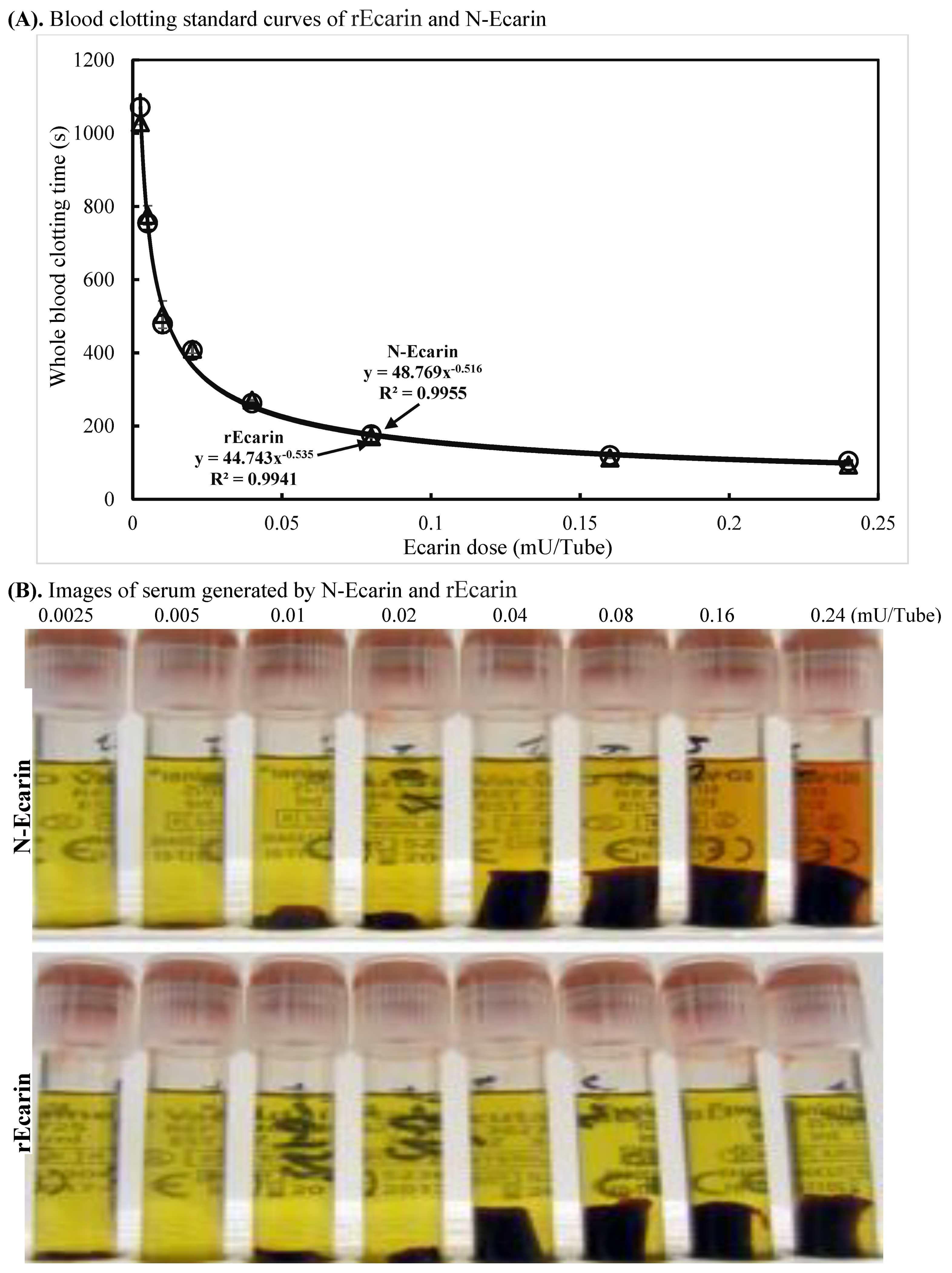
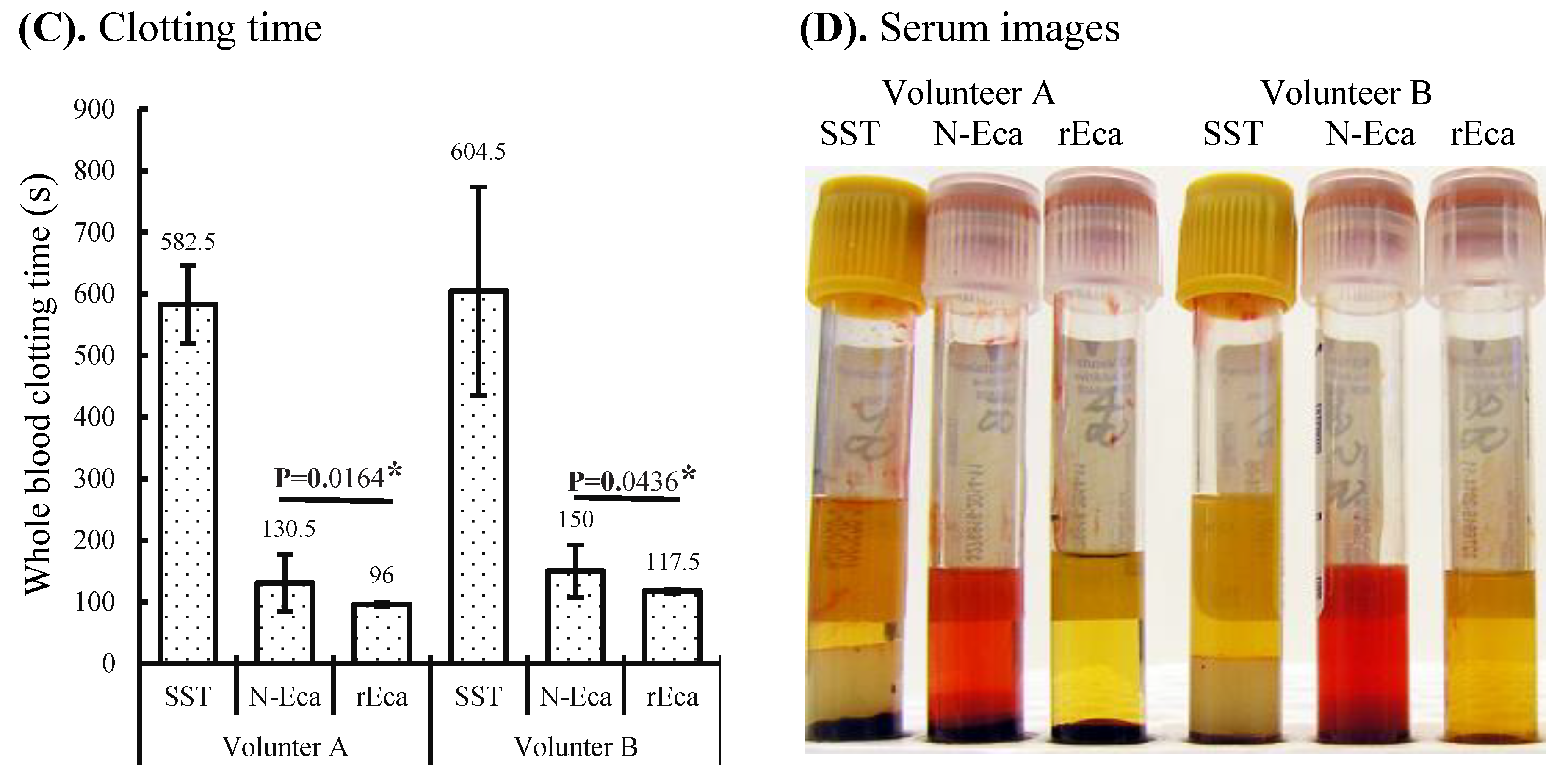
3.2. Blood Clotting Characterization of rEcarin Compared with N-Ecarin
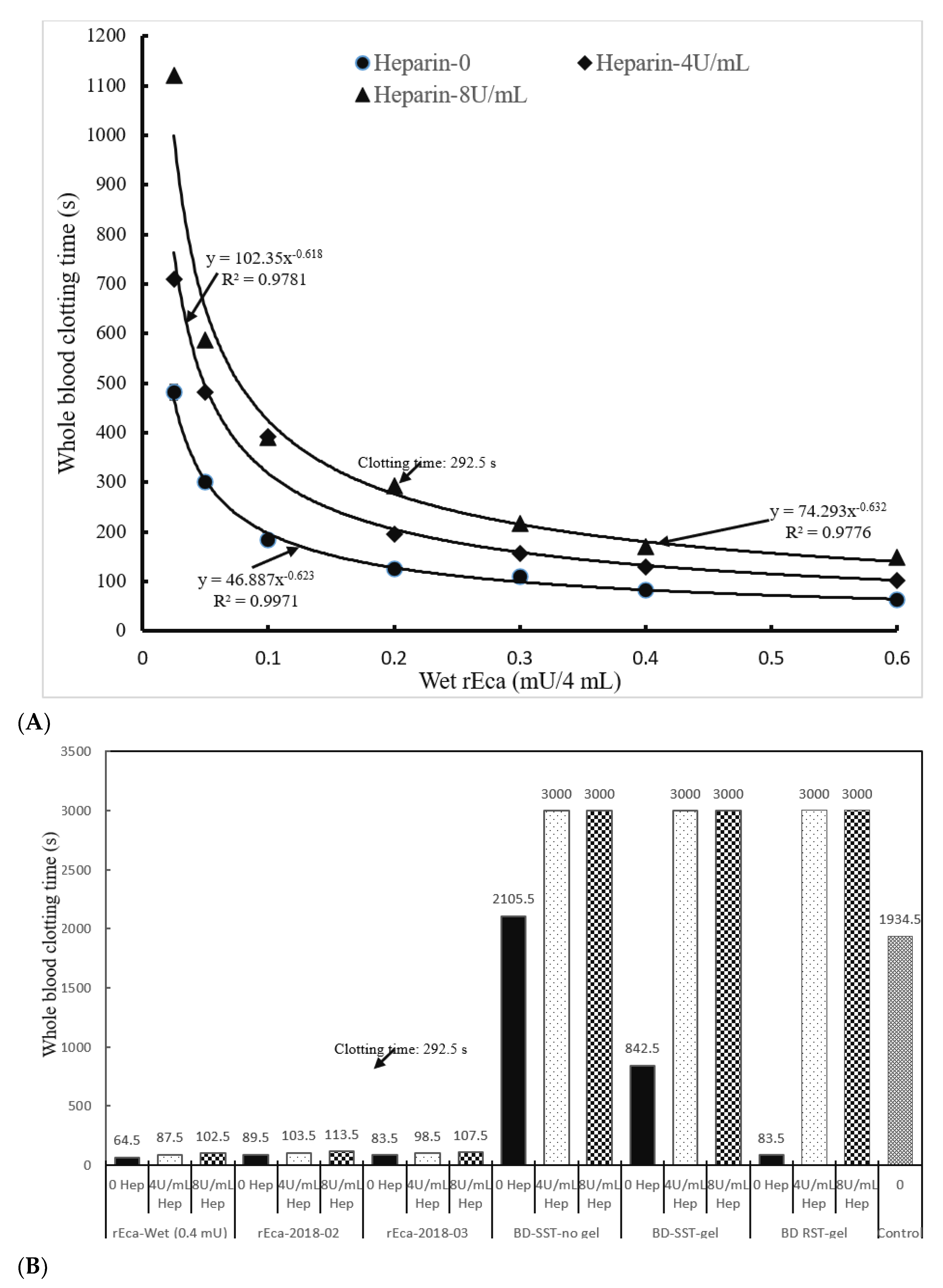
3.3. rEcarin Effectively Clots Anticoagulated Blood
3.3.1. Heparin
3.3.2. Warfarin
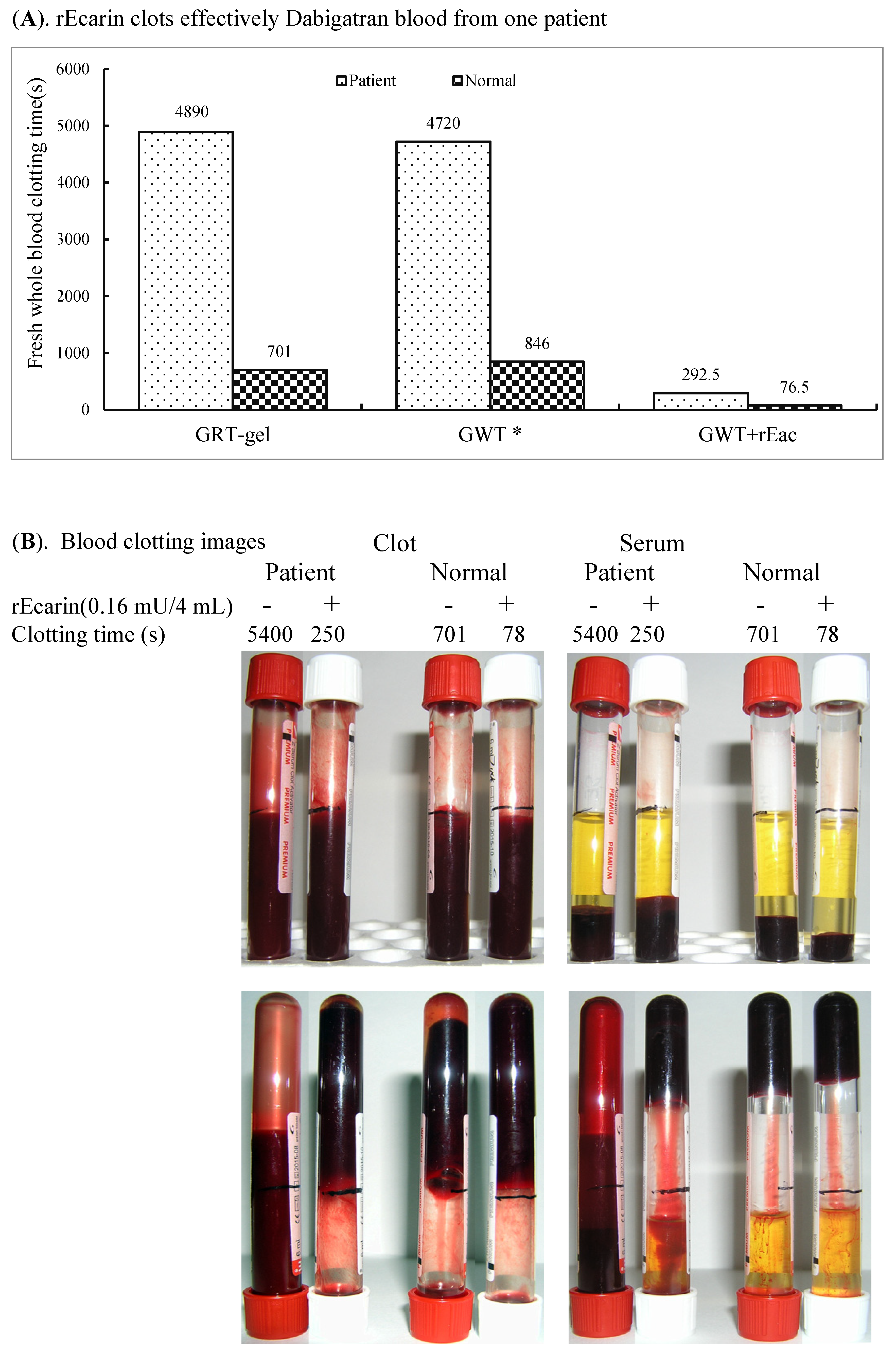
3.3.3. Dabigatran
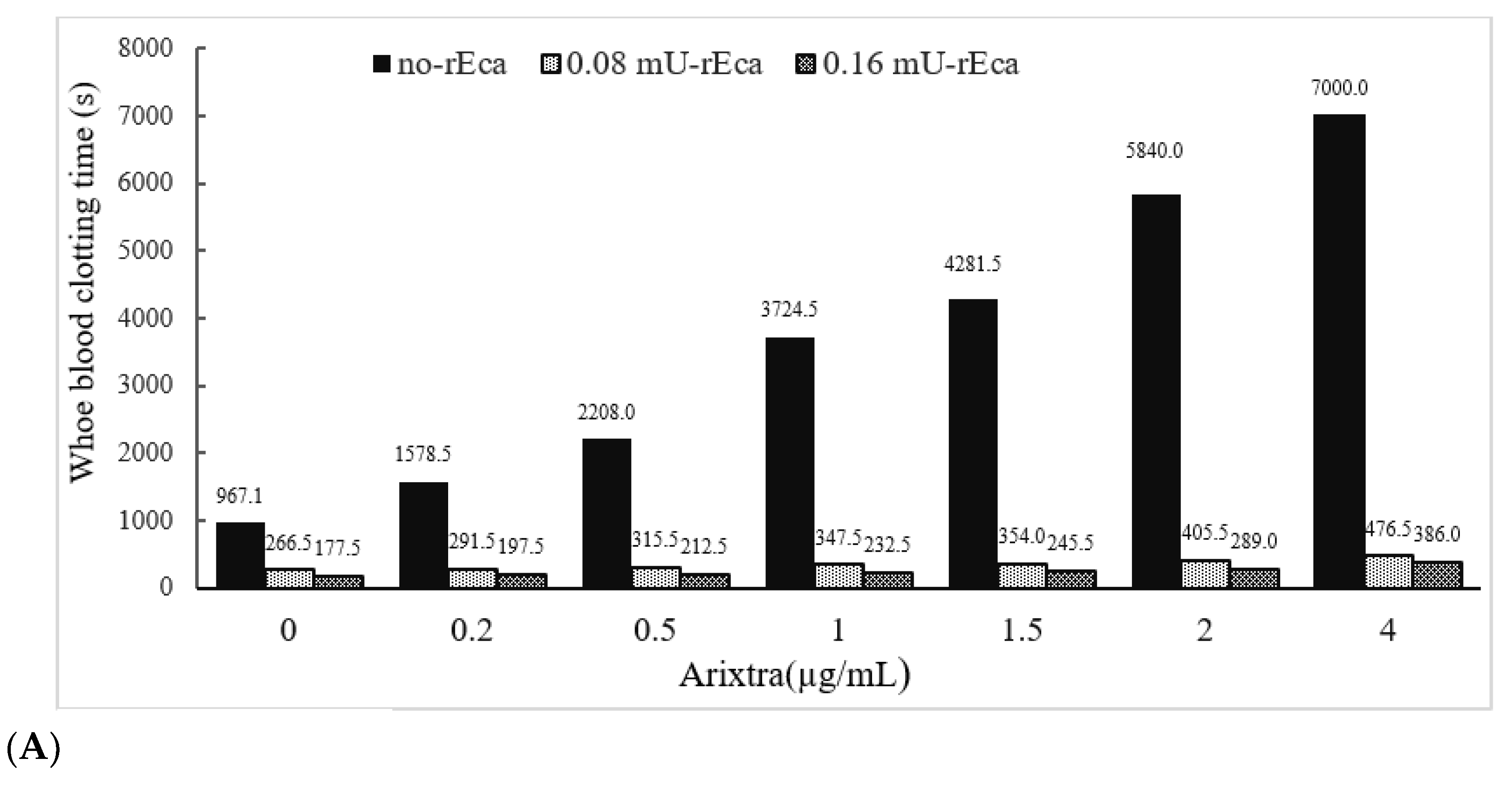
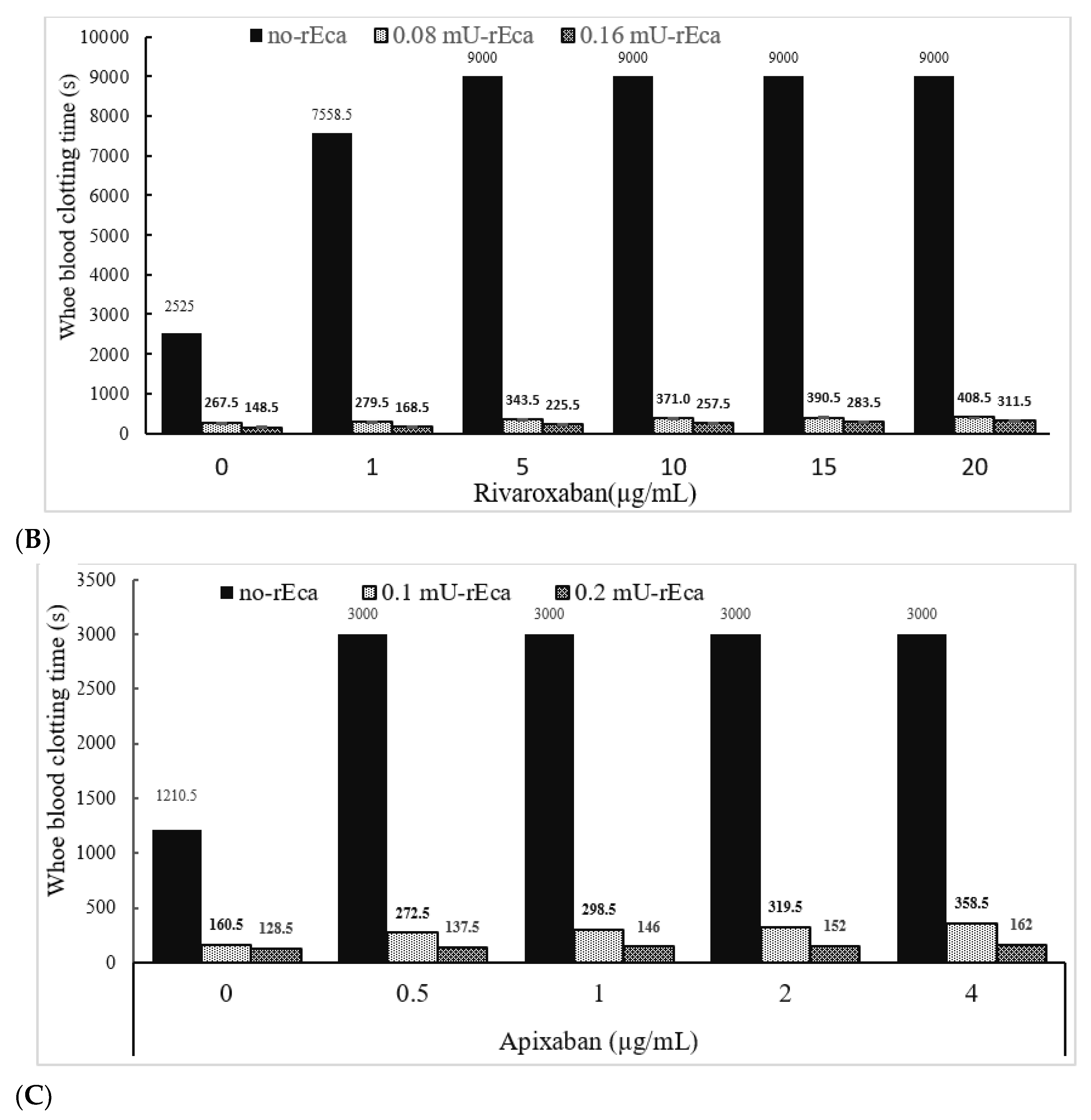
3.3.4. Other Anticoagulants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bos, M.H.; Camire, R.M. Blood coagulation factors V and VIII: Molecular Mechanisms of Procofactor Activation. J. Coagul. Disord. 2010, 2, 19–27. [Google Scholar] [PubMed]
- Bos, M.H.; Camire, R.M. Procoagulant adaptation of a blood coagulation prothrombinase-like enzyme complex in australian elapid venom. Toxins 2010, 2, 1554–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kini, R.M. The intriguing world of prothrombin activators from snake venom. Toxicon 2005, 45, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Speijer, H.; Govers-Riemslag, J.W.; Zwaal, R.F.; Rosing, J. Prothrombin activation by an activator from the venom of Oxyuranus scutellatus (Taipan snake). J. Biol. Chem. 1986, 261, 13258–13267. [Google Scholar] [CrossRef]
- Zhao, K.N.; Dimeski, G.; de Jersey, J.; Johnson, L.A.; Grant, M.; Masci, P.P.; Lavin, M.F. Rapid serum tube technology overcomes problems associated with use of anticoagulants. Biochem. Med. 2019, 29, 030706. [Google Scholar] [CrossRef]
- Zhao, K.N.; Dimeski, G.; de Jersey, J.; Johnson, L.A.; Grant, M.; Masci, P.P.; Lavin, M.F. Next-generation rapid serum tube technology using prothrombin activator coagulant: Fast, high-quality serum from normal samples. Clin. Chem. Lab. Med. 2019, 57, 483–497. [Google Scholar] [CrossRef] [Green Version]
- Bowen, R.A.; Remaley, A.T. Interferences from blood collection tube components on clinical chemistry assays. Biochem. Med. 2014, 24, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Eggenreich, B.; Scholz, E.; Wurm, D.J.; Forster, F.; Spadiut, O. The production of a recombinant tandem single chain fragment variable capable of binding prolamins triggering celiac disease. BMC Biotechnol. 2018, 18, 30. [Google Scholar] [CrossRef] [Green Version]
- Arsin, H.; Jasilionis, A.; Dahle, H.; Sandaa, R.A.; Stokke, R.; Nordberg Karlsson, E.; Steen, I.H. Exploring Codon Adjustment Strategies towards Escherichia coli-Based Production of Viral Proteins Encoded by HTH1, a Novel Prophage of the Marine Bacterium Hypnocyclicus thermotrophus. Viruses 2021, 13, 1215. [Google Scholar] [CrossRef]
- Kini, R.M.; Rao, V.S.; Joseph, J.S. Procoagulant proteins from snake venoms. Pathophysiol. Haemost. Thromb. 2001, 31, 218–224. [Google Scholar] [CrossRef]
- Filippovich, I.; Sorokina, N.; St Pierre, L.; Flight, S.; de Jersey, J.; Perry, N.; Masci, P.P.; Lavin, M.F. Cloning and functional expression of venom prothrombin activator protease from Pseudonaja textilis with whole blood procoagulant activity. Br. J. Haematol. 2005, 131, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.H.; Boltz, M.; St Pierre, L.; Masci, P.P.; de Jersey, J.; Lavin, M.F.; Camire, R.M. Venom factor V from the common brown snake escapes hemostatic regulation through procoagulant adaptations. Blood 2009, 114, 686–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonebring, A.; Lange, U.; Bucha, E.; Deinum, J.; Elg, M.; Lovgren, A. Expression and characterization of recombinant ecarin. Protein J. 2012, 31, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuelson, B.T.; Cuker, A. Measurement and reversal of the direct oral anticoagulants. Blood Rev. 2017, 31, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Moore, G.W.; Jones, P.O.; Platton, S.; Hussain, N.; White, D.; Thomas, W.; Rigano, J.; Pouplard, C.; Gray, E.; Devreese, K.M.J. International multicenter, multiplatform study to validate Taipan snake venom time as a lupus anticoagulant screening test with ecarin time as the confirmatory test: Communication from the ISTH SSC Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. J. Thromb. Haemost. 2021, 19, 3177–3192. [Google Scholar]
- Di Nisio, M.; Middeldorp, S.; Buller, H.R. Direct thrombin inhibitors. N. Engl. J. Med. 2005, 353, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, N.; Bandehpour, M.; Sotoodehnejadnematalahi, F.; Kazemi, B. Prokaryotic expression, evaluation, and prediction of the structure and function of the ecarin metalloproteinase domain. Proteins 2022, 90, 802–809. [Google Scholar] [CrossRef]
- Nishida, S.; Fujita, T.; Kohno, N.; Atoda, H.; Morita, T.; Takeya, H.; Kido, I.; Paine, M.J.; Kawabata, S.; Iwanaga, S. cDNA cloning and deduced amino acid sequence of prothrombin activator (ecarin) from Kenyan Echis carinatus venom. Biochemistry 1995, 34, 1771–1778. [Google Scholar] [CrossRef]
- Hasson, S.S.; Theakston, R.D.; Harrison, R.A. Cloning of a prothrombin activator-like metalloproteinase from the West African saw-scaled viper, Echis ocellatus. Toxicon 2003, 42, 629–634. [Google Scholar] [CrossRef]
- Dimeski, G.; Masci, P.P.; Trabi, M.; Lavin, M.F.; de Jersey, J. Evaluation of the Becton-Dickinson rapid serum tube: Does it provide a suitable alternative to lithium heparin plasma tubes? Clin. Chem. Lab. Med. 2010, 48, 651–657. [Google Scholar] [CrossRef] [Green Version]
- Kini, R.M.; Koh, C.Y. Metalloproteases Affecting Blood Coagulation, Fibrinolysis and Platelet Aggregation from Snake Venoms: Definition and Nomenclature of Interaction Sites. Toxins 2016, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; de Jersey, J.; Masci, P.P.; Zhao, K.N.; Bennett, N.C.; Dimeski, G.; Grant, M.; Lavin, M.F. Progress Curve Analysis of the one stage chromogenic assay for ecarin. Anal. Biochem. 2020, 608, 113907. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.; Bernard, A. Large-scale transient expression in mammalian cells for recombinant protein production. Curr. Opin. Biotechnol. 1999, 10, 156–159. [Google Scholar] [CrossRef]
- Zhao, K.N.; Frazer, I.H.; Liu, W.J.; Williams, M.; Zhou, J. Nucleotides 1506–1625 of bovine papillomavirus type 1 genome can enhance DNA packaging by L1/L2 capsids. Virology 1999, 259, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimeski, G.; Solano, C.; Petroff, M.K.; Hynd, M. Centrifugation protocols: Tests to determine optimal lithium heparin and citrate plasma sample quality. Ann. Clin. Biochem. 2011, 48 Pt 3, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, J.S.; Kallner, A.; Blomback, M. Measurement of blood and plasma coagulation time using free oscillating rheometry. Scand. J. Clin. Lab. Investig. 2002, 62, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Swallow, R.A.; Agarwala, R.A.; Dawkins, K.D.; Curzen, N.P. Thromboelastography: Potential bedside tool to assess the effects of antiplatelet therapy? Platelets 2006, 17, 385–392. [Google Scholar] [CrossRef]
- Maddrey, W.C.; Boitnott, J.K.; Bedine, M.S.; Weber, F.L., Jr.; Mezey, E.; White, R.I., Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978, 75, 193–199. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Danese, E.; Brocco, G.; Gelati, M.; Montagnana, M.; Sanchis-Gomar, F.; Favaloro, E.J. Serum Concentration of Growth Differentiation Factor-15 Is Independently Associated with Global Platelet Function and Higher Fibrinogen Values in Adult Healthy Subjects. Semin. Thromb. Hemost. 2017, 43, 621–628. [Google Scholar]
- Vorisek, C.N.; Sleeper, L.A.; Piekarski, B.; Lu, M.; Rogers, J.; Oladunjoye, O.O.; Emani, S.M. High-dose heparin is associated with higher bleeding and thrombosis rates in pediatric patients following cardiac surgery. J. Thorac. Cardiovasc. Surg. 2019, 158, 1199–1206. [Google Scholar] [CrossRef]
- Lee, W.; Chung, H.J.; Kim, S.; Jang, S.; Park, C.J.; Chi, H.S.; Chun, S.; Min, W.K. PIVKA-II is a candidate marker for monitoring the effects of the oral anticoagulant warfarin. Clin. Biochem. 2010, 43, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Ajmal, M.; Wolfson, A. Dabigatran in cardiovascular disease management: A comprehensive review. World J. Cardiol. 2021, 13, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Y.; Ponde, C.K.; Kumar, V.; Gaurav, K. Fondaparinux: A cornerstone drug in acute coronary syndromes. World J. Cardiol. 2022, 14, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Paramanathan, V. Rivaroxaban: Future in anticoagulation practice? Hematology 2008, 13, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.K.; Peng, C.C.; Lin, S.M.; Munir, K.M.; Chang, R.H.; Wu, B.B.; Liu, P.P.; Hsu, J.Y.; Loh, C.H.; Tu, Y.K. Fracture Risks in Patients Treated With Different Oral Anticoagulants: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e019618. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.C.; Chen, M.; Shi, Q.; Sun, R.; Wang, X. Chitosan/rectorite nanocomposite with injectable functionality for skin hemostasis. J. Mater. Chem. B 2018, 6, 6544–6549. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, Y.; Li, X.; Guo, D.; Zhou, Z.; Bai, G.; Li, J.; Huang, N.; Diao, J.; Li, Y.; et al. A Bionic Nano-Band-Aid Constructed by the Three-Stage Self-Assembly of Peptides for Rapid Liver Hemostasis. Nano Lett. 2021, 21, 7166–7174. [Google Scholar] [CrossRef] [PubMed]
- Currier, R.B.; Calvete, J.J.; Sanz, L.; Harrison, R.A.; Rowley, P.D.; Wagstaff, S.C. Unusual stability of messenger RNA in snake venom reveals gene expression dynamics of venom replenishment. PLoS ONE 2012, 7, e41888. [Google Scholar] [CrossRef] [Green Version]
- Wingfield, P.T. Overview of the purification of recombinant proteins. Curr. Protoc. Protein Sci. 2015, 80, 6.1.1–6.1.35. [Google Scholar] [CrossRef] [Green Version]
- Popovic, G.; Kirby, N.C.; Dement, T.C.; Peterson, K.M.; Daub, C.E.; Belcher, H.A.; Guthold, M.; Offenbacher, A.R.; Hudson, N.E. Development of Transient Recombinant Expression and Affinity Chromatography Systems for Human Fibrinogen. Int. J. Mol. Sci. 2022, 23, 1054. [Google Scholar] [CrossRef]
- Ramamurthy, N.; Baliga, N.; Wahr, J.A.; Schaller, U.; Yang, V.C.; Meyerhoff, M.E. Improved protamine-sensitive membrane electrode for monitoring heparin concentrations in whole blood via protamine titration. Clin. Chem. 1998, 44, 606–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, S.J.; Tillyer, M.L.; Keogh, J.; Hall, J.; Kelleher, A.A. Heparin concentrations in neonates during cardiopulmonary bypass. J. Thromb. Haemost. 2012, 10, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Baglin, T. The role of the laboratory in treatment with new oral anticoagulants. J. Thromb. Haemost. 2013, 11 (Suppl. S1), 122–128. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.C.; Douxfils, J. Ecarin based coagulation testing. Am. J. Hematol. 2020, 95, 863–869. [Google Scholar] [CrossRef]
- Davis, K.; Sebaaly, J.; Wooten, L.; Khouli, C.; Mihm, A.; Nisly, S.A. A Multicenter Retrospective Evaluation of Direct Oral Anticoagulants for the Treatment of Heparin-Induced Thrombocytopenia. Am. J. Cardiovasc. Drugs 2022, 22, 417–424. [Google Scholar] [CrossRef]
- Lippi, G.; Cadamuro, J. Novel Opportunities for Improving the Quality of Preanalytical Phase. A Glimpse to the Future? J. Med. Biochem. 2017, 36, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Plebani, M.; Favaloro, E.J. The Model List of Essential In Vitro Diagnostics: Nuisance or opportunity? Diagnosis 2019, 6, 187–188. [Google Scholar] [CrossRef]
- Hawkins, R.C. Laboratory turnaround time. Clin. Biochem. Rev. 2007, 28, 179–194. [Google Scholar]
- Lippi, G.; Betsou, F.; Cadamuro, J.; Cornes, M.; Fleischhacker, M.; Fruekilde, P.; Neumaier, M.; Nybo, M.; Padoan, A.; Plebani, M.; et al. Preanalytical challenges–time for solutions. Clin. Chem. Lab. Med. 2019, 57, 974–981. [Google Scholar] [CrossRef] [Green Version]
- von Meyer, A.; Lippi, G.; Simundic, A.M.; Cadamuro, J. Exact time of venous blood sample collection—An unresolved issue, on behalf of the European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working Group for Preanalytical Phase (WG-PRE). Clin. Chem. Lab. Med. 2020, 58, 1655–1662. [Google Scholar] [CrossRef]
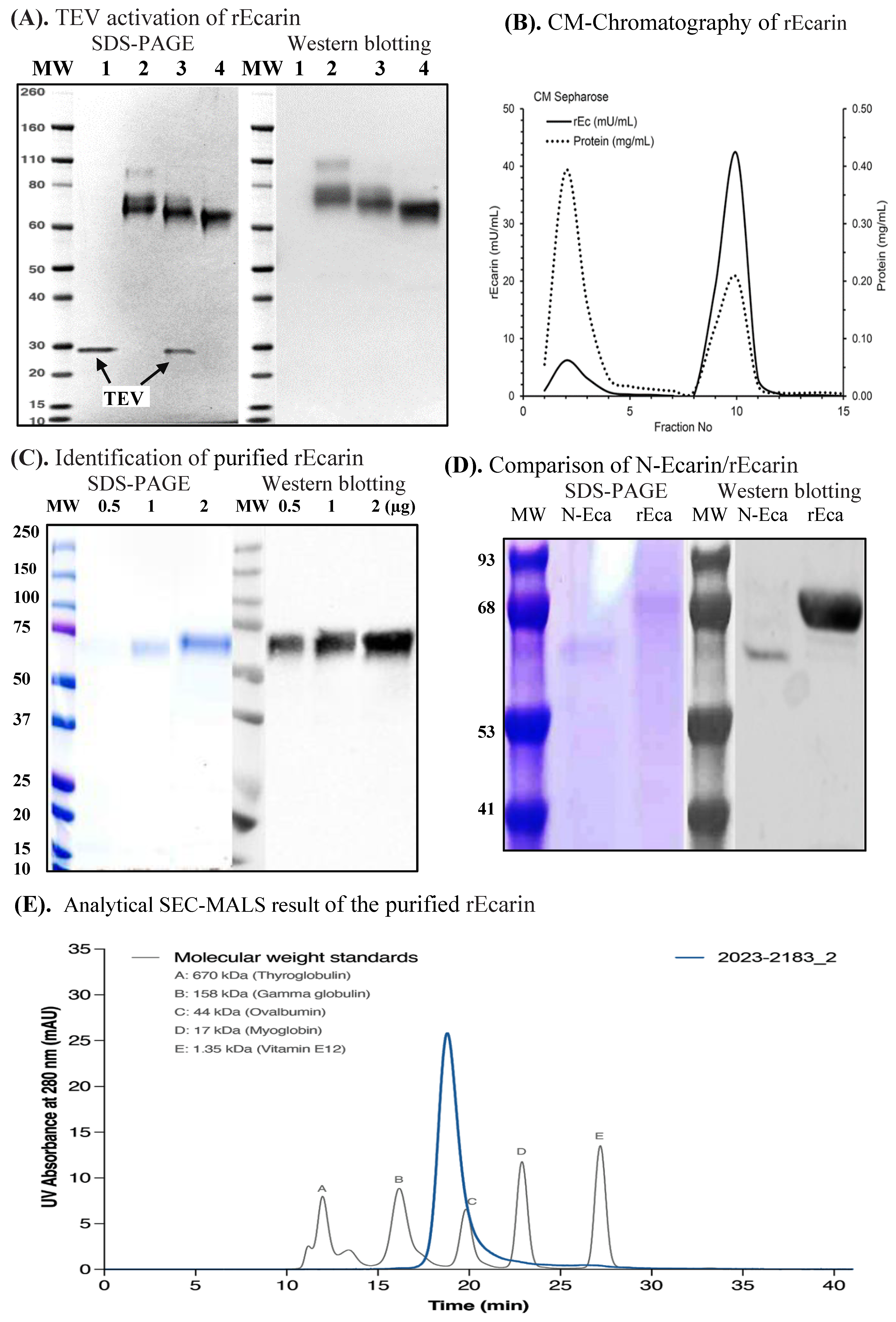

| Fraction | Volume | Protein | rEc Concentration | rEc | Specific Activity | Purification | Recovery |
|---|---|---|---|---|---|---|---|
| (Concentrates) | (mL) | (mg) | (mU/mL) | (mU) | (mU/mg) | (fold) | (%) |
| Cross Flow | 400 | 1687 | 80.5 | 32,219 | 19.1 | 1.0 | 100 |
| Q Sepharose | 252 | 703 | 131 | 32,927 | 46.9 | 2.5 | 102 |
| Hydroxyapatite | 32 | 239 | 845 | 27,040 | 113 | 5.9 | 84 |
| CM Sepharose | 150 | 75 | 163 | 24,403 | 323 | 17 | 76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.-N.; Masci, P.; Dimeski, G.; Johnson, L.; Grant, M.; de Jersey, J.; Lavin, M.F. Potential Application of Recombinant Snake Prothrombin Activator Ecarin in Blood Diagnostics. Biomolecules 2022, 12, 1704. https://doi.org/10.3390/biom12111704
Zhao K-N, Masci P, Dimeski G, Johnson L, Grant M, de Jersey J, Lavin MF. Potential Application of Recombinant Snake Prothrombin Activator Ecarin in Blood Diagnostics. Biomolecules. 2022; 12(11):1704. https://doi.org/10.3390/biom12111704
Chicago/Turabian StyleZhao, Kong-Nan, Paul Masci, Goce Dimeski, Lambro Johnson, Michael Grant, John de Jersey, and Martin F. Lavin. 2022. "Potential Application of Recombinant Snake Prothrombin Activator Ecarin in Blood Diagnostics" Biomolecules 12, no. 11: 1704. https://doi.org/10.3390/biom12111704
APA StyleZhao, K.-N., Masci, P., Dimeski, G., Johnson, L., Grant, M., de Jersey, J., & Lavin, M. F. (2022). Potential Application of Recombinant Snake Prothrombin Activator Ecarin in Blood Diagnostics. Biomolecules, 12(11), 1704. https://doi.org/10.3390/biom12111704








