Serum Metabolomic Profiling in Aging Mice Using Liquid Chromatography—Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice Husbandry and Serum Preparation
2.2. Sample Preparation
2.3. UPLC-Orbitrap MS Condition
2.4. Serum Metabolite Analysis
2.5. Pathway Analysis
2.6. Statistical Analysis
3. Results
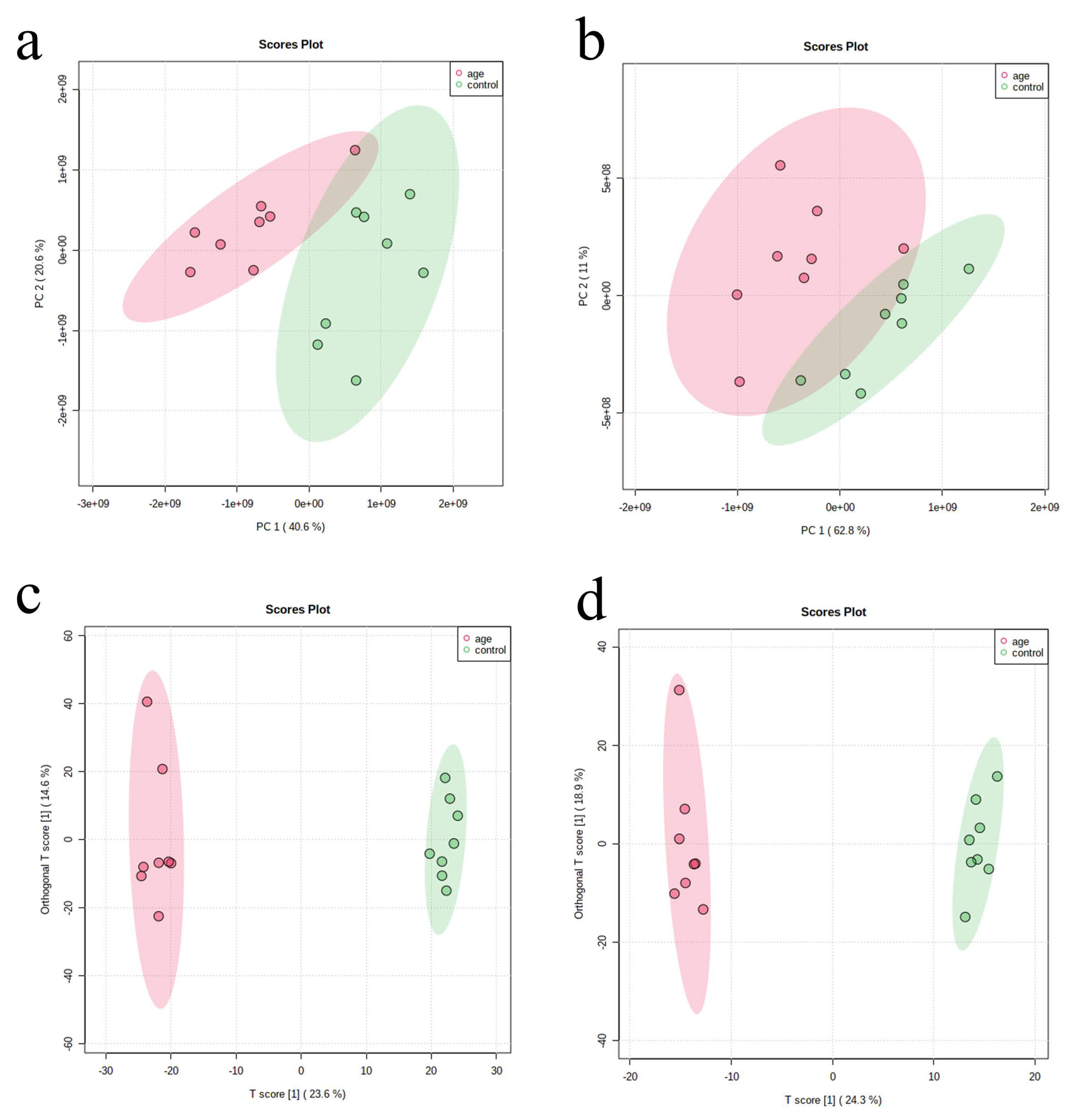
3.1. PCA and OPLS-DA of Serum Samples in Aging Mouse Models
3.2. Differential Metabolite Identification in Aging Mice
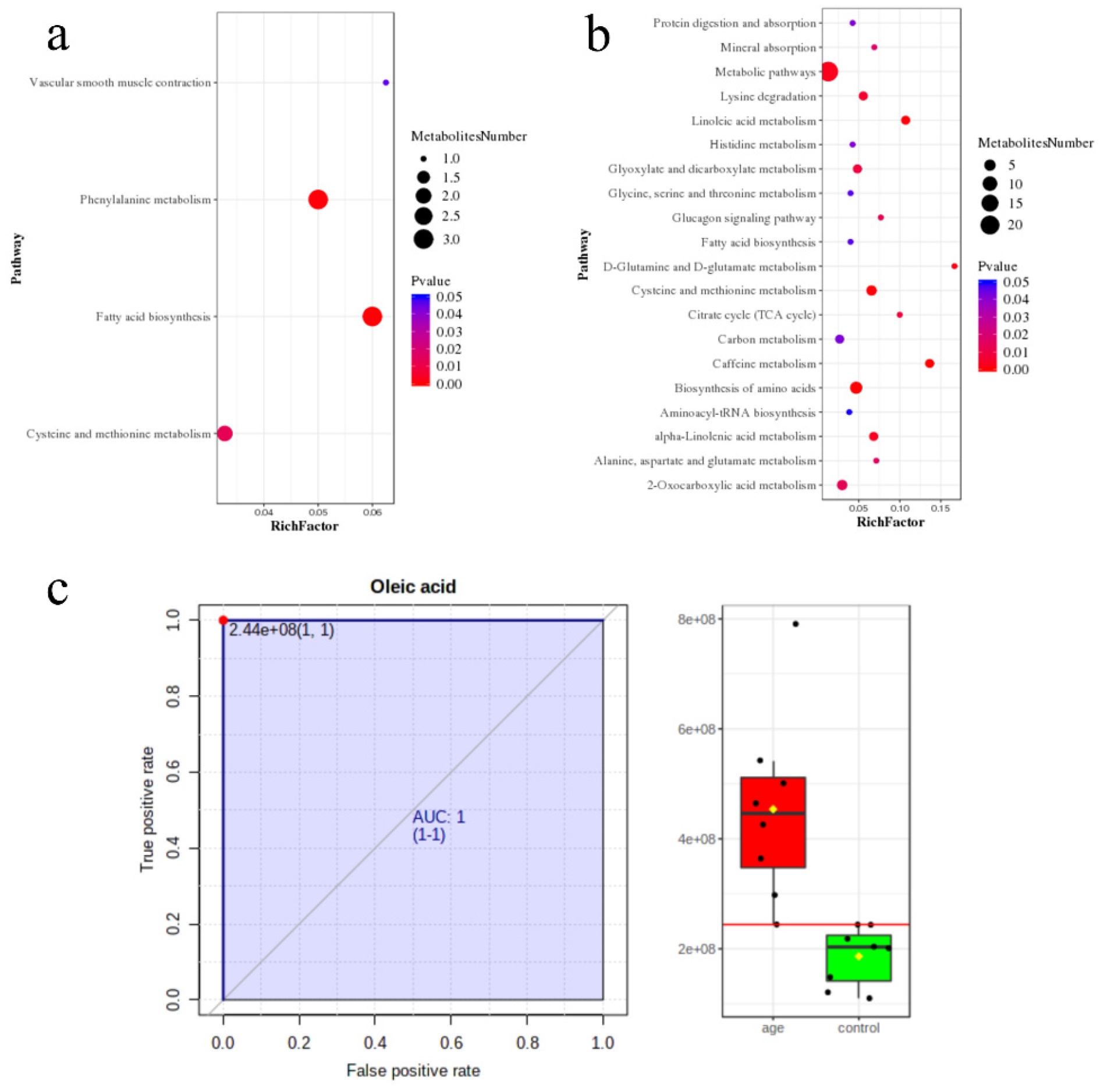
3.3. Pathway Enrichment Analysis
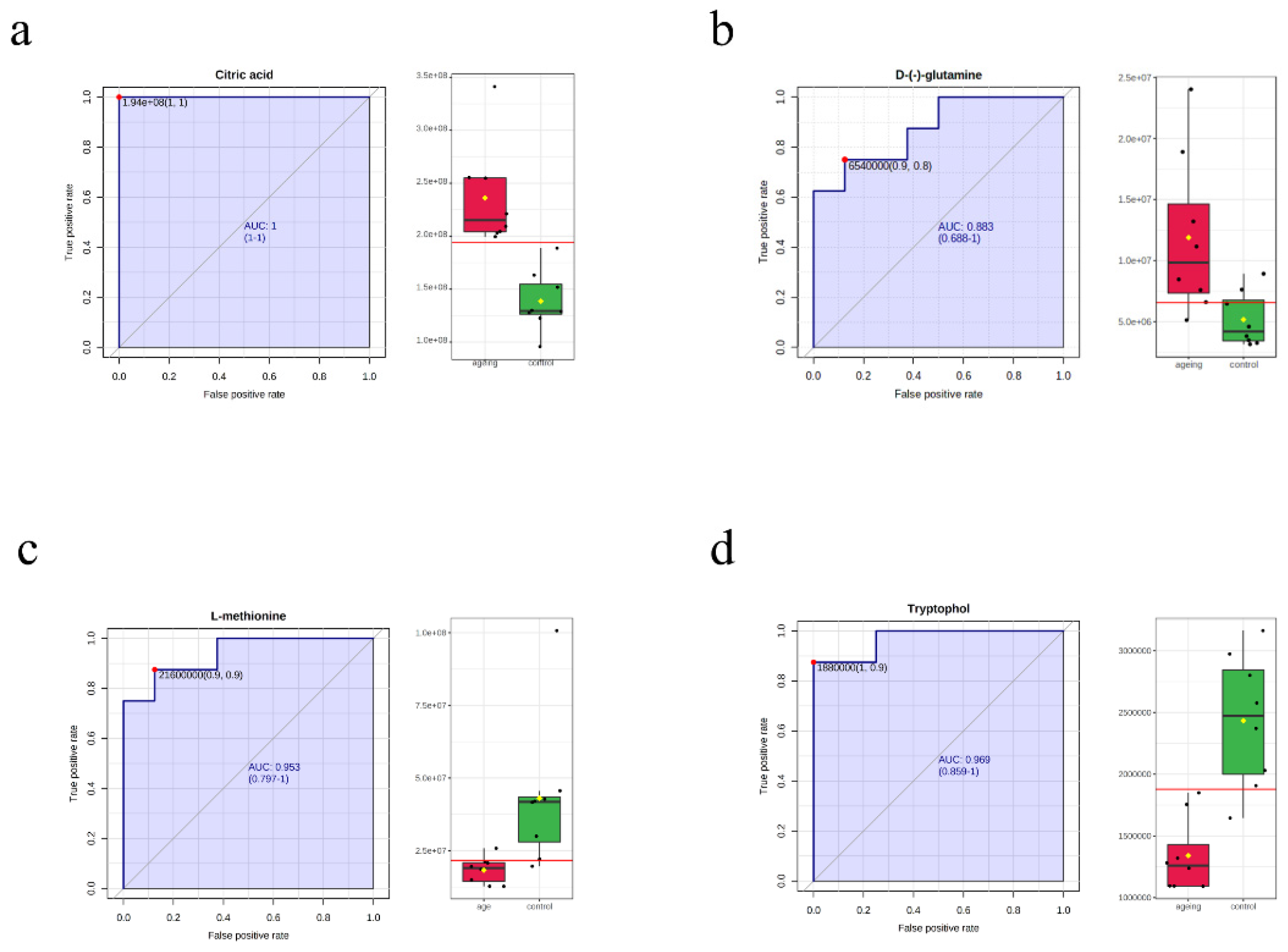
3.4. Machine Learning for the Candidate Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 2021, 22, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Alpert, A.; Pickman, Y.; Leipold, M.; Rosenberg-Hasson, Y.; Ji, X.; Gaujoux, R.; Rabani, H.; Starosvetsky, E.; Kveler, K.; Schaffert, S.; et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 2019, 25, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Segura, N.A.; Bello-Chavolla, O.Y.; Barrera-Vázquez, O.S.; Gutierrez-Robledo, L.M.; Gomez-Verjan, J.C. Promising biomarkers of human aging: In search of a multi-omics panel to understand the aging process from a multidimensional perspective. Ageing Res. Rev. 2020, 64, 101164. [Google Scholar] [CrossRef]
- Auro, K.; Joensuu, A.; Fischer, K.; Kettunen, J.; Salo, P.; Mattsson, H.; Niironen, M.; Kaprio, J.; Eriksson, J.G.; Lehtimaki, T.; et al. A metabolic view on menopause and ageing. Nat. Commun. 2014, 5, 4708. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Larson, M.G.; McCabe, E.L.; Murabito, J.M.; Rhee, E.P.; Ho, J.E.; Jacques, P.F.; Ghorbani, A.; Magnusson, M.; Souza, A.L.; et al. Distinct metabolomic signatures are associated with longevity in humans. Nat. Commun. 2015, 6, 6791. [Google Scholar] [CrossRef]
- Despres, J.P. Predicting longevity using metabolomics: A novel tool for precision lifestyle medicine? Nat. Rev. Cardiol. 2020, 17, 67–68. [Google Scholar] [CrossRef]
- Di Mauro, S.; Scamporrino, A.; Filippello, A.; Di Pino, A.; Scicali, R.; Malaguarnera, R.; Purrello, F.; Piro, S. Clinical and Molecular Biomarkers for Diagnosis and Staging of NAFLD. Int. J. Mol. Sci. 2021, 22, 11905. [Google Scholar] [CrossRef]
- Ashton, N.J.; Hye, A.; Rajkumar, A.P.; Leuzy, A.; Snowden, S.; Suárez-Calvet, M.; Karikari, T.K.; Schöll, M.; La Joie, R.; Rabinovici, G.D.; et al. An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat. Rev. Neurol. 2020, 16, 265–284. [Google Scholar] [CrossRef]
- Ussher, J.R.; Elmariah, S.; Gerszten, R.E.; Dyck, J.R. The Emerging Role of Metabolomics in the Diagnosis and Prognosis of Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 68, 2850–2870. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Almanza-Aguilera, E.; Llorach, R.; Vázquez-Fresno, R.; Estruch, R.; Corella, D.; Sorli, J.V.; Carmona, F.; Sanchez-Pla, A.; Salas-Salvadó, J.; et al. Non-targeted metabolomic biomarkers and metabotypes of type 2 diabetes: A cross-sectional study of PREDIMED trial participants. Diabetes Metab. 2019, 45, 167–174. [Google Scholar] [CrossRef]
- Deelen, J.; Kettunen, J.; Fischer, K.; van der Spek, A.; Trompet, S.; Kastenmuller, G.; Boyd, A.; Zierer, J.; van den Akker, E.B.; Ala-Korpela, M.; et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat. Commun. 2019, 10, 3346. [Google Scholar] [CrossRef] [PubMed]
- Chaleckis, R.; Murakami, I.; Takada, J.; Kondoh, H.; Yanagida, M. Individual variability in human blood metabolites identifies age-related differences. Proc. Natl. Acad. Sci. USA 2016, 113, 4252–4259. [Google Scholar] [CrossRef] [PubMed]
- Goecks, J.; Jalili, V.; Heiser, L.M.; Gray, J.W. How Machine Learning Will Transform Biomedicine. Cell 2020, 181, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Rist, M.J.; Roth, A.; Frommherz, L.; Weinert, C.H.; Krüger, R.; Merz, B.; Bunzel, D.; Mack, C.; Egert, B.; Bub, A.; et al. Metabolite patterns predicting sex and age in participants of the Karlsruhe Metabolomics and Nutrition (KarMeN) study. PLoS ONE 2017, 12, e0183228. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Jia, H.; Liu, C.; Li, D.; Huang, Q.; Liu, D.; Zhang, Y.; Ye, C.; Zhou, D.; Wang, Y.; Tan, Y.; et al. Metabolomic analyses reveals new stage-specific features of the COVID-19. Eur. Respir. J. 2021, 59, 2100284. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic. Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, health, and hallmarks of aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef]
- Pietri, P.; Stefanadis, C. Cardiovascular Aging and Longevity: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 189–204. [Google Scholar] [CrossRef]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 2020, 22, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.; Schoonjans, K. Molecular Physiology of Bile Acid Signaling in Health, Disease, and Aging. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef]
- Aon, M.A.; Bernier, M.; Mitchell, S.J.; Di Germanio, C.; Mattison, J.A.; Ehrlich, M.R.; Colman, R.J.; Anderson, R.M.; de Cabo, R. Untangling Determinants of Enhanced Health and Lifespan through a Multi-omics Approach in Mice. Cell Metab. 2020, 32, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.J.; Bernier, M.; Aon, M.A.; Cortassa, S.; Kim, E.Y.; Fang, E.F.; Palacios, H.H.; Ali, A.; Navas-Enamorado, I.; Di Francesco, A.; et al. Nicotinamide Improves Aspects of Healthspan, but Not Lifespan, in Mice. Cell Metab. 2018, 27, 667–676.e4. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Gross, A.L.; Carlson, M.C.; Chu, N.M.; McAdams-DeMarco, M.A.; Mungas, D.; Simonsick, E.M.; Varadhan, R.; Xue, Q.L.; Walston, J.; Bandeen-Roche, K. Derivation of a measure of physiological multisystem dysregulation: Results from WHAS and health ABC. Mech. Ageing Dev. 2020, 188, 111258. [Google Scholar] [CrossRef]
- Beheshti, I.; Nugent, S.; Potvin, O.; Duchesne, S. Disappearing metabolic youthfulness in the cognitively impaired female brain. Neurobiol. Aging 2021, 101, 224–229. [Google Scholar] [CrossRef]
- Palmer, D.; Fabris, F.; Doherty, A.; Freitas, A.A.; de Magalhães, J.P. Ageing transcriptome meta-analysis reveals similarities and differences between key mammalian tissues. Aging 2021, 13, 3313–3341. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Tan, Q.; Ruan, J.; Tian, Z.; Wang, X.; Liu, J.; Liu, X.; Liu, Z.; Zhang, Y.; Sun, C.; et al. Aging-related markers in rat urine revealed by dynamic metabolic profiling using machine learning. Aging (Albany NY) 2021, 13, 14322–14341. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; He, Y.; Chen, Y.; Yin, X.; Sha, X.; Wang, Y. Comparative Analysis of Multiple Neurodegenerative Diseases Based on Advanced Epigenetic Aging Brain. Front. Genet. 2021, 12, 657636. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.; Certo, M.; Lord, J.M.; Mauro, C.; Duggal, N.A. Understanding the role of host metabolites in the induction of immune senescence: Future strategies for keeping the ageing population healthy. Br. J. Pharmacol. 2021, 179, 1808–1824. [Google Scholar] [CrossRef]
- Hollander, D.; Dadufalza, V.D. Increased intestinal absorption of oleic acid with aging in the rat. Exp. Gerontol. 1983, 18, 287–292. [Google Scholar] [CrossRef]
- Ryan, M.; McInerney, D.; Owens, D.; Collins, P.; Johnson, A.; Tomkin, G.H. Diabetes and the Mediterranean diet: A beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium-dependent vasoreactivity. QJM 2000, 93, 85–91. [Google Scholar] [CrossRef]
- Soriguer, F.; Esteva, I.; Rojo-Martinez, G.; Ruiz de Adana, M.S.; Dobarganes, M.C.; García-Almeida, J.M.; Tinahones, F.; Beltrán, M.; González-Romero, S.; Olveira, G.; et al. Oleic acid from cooking oils is associated with lower insulin resistance in the general population (Pizarra study). Eur. J. Endocrinol. 2004, 150, 33–39. [Google Scholar] [CrossRef]
- Morin, S.J.; Gaziano, J.M.; Djoussé, L. Relation between plasma phospholipid oleic acid and risk of heart failure. Eur. J. Nutr. 2018, 57, 2937–2942. [Google Scholar] [CrossRef]
- Gaeini, Z.; Mirmiran, P.; Bahadoran, Z.; Aghayan, M.; Azizi, F. The association between dietary fats and the incidence risk of cardiovascular outcomes: Tehran Lipid and Glucose Study. Nutr. Metab. 2021, 18, 96. [Google Scholar] [CrossRef]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef]
- Zhu, J.; Berisa, M.; Schwörer, S.; Qin, W.; Cross, J.R.; Thompson, C.B. Transsulfuration Activity Can Support Cell Growth upon Extracellular Cysteine Limitation. Cell Metab. 2019, 30, 865–876.e5. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Li, W.; Kremer, D.M.; Sajjakulnukit, P.; Li, S.; Crespo, J.; Nwosu, Z.C.; Zhang, L.; Czerwonka, A.; Pawłowska, A.; et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature 2020, 585, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Navik, U.; Sheth, V.G.; Khurana, A.; Jawalekar, S.S.; Allawadhi, P.; Gaddam, R.R.; Bhatti, J.S.; Tikoo, K. Methionine as a double-edged sword in health and disease: Current perspective and future challenges. Ageing Res. Rev. 2021, 72, 101500. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Rouskin, S.; Dervishi, K.; McCormick, M.A.; Sasikumar, A.; Deng, C.; Chen, Z.; Kaeberlein, M.; Brem, R.B.; Polymenis, M.; et al. Life span extension by glucose restriction is abrogated by methionine supplementation: Cross-talk between glucose and methionine and implication of methionine as a key regulator of life span. Sci. Adv. 2020, 6, eaba1306. [Google Scholar] [CrossRef]
- Lees, E.K.; Król, E.; Grant, L.; Shearer, K.; Wyse, C.; Moncur, E.; Bykowska, A.S.; Mody, N.; Gettys, T.W.; Delibegovic, M. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 2014, 13, 817–827. [Google Scholar] [CrossRef]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A.; Treviño-Villarreal, J.H.; Mejia, P.; Ozaki, C.K.; et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef]
- Longchamp, A.; Mirabella, T.; Arduini, A.; MacArthur, M.R.; Das, A.; Treviño-Villarreal, J.H.; Hine, C.; Ben-Sahra, I.; Knudsen, N.H.; Brace, L.E.; et al. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H(2)S Production. Cell 2018, 173, 117–129.e14. [Google Scholar] [CrossRef]
- Jha, A.K.; Huang, S.C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef]
- Ariyoshi, M.; Katane, M.; Hamase, K.; Miyoshi, Y.; Nakane, M.; Hoshino, A.; Okawa, Y.; Mita, Y.; Kaimoto, S.; Uchihashi, M.; et al. (D)-Glutamate is metabolized in the heart mitochondria. Sci. Rep. 2017, 7, 43911. [Google Scholar] [CrossRef]
- Meynial-Denis, D. Glutamine metabolism in advanced age. Nutr. Rev. 2016, 74, 225–236. [Google Scholar] [CrossRef]
- Jiao, D.; Qi, L.; Hu, L.; Hu, D.; Li, X.; Li, G.; Li, Z.; Liu, S.; Zhao, C.; Wu, H. Changes in aging-induced kidney dysfunction in mice based on a metabolomics analysis. Front. Endocrinol. 2022, 13, 959311. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Bruno, D.; Nierenberg, J.; Marmar, C.R.; Zetterberg, H.; Blennow, K.; Pomara, N. Abnormality in glutamine-glutamate cycle in the cerebrospinal fluid of cognitively intact elderly individuals with major depressive disorder: A 3-year follow-up study. Transl. Psychiatry 2016, 6, e744. [Google Scholar] [CrossRef] [PubMed]
- Renick, P.J.; Mulgaonkar, A.; Co, C.M.; Wu, C.Y.; Zhou, N.; Velazquez, A.; Pennington, J.; Sherwood, A.; Dong, H.; Castellino, L.; et al. Imaging of Actively Proliferating Bacterial Infections by Targeting the Bacterial Metabolic Footprint with d-[5-(11)C]-Glutamine. ACS Infect. Dis 2021, 7, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Mo, Y.Y.; Feng, S.S.; Meng, M.W.; Chen, S.Y.; Huang, H.M.; Ling, X.; Song, H.; Liang, Y.H.; Ou, S.F.; et al. Urinary metabonomics study of anti-depressive mechanisms of Millettia speciosa Champ on rats with chronic unpredictable mild stress-induced depression. J. Pharm. Biomed. Anal. 2021, 205, 114338. [Google Scholar] [CrossRef] [PubMed]


| Metabolite | Fold Change | p-Value | q-Value | VIP | Label | Identification Level |
|---|---|---|---|---|---|---|
| Ophthalmic acid | 11.6266 | 0 | 0.0011 | 2.3414 | pos-up | Level 1 |
| Oleoyl ethanolamide | 3.0123 | 0 | 0 | 1.645 | pos-up | Level 2 |
| Oleate | 1.8467 | 0.0001 | 0.0014 | 1.175 | pos-up | Level 2 |
| Citric acid | 1.7038 | 0.0001 | 0.0016 | 1.1059 | pos-up | Level 4 |
| Alpha-ketoglutaric acid | 1.7726 | 0.0001 | 0.0013 | 1.1461 | pos-up | Level 4 |
| Linamarin | 12.4902 | 0 | 0.0001 | 2.5118 | pos-up | Level 4 |
| L-methionine | 0.4194 | 0.0001 | 0.0012 | 1.3447 | pos-down | Level 1 |
| Pantothenic acid | 0.4005 | 0 | 0.0006 | 1.4818 | pos-down | Level 1 |
| Formyl-l-methionyl peptide | 0.2699 | 0.0001 | 0.0018 | 1.7884 | pos-down | Level 1 |
| N-acetyl-l-phenylalanine | 0.5542 | 0 | 0.0004 | 1.1529 | pos-down | Level 1 |
| Genistein | 0.055 | 0 | 0.0008 | 2.7982 | pos-down | Level 2 |
| 9-oxo-10(e),12(e)-octadecadienoic acid | 0.1687 | 0 | 0.0001 | 2.1344 | pos-down | Level 2 |
| Gamma-linolenic acid | 0.1501 | 0.0001 | 0.0022 | 2.2762 | pos-down | Level 2 |
| Tryptophol | 0.5508 | 0.0001 | 0.0018 | 1.142 | pos-down | Level 4 |
| Palmitoleic acid2 | 4.7506 | 0 | 0.0002 | 1.7995 | neg-up | Level 2 |
| 4-hydroxybenzoic acid | 0.2634 | 0 | 0 | 0.0001 | neg-down | Level 2 |
| Fmet | 0.2899 | 0 | 0 | 0.001 | neg-down | Level 4 |
| 3-phenyllactic acid | 0.5047 | 0.0001 | 0.0001 | 0.0023 | neg-down | Level 2 |
| Pantothenic acid | 0.3525 | 0.0001 | 0.0001 | 0.0013 | neg-down | Level 2 |
| Genistein | 0.0826 | 0 | 0 | 0.0012 | neg-down | Level 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, T.; Tan, H.; Shi, Y.; Xu, M.; Luo, S.; Weng, J.; Xu, S. Serum Metabolomic Profiling in Aging Mice Using Liquid Chromatography—Mass Spectrometry. Biomolecules 2022, 12, 1594. https://doi.org/10.3390/biom12111594
Yue T, Tan H, Shi Y, Xu M, Luo S, Weng J, Xu S. Serum Metabolomic Profiling in Aging Mice Using Liquid Chromatography—Mass Spectrometry. Biomolecules. 2022; 12(11):1594. https://doi.org/10.3390/biom12111594
Chicago/Turabian StyleYue, Tong, Huiling Tan, Yu Shi, Mengyun Xu, Sihui Luo, Jianping Weng, and Suowen Xu. 2022. "Serum Metabolomic Profiling in Aging Mice Using Liquid Chromatography—Mass Spectrometry" Biomolecules 12, no. 11: 1594. https://doi.org/10.3390/biom12111594
APA StyleYue, T., Tan, H., Shi, Y., Xu, M., Luo, S., Weng, J., & Xu, S. (2022). Serum Metabolomic Profiling in Aging Mice Using Liquid Chromatography—Mass Spectrometry. Biomolecules, 12(11), 1594. https://doi.org/10.3390/biom12111594








