Abstract
Ayahuasca is a psychoactive brew traditionally used in indigenous and religious rituals and ceremonies in South America for its therapeutic, psychedelic, and entheogenic effects. It is usually prepared by lengthy boiling of the leaves of the bush Psychotria viridis and the mashed stalks of the vine Banisteriopsis caapi in water. The former contains the classical psychedelic N,N-dimethyltryptamine (DMT), which is thought to be the main psychoactive alkaloid present in the brew. The latter serves as a source for β-carbolines, known for their monoamine oxidase-inhibiting (MAOI) properties. Recent preliminary research has provided encouraging results investigating ayahuasca’s therapeutic potential, especially regarding its antidepressant effects. On a molecular level, pre-clinical and clinical evidence points to a complex pharmacological profile conveyed by the brew, including modulation of serotoninergic, glutamatergic, dopaminergic, and endocannabinoid systems. Its substances also interact with the vesicular monoamine transporter (VMAT), trace amine-associated receptor 1 (TAAR1), and sigma-1 receptors. Furthermore, ayahuasca’s components also seem to modulate levels of inflammatory and neurotrophic factors beneficially. On a biological level, this translates into neuroprotective and neuroplastic effects. Here we review the current knowledge regarding these molecular interactions and how they relate to the possible antidepressant effects ayahuasca seems to produce.
1. Introduction
Ayahuasca is a psychedelic preparation usually made by the decoction of Banisteriopsis caapi and Psychotria viridis, or Diplopterys cabrerana, plants endemic to the Amazonian Basin where the brew is traditionally used in ritualistic contexts [1,2,3]. B. caapi is known to contain a class of substances called β-carbolines or harmala alkaloids, mainly harmine, tetrahydroharmine (THH), and harmaline [4,5]. These substances are known to selectively and reversibly inhibit the enzyme monoamine oxidase type A (MAO-A), which is believed to be their main mechanism of action [5,6]. On the other hand, P. viridis is a source of DMT, a serotoninergic psychedelic belonging to the same pharmacological class of substances as lysergic acid diethylamide (LSD) and psilocybin [7]. The main mechanism of action for DMT and related psychedelic substances is widely accepted to be agonism at the serotonin receptors 5-HT1A,2A,2C, with the 2A subtype being the primary molecular target and its activation dose-dependently related to the psychoactive effects these substances cause [8,9,10,11]. In the case of ayahuasca, the presence of β-carbolines inhibits the main DMT metabolic pathway via MAO-A inactivation, which in turn allows for DMT to be orally active [11,12,13].
In the last several decades, scientific research on ayahuasca consumption and administration has been conducted to investigate its possible therapeutic effects. From pre-clinical investigations [14,15,16,17,18,19,20,21,22] to observational studies [13,23,24,25,26,27,28,29,30,31,32,33], clinical trials [11,34,35,36,37,38,39,40,41], reviews, and meta-analyses [2,42,43,44,45,46,47,48,49,50], there is accumulating evidence that ayahuasca and its isolated substances may present neuroprotective, neuroplastic, immunomodulatory, anticancer, anti-addictive, anxiolytic, and, most notably, antidepressant properties, while also increasing well-being and quality of life and improving emotion regulation. Investigations on the therapeutic properties of psychedelics are exploring possibilities to use these substances for treating several psychiatric disorders, especially depression [43,46,51]. Clinical evidence already points to possible antidepressant and anxiolytic properties in psilocybin, LSD, ayahuasca, and DMT [38,43,52,53]. Additionally, ketamine, a non-classic psychedelic, has recently been approved as an alternative medication for treatment-resistant depression [54].
Besides the most prominent mechanisms of action cited above, ayahuasca’s substances seem to act in synergy when consumed together, directly or indirectly modulating many other molecular targets which may include not only serotoninergic receptors but also glutamatergic [55,56], dopaminergic [57,58], and endocannabinoid [35,59] systems, the serotonin transporter (SERT) [60,61], the VMAT [60], the TAAR [62], and the sigma-1 receptor [63,64]. With this complex pharmacological profile [65] in evidence, this review sought to analyze the molecular targets of ayahuasca and its substances and relate their modulation to the possible therapeutic effects the brew seems to provide.
2. A Brief Review of the Current Evidence for the Antidepressant Effects of Ayahuasca in Humans
As with other classical psychedelics such as psilocybin and LSD, published research regarding the therapeutic effects of ayahuasca has grown steadily in the last two decades. Although preliminary and still lacking further and more thorough evaluation, results so far have been promising, especially regarding the antidepressant and anxiolytic effects the brew seems to exert. Grob et al. (1996) [66] performed the first observational study with frequent ritual ayahuasca practitioners that reported possible antidepressant effects of the brew. Similar results have been demonstrated in observational studies in the following years by other groups [24,29,32,67,68]. The first preliminary open-label clinical trial investigating the antidepressant effects of ayahuasca was published by our group in 2015 [37], which was followed by a complementary study that expanded the sample in 2016 [40]. The first randomized, placebo-controlled trial (RCT) investigating the antidepressant effects of ayahuasca replicated the positive results found in the preliminary clinical trial [38]. Nevertheless, small sample sizes, single-dose administration, short time assessments, and alkaloid dose standardization are limitations that still need to be addressed in future research on the topic. Table 1 below summarizes the current evidence regarding ayahuasca’s antidepressant effects in depressed patients, which was basically derived from two clinical trials (one open and one placebo-controlled).

Table 1.
Clinical trials that investigated ayahuasca’s potential for the treatment of depression and related research.
3. Safety and Tolerability of Ayahuasca Administration
Although there is no formal report of deaths caused by ayahuasca intake, the lack of regulation over its production and consumption in some countries can result in medical tourism scenarios [72]. Excessive optimism of psychedelics’ therapeutic properties portraited in mediatic coverage can mask the consequences of reckless use, namely interaction with other psychoactive substances (such as Selective Serotonin Reuptake Inhibitors (SSRIs)) and with previous medical conditions, which can produce lifelong health impairments [72,73].
Ayahuasca consumption at reported doses in observational studies and clinical trials causes a wide variety of effects, of which some are desired, some are unwanted, and/or can be considered adverse events (AEs). Regarding randomized, blinded (single or double), and placebo-controlled trials, a recent review evaluated the occurrence of adverse events following ayahuasca administration to healthy volunteers and treatment-resistant depressive patients in 11 distinct trials (n = 108 ayahuasca administrations) [74]. On one hand, most common AEs reported were gastrointestinal malaise, nausea, vomiting, headaches, and mild-to-moderate transient increases in heart rate and blood pressure [74]. These AEs were all expected and known to occur with ayahuasca and other psychedelic administration such as LSD and psilocybin [74,75]. On the other hand, there were more clinically significant reports of anxiety, confusion, emotional distress, depersonalization, and dysphoric state manifestations after ayahuasca administration, although these are much less common. However, there are currently no reports of psychotic states, lasting AEs, trial dropouts, or the need for medical interventions in any case, even in more significant situations. All reported AEs were transient and resolved on their own with researcher’s psychological support [74].
Regarding the occurrence of more significant adverse events in clinical trials with ayahuasca performed by our group, there is a detailed report of the two instances where these effects had to be managed by our research team, how the situation was resolved, and an initial nine step guideline on managing this kind of occurrence [76]. Overall, it seems that as with other psychedelic administration, a cautious and detailed selection of the volunteers who are allowed to participate in the trials and a supportive setting constructed by researchers has been demonstrated to be enough to reduce and manage the occurrence of AEs in clinical trials. Nevertheless, there is still the need for further research with bigger samples to confirm the preliminary findings we have so far for the occurrence of both positive and negative effects.
4. Ayahuasca’s Alkaloid Content
The concentrations of psychoactive alkaloids in ayahuasca vary greatly due to the lack of standardization in the quantity and quality of the plants used in its production, the region where it is produced, cultural aspects related to its use, and the desired final concentration of the drink [77]. Previous studies that quantified the alkaloid levels present in diverse ayahuasca samples reported a wide range of concentrations. For example, the content of alkaloids reported by McKenna et al. (1984) [5] ranged from 0.15 mg/mL of harmine, 0.05 mg/mL of THH, and 0.125 mg/mL of DMT, to 4.67 mg/mL of harmine, 1.60 mg/mL of THH, 0.41 mg/mL of harmaline, and 0.60 mg/mL of DMT in a dose. Callaway (2005) [78] measured the levels of alkaloids in 29 samples of ayahuasca from different religions where it is consumed. Again, a wide variation in alkaloid levels was demonstrated in the different samples, with DMT ranging from 0 to 14.15 mg/mL, harmine from 0.45 to 22.85 mg/mL, THH from 0.48 to 23.8 mg/mL, and harmaline from <0.01 to 0.9 mg/mL [78]. Santos et al. (2017) [79] validated a solid-phase extraction technique to quantify the alkaloids of 20 ayahuasca samples, where the levels of DMT, harmine, harmaline, THH, tryptamine, and harmalol were evaluated in concentrations ranging from 0.3 to 36,7 mg/ml. Souza et al. (2019) [80] quantified DMT, harmine, THH, and harmaline in 38 ayahuasca samples. DMT was reported in concentrations from 0.62 to 3.4 mg/mL, harmine from 4.14 to 18.16 mg/mL, THH from 4.02 to 30.88 mg/mL, and for harmaline from 0.4 to 3.92 mg/mL. Table 2 below summarizes the data for studies that evaluated alkaloid levels in at least eight ayahuasca samples.

Table 2.
Ayahuasca’s alkaloid concentration reported in studies with at least 8 different samples.
Even across clinical trials with healthy and depressed patients, there is still a lack of standardization of the dosage administered to participants. Table 3 below illustrates this variation.

Table 3.
Ayahuasca’s alkaloid concentrations and dosages from clinical trials with ayahuasca.
This variation in alkaloid administration in investigations where ayahuasca is used is one of the major challenges associated with its possible use as an antidepressant. Although clinical research with ayahuasca is still in its infancy, the necessity for standardizing dosages given in different trials is becoming more apparent. In the current state, it is difficult to directly compare results amongst the published research. Furthermore, with the current published data it is very challenging to determine the optimal DMT to β-carboline ratio and dosages that maximize the therapeutic effects while also minimizing the occurrence of AEs. This and other challenges associated with standardized ayahuasca medicinal use (and other psychedelics) go beyond the main focus of this article and have been thoroughly discussed elsewhere [74,75,76,87].
5. Pharmacokinetics of Ayahuasca
The human pharmacokinetics of ayahuasca were evaluated for the first time in a study by Callaway et al. (1999) [88] after the administration of 2 mL/kg of ayahuasca (with alkaloid concentrations: harmine 1.70 mg/mL, harmaline 0.20 mg/mL, THH 1.07 mg/mL, and DMT 0.24 mg/mL). In this study, the following values of maximum concentration (CMAX) and time to reach CMAX (TMAX) were found in blood analysis: DMT—CMAX 15.8 ± 4.4 ng/mL, TMAX 107.5 ± 32.5 min; harmine—CMAX 114.8 ± 61.7 ng/mL, TMAX 102.0 ± 58.3 min; THH—CMAX 91.0 ± 22.0 ng/mL, TMAX 174.0 ± 39.6 min; harmaline—CMAX 6.3 ± 3.1 ng/mL, TMAX 145.0 ± 66.9 min. Another investigation looking at plasma alkaloid levels after administration of a low dose (standardized at 0.6 mg/kg DMT) and a high dose (standardized at 0.85 mg/kg DMT) of lyophilized ayahuasca was performed by Riba et al. (2003) [11]. In this study, CMAX values were found for DMT of 12.14 ng/mL and 17.44 ng/mL for low and high doses, respectively, with TMAX of 1.5 hours after administration for both doses. Dos Santos et al. (2011) [1] measured plasma DMT levels after administration of placebo, one dose and two consecutive doses of ayahuasca four hours apart, at a standardized dose of 0.75 mg/kg DMT. The mean ± SD of maximum concentration values was 13.97 ± 9.35 ng/mL for ayahuasca preceded by placebo and 32.57 ± 20.96 ng/mL for ayahuasca preceded by ayahuasca, suggesting a non-linear increase in DMT levels after administration of two consecutive doses of ayahuasca, likely due to prolonged peripheral MAO-A inhibition [1]. Another study by the same research group demonstrated a comparison with a single dose of ayahuasca (standardized at 1 mg/kg of DMT) and 20 mg of dextroamphetamine [84]. The mean ± SD of maximum plasma DMT concentration values was 11.8 ± 6.4 ng/mL. The median time at which CMAX was reached was 1.8 hours (range 1 to 4.5 hours) after administration [84].
According to these data, we can estimate the nM levels of the alkaloids at CMAX. Considering a single ayahuasca administration, the mean CMAX for DMT of the cited studies where CMAX was measured was (15.8 + 12.14 + 17.44 + 13.97 + 11.8)/5 = 14.23 ng/mL. The molar mass of DMT is 188.274, which results in a 75.58 nM mean maximum plasma concentration. At first this concentration appears to be lower than the required to interact with certain molecular targets, but there is an evidence-based proposed mechanism through which DMT can reach high local concentrations within neurons of the CNS [60,89]. Briefly, this three-step mechanism starts with the crossing of DMT through the blood-brain barrier (BBB) via uptake across the endothelial plasma membrane. To cross the BBB, DMT is actively transported through the endothelial plasma membrane via Mg2+ and ATP-dependent uptake [90,91,92]. Although we are not aware of an investigation regarding the precise mechanism by which this accomplished, it is possible that the Organic Cation Transporter (OTC) family of transporters is involved, since it can transport other closely related monoamines, such as serotonin [93].
This is corroborated to happen given the results from studies that verified the accumulation of DMT and other tryptamines in the brain after peripheral administration [91,94,95,96]. It has been shown that DMT promptly enters the CNS and is kept there after excretion in urine has stopped (24 hours), being detected up to 7 days after administration [97]. Next, cell-membrane SERT uptakes DMT to inside the neurons, where VMAT2 promotes its sequestration and accumulation within vesicles [89]. Here DMT is protected from MAO degradation and can be stored several days before being released when the correct stimuli are given [97]. The proposed mechanism and investigations that support it are evidence of DMT’s role as an important cellular messenger, since there is a considerable physiological effort and prioritization to transport, accumulate, and store it within the CNS [89]. Furthermore, it shows that DMT must have molecular activity endogenously in a much lower average concentration environment inside the human body, in the absence of exogenous intake [89]. If this molecule is active within this relatively hostile environment to its existence, exogenously administered DMT in tandem with peripheral degradation inhibition provided by MAO-A deactivation from β-carbolines surely augments its concentrations to levels high enough to influence other molecular targets it would otherwise not be capable of influencing. The current evidence for the action of DMT and β-carbolines within these possible targets is discussed with more detail in the next sections.
6. Ayahuasca’s Molecular Targets and Their Relation to Possible Antidepressant Effects
The following paragraphs will focus on the molecular targets of ayahuasca and its constituent substances in several neurotransmission systems. Figure 1 below summarizes the main effects described.
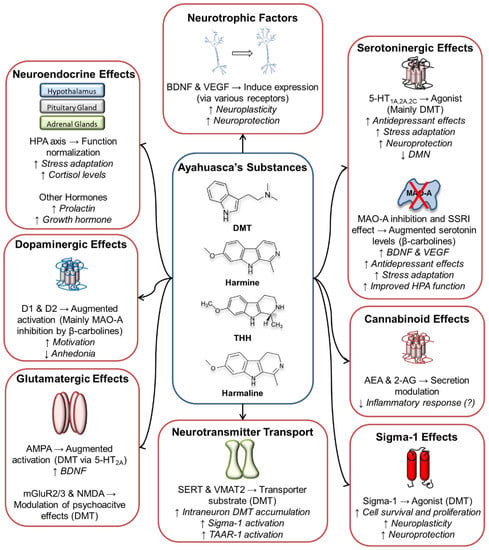
Figure 1.
Main molecular targets and effects of ayahuasca’s alkaloids. 2-AG: 2-Arachidonoylglycerol; 5-HT: Serotonin; AEA: Anandamide; AMPA: α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; BDNF: Brain-Derived Neurotrophic Factor; D1 and D2: Dopamine receptors 1 and 2; DMN: Default Mode Network; DMT: N,N-dimethyltryptamine; HPA axis: Hypothalamic-Pituitary-Adrenal axis; mGluR: Metabotropic Glutamate Receptor; MAO-A: Monoamine Oxidase A; NMDA: N-Methyl-D-aspartate receptor; SERT: Serotonin Transporter SSRI: Selective Serotonin Reuptake Inhibitor; TAAR-1: Trace Amine-Associated Receptor 1; THH: Tetrahydroharmine; VEGF: Vascular Endothelial Growth Factor; VMAT2: Vesicular Monoamine Transporter 2.
6.1. Serotoninergic System
As the main target of action of DMT and modulated by MAO-A inhibition via β-carbolines, the serotoninergic system is greatly affected by the ingestion of ayahuasca. DMT binds to multiple serotonergic receptor subtypes including 5-HT1A,1B,1D,2A,2B,2C,5A,6,7 with varying degrees of affinity [98,99,100,101,102,103] where it is believed to act as a partial or full agonist [102,104]. This system has been a primary target for antidepressant drugs for many decades, with MAO-inhibiting drugs being first introduced in the 1950s [105]. The β-carboline harmane has been shown to increase serotonin release in vitro [106], and acute peripheral harmane injection in rats resulted in increased levels of serotonin in the hippocampus, amygdala, the prefrontal cortex (PFC), and hypothalamus [107]. Furthermore, THH has been shown to possess an SSRI effect [108]. Considering many antidepressants and anxiolytic drugs act through similar mechanisms (i.e., increasing serotonin neurotransmission), the direct interaction with serotoninergic receptors by ayahuasca’s alkaloids and augmented levels of circulating serotonin via MAO-A inhibition are thought to be important protagonists in these possible antidepressant effects of the brew.
Although ayahuasca seems capable of influencing many serotonin receptors concomitantly, human evidence suggests that the 5-HT2A receptor is responsible for most of the subjective and neurophysiological effects of its administration [109]. Furthermore, this has been corroborated by similar research with LSD [9,110,111] and psilocybin [112,113]. Furthermore, while other serotonin receptors besides 5-HT1A,2A,2C may also influence ayahuasca’s effects, research that regards these receptors’ specific roles after DMT/β-carbolines/ayahuasca administration does not exist to the best of our knowledge. Taking these points in consideration, the next paragraphs will focus on the discussion of the activation of 5-HT1A,2A,2C receptors.
The 5-HT1A Gi/Go-protein coupled receptors are one of the most prominent targets for currently approved antidepressant drugs [114,115] and are believed to have a key role in the etiology and treatment of depression [116]. Whether through direct or indirect mechanisms, SSRIs, tricyclic antidepressants (TCAs), MAOI, other antidepressant drugs and therapies increase 5-HT1A post-synaptic signaling [117,118,119,120]. Furthermore, the therapeutic effects of antidepressants have been related to the neurogenesis associated with the activation of this receptor [121,122].
These receptors are involved in the molecular cascades of phospholipase-C activity regulation, inhibition of cAMP accumulation, adenylyl cyclase inhibition, and calcium current reduction [114,123,124] and are abundant in the hippocampus, hypothalamus, amygdala, cingulate, and infralimbic cortex, raphe nuclei, and layers I-II and to a lesser extent V-VI of the cerebral cortex [115,116]. In cortico-limbic areas, 5-HT1A receptors are located post-synaptically, while in the raphe nuclei (where the serotoninergic neurons project from) they are found as autoreceptors responsible for controlling serotonin release, activity, and neuronal firing to projected areas [117].
Ayahuasca’s alkaloids concomitantly modulate these receptors, with DMT being a 5-HT1A agonist [125] and β-carbolines enhancing their activation via the SSRI effect of THH and augmented serotonin neurotransmission by inhibiting MAO-A-induced serotonin degradation, a process which also occurs with other MAO-inhibiting antidepressant drugs [126]. It has previously been shown that harmaline-induced hypothermia was attenuated by co-administration with a 5-HT1A receptor antagonist, although the exact mechanism by which this occurs is not clear [127]. In humans, administration of pindolol (a 5-HT1A antagonist) significantly increased psychological responses to i.v. DMT, suggesting a buffering effect of 5-HT1A agonism on 5-HT2A-mediated effects [102]. Agonistic activation of this receptor has been proposed to improve stress adaptation [128,129,130], positively modulate the HPA axis [129,131], and possibly be related to neuroplastic effects [132,133], processes related to the remission of depressive symptoms. Preclinical behavioral investigations have shown that the deletion of 5-HT1A heteroreceptors in the dentate gyrus granule cells of the hippocampus abolished the positive effects of SSRIs in various tasks and attenuated the neuroplastic effects associated with their administration via a reduction in the expression of the brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) [122], which further demonstrates their importance for treatment of depression.
Arguably the most important molecular target for the effects of ayahuasca is the 5-HT2A Gq/G11 protein-coupled receptor. Neurons expressing 5-HT2A receptors are located mainly on layer V of pyramidal neurons from the neocortex, but also in limbic and basal ganglia structures such as the mammillary bodies of the hypothalamus, hippocampus, nucleus accumbens (NAc), amygdala, caudate, and putamen [134,135]. On a molecular level, the activation of these receptors induces intracellular signaling cascades which include phosphoinositide-specific phospholipase C (PLC)-induced increase in inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), extracellular signal-regulated kinases (ERK), tyrosine kinase pathways, and a β-arrestin2-mediated pathway that results from serotonin binding [135,136,137,138]. DMT binding to these receptors activates the phospholipase A2 signal transduction pathway, leading to more arachidonic acid release and less inositol phosphate formation [104,139].While 5-HT2A modulation by DMT is considered to be the main mechanism of action from ayahuasca, β-carbolines can also act as serotonin 5-HT2A receptor agonists, although generally with less affinity (Ki 230 nM for harmine, 7780 nM for harmaline, and >10,000 nM for THH) [140,141].
Preclinical evidence has demonstrated that chronic daily treatment with ayahuasca (28 days) in rats resulted in increased hippocampal BDNF levels in females [142]. Corroborating these findings, results from an RCT where ayahuasca was administered to treatment-resistant depressive patients revealed higher serum BDNF levels 2 days after treatment in both patients and controls when compared with the placebo [71]. Activation of 5-HT2A receptors has also been implicated in neuroprotective and neuroplastic effects [12,143]. It has been demonstrated that agonists at these receptors prevented NMDA antagonist neurotoxicity in the rat brain [144]. The activation of these receptors through administration of ayahuasca and other psychedelics has also been related to reduced activity of the default mode network (DMN), a brain-wide network that is overactive in depressed patients and related to rumination occurrence [145,146]. Indeed, there is evidence of neuroplastic effects in the PCC (a key node of the DMN) associated with long-term ritual ayahuasca use [25]. Ritual users showed increased cortical thinning in the PCC, which was inversely correlated with the intensity and duration of prior use of ayahuasca and with scores on the personality trait of self-transcendence. Users also showed increased cortical thickness in the ACC, a region prominent in the DMN that is related to emotional regulation and response to antidepressants [147].
Importantly, it is not yet known if the psychedelic effects induced by 5-HT2A receptor activation are necessary for the antidepressant or other therapeutic effects to occur. Although there is pre-clinical evidence that they are not [148] and that ayahuasca acts through many other pathways to exert its effects, studies on the concomitant administration of 5-HT2A receptor antagonists (such as ketanserin) with ayahuasca (and other psychedelics) that evaluate antidepressant effects in clinical populations have not been published to date. In healthy individuals, studies involving the concomitant administration of ketanserin with psilocybin [149], LSD [110], and ayahuasca [109] have shown that this drug blocks most of the subjective effects of these compounds.
On the other hand, we do have preliminary evidence for the antidepressant effect of psilocybin, which is thought to be much more specific in its central nervous system (CNS) modulation effects by acting primarily and mostly on 5-HT1A/2A/2C receptors [150,151]. In a first analysis, this would indicate that these receptors’ agonism is sufficient for the antidepressant effects to occur, but these investigations where psilocybin was administered to depressed patients also provided psychological support, which was not given in antidepressant effects investigations with ayahuasca and must account at least partially for the results reported. Furthermore, to the best of our knowledge there are no available drugs to specifically activate 5-HT2A receptors that could be used to verify the extent to which they participate in the possible therapeutic effects of ayahuasca and other psychedelics.
Another relevant serotoninergic target of ayahuasca’s effects is the 5-HT2C Gq/G11 protein-coupled receptor. The 5-HT2C receptors are abundant in choroid plexus epithelial cells and parvalbumin GABAergic neurons in the prelimbic PFC but are also expressed in many other limbic, cortical, and basal ganglia brain regions [114]. These receptors are intracellular messengers via phospholipase C, which triggers cell-signaling pathways related to the transcription of immediate early genes (IEGs) [114]. Being from the same family of receptors as 5-HT2A, they are also involved in IP3 and DAG intracellular accumulation via Gq/G11-protein signaling, ERK signaling, and arachidonic acid release via phospholipase A2 [114,152]. Depending on the agonist binding, these receptors will modulate which signaling pathway is activated, with psychedelic drugs including LSD, 2,5-dimethoxy-4-iodoamphetamine (DOI), and bufotenin (closely related to DMT) preferably activating the arachidonic acid/phospholipase A2 pathway [153].
Both DMT and harmine can bind at 5-HT2C receptors with low affinity [140]. Although several classes of antidepressants modulate 5-HT2C receptors, there is a need for further research to define their role in depression. Preclinical studies have provided conflicting evidence with regard to the agonism and antagonism at these receptors, as both types of modulation have resulted in antidepressant-like effects in preclinical investigations [154,155,156].
6.2. Glutamatergic System
The glutamatergic system is the main excitatory neurotransmitter system within the brain and is composed of several metabotropic glutamate G protein-coupled receptors (mGlusRs) and ionotropic glutamate receptors (iGluRs) [157]. Of special relation to ayahuasca’s effects are the AMPA receptors, ionotropic receptors distributed widely within the CNS, being found in the hippocampus, outer layer of the cortex, basal ganglia, lateral septum, amygdala, cerebellum, thalamus, and brain stem depending on the subunits expressed [158]. The interplay between 5-HT and AMPA receptors is thought to have an important role in the antidepressant and neuroplastic effects of ayahuasca, other psychedelics, and ketamine [159,160].
In this regard, DMT binding at 5-HT2A receptors seems to modulate glutamatergic activity [100,161,162,163], which results in the activation of AMPA receptors. This in turn leads to the expression of BDNF, a ligand to tyrosine receptor Kinase B (TrkB) receptors which promotes the activation of the mechanistic target of the rapamycin complex-1 (mTORC1) pathway [160,164]. This mechanism seems to induce neuronal synaptic and structural plasticity [160,165] and could be a possible explanation for psychedelics' long-lasting therapeutic effects. As BDNF can facilitate glutamate release, this pathway results in sustained activation [166]. Furthermore, the activation of 5-HT1A receptors located in the medial PFC has also been linked to the stimulation of the same pathway, which implicates not only DMT but also β-carbolines as protagonists in the neuroplastic effects of ayahuasca [159].
There are also further interactions between serotoninergic and glutamatergic systems that have been reported. Metabotropic glutamatergic receptors 2/3 (mGluR2/3) are modulated in the presence of 5-HT2A agonists, and there is evidence that the 5-HT2A-mGluR2 heterodimer receptor is related to the effects of serotonergic psychedelics [56]. Preclinical studies have also demonstrated that concomitant administration of a 5-HT2C receptor antagonist with DMT modestly attenuated DMT’s effects, while a mGluR2/3 receptor agonist potently blocked some of DMT’s effects and an antagonist at the same receptors facilitated DMT’s effects [56,167,168]. Furthermore, mGluR2 knockout mice had a greatly attenuated head twitch after DOI administration, which implicates these receptors in the effects of serotoninergic psychedelics [56]. Lastly, there is a study that implicated NMDA glutamatergic receptors as modulators of DMT’s effects. In this investigation, the effects of phencyclidine, an NMDA antagonist, were partially blocked by DMT administration [169]. However, the relationship of the above-cited interactions to the treatment of depression still warrants further research.
6.3. Dopaminergic System
Dopamine is a catecholamine related to multiple cognitive and behavioral functions, such as the reward system and working memory [170,171]. Dopaminergic receptors can be divided into two families, D1-like and D2-like receptors, which are coupled to stimulatory and inhibitory G proteins, respectively [172,173,174]. The D1-like receptor signaling pathway leads to the activation of adenylyl cyclase (AC), which promotes increased levels of cAMP, a second messenger that activates the protein kinase A. This pathway results in enhanced dopamine-activated phosphoprotein with 32kD (DARPP-32) expression and inhibits the protein phosphatase 1, responsible for the dephosphorylation of multiple cell proteins [174]. On the other hand, D2-like receptors inhibit AC expression, downregulating the D1 receptor signaling path [172].
The dopaminergic system has three main pathways. The substantia nigra projects neurons to the striatum, and this is the nigrostriatal pathway, involved in motor behavior [175]. Inputs from the ventral tegmental area (VTA) to the prefrontal cortex compose the mesocortical pathway, related to cognitive functions [176], and the VTA to NAc projections form the mesolimbic pathway, commonly referred to as the reward system [177]. Although depression could result in altered functioning in all dopaminergic pathways [178], mesolimbic projections can be directly linked to anhedonia, commonly observed in studies of depressive symptoms [20,179]. As for the mesocortical pathway, impaired functioning can result in the alterations in motivational behavior usually present in depressed individuals [176].
Ayahuasca modulation of the dopaminergic system may be primarily caused by β-carbolines’ ability to inhibit MAO-A [180], and, although β-carbolines do not seem to possess an affinity for dopaminergic receptors [99], the increase in the availability of monoamines caused by this inhibition can affect dopaminergic signaling, in a mechanism similar to that of the first antidepressants, the MAO inhibitors [181]. A study by Brierley and Davidson [57] demonstrated that harmine increases dopamine levels on the surface of the NAc. In this study, a comparison with moclobemide (a reversible, selective MAO-A inhibitor) did not cause the same effect, suggesting that the increase in dopamine concentrations in NAc may be related to its modulation by 5-HT2A receptors, a mechanism of action still little explored [57]. Even though ayahuasca components are not effective ligands at dopamine receptors [99], DMT could interfere with dopaminergic signaling through sigma-1 receptors since these receptors can be expressed on dopaminergic neurons [175]. Furthermore, DMT has been shown to cause dopamine release from presynaptic stores, possibly related to modulation in dopamine turnover and striatal synthesis, affecting endogenous levels of dopamine metabolites [104].
Ayahuasca administration to rats also resulted in enhanced amygdala DA concentrations [182] and treating animals with β-carbolines can improve depressive-like symptoms caused by chronic unpredictable stress [20]. Additionally, D2 receptors can form heterodimers with 5-HT2A receptors [183]. While psychedelics can increase excitability in cortical structures [161], the activation of D1-like receptors by an agonist is able to prevent DOI-driven excitability in rat brain slices, through cAMP signaling [184]. It is possible then that D2 receptors can play a role in enabling psychedelic effects by reducing cAMP signaling in the PFC. Furthermore, activation of D2 receptors in the PFC seems to improve depressive-like behavior in mice submitted to the tail suspension test [176].
The psychedelics’ effects on NAc activity are still not well understood, but LSD administration to rats resulted in increased c-Fos expression in the NAc [185], and ayahuasca and psilocybin intake increased the activity in the NAc of volunteers [40,186]. Since D1 and D2 activity result in opposite effects, it is possible that psychedelics might promote a preferential D2 receptor activation through modulation of cortical inputs to the NAc, consequently reducing the expression of the cAMP response element-binding protein (CREB).
6.4. Endocannabinoid System
The endocannabinoid receptors CB1 and CB2 are class A G protein-coupled receptors (GPCRs) that are widely distributed throughout the human body and are believed to be important in regulating endocrine, neurotransmission, neuroprotective, developmental, and immune processes [187,188,189]. These receptors are primarily involved in retrograde neurotransmission signaling and are thought to be important protagonists in the regulation and modulation of neuronal excitability and signal transmission [190]. CB1 receptor agonists inhibit forskolin-stimulated adenylyl cyclase, negatively modulate calcium channels (N-, P-, and Q-type), and stimulate potassium intracellular influx [189,191,192]. The CB2 receptor’s intracellular pathways include cyclooxygenase-2 (COX-2), mitogen-activated protein kinase and phosphoinositide 3-kinase pathways induction, and inhibition of adenylyl cyclase [193,194,195]. CB2 receptors are found in particularly high amounts in the immune system, and thus may provide a viable pathway to positively modulate inflammatory processes within the CNS which may be related to depressive symptoms [196]. Besides physiological functions, this system is also implicated in mood regulation, emotion processing and recognition, cognitive processes such as memory and learning, pain, and other homeostatic functions [188,197]. The most studied endocannabinoids which modulate this system are anandamide (AEA) and 2-arachidonoyl glycerol (2-AG). Both substances are thought to be important modulators of the biological processes cited above, within, and outside the CNS [198].
Preclinical evidence has shown that 5-HT2A receptor activation is linked to 2-AG secretion [199,200] and that stereotypic behavior induced by 5-HT2A receptor agonism is inhibited by the presence of CB1 receptor antagonists [201]. Furthermore, evidence supports the presence of heterodimer CB1+5-HT2A receptors in brain regions involved in memory formation [202]. Although preliminary, there is evidence that this system is modulated by ayahuasca ingestion, albeit indirectly through changes in circulating levels of AEA and 2-AG [35,59]. The first study showed a decrease in anandamide and a slight increase in 2-AG plasma levels 4 hours after ayahuasca intake by one healthy volunteer [59]. The second showed acutely evaluated changes in AEA and 2-AG in nine healthy volunteers and five volunteers with social anxiety disorder (SAD) from two randomized, placebo-controlled trials after a single ayahuasca administration [35]. This preliminary evidence pointed to an increase (90 minutes after ayahuasca administration) followed by a decrease (240 minutes after ayahuasca administration) in the levels of AEA in SAD patients, with no significant changes in healthy volunteers. Nevertheless, the limited samples in RCTs to date preclude solid conclusions in regard to the modulation of the endocannabinoid system by ayahuasca and its possible role in the therapeutic effects of the brew.
6.5. Sigma-1 Receptors
The sigma-1 receptor is distributed widely throughout the human body within the CNS and vital organs and is believed to have a considerable role in the etiology of depression and other diseases [203,204,205]. These receptors are localized between the endoplasmic reticulum (ER) and mitochondria, and agonists at these receptors cause them to disassociate from ER chaperones which transforms them into chaperones to IP3 receptors [104]. The sigma-1 receptors are involved in important molecular biological processes such as calcium signaling from ER to mitochondria, regulation of the citric acid cycle and ATP production, ion channel regulation, cell survival, and the proliferation and potentiation of NMDA receptors [60,104,206].
Regarding the effects of ayahuasca, DMT is believed to be an agonist at sigma-1 receptors and its action has been related to the modulation of the immune system and neuroprotective effect against hypoxia [63,100,207,208]. DMT’s activation of these receptors has also been proposed to be a possible therapeutic route for the extinction of traumatic memories, which may be beneficial to the treatment of post-traumatic stress disorder but also aversive memories in depression [209]. Furthermore, a recent in vitro investigation has shown that treatment of neural stem cells with DMT increased neurogenic processes (e.g., proliferation, migration, and cell differentiation), resulting in increased neuronal and glial populations and better performance in episodic memory tasks [210]. These effects were prevented when treatment was combined with a sigma-1 receptor antagonist, but not when it was combined with 5-HT1A/2A receptor antagonists, suggesting that the neurogenic enhancement promoted by DMT might not be dependent on serotonergic signaling [210]. There is also a possible association between DMT’s effects on synaptic plasticity and increased BDNF expression and its effect at sigma-1 receptors [211]. In fact, the combination of DMT's effects on serotoninergic and sigma-1 receptors seems to imbue this substance with a singular pharmacological profile, resulting in unique capabilities of neuroprotection and immune modulation [100,208]. To the best of our knowledge, no specific role of β-carbolines at these receptors has been reported to date.
6.6. Neuroendocrine System
The etiology of depression has been related to neuroendocrine changes for many decades. Stress-related changes in HPA axis function which result in increased hypothalamic corticotropin-releasing hormone (CRH) and consequently cortisol secretion have consistently been associated with the emergence of depressive symptoms, although it is not yet understood if this contributes as a cause to depressive symptoms or is an epiphenomenon [212,213]. Preclinical evidence has indicated that antidepressant medications facilitate the negative feedback by endogenous glucocorticoids, which in turn reduces HPA axis activity [214]. Dysfunctions in thyroxine (T4), thyroid-stimulating hormone (TSH), and growth hormone (GH) secretion have also been reported in depressive patients and correlated with emergence of depressive symptoms [215,216,217,218,219]. It is also known that many receptors influenced by ayahuasca are expressed in the hypothalamus, including 5-HT1A/2A/2C and sigma-1 receptors (reviewed in Schindler et al., 2018 [220]). Furthermore, preclinical evidence has previously shown that 5-HT1A/2A signaling is involved in modulating CRH [221], adrenocorticotropic hormone (ACTH) and corticosterone [222,223,224] release, while clinical evidence with LSD and psilocybin administration demonstrates increases in ACTH and cortisol after drug exposure [225,226,227].
Clinical trials with ayahuasca have shown it is capable of modulating neuroendocrine variables in healthy and depressed patients. Regarding healthy volunteers, ayahuasca administration (1.0 mg DMT/kg body weight) significantly increased prolactin and cortisol secretion when compared with baseline [1]. Another study related the administration of two ayahuasca doses (0.75 mg DMT/kg body weight) 4 hours apart with significant increases in serum prolactin, GH, and cortisol when compared with placebo [84]. In depressive patients with hypocortisolemia, a normalization of salivary cortisol levels 48 hours after a single ayahuasca administration (0.36 mg DMT/kg body weight), was observed, although no significant changes were reported for plasma cortisol levels [69].
6.7. Other Molecular Targets/Systems
DMT interacts with other proteins implicated in monoaminergic neurotransmission including SERT and neuronal VMAT2 [60,98,100]. DMT is a substrate for these transporters, a mechanism which is thought to be responsible for gathering sufficient vesicular concentrations of the alkaloid (where it can remain stored for at least 1 week) for it to become endogenously active, supposedly at sigma-1 receptors and TAAR1s [104]. DMT’s agonism (and that of other psychedelics including LSD, DOI and 5-MeO-DMT) at TAAR-1s has been shown to activate adenylyl cyclase and promote cAMP accumulation, although there is still no research regarding the possible role (if any) of this in the alkaloid’s effects [104,211]. These accumulation and storage mechanisms are thought to be related to the influence DMT (and thus ayahuasca) has on the CNS [104].
With regard to β-carbolines, harmine demonstrates a high affinity for tyrosine-phosphorylation-regulated protein kinase of dual specificity 1A (DYRK1A) and moderate affinity for and imidazoline I2 receptors [228,229,230]. The ability of harmine to inhibit intracellular protein aggregation has also been previously observed alongside its antioxidant potential [231]. Furthermore, harmine has also been found to influence the basal amygdala and projection neurons through their modulation of GABAergic neurons and its low-intensity agonism to GABA-A receptors in rats [232]. Harmaline and harmine seem to have an antagonistic effect on alpha-1 adrenergic receptors in a non-competitive manner [233] and possible acetylcholinesterase inhibition properties [160]. Furthermore, β-carbolines have also been shown to stimulate adult neurogenesis in vitro, although the exact mechanism by which they achieve this has not yet been fully elucidated [108].
Concerning ayahuasca as a whole, there is one report that described reductions in C-reactive protein in healthy controls and depressed patients alike, with this reduction correlated with suppression of depressive symptoms in patients, although there were no significant results regarding interleukin-6 [36]. C-reactive protein is a biomarker primarily produced by the liver that has been related to many pro-inflammatory processes within the body, including the production of other pro-inflammatory biomarkers such as interleukin-6 and tumor necrosis factor-α [234]. Reductions in the levels of such biomarkers are related to reductions in the inflammatory response and are hypothesized to be beneficial for depressed patients, especially those with an augmented inflammatory state [235,236]. Nevertheless, these and other inflammatory biomarkers have not yet been found to be reliable as indicators of response to rapid-acting antidepressants (such as psychedelics and ketamine), and the mechanism by which ayahuasca modulates their levels is not fully understood [75,237]. In fact, all these ayahuasca interactions have not been thoroughly investigated and it is yet to be shown how much they contribute to the psychoactive and possible therapeutic effects of ayahuasca in humans.
7. Final Remarks
As evidenced in this review, the psychoactive and possible therapeutic effects of ayahuasca cannot be explained by a single receptor or neurotransmitter system. Given the complex combination of alkaloids contained in ayahuasca, the physiological and consequently mental effects of the brew probably arise from the modulation of multiple targets at once, in an intricate molecular symphony that gives ayahuasca a difficult but unique pharmacological profile. Preclinical and clinical studies that focus on the effects of its separate substances will continue to help unfold the effects of particular molecular targets, but ultimately future investigations should focus on researching how these effects interact with each other to fully elucidate ayahuasca’s possible therapeutic potential.
Author Contributions
Writing—original draft preparation, G.N.R., L.T.L.G. and R.G.d.S.; writing—review and editing, G.B.B., S.M.D., J.C.B.S., J.E.C.H. and R.G.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
G.N.R. received funding from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). L.T.L.G. received funding from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil). J.E.C.H. is recipient of CNPq 1A productivity fellowship. R.G.S. is Fellow of the Programa Nacional de Pós-Doutorado, Brazil (PNPD/CAPES).
Acknowledgments
We thank the main science-supporting agencies (CAPES, CNPq and FAPESP) for the resources provided to fund this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- dos Santos, R.G.; Valle, M.; Bouso, J.C.; Nomdedéu, J.F.; Rodríguez-Espinosa, J.; McIlhenny, E.H.; Barker, S.A.; Barbanoj, M.J.; Riba, J. Autonomic, Neuroendocrine, and Immunological Effects of Ayahuasca: A Comparative Study With d-Amphetamine. J. Clin. Psychopharmacol. 2011, 31, 717–726. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Osório, F.L.; Crippa, J.A.; Hallak, J.E. Antidepressive and Anxiolytic Effects of Ayahuasca: A Systematic Literature Review of Animal and Human Studies. Braz. J. Psychiatry 2016, 38, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Schultes, R.E.; Ceballos, L.F.; Castillo, A. El desarrollo histórico de la identificación de las malpigiáceas empleadas como alucinógenos. Am. Indig. 1986, 46, 9–47. [Google Scholar] [PubMed]
- Cata-Preta, E.G.; Serra, Y.A.; Moreira-Junior, E.D.C.; Reis, H.S.; Kisaki, N.D.; Libarino-Santos, M.; Silva, R.R.R.; Barros-Santos, T.; Santos, L.C.; Barbosa, P.C.R.; et al. Ayahuasca and Its DMT- and β-Carbolines—Containing Ingredients Block the Expression of Ethanol-Induced Conditioned Place Preference in Mice: Role of the Treatment Environment. Front. Pharmacol. 2018, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.J.; Towers, G.H.; Abbott, F. Monoamine Oxidase Inhibitors in South American Hallucinogenic Plants: Tryptamine and Beta-Carboline Constituents of Ayahuasca. J. Ethnopharmacol. 1984, 10, 195–223. [Google Scholar] [CrossRef]
- Buckholtz, N.S.; Boggan, W.O. Monoamine Oxidase Inhibition in Brain and Liver Produced by Beta-Carbolines: Structure-Activity Relationships and Substrate Specificity. Biochem. Pharmacol. 1977, 26, 1991–1996. [Google Scholar] [CrossRef]
- Knudsen, G.M. Sustained Effects of Single Doses of Classical Psychedelics in Humans. Neuropsychopharm 2022, 1–6. [Google Scholar] [CrossRef]
- Hirschfeld, T.; Schmidt, T.T. Dose-Response Relationships of Psilocybin-Induced Subjective Experiences in Humans. J. Psychopharmacol. 2021, 35, 384–397. [Google Scholar] [CrossRef]
- Holze, F.; Avedisian, I.; Varghese, N.; Eckert, A.; Liechti, M.E. Role of the 5-HT2A Receptor in Acute Effects of LSD on Empathy and Circulating Oxytocin. Front. Pharmacol. 2021, 12, 711255. [Google Scholar] [CrossRef]
- Nichols, D.E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. [Google Scholar] [CrossRef]
- Riba, J.; Valle, M.; Urbano, G.; Yritia, M.; Morte, A.; Barbanoj, M.J. Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion, and Pharmacokinetics. J. Pharmacol. Exp. Ther. 2003, 306, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A.; Monti, J.A.; Christian, S.T. Metabolism of the Hallucinogen N,N-Dimethyltryptamine in Rat Brain Homogenates. Biochem. Pharmacol. 1980, 29, 1049–1057. [Google Scholar] [CrossRef]
- Riba, J.; McIlhenny, E.H.; Bouso, J.C.; Barker, S.A. Metabolism and Urinary Disposition of N,N-Dimethyltryptamine after Oral and Smoked Administration: A Comparative Study. Drug Test. Anal. 2015, 7, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Abelaira, H.M.; Réus, G.Z.; Scaini, G.; Streck, E.L.; Crippa, J.A.; Quevedo, J. β-Carboline Harmine Reverses the Effects Induced by Stress on Behaviour and Citrate Synthase Activity in the Rat Prefrontal Cortex. Acta Neuropsychiatr. 2013, 25, 328–333. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.G.; Daros, G.C.; Santos, F.P.; Yonamine, M.; de Bitencourt, R.M. Antidepressant and Anxiolytic-like Effects of Ayahuasca in Rats Subjected to LPS-Induced Neuroinflammation. Behav. Brain Res. 2022, 434, 114007. [Google Scholar] [CrossRef]
- Daldegan-Bueno, D.; Favaro, V.M.; Morais, P.R.; Sussulini, A.; Oliveira, M.G.M. Effects of Repeated Ayahuasca Administration on Behaviour and C-Fos Expression in Male Rats Exposed to the Open Field. Behav. Brain Res. 2022, 427, 113878. [Google Scholar] [CrossRef]
- Farzin, D.; Mansouri, N. Antidepressant-like Effect of Harmane and Other β-Carbolines in the Mouse Forced Swim Test. Eur. Neuropsychopharmacol. 2006, 16, 324–328. [Google Scholar] [CrossRef]
- Fortunato, J.J.; Réus, G.Z.; Kirsch, T.R.; Stringari, R.B.; Stertz, L.; Kapczinski, F.; Pinto, J.P.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; et al. Acute Harmine Administration Induces Antidepressive-like Effects and Increases BDNF Levels in the Rat Hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1425–1430. [Google Scholar] [CrossRef]
- Fortunato, J.J.; Réus, G.Z.; Kirsch, T.R.; Stringari, R.B.; Fries, G.R.; Kapczinski, F.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Quevedo, J. Effects of β-Carboline Harmine on Behavioral and Physiological Parameters Observed in the Chronic Mild Stress Model: Further Evidence of Antidepressant Properties. Brain Res. Bull. 2010, 81, 491–496. [Google Scholar] [CrossRef]
- Fortunato, J.J.; Réus, G.Z.; Kirsch, T.R.; Stringari, R.B.; Fries, G.R.; Kapczinski, F.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Quevedo, J. Chronic Administration of Harmine Elicits Antidepressant-like Effects and Increases BDNF Levels in Rat Hippocampus. J. Neural Transm. 2010, 117, 1131–1137. [Google Scholar] [CrossRef]
- Hilber, P.; Chapillon, P. Effects of Harmaline on Anxiety-Related Behavior in Mice. Physiol. Behav. 2005, 86, 164–167. [Google Scholar] [CrossRef]
- Réus, G.Z.; Stringari, R.B.; de Souza, B.; Petronilho, F.; Dal-Pizzol, F.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Quevedo, J. Harmine and Imipramine Promote Antioxidant Activities in Prefrontal Cortex and Hippocampus. Oxidative Med. Cell. Longev. 2010, 3, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Argento, E.; Capler, R.; Thomas, G.; Lucas, P.; Tupper, K.W. Exploring Ayahuasca-Assisted Therapy for Addiction: A Qualitative Analysis of Preliminary Findings among an Indigenous Community in Canada. Drug Alcohol Rev. 2019, 38, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Bouso, J.C.; González, D.; Fondevila, S.; Cutchet, M.; Fernández, X.; Ribeiro Barbosa, P.C.; Alcázar-Córcoles, M.; Araújo, W.S.; Barbanoj, M.J.; Fábregas, J.M.; et al. Personality, Psychopathology, Life Attitudes and Neuropsychological Performance among Ritual Users of Ayahuasca: A Longitudinal Study. PLoS ONE 2012, 7, e42421. [Google Scholar] [CrossRef] [PubMed]
- Bouso, J.C.; Palhano-Fontes, F.; Rodríguez-Fornells, A.; Ribeiro, S.; Sanches, R.; Crippa, J.A.S.; Hallak, J.E.C.; de Araujo, D.B.; Riba, J. Long-Term Use of Psychedelic Drugs Is Associated with Differences in Brain Structure and Personality in Humans. Eur. Neuropsychopharmacol. 2015, 25, 483–492. [Google Scholar] [CrossRef]
- Domínguez-Clavé, E.; Soler, J.; Pascual, J.C.; Elices, M.; Franquesa, A.; Valle, M.; Alvarez, E.; Riba, J. Ayahuasca Improves Emotion Dysregulation in a Community Sample and in Individuals with Borderline-like Traits. Psychopharmacology 2019, 236, 573–580. [Google Scholar] [CrossRef]
- Gonzalez, D.; Cantillo, J.; Perez, I.; Carvalho, M.; Aronovich, A.; Farre, M.; Feilding, A.; Obiols, J.E.; Bouso, J.C. The Shipibo Ceremonial Use of Ayahuasca to Promote Well-Being: An Observational Study. Front. Pharmacol. 2021, 12, 623923. [Google Scholar] [CrossRef]
- González, D.; Cantillo, J.; Pérez, I.; Farré, M.; Feilding, A.; Obiols, J.E.; Bouso, J.C. Therapeutic Potential of Ayahuasca in Grief: A Prospective, Observational Study. Psychopharmacology 2020, 237, 1171–1182. [Google Scholar] [CrossRef]
- Jiménez-Garrido, D.F.; Gómez-Sousa, M.; Ona, G.; Dos Santos, R.G.; Hallak, J.E.C.; Alcázar-Córcoles, M.; Bouso, J.C. Effects of Ayahuasca on Mental Health and Quality of Life in Naïve Users: A Longitudinal and Cross-Sectional Study Combination. Sci. Rep. 2020, 10, 4075. [Google Scholar] [CrossRef]
- Kohek, M.; Ona, G.; van Elk, M.; dos Santos, R.G.; Hallak, J.E.C.; Alcázar-Córcoles, M.; Bouso, J.C. Ayahuasca and Public Health II: Health Status in a Large Sample of Ayahuasca-Ceremony Participants in the Netherlands. J. Psychoact. Drugs 2022, 1–12. [Google Scholar] [CrossRef]
- Thomas, G.; Lucas, P.; Capler, N.R.; Tupper, K.W.; Martin, G. Ayahuasca-Assisted Therapy for Addiction: Results from a Preliminary Observational Study in Canada. Curr. Drug Abuse Rev. 2013, 6, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Uthaug, M.V.; van Oorsouw, K.; Kuypers, K.P.C.; van Boxtel, M.; Broers, N.J.; Mason, N.L.; Toennes, S.W.; Riba, J.; Ramaekers, J.G. Sub-Acute and Long-Term Effects of Ayahuasca on Affect and Cognitive Thinking Style and Their Association with Ego Dissolution. Psychopharmacology 2018, 235, 2979–2989. [Google Scholar] [CrossRef] [PubMed]
- van Oorsouw, K.; Toennes, S.W.; Ramaekers, J.G. Therapeutic Effect of an Ayahuasca Analogue in Clinically Depressed Patients: A Longitudinal Observational Study. Psychopharmacology 2022, 239, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.G.; Landeira-Fernandez, J.; Strassman, R.J.; Motta, V.; Cruz, A.P. Effects of Ayahuasca on Psychometric Measures of Anxiety, Panic-like and Hopelessness in Santo Daime Members. J. Ethnopharmacol. 2007, 112, 507–513. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Rocha, J.M.; Rossi, G.N.; Osório, F.L.; Ona, G.; Bouso, J.C.; Silveira, G.O.; Yonamine, M.; Marchioni, C.; Crevelin, E.J.; et al. Effects of Ayahuasca on the Endocannabinoid System of Healthy Volunteers and in Volunteers with Social Anxiety Disorder: Results from Two Pilot, Proof-of-Concept, Randomized, Placebo-Controlled Trials. Hum. Psychopharmacol. 2022, 37, e2834. [Google Scholar] [CrossRef]
- Galvão-Coelho, N.L.; de Menezes Galvão, A.C.; de Almeida, R.N.; Palhano-Fontes, F.; Campos Braga, I.; Lobão Soares, B.; Maia-de-Oliveira, J.P.; Perkins, D.; Sarris, J.; de Araujo, D.B. Changes in Inflammatory Biomarkers Are Related to the Antidepressant Effects of Ayahuasca. J. Psychopharmacol. 2020, 34, 1125–1133. [Google Scholar] [CrossRef]
- Osório Fde, L.; Sanches, R.F.; Macedo, L.R.; Santos, R.G.; Maia-de-Oliveira, J.P.; Wichert-Ana, L.; Araujo, D.B.; Riba, J.; Crippa, J.A.; Hallak, J.E. Antidepressant Effects of a Single Dose of Ayahuasca in Patients with Recurrent Depression: A Preliminary Report. Braz. J. Psychiatry 2015, 37, 13–20. [Google Scholar] [CrossRef]
- Palhano-Fontes, F.; Barreto, D.; Onias, H.; Andrade, K.C.; Novaes, M.M.; Pessoa, J.A.; Mota-Rolim, S.A.; Osório, F.L.; Sanches, R.; Dos Santos, R.G.; et al. Rapid Antidepressant Effects of the Psychedelic Ayahuasca in Treatment-Resistant Depression: A Randomized Placebo-Controlled Trial. Psychol. Med. 2019, 49, 655–663. [Google Scholar] [CrossRef]
- Riba, J.; Romero, S.; Grasa, E.; Mena, E.; Carrió, I.; Barbanoj, M.J. Increased Frontal and Paralimbic Activation Following Ayahuasca, the Pan-Amazonian Inebriant. Psychopharmacology 2006, 186, 93–98. [Google Scholar] [CrossRef]
- Sanches, R.F.; Osório, F.L.; dos Santos, R.G.; Macedo, L.R.H.; Maia-de-Oliveira, J.P.; Wichert-Ana, L.; de Araujo, D.B.; Riba, J.; Crippa, J.A.S.; Hallak, J.E.C. Antidepressant Effects of a Single Dose of Ayahuasca in Patients with Recurrent Depression: A SPECT Study. J. Clin. Psychopharmacol. 2016, 36, 77–81. [Google Scholar] [CrossRef]
- Zeifman, R.J.; Singhal, N.; Dos Santos, R.G.; Sanches, R.F.; de Lima Osório, F.; Hallak, J.E.C.; Weissman, C.R. Rapid and Sustained Decreases in Suicidality Following a Single Dose of Ayahuasca among Individuals with Recurrent Major Depressive Disorder: Results from an Open-Label Trial. Psychopharmacology 2021, 238, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Aaghaz, S.; Sharma, K.; Jain, R.; Kamal, A. β-Carbolines as Potential Anticancer Agents. Eur. J. Med. Chem. 2021, 216, 113321. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Osório, F.L.; Crippa, J.A.; Riba, J.; Zuardi, A.W.; Hallak, J.E. Antidepressive, Anxiolytic, and Antiaddictive Effects of Ayahuasca, Psilocybin and Lysergic Acid Diethylamide (LSD): A Systematic Review of Clinical Trials Published in the Last 25 Years. Ther. Adv. Psychopharmacol. 2016, 6, 193–213. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.G.; Hallak, J.E.C. Effects of the Natural β-Carboline Alkaloid Harmine, a Main Constituent of Ayahuasca, in Memory and in the Hippocampus: A Systematic Literature Review of Preclinical Studies. J. Psychoact. Drugs 2017, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.G.; Hallak, J.E.C. Ayahuasca, an Ancient Substance with Traditional and Contemporary Use in Neuropsychiatry and Neuroscience. Epilepsy Behav. 2021, 121, 106300. [Google Scholar] [CrossRef]
- Galvão-Coelho, N.L.; Marx, W.; Gonzalez, M.; Sinclair, J.; de Manincor, M.; Perkins, D.; Sarris, J. Classic Serotonergic Psychedelics for Mood and Depressive Symptoms: A Meta-Analysis of Mood Disorder Patients and Healthy Participants. Psychopharmacology 2021, 238, 341–354. [Google Scholar] [CrossRef]
- Hamill, J.; Hallak, J.; Dursun, S.M.; Baker, G. Ayahuasca: Psychological and Physiologic Effects, Pharmacology and Potential Uses in Addiction and Mental Illness. Curr. Neuropharmacol. 2019, 17, 108–128. [Google Scholar] [CrossRef]
- Nunes, A.A.; Dos Santos, R.G.; Osório, F.L.; Sanches, R.F.; Crippa, J.A.; Hallak, J.E. Effects of Ayahuasca and Its Alkaloids on Drug Dependence: A Systematic Literature Review of Quantitative Studies in Animals and Humans. J. Psychoact. Drugs 2016, 48, 195–205. [Google Scholar] [CrossRef]
- Rodrigues, L.S.; Rossi, G.N.; Rocha, J.M.; Osório, F.L.; Bouso, J.C.; Hallak, J.E.C.; Dos Santos, R.G. Effects of Ayahuasca and Its Alkaloids on Substance Use Disorders: An Updated (2016–2020) Systematic Review of Preclinical and Human Studies. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 541–556. [Google Scholar] [CrossRef]
- Calder, A.E.; Hasler, G. Towards an Understanding of Psychedelic-Induced Neuroplasticity. Neuropsychopharmacology 2022, 1–9. [Google Scholar] [CrossRef]
- Wolf, M.E.; Abi-Dargham, A. Synaptic Plasticity as a Therapeutic Target to Modulate Circuits in Psychiatric Disorders. Neuropsychopharmacology 2022, 1–2. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.C.; Syed, S.A.; Flynn, L.T.; Safi-Aghdam, H.; Cozzi, N.V.; Ranganathan, M. Exploratory Study of the Dose-Related Safety, Tolerability, and Efficacy of Dimethyltryptamine (DMT) in Healthy Volunteers and Major Depressive Disorder. Neuropsychopharmacology 2022, 47, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.; Holstein, D.; Michel, Y.; Doblin, R.; Yazar-Klosinski, B.; Passie, T.; Brenneisen, R. Safety and Efficacy of Lysergic Acid Diethylamide-Assisted Psychotherapy for Anxiety Associated With Life-Threatening Diseases. J. Nerv. Ment. Dis. 2014, 202, 513–520. [Google Scholar] [CrossRef]
- Jelen, L.A.; Stone, J.M. Ketamine for Depression. Int. Rev. Psychiatry 2021, 33, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Delille, H.K.; Becker, J.M.; Burkhardt, S.; Bleher, B.; Terstappen, G.C.; Schmidt, M.; Meyer, A.H.; Unger, L.; Marek, G.J.; Mezler, M. Heterocomplex Formation of 5-HT2A-MGlu2 and Its Relevance for Cellular Signaling Cascades. Neuropsychopharmacology 2012, 62, 2184–2191. [Google Scholar] [CrossRef]
- Moreno, J.L.; Holloway, T.; Albizu, L.; Sealfon, S.C.; González-Maeso, J. Metabotropic Glutamate MGlu2 Receptor Is Necessary for the Pharmacological and Behavioral Effects Induced by Hallucinogenic 5-HT2A Receptor Agonists. Neurosci. Lett. 2011, 493, 76–79. [Google Scholar] [CrossRef]
- Brierley, D.I.; Davidson, C. Harmine Augments Electrically Evoked Dopamine Efflux in the Nucleus Accumbens Shell. J. Psychopharmacol. 2013, 27, 98–108. [Google Scholar] [CrossRef]
- Iurlo, M.; Leone, G.; Schilström, B.; Linnér, L.; Nomikos, G.; Hertel, P.; Silvestrini, B.; Svensson, H. Effects of Harmine on Dopamine Output and Metabolism in Rat Striatum: Role of Monoamine Oxidase-A Inhibition. Psychopharmacology 2001, 159, 98–104. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Crippa, J.A.; Osório, F.L.; Rocha, J.M.; Rossi, G.N.; Marchioni, C.; Queiroz, M.E.C.; Silveira, G.O.; Yonamine, M.; Hallak, J.E.C. Possible Interactions Between 5-HT2A Receptors and the Endocannabinoid System in Humans: Preliminary Evidence of Interactive Effects of Ayahuasca and Endocannabinoids in a Healthy Human Subject. J. Clin. Psychopharmacol. 2018, 38, 644–646. [Google Scholar] [CrossRef]
- Cozzi, N.V.; Gopalakrishnan, A.; Anderson, L.L.; Feih, J.T.; Shulgin, A.T.; Daley, P.F.; Ruoho, A.E. Dimethyltryptamine and Other Hallucinogenic Tryptamines Exhibit Substrate Behavior at the Serotonin Uptake Transporter and the Vesicle Monoamine Transporter. J. Neural Transm. 2009, 116, 1591–1599. [Google Scholar] [CrossRef]
- Nagai, F.; Nonaka, R.; Satoh Hisashi Kamimura, K. The Effects of Non-Medically Used Psychoactive Drugs on Monoamine Neurotransmission in Rat Brain. Eur. J. Pharmacol. 2007, 559, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-Methylenedioxymethamphetamine, Lysergic Acid Diethylamide, and Metabolites of the Catecholamine Neurotransmitters Are Agonists of a Rat Trace Amine Receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The Hallucinogen N,N -Dimethyltryptamine (DMT) Is an Endogenous Sigma-1 Receptor Regulator. Science 2009, 323, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Su, T.P.; Hayashi, T.; Vaupel, D.B. When the Endogenous Hallucinogenic Trace Amine N,N-Dimethyltryptamine Meets the Sigma-1 Receptor. Sci. Signal. 2009, 2, pe12. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, S.; Netzband, N.; Bird, C.; Young, A.H.; Juruena, M.F. The Pharmacological Interaction of Compounds in Ayahuasca: A Systematic Review. Braz. J. Psychiatry 2020, 42, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Grob, C.S.; McKenna, D.J.; Callaway, J.C.; Brito, G.S.; Neves, E.S.; Oberlaender, G.; Saide, O.L.; Labigalini, E.; Tacla, C.; Miranda, C.T.; et al. Human Psychopharmacology of Hoasca, a Plant Hallucinogen Used in Ritual Context in Brazil. J. Nerv. Ment. Dis. 1996, 184, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, D.X.; Grob, C.S.; de Rios, M.D.; Lopez, E.; Alonso, L.K.; Tacla, C.; Doering-Silveira, E. Ayahuasca in Adolescence: A Preliminary Psychiatric Assessment. J. Psychoact. Drugs 2005, 37, 129–133. [Google Scholar] [CrossRef]
- Sarris, J.; Perkins, D.; Cribb, L.; Schubert, V.; Opaleye, E.; Bouso, J.C.; Scheidegger, M.; Aicher, H.; Simonova, H.; Horák, M.; et al. Ayahuasca Use and Reported Effects on Depression and Anxiety Symptoms: An International Cross-Sectional Study of 11,912 Consumers. J. Affect. Disord. Rep. 2021, 4, 100098. [Google Scholar] [CrossRef]
- Galvão, A.C.M.; de Almeida, R.N.; Silva, E.; Freire, F.A.M.; Palhano-Fontes, F.; Onias, H.; Arcoverde, E.; Maia-de-Oliveira, J.P.; de Araújo, D.B.; Lobão-Soares, B.; et al. Cortisol Modulation by Ayahuasca in Patients With Treatment Resistant Depression and Healthy Controls. Front. Psychiatry 2018, 9, 185. [Google Scholar] [CrossRef]
- Zeifman, R.J.; Palhano-Fontes, F.; Hallak, J.; Arcoverde, E.; Maia-Oliveira, J.P.; Araujo, D.B. The Impact of Ayahuasca on Suicidality: Results From a Randomized Controlled Trial. Front. Pharmacol. 2019, 10, 1325. [Google Scholar] [CrossRef]
- de Almeida, R.N.; Galvão, A.C.M.; da Silva, F.S.; Silva, E.; Palhano-Fontes, F.; Maia-de-Oliveira, J.P.; de Araújo, L.B.; Lobão-Soares, B.; Galvão-Coelho, N.L. Modulation of Serum Brain-Derived Neurotrophic Factor by a Single Dose of Ayahuasca: Observation From a Randomized Controlled Trial. Front. Psychol. 2019, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.L. Ayahuasca: A risk for travellers? Travel Med. Infect. Dis. 2018, 21, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Callaway, J.C.; Grob, C.S. Ayahuasca Preparations and Serotonin Reuptake Inhibitors: A Potential Combination for Severe Adverse Interactions. J. Psychoact. Drugs 1998, 30, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.N.; Dias, I.C.D.S.; Baker, G.; Bouso Saiz, J.C.; Dursun, S.M.; Hallak, J.E.C.; Dos Santos, R.G. Ayahuasca, a Potentially Rapid Acting Antidepressant: Focus on Safety and Tolerability. Expert Opin. Drug Saf. 2022, 21, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.N.; Hallak, J.E.C.; Baker, G.; Dursun, S.M.; Dos Santos, R.G. The Effects of Ketamine and Classic Hallucinogens on Neurotrophic and Inflammatory Markers in Unipolar Treatment-Resistant Depression: A Systematic Review of Clinical Trials. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.M.; Rossi, G.N.; Osório, F.L.; Hallak, J.E.C.; Dos Santos, R.G. Adverse Effects After Ayahuasca Administration in the Clinical Setting. J. Clin. Psychopharmacol. 2022, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.J. Clinical Investigations of the Therapeutic Potential of Ayahuasca: Rationale and Regulatory Challenges. Pharmacol. Ther. 2004, 102, 111–129. [Google Scholar] [CrossRef]
- Callaway, J.C. Various Alkaloid Profiles in Decoctions of Banisteriopsis caapi. J. Psychoact. Drugs 2005, 37, 151–155. [Google Scholar] [CrossRef]
- Santos, M.C.; Navickiene, S.; Gaujac, A. Determination of Tryptamines and β-Carbolines in Ayahuasca Beverage Consumed During Brazilian Religious Ceremonies. J. AOAC Int. 2017, 100, 820–824. [Google Scholar] [CrossRef][Green Version]
- Souza, R.C.Z.; Zandonadi, F.S.; Freitas, D.P.; Tófoli, L.F.F.; Sussulini, A. Validation of an Analytical Method for the Determination of the Main Ayahuasca Active Compounds and Application to Real Ayahuasca Samples from Brazil. J. Chromatogr. B 2019, 1124, 197–203. [Google Scholar] [CrossRef]
- Santos, B.W.L.; de Oliveira, R.C.; Sonsin-Oliveira, J.; Fagg, C.W.; Barbosa, J.B.F.; Caldas, E.D. Biodiversity of β-Carboline Profile of Banisteriopsis Caapi and Ayahuasca, a Plant and a Brew with Neuropharmacological Potential. Plants 2020, 9, 870. [Google Scholar] [CrossRef] [PubMed]
- Kaasik, H.; Souza, R.C.Z.; Zandonadi, F.S.; Tófoli, L.F.; Sussulini, A. Chemical Composition of Traditional and Analog Ayahuasca. J. Psychoact. Drugs 2021, 53, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Riba, J.; Rodríguez-Fornells, A.; Urbano, G.; Morte, A.; Antonijoan, R.; Montero, M.; Callaway, J.C.; Barbanoj, M.J. Subjective Effects and Tolerability of the South American Psychoactive Beverage Ayahuasca in Healthy Volunteers. Psychopharmacology 2001, 154, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.G.; Grasa, E.; Valle, M.; Ballester, M.R.; Bouso, J.C.; Nomdedéu, J.F.; Homs, R.; Barbanoj, M.J.; Riba, J. Pharmacology of Ayahuasca Administered in Two Repeated Doses. Psychopharmacology 2012, 219, 1039–1053. [Google Scholar] [CrossRef]
- Rocha, J.M.; Rossi, G.N.; de Lima Osório, F.; Bouso, J.C.; de Oliveira Silveira, G.; Yonamine, M.; Campos, A.C.; Bertozi, G.; Cecílio Hallak, J.E.; Dos Santos, R.G. Effects of Ayahuasca on the Recognition of Facial Expressions of Emotions in Naive Healthy Volunteers: A Pilot, Proof-of-Concept, Randomized Controlled Trial. J. Clin. Psychopharmacol. 2021, 41, 267–274. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Osório, F.D.L.; Rocha, J.M.; Rossi, G.N.; Bouso, J.C.; Rodrigues, L.S.; Silveira, G.D.O.; Yonamine, M.; Hallak, J.E.C. Ayahuasca improves self-perception of speech performance in subjects with social anxiety disorder. J. Clin. Psychopharmacol. 2021, 41, 540–550. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Bouso, J.C.; Rocha, J.M.; Rossi, G.N.; Hallak, J.E. The Use of Classic Hallucinogens/Psychedelics in a Therapeutic Context: Healthcare Policy Opportunities and Challenges. Risk Manag. Healthc. Policy 2021, 14, 901–910. [Google Scholar] [CrossRef]
- Callaway, J.C.; McKenna, D.J.; Grob, C.S.; Brito, G.S.; Raymon, L.P.; Poland, R.E.; Andrade, E.N.; Andrade, E.O.; Mash, D.C. Pharmacokinetics of Hoasca Alkaloids in Healthy Humans. J. Ethnopharmacol. 1999, 65, 243–256. [Google Scholar] [CrossRef]
- Frecska, E.; Szabo, A.; Winkelman, M.J.; Luna, L.E.; McKenna, D.J. A Possibly Sigma-1 Receptor Mediated Role of Dimethyltryptamine in Tissue Protection, Regeneration, and Immunity. J. Neural Transm. 2013, 120, 1295–1303. [Google Scholar] [CrossRef]
- Cohen, I.; Vogel, W.H. Determination and Physiological Disposition of Dimethyltryptamine and Diethyltryptamine in Rat Brain, Liver and Plasma. Biochem. Pharmacol. 1972, 21, 1214–1216. [Google Scholar] [CrossRef]
- Barker, S.A.; Beaton, J.M.; Christian, S.T.; Monti, J.A.; Morris, P.E. Comparison of the Brain Levels of N,N-Dimethyltryptamine and Alpha, Alpha, Beta, Beta-Tetradeutero-N-N-Dimethyltryptamine Following Intraperitoneal Injection. The in Vivo Kinetic Isotope Effect. Biochem. Pharmacol. 1982, 31, 2513–2516. [Google Scholar] [CrossRef]
- Christian, S.T.; Harrison, R.; Quayle, E.; Pagel, J.; Monti, J. The in Vitro Identification of Dimethyltryptamine (DMT) in Mammalian Brain and Its Characterization as a Possible Endogenous Neuroregulatory Agent. Biochem. Med. 1977, 18, 164–183. [Google Scholar] [CrossRef]
- Busch, A.E.; Karbach, U.; Miska, D.; Gorboulev, V.; Akhoundova, A.; Volk, C.; Arndt, P.; Ulzheimer, J.C.; Sonders, M.S.; Baumann, C.; et al. Human Neurons Express the Polyspecific Cation Transporter HOCT2, Which Translocates Monoamine Neurotransmitters, Amantadine, and Memantine. Mol. Pharmacol. 1998, 54, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, B.R.; Lockett, L.; Talomsin, R.; Blackman, G.L.; McLeod, W.R. In Vivo Metabolism of 5-Methoxy-N,N-Dimethyltryptamine and N,N-Dimethyltryptamine in the Rat. Biochem. Pharmacol. 1987, 36, 1509–1512. [Google Scholar] [CrossRef]
- Takahashi, T.; Takahashi, K.; Ido, T.; Yanai, K.; Iwata, R.; Ishiwata, K.; Nozoe, S. 11C-Labeling of Indolealkylamine Alkaloids and the Comparative Study of Their Tissue Distributions. Int. J. Appl. Radiat. Isot. 1985, 36, 965–969. [Google Scholar] [CrossRef]
- Yanai, K.; Ido, T.; Ishiwata, K.; Hatazawa, J.; Takahashi, T.; Iwata, R.; Matsuzawa, T. In Vivo Kinetics and Displacement Study of a Carbon-11-Labeled Hallucinogen, N,N-[11C]Dimethyltryptamine. Eur. J. Nucl. Med. 1986, 12, 141–146. [Google Scholar] [CrossRef]
- Vitale, A.A.; Pomilio, A.B.; Cañellas, C.O.; Vitale, M.G.; Putz, E.M.; Ciprian-Ollivier, J. In Vivo Long-Term Kinetics of Radiolabeled n,n-Dimethyltryptamine and Tryptamine. J. Nucl. Med. 2011, 52, 970–977. [Google Scholar] [CrossRef]
- Blough, B.E.; Landavazo, A.; Decker, A.M.; Partilla, J.S.; Baumann, M.H.; Rothman, R.B. Interaction of Psychoactive Tryptamines with Biogenic Amine Transporters and Serotonin Receptor Subtypes. Psychopharmacology 2014, 231, 4135–4144. [Google Scholar] [CrossRef]
- Glennon, R.A.; Dukat, M.; Grella, B.; Hong, S.-S.; Costantino, L.; Teitler, M.; Smith, C.; Egan, C.; Davis, K.; Mattson, M.V. Binding of β-Carbolines and Related Agents at Serotonin (5-HT2 and 5-HT1A), Dopamine (D2) and Benzodiazepine Receptors. Drug Alcohol Depend. 2000, 60, 121–132. [Google Scholar] [CrossRef]
- Jiménez, J.H.; Bouso, J.C. Significance of Mammalian N, N-Dimethyltryptamine (DMT): A 60-Year-Old Debate. J. Psychopharmacol. 2022, 36, 905–919. [Google Scholar] [CrossRef]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.I.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting New Molecular Targets for Known Drugs. Nature 2009, 462, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Strassman, R.J. Human Psychopharmacology of N,N-Dimethyltryptamine. Behav. Brain Res. 1995, 73, 121–124. [Google Scholar] [CrossRef]
- Ray, T.S. Psychedelics and the Human Receptorome. PLoS ONE 2010, 5, e9019. [Google Scholar] [CrossRef]
- Carbonaro, T.M.; Gatch, M.B. Neuropharmacology of N,N-Dimethyltryptamine. Brain Res. Bull. 2016, 126, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Sub Laban, T.; Saadabadi, A. Monoamine Oxidase Inhibitors (MAOI); StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Abu Ghazaleh, H.; Lalies, M.D.; Nutt, D.J.; Hudson, A.L. The Modulatory Action of Harmane on Serotonergic Neurotransmission in Rat Brain. Brain Res. 2015, 1597, 57–64. [Google Scholar] [CrossRef]
- Smith, K.L.; Ford, G.K.; Jessop, D.S.; Finn, D.P. Behavioural, Neurochemical and Neuroendocrine Effects of the Endogenous β-Carboline Harmane in Fear-Conditioned Rats. J. Psychopharmacol. 2013, 27, 162–170. [Google Scholar] [CrossRef]
- Morales-García, J.A.; de la Fuente Revenga, M.; Alonso-Gil, S.; Rodríguez-Franco, M.I.; Feilding, A.; Perez-Castillo, A.; Riba, J. The Alkaloids of Banisteriopsis Caapi, the Plant Source of the Amazonian Hallucinogen Ayahuasca, Stimulate Adult Neurogenesis in Vitro. Sci. Rep. 2017, 7, 5309. [Google Scholar] [CrossRef]
- Valle, M.; Maqueda, A.E.; Rabella, M.; Rodríguez-Pujadas, A.; Antonijoan, R.M.; Romero, S.; Alonso, J.F.; Mañanas, M.À.; Barker, S.; Friedlander, P.; et al. Inhibition of Alpha Oscillations through Serotonin-2A Receptor Activation Underlies the Visual Effects of Ayahuasca in Humans. Eur. Neuropsychopharmacol. 2016, 26, 1161–1175. [Google Scholar] [CrossRef]
- Preller, K.H.; Burt, J.B.; Ji, J.L.; Schleifer, C.H.; Adkinson, B.D.; Stämpfli, P.; Seifritz, E.; Repovs, G.; Krystal, J.H.; Murray, J.D.; et al. Changes in Global and Thalamic Brain Connectivity in LSD-Induced Altered States of Consciousness Are Attributable to the 5-HT2A Receptor. Elife 2018, 7, e35082. [Google Scholar] [CrossRef]
- Olbrich, S.; Preller, K.H.; Vollenweider, F.X. LSD and Ketanserin and Their Impact on the Human Autonomic Nervous System. Psychophysiology 2021, 58, e13822. [Google Scholar] [CrossRef]
- Quednow, B.B.; Kometer, M.; Geyer, M.A.; Vollenweider, F.X. Psilocybin-Induced Deficits in Automatic and Controlled Inhibition Are Attenuated by Ketanserin in Healthy Human Volunteers. Neuropsychopharmacology 2012, 37, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Kometer, M.; Schmidt, A.; Jäncke, L.; Vollenweider, F.X. Activation of Serotonin 2A Receptors Underlies the Psilocybin-Induced Effects on α Oscillations, N170 Visual-Evoked Potentials, and Visual Hallucinations. J. Neurosci. 2013, 33, 10544–10551. [Google Scholar] [CrossRef] [PubMed]
- Chagraoui, A.; Thibaut, F.; Skiba, M.; Thuillez, C.; Bourin, M. 5-HT2C Receptors in Psychiatric Disorders: A Review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 66, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; DeLorenzo, C.; Choudhury, S.; Parsey, R.V. The 5-HT1A Receptor in Major Depressive Disorder. Eur. Neuropsychopharmacol. 2016, 26, 397–410. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.L.; Newman-Tancredi, A.; Leonardo, E.D. 5-HT(1A) [Corrected] Receptors in Mood and Anxiety: Recent Insights into Autoreceptor versus Heteroreceptor Function. Psychopharmacology 2014, 231, 623–636. [Google Scholar] [CrossRef]
- Savitz, J.; Lucki, I.; Drevets, W.C. 5-HT(1A) Receptor Function in Major Depressive Disorder. Prog. Neurobiol. 2009, 88, 17–31. [Google Scholar] [CrossRef]
- Kreiss, D.S.; Lucki, I. Effects of Acute and Repeated Administration of Antidepressant Drugs on Extracellular Levels of 5-Hydroxytryptamine Measured in Vivo. J. Pharmacol. Exp. Ther. 1995, 274, 866–876. [Google Scholar]
- Rausch, J.L.; Johnson, M.E.; Kasik, K.E.; Stahl, S.M. Temperature Regulation in Depression: Functional 5HT1A Receptor Adaptation Differentiates Antidepressant Response. Neuropsychopharmacology 2006, 31, 2274–2280. [Google Scholar] [CrossRef][Green Version]
- Giovacchini, G.; Lang, L.; Ma, Y.; Herscovitch, P.; Eckelman, W.C.; Carson, R.E. Differential Effects of Paroxetine on Raphe and Cortical 5-HT1A Binding: A PET Study in Monkeys. Neuroimage 2005, 28, 238–248. [Google Scholar] [CrossRef]
- Fricker, A.D.; Rios, C.; Devi, L.A.; Gomes, I. Serotonin Receptor Activation Leads to Neurite Outgrowth and Neuronal Survival. Brain Res. Mol. Brain Res. 2005, 138, 228–235. [Google Scholar] [CrossRef]
- Samuels, B.A.; Anacker, C.; Hu, A.; Levinstein, M.R.; Pickenhagen, A.; Tsetsenis, T.; Madroñal, N.; Donaldson, Z.R.; Drew, L.J.; Dranovsky, A.; et al. 5-HT1A Receptors on Mature Dentate Gyrus Granule Cells Are Critical for the Antidepressant Response. Nat. Neurosci. 2015, 18, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Claustre, Y.; Benavides, J.; Scatton, B. Potential Mechanisms Involved in the Negative Coupling between Serotonin 5-HT1A Receptors and Carbachol-Stimulated Phosphoinositide Turnover in the Rat Hippocampus. J. Neurochem. 1991, 56, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.R.; Mukhin, Y.V.; Gelasco, A.; Turner, J.; Collinsworth, G.; Gettys, T.W.; Grewal, J.S.; Garnovskaya, M.N. Multiplicity of Mechanisms of Serotonin Receptor Signal Transduction. Pharmacol. Ther. 2001, 92, 179–212. [Google Scholar] [CrossRef]
- Deliganis, A.V.; Pierce, P.A.; Peroutka, S.J. Differential Interactions of Dimethyltryptamine (DMT) with 5-HT1A and 5-HT2 Receptors. Biochem. Pharmacol. 1991, 41, 1739–1744. [Google Scholar] [CrossRef]
- Chenu, F.; Mansari, M.E.; Blier, P. Long-Term Administration of Monoamine Oxidase Inhibitors Alters the Firing Rate and Pattern of Dopamine Neurons in the Ventral Tegmental Area. Int. J. Neuropsychopharmacol. 2009, 12, 475–485. [Google Scholar] [CrossRef]
- Jiang, X.L.; Shen, H.W.; Yu, A.M. Potentiation of 5-Methoxy-N,N-Dimethyltryptamine-Induced Hyperthermia by Harmaline and the Involvement of Activation of 5-HT1A and 5-HT2A Receptors. Neuropharmacology 2015, 89, 342–351. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and Brain Function: A Tale of Two Receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef]
- Stamper, C.E.; Hassell, J.E.; Kapitz, A.J.; Renner, K.J.; Orchinik, M.; Lowry, C.A. Activation of 5-HT(1A) Receptors in the Rat Dorsomedial Hypothalamus Inhibits Stress-Induced Activation of the Hypothalamic-Pituitary-Adrenal Axis. Stress 2017, 20, 223–230. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, X.; Mar, A.C.; Ding, Y.Q.; Wang, X.; Li, Q.; Li, L. Activation of Postsynaptic 5-HT1A Receptors Improve Stress Adaptation. Psychopharmacology 2014, 231, 2067–2075. [Google Scholar] [CrossRef]
- Tafet, G.E.; Nemeroff, C.B. Pharmacological Treatment of Anxiety Disorders: The Role of the HPA Axis. Front. Psychiatry 2020, 11, 443. [Google Scholar] [CrossRef]
- Jaster, A.M.; de la Fuente Revenga, M.; González-Maeso, J. Molecular Targets of Psychedelic-induced Plasticity. J. Neurochem. 2022, 162, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Rojas, P.S.; Neira, D.; Muñoz, M.; Lavandero, S.; Fiedler, J.L. Serotonin (5-HT) Regulates Neurite Outgrowth through 5-HT1A and 5-HT7 Receptors in Cultured Hippocampal Neurons. J. Neurosci. Res. 2014, 92, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Cornea-Hébert, V.; Riad, M.; Wu, C.; Singh, S.K.; Descarries, L. Cellular and Subcellular Distribution of the Serotonin 5-HT2A Receptor in the Central Nervous System of Adult Rat. J. Comp. Neurol. 1999, 409, 187–209. [Google Scholar] [CrossRef]
- Zhang, G.; Stackman, R.W. The Role of Serotonin 5-HT2A Receptors in Memory and Cognition. Front. Pharmacol. 2015, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.; Emerit, M.B.; Hamon, M.; Darmon, M. Serotonergic Signaling: Multiple Effectors and Pleiotropic Effects. Wiley Interdiscip. Rev.: Membr. Transp. Signal. 2012, 1, 685–713. [Google Scholar] [CrossRef]
- Schmid, C.L.; Bohn, L.M. Serotonin, But Not N-Methyltryptamines, Activates the Serotonin 2A Receptor via a β-Arrestin2/Src/Akt Signaling Complex In Vivo. J. Neurosci. 2010, 30, 13513–13524. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.A.; Vaidya, V.A. Differential Signaling Signatures Evoked by DOI versus Lisuride Stimulation of the 5-HT2A Receptor. Biochem. Biophys. Res. Commun. 2020, 531, 609–614. [Google Scholar] [CrossRef]
- Eshleman, A.J.; Forster, M.J.; Wolfrum, K.M.; Johnson, R.A.; Janowsky, A.; Gatch, M.B. Behavioral and Neurochemical Pharmacology of Six Psychoactive Substituted Phenethylamines: Mouse Locomotion, Rat Drug Discrimination and in Vitro Receptor and Transporter Binding and Function. Psychopharmacology 2014, 231, 875–888. [Google Scholar] [CrossRef]
- Grella, B.; Dukat, M.; Young, R.; Teitler, M.; Herrick-Davis, K.; Gauthier, C.B.; Glennon, R.A. Investigation of Hallucinogenic and Related Beta-Carbolines. Drug Alcohol Depend. 1998, 50, 99–107. [Google Scholar] [CrossRef]
- Grella, B.; Teitler, M.; Smith, C.; Herrick-Davis, K.; Glennon, R.A. Binding of β-Carbolines at 5-HT2 Serotonin Receptors. Bioorganic Med. Chem. Lett. 2003, 13, 4421–4425. [Google Scholar] [CrossRef]
- Colaço, C.S.; Alves, S.S.; Nolli, L.M.; Pinheiro, W.O.; de Oliveira, D.G.R.; Santos, B.W.L.; Pic-Taylor, A.; Mortari, M.R.; Caldas, E.D. Toxicity of Ayahuasca after 28 Days Daily Exposure and Effects on Monoamines and Brain-Derived Neurotrophic Factor (BDNF) in Brain of Wistar Rats. Metab. Brain Dis. 2020, 35, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.R.; Johansen, A.; Donovan, L.L.; Ros, N.F.; Ozenne, B.; Hansen, H.D.; Knudsen, G.M. A Single Dose of Psilocybin Increases Synaptic Density and Decreases 5-HT(2A) Receptor Density in the Pig Brain. Int. J. Mol. Sci. 2021, 22, 835. [Google Scholar] [CrossRef] [PubMed]
- Farber, N.B.; Hanslick, J.; Kirby, C.; McWilliams, L.; Olney, J.W. Serotonergic Agents That Activate 5HT2A Receptors Prevent NMDA Antagonist Neurotoxicity. Neuropsychopharmacology 1998, 18, 57–62. [Google Scholar] [CrossRef]
- Nutt, D. 5HT2a Receptors—A New Target for Depression? Eur. Psychiatry 2015, 30, 35. [Google Scholar] [CrossRef]
- Palhano-Fontes, F.; Andrade, K.C.; Tofoli, L.F.; Santos, A.C.; Crippa, J.A.; Hallak, J.E.; Ribeiro, S.; de Araujo, D.B. The Psychedelic State Induced by Ayahuasca Modulates the Activity and Connectivity of the Default Mode Network. PLoS ONE 2015, 10, e0118143. [Google Scholar] [CrossRef] [PubMed]
- Downey, D.; Dutta, A.; McKie, S.; Dawson, G.R.; Dourish, C.T.; Craig, K.; Smith, M.A.; McCarthy, D.J.; Harmer, C.J.; Goodwin, G.M.; et al. Comparing the Actions of Lanicemine and Ketamine in Depression: Key Role of the Anterior Cingulate. Eur. Neuropsychopharmacol. 2016, 26, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Hesselgrave, N.; Troppoli, T.A.; Wulff, A.B.; Cole, A.B.; Thompson, S.M. Harnessing Psilocybin: Antidepressant-like Behavioral and Synaptic Actions of Psilocybin Are Independent of 5-HT2R Activation in Mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2022489118. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Vollenweider-Scherpenhuyzen, M.F.; Bäbler, A.; Vogel, H.; Hell, D. Psilocybin Induces Schizophrenia-like Psychosis in Humans via a Serotonin-2 Agonist Action. Neuroreport 1998, 9, 3897–3902. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef]
- McGrew, L.; Chang, M.S.; Sanders-Bush, E. Phospholipase D Activation by Endogenous 5-Hydroxytryptamine 2C Receptors Is Mediated by Galpha13 and Pertussis Toxin-Insensitive Gbetagamma Subunits. Mol. Pharmacol. 2002, 62, 1339–1343. [Google Scholar] [CrossRef]
- Berg, K.A.; Maayani, S.; Goldfarb, J.; Scaramellini, C.; Leff, P.; Clarke, W.P. Effector Pathway-Dependent Relative Efficacy at Serotonin Type 2A and 2C Receptors: Evidence for Agonist-Directed Trafficking of Receptor Stimulus. Mol. Pharmacol. 1998, 54, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Ślifirski, G.; Król, M.; Turło, J. 5-HT Receptors and the Development of New Antidepressants. Int. J. Mol. Sci. 2021, 22, 9015. [Google Scholar] [CrossRef] [PubMed]
- Dekeyne, A.; Mannoury la Cour, C.; Gobert, A.; Brocco, M.; Lejeune, F.; Serres, F.; Sharp, T.; Daszuta, A.; Soumier, A.; Papp, M.; et al. S32006, a Novel 5-HT2C Receptor Antagonist Displaying Broad-Based Antidepressant and Anxiolytic Properties in Rodent Models. Psychopharmacology 2008, 199, 549–568. [Google Scholar] [CrossRef] [PubMed]
- Dekeyne, A.; Brocco, M.; Loiseau, F.; Gobert, A.; Rivet, J.-M.; Di Cara, B.; Cremers, T.I.; Flik, G.; Fone, K.C.F.; Watson, D.J.G.; et al. S32212, a Novel Serotonin Type 2C Receptor Inverse Agonist/A2-Adrenoceptor Antagonist and Potential Antidepressant: II. A Behavioral, Neurochemical, and Electrophysiological Characterization. J. Pharmacol. Exp. Ther. 2012, 340, 765–780. [Google Scholar] [CrossRef]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 298–487. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Dravid, S.M.; Yuan, H.; Traynelis, S.F. AMPA Receptors: Molecular Biology and Pharmacology. In The Curated Reference Collection in Neuroscience and Biobehavioral Psychology; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2017; pp. 311–318. [Google Scholar] [CrossRef]
- Fukumoto, K.; Fogaça, M.V.; Liu, R.J.; Duman, C.H.; Li, X.Y.; Chaki, S.; Duman, R.S. Medial PFC AMPA Receptor and BDNF Signaling Are Required for the Rapid and Sustained Antidepressant-like Effects of 5-HT(1A) Receptor Stimulation. Neuropsychopharmacology 2020, 45, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Berthoux, C.; De Bundel, D.; Valjent, E.; Bockaert, J.; Marin, P.; Bécamel, C. Presynaptic Serotonin 2A Receptors Modulate Thalamocortical Plasticity and Associative Learning. Proc. Natl. Acad. Sci. USA 2016, 113, E1382–E1391. [Google Scholar] [CrossRef]
- Berthoux, C.; Barre, A.; Bockaert, J.; Marin, P.; Bécamel, C. Sustained Activation of Postsynaptic 5-HT2A Receptors Gates Plasticity at Prefrontal Cortex Synapses. Cereb. Cortex 2019, 29, 1659–1669. [Google Scholar] [CrossRef]
- Riga, M.S.; Lladó-Pelfort, L.; Artigas, F.; Celada, P. The Serotonin Hallucinogen 5-MeO-DMT Alters Cortico-Thalamic Activity in Freely Moving Mice: Regionally-Selective Involvement of 5-HT1A and 5-HT2A Receptors. Neuropharmacology 2018, 142, 219–230. [Google Scholar] [CrossRef]
- Scruggs, J.L.; Patel, S.; Bubser, M.; Deutch, A.Y. DOI-Induced Activation of the Cortex: Dependence on 5-HT 2A Heteroceptors on Thalamocortical Glutamatergic Neurons. J. Neurosci. 2000, 20, 8846–8852. [Google Scholar] [CrossRef]
- Riga, M.S.; Soria, G.; Tudela, R.; Artigas, F.; Celada, P. The Natural Hallucinogen 5-MeO-DMT, Component of Ayahuasca, Disrupts Cortical Function in Rats: Reversal by Antipsychotic Drugs. Int. J. Neuropharmacol. 2014, 17, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Takei, N.; Numakawa, T.; Kozaki, S.; Sakai, N.; Endo, Y.; Takahashi, M.; Hatanaka, H. Brain-Derived Neurotrophic Factor Induces Rapid and Transient Release of Glutamate through the Non-Exocytotic Pathway from Cortical Neurons. J. Biol. Chem. 1998, 273, 27620–27624. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, T.M.; Eshleman, A.J.; Forster, M.J.; Cheng, K.; Rice, K.C.; Gatch, M.B. The Role of 5-HT2A, 5-HT 2C and MGlu2 Receptors in the Behavioral Effects of Tryptamine Hallucinogens N,N-Dimethyltryptamine and N,N-Diisopropyltryptamine in Rats and Mice. Psychopharmacology 2015, 232, 275–284. [Google Scholar] [CrossRef] [PubMed]
- González-Maeso, J.; Ang, R.L.; Yuen, T.; Chan, P.; Weisstaub, N.V.; López-Giménez, J.F.; Zhou, M.; Okawa, Y.; Callado, L.F.; Milligan, G.; et al. Identification of a Serotonin/Glutamate Receptor Complex Implicated in Psychosis. Nature 2008, 452, 93–97. [Google Scholar] [CrossRef] [PubMed]
- West, W.B.; Lou, A.; Pechersky, K.; Chachich, M.E.; Appel, J.B. Antagonism of a PCP Drug Discrimination by Hallucinogens and Related Drugs. Neuropsychopharmacology 2000, 22, 618–625. [Google Scholar] [CrossRef][Green Version]
- Bäckman, L.; Nyberg, L.; Lindenberger, U.; Li, S.-C.; Farde, L. The Correlative Triad among Aging, Dopamine, and Cognition: Current Status and Future Prospects. Neurosci. Biobehav. Rev. 2006, 30, 791–807. [Google Scholar] [CrossRef]
- Nestler, E.J.; Carlezon, W.A. The Mesolimbic Dopamine Reward Circuit in Depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Jin, M.; Min, C.; Zheng, M.; Cho, D.-I.; Cheong, S.-J.; Kurose, H.; Kim, K.-M. Multiple Signaling Routes Involved in the Regulation of Adenylyl Cyclase and Extracellular Regulated Kinase by Dopamine D2 and D3 Receptors. Pharmacol. Res. 2013, 67, 31–41. [Google Scholar] [CrossRef]
- Lobo, M.K.; Covington, H.E.; Chaudhury, D.; Friedman, A.K.; Sun, H.; Damez-Werno, D.; Dietz, D.M.; Zaman, S.; Koo, J.W.; Kennedy, P.J.; et al. Cell Type–Specific Loss of BDNF Signaling Mimics Optogenetic Control of Cocaine Reward. Science 2010, 330, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Undieh, A.S. Pharmacology of Signaling Induced by Dopamine D1-like Receptor Activation. Pharmacol. Ther. 2010, 128, 37–60. [Google Scholar] [CrossRef] [PubMed]
- Francardo, V.; Bez, F.; Wieloch, T.; Nissbrandt, H.; Ruscher, K.; Cenci, M.A. Pharmacological Stimulation of Sigma-1 Receptors Has Neurorestorative Effects in Experimental Parkinsonism. Brain 2014, 137, 1998–2014. [Google Scholar] [CrossRef]
- Wilke, S.A.; Lavi, K.; Byeon, S.; Donohue, K.C.; Sohal, V.S. Convergence of Clinically Relevant Manipulations on Dopamine-Regulated Prefrontal Activity Underlying Stress Coping Responses. Biol. Psychiatry 2022, 91, 810–820. [Google Scholar] [CrossRef]
- Bergamini, G.; Sigrist, H.; Ferger, B.; Singewald, N.; Seifritz, E.; Pryce, C.R. Depletion of Nucleus Accumbens Dopamine Leads to Impaired Reward and Aversion Processing in Mice: Relevance to Motivation Pathologies. Neuropharmacology 2016, 109, 306–319. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M.; Yohn, S.; Lopez Cruz, L.; San Miguel, N.; Alatorre, L. The Pharmacology of Effort-Related Choice Behavior: Dopamine, Depression, and Individual Differences. Behav. Process. 2016, 127, 3–17. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Chen, X.; Zhang, Z.; Xiao, L.; Zhou, Y. Anhedonia Correlates with Functional Connectivity of the Nucleus Accumbens Subregions in Patients with Major Depressive Disorder. NeuroImage: Clin. 2021, 30, 102599. [Google Scholar] [CrossRef]
- Kim, H.; Sablin, S.O.; Ramsay, R.R. Inhibition of Monoamine Oxidase A by Beta-Carboline Derivatives. Arch. Biochem. Biophys. 1997, 337, 137–142. [Google Scholar] [CrossRef]
- Elhwuegi, A.S. Central Monoamines and Their Role in Major Depression. Prog. Neuro-Psychopharm Biol. Psychiatry 2004, 28, 435–451. [Google Scholar] [CrossRef]
- De Castro-Neto, E.F.; da Cunha, R.H.; da Silveira, D.X.; Yonamine, M.; Gouveia, T.L.F.; Cavalheiro, E.A.; Amado, D.; Naffah-Mazzacoratti, M.D.G. Changes in Aminoacidergic and Monoaminergic Neurotransmission in the Hippocampus and Amygdala of Rats after Ayahuasca Ingestion. World J. Biol. Chem. 2013, 4, 141. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Polit, A.; Kędracka-Krok, S.; Wędzony, K.; Maćkowiak, M.; Dziedzicka-Wasylewska, M. Hetero-Dimerization of Serotonin 5-HT2A and Dopamine D2 Receptors. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2010, 1803, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Lambe, E.K.; Aghajanian, G.K. Prefrontal Cortical Network Activity: Opposite Effects of Psychedelic Hallucinogens and D1/D5 Dopamine Receptor Activation. Neuroscience 2007, 145, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Frankel, P.S.; Cunningham, K.A. The Hallucinogen D-Lysergic Acid Diethylamide (d-LSD) Induces the Immediate-Early Gene c-Fos in Rat Forebrain. Brain Res. 2002, 958, 251–260. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Leech, R.; Williams, T.M.; Erritzoe, D.; Abbasi, N.; Bargiotas, T.; Hobden, P.; Sharp, D.J.; Evans, J.; Feilding, A.; et al. Implications for Psychedelic-Assisted Psychotherapy: Functional Magnetic Resonance Imaging Study with Psilocybin. Br. J. Psychiatry 2012, 200, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, J.; Stepień, H. The role of the endocannabinoid system in the regulation of endocrine function and in the control of energy balance in humans. Postep. Hig. Med. Dosw. 2007, 61, 99–105. [Google Scholar]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef] [PubMed]
- Reggio, P.H. Endocannabinoid Binding to the Cannabinoid Receptors: What Is Known and What Remains Unknown. Curr. Med. Chem. 2010, 17, 1468–1486. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Felder, C.C.; Joyce, K.E.; Briley, E.M.; Mansouri, J.; Mackie, K.; Blond, O.; Lai, Y.; Ma, A.L.; Mitchell, R.L. Comparison of the Pharmacology and Signal Transduction of the Human Cannabinoid CB1 and CB2 Receptors. Mol. Pharmacol. 1995, 48, 443–450. [Google Scholar]
- Mackie, K.; Lai, Y.; Westenbroek, R.; Mitchell, R. Cannabinoids Activate an Inwardly Rectifying Potassium Conductance and Inhibit Q-Type Calcium Currents in AtT20 Cells Transfected with Rat Brain Cannabinoid Receptor. J. Neurosci. 1995, 15, 6552–6561. [Google Scholar] [CrossRef]
- Bouaboula, M.; Poinot-Chazel, C.; Marchand, J.; Canat, X.; Bourrié, B.; Rinaldi-Carmona, M.; Calandra, B.; Le Fur, G.; Casellas, P. Signaling Pathway Associated with Stimulation of CB2 Peripheral Cannabinoid Receptor. Involvement of Both Mitogen-Activated Protein Kinase and Induction of Krox-24 Expression. Eur. J. Biochem. 1996, 237, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Bouaboula, M.; Desnoyer, N.; Carayon, P.; Combes, T.; Casellas, P. Gi Protein Modulation Induced by a Selective Inverse Agonist for the Peripheral Cannabinoid Receptor CB2: Implication for Intracellular Signalization Cross-Regulation. Mol. Pharmacol. 1999, 55, 473–480. [Google Scholar] [PubMed]
- Guzmán, M.; Sánchez, C.; Galve-Roperh, I. Control of the Cell Survival/Death Decision by Cannabinoids. J. Mol. Med. 2001, 78, 613–625. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Functional Expression of Brain Neuronal CB2 Cannabinoid Receptors Are Involved in the Effects of Drugs of Abuse and in Depression. Ann. N. Y. Acad. Sci. 2008, 1139, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.N.; Osorio, F.L.; Morgan, C.J.A.; Crippa, J.A.S.; Bouso, J.C.; Rocha, J.M.; Zuardi, A.W.; Hallak, J.E.C.; Santos, R.G.D. The Effects of Cannabidiol (CBD) and Delta-9-Tetrahydrocannabinol (THC) on the Recognition of Emotions in Facial Expressions: A Systematic Review of Randomized Controlled Trials. Neurosci. Biobehav. Rev. 2020, 118, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Scherma, M.; Masia, P.; Satta, V.; Fratta, W.; Fadda, P.; Tanda, G. Brain Activity of Anandamide: A Rewarding Bliss? Acta Pharmacol. Sin. 2019, 40, 309–323. [Google Scholar] [CrossRef]
- Best, A.R.; Regehr, W.G. Serotonin Evokes Endocannabinoid Release and Retrogradely Suppresses Excitatory Synapses. J. Neurosci. 2008, 28, 6508–6515. [Google Scholar] [CrossRef]
- Parrish, J.C.; Nichols, D.E. Serotonin 5-HT(2A) Receptor Activation Induces 2-Arachidonoylglycerol Release through a Phospholipase c-Dependent Mechanism. J. Neurochem. 2006, 99, 1164–1175. [Google Scholar] [CrossRef]
- Gorzalka, B.B.; Hill, M.N.; Sun, J.C. Functional Role of the Endocannabinoid System and AMPA/Kainate Receptors in 5-HT2A Receptor-Mediated Wet Dog Shakes. Eur. J. Pharmacol. 2005, 516, 28–33. [Google Scholar] [CrossRef]
- Viñals, X.; Moreno, E.; Lanfumey, L.; Cordomí, A.; Pastor, A.; de La Torre, R.; Gasperini, P.; Navarro, G.; Howell, L.A.; Pardo, L.; et al. Cognitive Impairment Induced by Delta9-Tetrahydrocannabinol Occurs through Heteromers between Cannabinoid CB1 and Serotonin 5-HT2A Receptors. PLoS Biol. 2015, 13, e1002194. [Google Scholar] [CrossRef]
- Hashimoto, K. Sigma-1 Receptor Chaperone and Brain-Derived Neurotrophic Factor: Emerging Links between Cardiovascular Disease and Depression. Prog. Neurobiol. 2013, 100, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.K.; Dhir, A. Sigma-1 Receptors in Major Depression and Anxiety. Expert Rev. Neurother. 2009, 9, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Sałaciak, K.; Pytka, K. Revisiting the Sigma-1 Receptor as a Biological Target to Treat Affective and Cognitive Disorders. Neurosci. Biobehav. Rev. 2022, 132, 1114–1136. [Google Scholar] [CrossRef] [PubMed]
- Collina, S.; Gaggeri, R.; Marra, A.; Bassi, A.; Negrinotti, S.; Negri, F.; Rossi, D. Sigma Receptor Modulators: A Patent Review. Expert Opin. Ther. Pat. 2013, 23, 597–613. [Google Scholar] [CrossRef]
- Szabo, A.; Kovacs, A.; Frecska, E.; Rajnavolgyi, E. Psychedelic N,N-Dimethyltryptamine and 5-Methoxy-N,N-Dimethyltryptamine Modulate Innate and Adaptive Inflammatory Responses through the Sigma-1 Receptor of Human Monocyte-Derived Dendritic Cells. PLoS ONE 2014, 9, e106533. [Google Scholar] [CrossRef]
- Szabo, A.; Frecska, E. Dimethyltryptamine (DMT): A Biochemical Swiss Army Knife in Neuroinflammation and Neuroprotection? Neural Regen. Res. 2016, 11, 396–397. [Google Scholar] [CrossRef]
- Inserra, A. Hypothesis: The Psychedelic Ayahuasca Heals Traumatic Memories via a Sigma 1 Receptor-Mediated Epigenetic-Mnemonic Process. Front. Pharmacol. 2018, 9, 330. [Google Scholar] [CrossRef]
- Morales-Garcia, J.A.; Calleja-Conde, J.; Lopez-Moreno, J.A.; Alonso-Gil, S.; Sanz-SanCristobal, M.; Riba, J.; Perez-Castillo, A. N,N-Dimethyltryptamine Compound Found in the Hallucinogenic Tea Ayahuasca, Regulates Adult Neurogenesis in Vitro and in Vivo. Transl. Psychiatry 2020, 10, 331. [Google Scholar] [CrossRef]
- Barker, S.A. N,N-Dimethyltryptamine (DMT), an Endogenous Hallucinogen: Past, Present, and Future Research to Determine Its Role and Function. Front. Neurosci. 2018, 12, 536. [Google Scholar] [CrossRef]
- Duval, F.; Mokrani, M.C.; Ortiz, J.A.; Schulz, P.; Champeval, C.; Macher, J.P. Neuroendocrine Predictors of the Evolution of Depression. Dialogues Clin. Neurosci. 2005, 7, 273–282. [Google Scholar] [CrossRef]
- Joyce, P.R. Neuroendocrine Changes in Depression. Aust. N. Z. J. Psychiatry 1985, 19, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Thomas, S.A.; Lovestone, S.; Makoff, A.; Kerwin, R.W. Do Antidepressants Regulate How Cortisol Affects the Brain? Psychoneuroendocrinology 2004, 29, 423–447. [Google Scholar] [CrossRef] [PubMed]
- Akaltun, İ.; Çayır, A.; Kara, T.; Ayaydın, H. Is Growth Hormone Deficiency Associated with Anxiety Disorder and Depressive Symptoms in Children and Adolescents?: A Case-Control Study. Growth Horm. IGF Res. 2018, 41, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Algahtany, M.; Sharma, S.; Fahoum, K.; Jing, R.; Zhang, S.; Kovacs, K.; Rotondo, F.; Lee, J.; Vanek, I.; Cusimano, M.D. The Role of Growth Hormone in Depression: A Human Model. Front. Neurosci. 2021, 15, 661819. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.M. The Thyroid Axis and Depression. Thyroid 1998, 8, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, T.; Crown, A.; Checkley, S.; Farmer, A.; Lightman, S. Atypical Depression in Growth Hormone Deficient Adults, and the Beneficial Effects of Growth Hormone Treatment on Depression and Quality of Life. Eur. J. Endocrinol. 2004, 151, 325–332. [Google Scholar] [CrossRef][Green Version]
- Sullivan, P.F.; Wilson, D.A.; Mulder, R.T.; Joyce, P.R. The Hypothalamic-Pituitary-Thyroid Axis in Major Depression. Acta Psychiatr. Scand. 1997, 95, 370–378. [Google Scholar] [CrossRef]
- Schindler, E.A.D.; Wallace, R.M.; Sloshower, J.A.; D’Souza, D.C. Neuroendocrine Associations Underlying the Persistent Therapeutic Effects of Classic Serotonergic Psychedelics. Front. Pharmacol. 2018, 9, 177. [Google Scholar] [CrossRef]
- Calogero, A.E.; Bernardini, R.; Margioris, A.N.; Bagdy, G.; Gallucci, W.T.; Munson, P.J.; Tamarkin, L.; Tomai, T.P.; Brady, L.; Gold, P.W.; et al. Effects of Serotonergic Agonists and Antagonists on Corticotropin-Releasing Hormone Secretion by Explanted Rat Hypothalami. Peptides 1989, 10, 189–200. [Google Scholar] [CrossRef]
- Hemrick-Luecke, S.K.; Evans, D.C. Comparison of the Potency of MDL 100,907 and SB 242084 in Blocking the Serotonin (5-HT)(2) Receptor Agonist-Induced Increases in Rat Serum Corticosterone Concentrations: Evidence for 5-HT(2A) Receptor Mediation of the HPA Axis. Neuropharmacology 2002, 42, 162–169. [Google Scholar] [CrossRef]
- Mikkelsen, J.D.; Hay-Schmidt, A.; Kiss, A. Serotonergic Stimulation of the Rat Hypothalamo-Pituitary-Adrenal Axis: Interaction between 5-HT1A and 5-HT2A Receptors. Ann. N. Y. Acad. Sci. 2004, 1018, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Owens, M.J.; Knight, D.L.; Ritchie, J.C.; Nemeroff, C.B. The 5-Hydroxytryptamine2 Agonist, (+-)-1-(2,5-Dimethoxy-4-Bromophenyl)-2-Aminopropane Stimulates the Hypothalamic-Pituitary-Adrenal (HPA) Axis. I. Acute Effects on HPA Axis Activity and Corticotropin-Releasing Factor-Containing Neurons in the Rat Brain. J. Pharmacol. Exp. Ther. 1991, 256, 787–794. [Google Scholar] [PubMed]
- Hasler, F.; Grimberg, U.; Benz, M.A.; Huber, T.; Vollenweider, F.X. Acute Psychological and Physiological Effects of Psilocybin in Healthy Humans: A Double-Blind, Placebo-Controlled Dose-Effect Study. Psychopharmacology 2004, 172, 145–156. [Google Scholar] [CrossRef]
- Schmid, Y.; Enzler, F.; Gasser, P.; Grouzmann, E.; Preller, K.H.; Vollenweider, F.X.; Brenneisen, R.; Müller, F.; Borgwardt, S.; Liechti, M.E. Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol. Psychiatry 2015, 78, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Strajhar, P.; Schmid, Y.; Liakoni, E.; Dolder, P.C.; Rentsch, K.M.; Kratschmar, D.V.; Odermatt, A.; Liechti, M.E. Acute Effects of Lysergic Acid Diethylamide on Circulating Steroid Levels in Healthy Subjects. J. Neuroendocrinol. 2016, 28, 12374. [Google Scholar] [CrossRef]
- Brierley, D.I.; Davidson, C. Developments in Harmine Pharmacology—Implications for Ayahuasca Use and Drug-Dependence Treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 263–272. [Google Scholar] [CrossRef]
- Miralles, A.; Esteban, S.; Sastre-Coll, A.; Moranta, D.; Asensio, V.J.; García-Sevilla, J.A. High-Affinity Binding of Beta-Carbolines to Imidazoline I2B Receptors and MAO-A in Rat Tissues: Norharman Blocks the Effect of Morphine Withdrawal on DOPA/Noradrenaline Synthesis in the Brain. Eur. J. Pharmacol. 2005, 518, 234–242. [Google Scholar] [CrossRef]
- Göckler, N.; Jofre, G.; Papadopoulos, C.; Soppa, U.; Tejedor, F.J.; Becker, W. Harmine Specifically Inhibits Protein Kinase DYRK1A and Interferes with Neurite Formation. FEBS J. 2009, 276, 6324–6337. [Google Scholar] [CrossRef]
- Jain, S.; Panuganti, V.; Jha, S.; Roy, I. Harmine Acts as an Indirect Inhibitor of Intracellular Protein Aggregation. ACS Omega 2020, 5, 5620–5628. [Google Scholar] [CrossRef]
- Weiss, M.; Buldakova, S.; Dutova, E. Interaction of the Beta-Carboline Harmaline with a GABA-Benzodiazepine Mechanism: An Electrophysiological Investigation on Rat Hippocampal Slices. Brain Res. 1995, 695, 105–109. [Google Scholar] [CrossRef]
- Berrougui, H.; Martín-Cordero, C.; Khalil, A.; Hmamouchi, M.; Ettaib, A.; Marhuenda, E.; Herrera, M.D. Vasorelaxant Effects of Harmine and Harmaline Extracted from Peganum harmala, L. Seeds in Isolated Rat Aorta. Pharmacol. Res. 2006, 54, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, L.; Pompili, S.; Tempia Valenta, S.; Salvi, V.; Volpe, U. C-Reactive Protein as a Biomarker for Major Depressive Disorder? Int. J. Mol. Sci. 2022, 23, 1616. [Google Scholar] [CrossRef] [PubMed]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and Depression: A Review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, G.C.; Gould, T.D.; Prueitt, W.L.; Nanavati, J.; Grunebaum, M.F.; Farber, N.B.; Singh, B.; Selvaraj, S.; Machado-Vieira, R.; Achtyes, E.D.; et al. Blood-Based Biomarkers of Antidepressant Response to Ketamine and Esketamine: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).