A Promising Candidate in Tendon Healing Events—PDGF-BB
Abstract
:1. Introduction
2. PDGF-BB in Tendon Healing: Experimental Studies
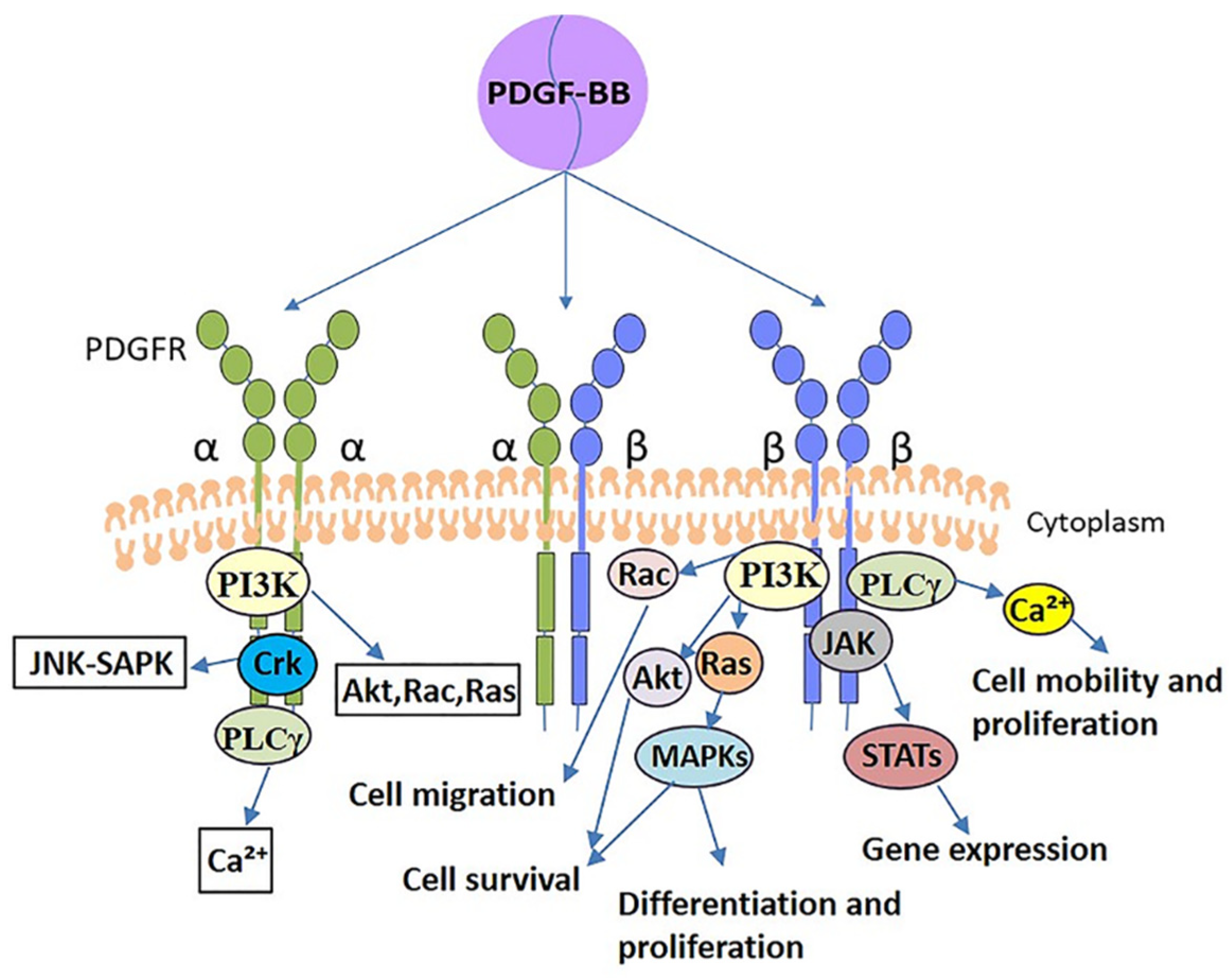
3. The Basis of PDGF-BB Efforts: PDGFRs
4. The Mechanisms of PDGF-BB in Tendon Healing
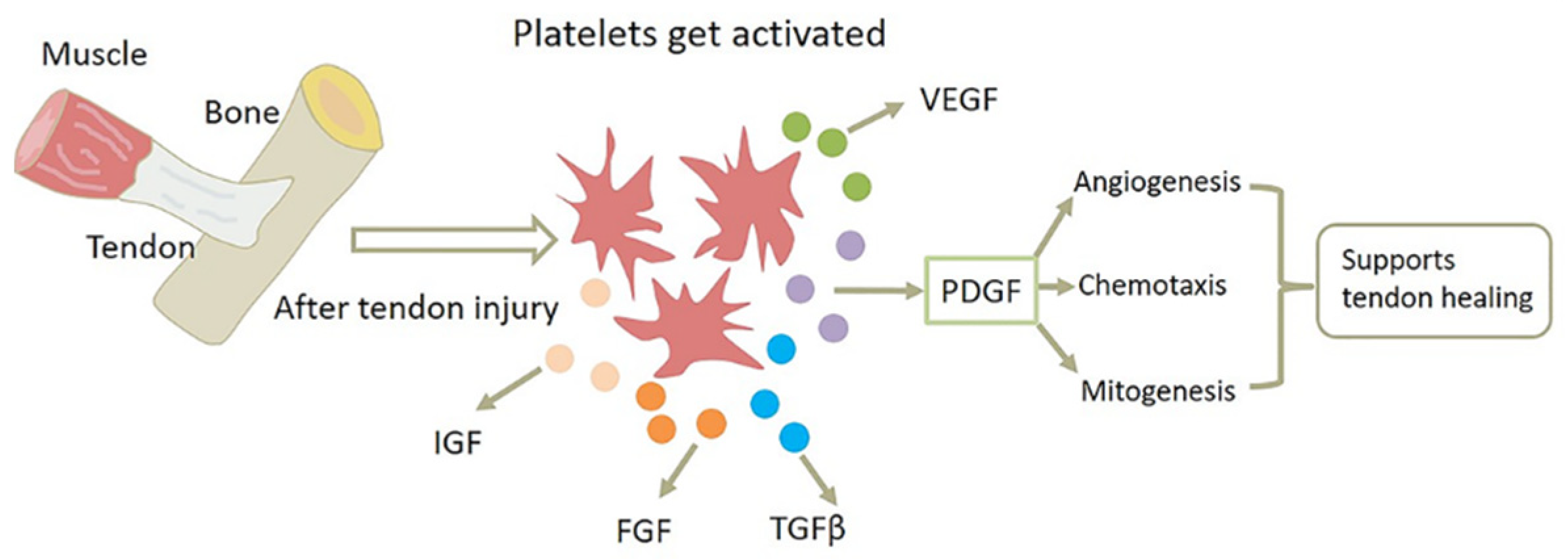
4.1. PDGF-BB Enhances Inflammatory Response
4.2. PDGF-BB Speeds Up Angiogenesis
4.3. PDGF-BB Stimulates Tendon Cell Proliferation and Increases Collagen Synthesis
4.4. PDGF-BB Induces Cell Differentiation
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, W.; Zhang, M.; Yao, J.; Wang, L.; Siu, K.C. Biomechanics in Musculoskeletal Health. J. Healthc. Eng. 2017, 2017, 8916431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilaltdinov, A.W.; Gong, Y.; Leong, D.J.; Gruson, K.I.; Zheng, D.; Fung, D.T.; Sun, L.; Sun, H.B. Advances in the development of gene therapy, noncoding RNA, and exosome-based treatments for tendinopathy. Ann. N. Y. Acad. Sci. 2021, 1490, 3–12. [Google Scholar] [CrossRef]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Prim. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, S.; Das, R.; Sakiyama-Elbert, S.; Silva, M.J.; Charlton, N.; Gelberman, R.H. bFGF and PDGF-BB for tendon repair: Controlled release and biologic activity by tendon fibroblasts in vitro. Ann. Biomed. Eng. 2010, 38, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Evrova, O.; Kellenberger, D.; Calcagni, M.; Vogel, V.; Buschmann, J. Supporting Cell-Based Tendon Therapy: Effect of PDGF-BB and Ascorbic Acid on Rabbit Achilles Tenocytes in Vitro. Int. J. Mol. Sci. 2020, 21, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, H.; Tholema, N.; Petersen, W.; Raschke, M.J.; Stange, R. Impaired biomechanical properties correlate with neoangiogenesis as well as VEGF and MMP-3 expression during rat patellar tendon healing. J. Orthop. Res. 2012, 30, 1952–1957. [Google Scholar] [CrossRef] [PubMed]
- Vercoutter-Edouart, A.S.; Dubreucq, G.; Vanhoecke, B.; Rigaut, C.; Renaux, F.; Dahri-Correia, L.; Lemoine, J.; Bracke, M.; Michalski, J.C.; Correia, J. Enhancement of PDGF-BB mitogenic activity on human dermal fibroblasts by biospecific dextran derivatives. Biomaterials 2008, 29, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, D.; Gulotta, L.V.; Ying, L.; Ehteshami, J.R.; Deng, X.H.; Rodeo, S.A. rhPDGF-BB promotes early healing in a rat rotator cuff repair model. Clin. Orthop. Relat. Res. 2015, 473, 1644–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evrova, O.; Burgisser, G.M.; Ebnother, C.; Adathala, A.; Calcagni, M.; Bachmann, E.; Snedeker, J.G.; Scalera, C.; Giovanoli, P.; Vogel, V.; et al. Elastic and surgeon friendly electrospun tubes delivering PDGF-BB positively impact tendon rupture healing in a rabbit Achilles tendon model. Biomaterials 2020, 232, 119722. [Google Scholar] [CrossRef] [PubMed]
- Ucuzian, A.A.; Brewster, L.P.; East, A.T.; Pang, Y.; Gassman, A.A.; Greisler, H.P. Characterization of the chemotactic and mitogenic response of SMCs to PDGF-BB and FGF-2 in fibrin hydrogels. J. Biomed. Mater. Res. A 2010, 94, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Evrova, O.; Buschmann, J. In vitro and in vivo effects of PDGF-BB delivery strategies on tendon healing: A review. Eur. Cells Mater. 2017, 34, 15–39. [Google Scholar] [CrossRef]
- Agoulnik, I.; Wang, H.; Yin, Y.; Li, W.; Zhao, X.; Yu, Y.; Zhu, J.; Qin, Z.; Wang, Q.; Wang, K.; et al. Over-Expression of PDGFR-β Promotes PDGF-Induced Proliferation, Migration, and Angiogenesis of EPCs through PI3K/Akt Signaling Pathway. PLoS ONE 2012, 7, e30503. [Google Scholar] [CrossRef] [Green Version]
- Meier Burgisser, G.; Evrova, O.; Calcagni, M.; Scalera, C.; Giovanoli, P.; Buschmann, J. Impact of PDGF-BB on cellular distribution and extracellular matrix in the healing rabbit Achilles tendon three weeks post-operation. FEBS Open Bio 2020, 10, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Borena, B.M.; Martens, A.; Broeckx, S.Y.; Meyer, E.; Chiers, K.; Duchateau, L.; Spaas, J.H. Regenerative Skin Wound Healing in Mammals: State-of-the-Art on Growth Factor and Stem Cell Based Treatments. Cell. Physiol. Biochem. 2015, 36, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, S.; Zaegel, M.; Das, R.; Harwood, F.L.; Silva, M.J.; Amiel, D.; Sakiyama-Elbert, S.; Gelberman, R.H. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J. Orthop. Res. 2007, 25, 1358–1368. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; De la Fuente, M.; Zalduendo, M.M.; Orive, G. Plasma rich in growth factors (PRGF-Endoret) stimulates tendon and synovial fibroblasts migration and improves the biological properties of hyaluronic acid. Knee Surg. Sport. Traumatol. Arthrosc. 2011, 20, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Abrahamsson, S.O. Dose-related cellular effects of platelet-derived growth factor-BB differ in various types of rabbit tendons in vitro. Acta Orthop. Scand. 2001, 72, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, S.; Harwood, F.L.; Silva, M.J.; Amiel, D.; Gelberman, R.H. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J. Hand Surg. Am. 2005, 30, 441–447. [Google Scholar] [CrossRef]
- Haupt, J.L.; Donnelly, B.P.; Nixon, A.J. Effects of platelet-derived growth factor-BB on the metabolic function and morphologic features of equine tendon in explant culture. Am. J. Vet. Res. 2006, 67, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, K.A.; Woo, S.L.Y.; Smith, D.W.; Allen, C.R.; Deie, M.; Taylor, B.J.; Schmidt, C.C. The Effects of Platelet-Derived Growth Factor-BB on Healing of the Rabbit Medial Collateral Ligament. Am. J. Sport. Med. 2016, 26, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Younesi, M.; Knapik, D.M.; Cumsky, J.; Donmez, B.O.; He, P.; Islam, A.; Learn, G.; McClellan, P.; Bohl, M.; Gillespie, R.J.; et al. Effects of PDGF-BB delivery from heparinized collagen sutures on the healing of lacerated chicken flexor tendon in vivo. Acta Biomater. 2017, 63, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Harwood, F.L.; Goomer, R.S.; Gelberman, R.H.; Silva, M.J.; Amiel, D. Regulation of alpha(v)beta3 and alpha5beta1 integrin receptors by basic fibroblast growth factor and platelet-derived growth factor-BB in intrasynovial flexor tendon cells. Wound Repair Regen. 1999, 7, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Chen, A.C.; Yuan, L.J.; Lin, S.S.; Yang, C.Y.; Lee, M.S.; Ueng, S.W. Effects of hyperbaric oxygen and platelet derived growth factor on medial collateral ligament fibroblasts. Undersea Hyperb. Med. J. Undersea Hyperb. Med. Soc. Inc. 2007, 34, 181–190. [Google Scholar]
- Banes, A.J.; Tsuzaki, M.; Hu, P.; Brigman, B.; Brown, T.; Almekinders, L.; Lawrence, W.T.; Fischer, T. PDGF-BB, IGF-I and mechanical load stimulate DNA synthesis in avian tendon fibroblasts in vitro. J. Biomech. 1995, 28, 1505–1513. [Google Scholar] [CrossRef]
- Kang, H.J.; Kang, E.S. Ideal concentration of growth factors in rabbit’s flexor tendon culture. Yonsei Med. J. 1999, 40, 26–29. [Google Scholar] [CrossRef]
- Liang, H.W.; Lin, Y.; Li, Y.J.; Chen, X.; Zhou, H.R.; Tan, Q. Effect of platelet-derived growth factor-BB on proliferation of tendon cells cultured in vitro. Zhonghua Shao Shang Za Zhi Zhonghua Shaoshang Zazhi Chin. J. Burn. 2009, 25, 298–300. [Google Scholar]
- Ren, Y.; Li, W.; Liu, X. Effect of platelet derived growth factor BB on healing of medial collateral ligament in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi = Zhongguo Xiufu Chongjian Waike Zazhi = Chin. J. Reparative Reconstr. Surg. 2006, 20, 616–619. [Google Scholar]
- Kobayashi, K.; Healey, R.M.; Sah, R.L.; Clark, J.J.; Tu, B.P.; Goomer, R.S.; Akeson, W.H.; Moriya, H.; Amiel, D. Novel method for the quantitative assessment of cell migration: A study on the motility of rabbit anterior cruciate (ACL) and medial collateral ligament (MCL) cells. Tissue Eng. 2000, 6, 29–38. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [Green Version]
- Heldin, C.H.; Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, A.; Siegbahn, A.; Westermark, B.; Heldin, C.H.; Claesson-Welsh, L. PDGF alpha- and beta-receptors activate unique and common signal transduction pathways. EMBO J. 1992, 11, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Siegbahn, A.; Hammacher, A.; Westermark, B.; Heldin, C.H. Differential effects of the various isoforms of platelet-derived growth factor on chemotaxis of fibroblasts, monocytes, and granulocytes. J. Clin. Investig. 1990, 85, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Rolf, C.G.; Fu, B.S.; Pau, A.; Wang, W.; Chan, B. Increased cell proliferation and associated expression of PDGFRbeta causing hypercellularity in patellar tendinosis. Rheumatology 2001, 40, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Cao, Y.; Wu, Y.F.; Bais, A.J.; Gao, J.S.; Tang, J.B. Tendon Healing In Vivo: Gene Expression and Production of Multiple Growth Factors in Early Tendon Healing Period. J. Hand Surg. 2008, 33, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Deuel, T.F.; Senior, R.M.; Huang, J.S.; Griffin, G.L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J. Clin. Investig. 1982, 69, 1046–1049. [Google Scholar] [CrossRef] [Green Version]
- Dakin, S.G.; Dudhia, J.; Smith, R.K.W. Resolving an inflammatory concept: The importance of inflammation and resolution in tendinopathy. Vet. Immunol. Immunopathol. 2014, 158, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Chen, Y.; Huang, J.; Zhao, K.; Chen, X.; Yin, Z.; Heng, B.C.; Chen, W.; Shen, W. The roles of inflammatory mediators and immunocytes in tendinopathy. J. Orthop. Transl. 2018, 14, 23–33. [Google Scholar] [CrossRef]
- Pierce, G.F.; Mustoe, T.A.; Altrock, B.W.; Deuel, T.F.; Thomason, A. Role of platelet-derived growth factor in wound healing. J. Cell. Biochem. 1991, 45, 319–326. [Google Scholar] [CrossRef]
- Arvind, V.; Huang, A.H. Reparative and Maladaptive Inflammation in Tendon Healing. Front. Bioeng. Biotechnol. 2021, 9, 625. [Google Scholar] [CrossRef]
- Pierce, G.F.J.A.J.P. Platelet-derived growth factor (BB Homo-dimer), transforming growth factor-β1, and basic fibroblast growth factor in dermal wound healing. Am. J. Pathol. 1992, 140, 1375. [Google Scholar]
- Yang, Q.Q.; Zhang, L.; Zhou, Y.L.; Tang, J.B. Morphological changes of macrophages and their potential contribution to tendon healing. Colloids Surf. B Biointerfaces 2022, 209, 112145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gong, Y.; Zheng, X.; Qiu, J.; Jiang, T.; Chen, L.; Lu, F.; Wu, X.; Cheng, F.; Hong, Z. Platelet-Derived Growth Factor-BB Inhibits Intervertebral Disc Degeneration via Suppressing Pyroptosis and Activating the MAPK Signaling Pathway. Front. Pharm. 2021, 12, 799130. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Angiogenic growth factors. Prog. Growth Factor Res. 1990, 2, 71–79. [Google Scholar] [CrossRef]
- van Roeyen, C.R.C.; Ostendorf, T.; Denecke, B.; Bokemeyer, D.; Behrmann, I.; Strutz, F.; Lichenstein, H.S.; LaRochelle, W.J.; Pena, C.E.; Chaudhuri, A.; et al. Biological responses to PDGF-BB versus PDGF-DD in human mesangial cells. Kidney Int. 2006, 69, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H.; Östman, A.; Rönnstrand, L. Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta (BBA) Rev. Cancer 1998, 1378, F79–F113. [Google Scholar] [CrossRef]
- Cantley, L.C. The Phosphoinositide 3-Kinase Pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Lu, Y.; Azad, N.; Wang, L.; Iyer, A.K.; Castranova, V.; Jiang, B.H.; Rojanasakul, Y. Phosphatidylinositol-3-kinase/akt regulates bleomycin-induced fibroblast proliferation and collagen production. Am. J. Respir. Cell Mol. Biol. 2010, 42, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.H.; Jiang, G.; Zheng, J.Z.; Lu, Z.; Hunter, T.; Vogt, P.K. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 2001, 12, 363–369. [Google Scholar]
- Gianni-Barrera, R.; Butschkau, A.; Uccelli, A.; Certelli, A.; Valente, P.; Bartolomeo, M.; Groppa, E.; Burger, M.G.; Hlushchuk, R.; Heberer, M.; et al. PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation. Angiogenesis 2018, 21, 883–900. [Google Scholar] [CrossRef] [Green Version]
- Battegay, E.J.; Rupp, J.; Iruela-Arispe, L.; Sage, E.H.; Pech, M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J. Cell Biol. 1994, 125, 917–928. [Google Scholar] [CrossRef]
- Folkman, J.; Shing, Y. Angiogenesis. J. Biol. Chem. 1992, 267, 10931–10934. [Google Scholar] [CrossRef]
- Thommen, R.; Humar, R.; Misevic, G.; Pepper, M.S.; Hahn, A.W.; John, M.; Battegay, E.J. PDGF-BB increases endothelial migration on cord movements during angiogenesis in vitro. J. Cell. Biochem. 1997, 64, 403–413. [Google Scholar] [CrossRef]
- Bar, R.S.; Boes, M.; Booth, B.A.; Dake, B.L.; Henley, S.; Hart, M.N. The Effects of Platelet-Derived Growth Factor in Cultured Microvessel Endothelial Cells*. Endocrinology 1989, 124, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.; Hermansson, M.; Nister, M.; Karnushina, I.; Heldin, C.-H.; Westermark, B.; Funa, K. Rat Brain Capillary Endothelial Cells Express Functional PDGF B-Type Receptors. Growth Factors 2009, 2, 1–8. [Google Scholar] [CrossRef]
- Reuterdahl, C.; Sundberg, C.; Rubin, K.; Funa, K.; Gerdin, B. Tissue localization of beta receptors for platelet-derived growth factor and platelet-derived growth factor B chain during wound repair in humans. J. Clin. Investig. 1993, 91, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Lim, S.; Yang, Y.; Wang, Z.; Jensen, L.D.E.; Hedlund, E.-M.; Andersson, P.; Sasahara, M.; Larsson, O.; Galter, D.; et al. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat. Med. 2011, 18, 100–110. [Google Scholar] [CrossRef]
- Uslu, M.; Kaya, E.; Yaykasli, K.O.; Oktay, M.; Inanmaz, M.E.; Isik, C.; Erdem, H.; Erkan, M.E.; Kandis, H. Erythropoietin stimulates patellar tendon healing in rats. Knee 2015, 22, 461–468. [Google Scholar] [CrossRef]
- Bahlmann, F.H.; De Groot, K.; Spandau, J.M.; Landry, A.L.; Hertel, B.; Duckert, T.; Boehm, S.M.; Menne, J.; Haller, H.; Fliser, D. Erythropoietin regulates endothelial progenitor cells. Blood 2004, 103, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Naito, T.; Shun, M.; Nishimura, H.; Gibo, T.; Tosaka, M.; Kawashima, M.; Ando, A.; Ogawa, T.; Sanaka, T.; Nitta, K. Pleiotropic effect of erythropoiesis-stimulating agents on circulating endothelial progenitor cells in dialysis patients. Clin. Exp. Nephrol. 2021, 25, 1111–1120. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, K.; Wang, L.; Lu, T.; Zhou, C.; Ge, Y.; Wu, R.; Jia, R.; Zheng, C. Erythropoietin Preconditioning Mobilizes Endothelial Progenitor Cells to Attenuate Nephron-Sparing Surgery-Induced Ischemia-Reperfusion Injury. Transpl. Proc. 2020, 52, 2955–2963. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A.; Roccaro, A.M.; Crivellato, E.; Presta, M. Erythropoietin as an angiogenic factor. Eur. J. Clin. Investig. 2003, 33, 891–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiftsoglou, A.S. Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells 2021, 10, 2140. [Google Scholar] [CrossRef] [PubMed]
- Trincavelli, M.L.; Da Pozzo, E.; Ciampi, O.; Cuboni, S.; Daniele, S.; Abbracchio, M.P.; Martini, C. Regulation of Erythropoietin Receptor Activity in Endothelial Cells by Different Erythropoietin (EPO) Derivatives: An in Vitro Study. Int. J. Mol. Sci. 2013, 14, 2258–2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltaneri, R.E.; Schiappacasse, A.; Chamorro, M.E.; Nesse, A.B.; Vittori, D.C. Aquaporin-1 plays a key role in erythropoietin-induced endothelial cell migration. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118569. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Connor, K.M.; Aderman, C.M.; Smith, L.E. Erythropoietin deficiency decreases vascular stability in mice. J. Clin. Investig. 2008, 118, 526–533. [Google Scholar] [CrossRef]
- Brooks, P.C.; Clark, R.A.; Cheresh, D.A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994, 264, 569–571. [Google Scholar] [CrossRef]
- Wang, J.; You, J.; Gong, D.; Xu, Y.; Yang, B.; Jiang, C. PDGF-BB induces conversion, proliferation, migration, and collagen synthesis of oral mucosal fibroblasts through PDGFR-beta/PI3K/ AKT signaling pathway. Cancer Biomark. 2021, 30, 407–415. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Liang, C.; Yang, W. CCN5 overexpression inhibits profibrotic phenotypes via the PI3K/Akt signaling pathway in lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis and in an in vivo model of lung fibrosis. Int. J. Mol. Med. 2014, 33, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Bajraszewski, N.; Wu, E.; Wang, H.; Moseman, A.P.; Dabora, S.L.; Griffin, J.D.; Kwiatkowski, D.J. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J. Clin. Investig. 2007, 117, 730–738. [Google Scholar] [CrossRef] [Green Version]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1032. [Google Scholar] [CrossRef]
- Danen, E.H.; Yamada, K.M. Fibronectin, integrins, and growth control. J. Cell Physiol. 2001, 189, 1–13. [Google Scholar] [CrossRef]
- Hu, Y.; Zou, Y.; Dietrich, H.; Wick, G.; Xu, Q. Inhibition of neointima hyperplasia of mouse vein grafts by locally applied suramin. Circulation 1999, 100, 861–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagami, S.; Urushihara, M.; Kitamura, A.; Kondo, S.; Hisayama, T.; Kitamura, M.; Loster, K.; Reutter, W.; Kuroda, Y. PDGF-BB enhances alpha1beta1 integrin-mediated activation of the ERK/AP-1 pathway involved in collagen matrix remodeling by rat mesangial cells. J. Cell Physiol. 2004, 198, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Munoz, P.; Ibares-Frias, L.; Garrote, J.A.; Valsero-Blanco, M.C.; Cantalapiedra-Rodriguez, R.; Merayo-Lloves, J.; Carmen Martinez-Garcia, M. Human corneal fibroblast migration and extracellular matrix synthesis during stromal repair: Role played by platelet-derived growth factor-BB, basic fibroblast growth factor, and transforming growth factor-beta1. J. Tissue Eng. Regen. Med. 2018, 12, e737–e746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oates, T.W.; Rouse, C.A.; Cochran, D.L. Mitogenic effects of growth factors on human periodontal ligament cells in vitro. J. Periodontol. 1993, 64, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Millette, E.; Rauch, B.H.; Kenagy, R.D.; Daum, G.; Clowes, A.W. Platelet-derived growth factor-BB transactivates the fibroblast growth factor receptor to induce proliferation in human smooth muscle cells. Trends Cardiovasc. Med. 2006, 16, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Boyan, L.A.; Bhargava, G.; Nishimura, F.; Orman, R.; Price, R.; Terranova, V.P. Mitogenic and chemotactic responses of human periodontal ligament cells to the different isoforms of platelet-derived growth factor. J. Dent. Res. 1994, 73, 1593–1600. [Google Scholar] [CrossRef]
- Pham, Q.L.; Tong, A.; Rodrigues, L.N.; Zhao, Y.; Surblyte, M.; Ramos, D.; Brito, J.; Rahematpura, A.; Voronov, R.S. Ranking migration cue contributions to guiding individual fibroblasts faced with a directional decision in simple microfluidic bifurcations. Integr. Biol. Quant. Biosci. Nano Macro 2019, 11, 208–220. [Google Scholar] [CrossRef]
- Porsch, H.; Mehić, M.; Olofsson, B.; Heldin, P.; Heldin, C.H. Platelet-derived growth factor β-receptor, transforming growth factor β type I receptor, and CD44 protein modulate each other’s signaling and stability. J. Biol. Chem. 2014, 289, 19747–19757. [Google Scholar] [CrossRef] [Green Version]
- Javanshir, S.; Younesi Soltani, F.; Dowlati, G.; Parham, A.; Naderi-Meshkin, H. Induction of tenogenic differentiation of equine adipose-derived mesenchymal stem cells by platelet-derived growth factor-BB and growth differentiation factor-6. Mol. Biol. Rep. 2020, 47, 6855–6862. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Li, Z. PDGF-BB promotes the differentiation and proliferation of MC3T3-E1 cells through the Src/JAK2 signaling pathway. Mol. Med. Rep. 2018, 18, 3719–3726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Yu, J.C.; Michieli, P.; Beeler, J.F.; Ellmore, N.; Heidaran, M.A.; Pierce, J.H. Stimulation of the platelet-derived growth factor beta receptor signaling pathway activates protein kinase C-delta. Mol. Cell. Biol. 1994, 14, 6727–6735. [Google Scholar] [CrossRef] [PubMed]
- Madhurakkat Perikamana, S.K.; Lee, J.; Ahmad, T.; Kim, E.M.; Byun, H.; Lee, S.; Shin, H. Harnessing biochemical and structural cues for tenogenic differentiation of adipose derived stem cells (ADSCs) and development of an in vitro tissue interface mimicking tendon-bone insertion graft. Biomaterials 2018, 165, 79–93. [Google Scholar] [CrossRef]
- Younesi Soltani, F.; Javanshir, S.; Dowlati, G.; Parham, A.; Naderi-Meshkin, H. Differentiation of human adipose-derived mesenchymal stem cells toward tenocyte by platelet-derived growth factor-BB and growth differentiation factor-6. Cell Tissue Bank. 2021, 23, 237–246. [Google Scholar] [CrossRef]
- Norelli, J.B.; Plaza, D.P.; Stal, D.N.; Varghese, A.M.; Liang, H.; Grande, D.A. Tenogenically differentiated adipose-derived stem cells are effective in Achilles tendon repair in vivo. J. Tissue Eng. 2018, 9, 2041731418811183. [Google Scholar] [CrossRef] [PubMed]
- Evrova, O.; Houska, J.; Welti, M.; Bonavoglia, E.; Calcagni, M.; Giovanoli, P.; Vogel, V.; Buschmann, J. Bioactive, Elastic, and Biodegradable Emulsion Electrospun DegraPol Tube Delivering PDGF-BB for Tendon Rupture Repair. Macromol. Biosci. 2016, 16, 1048–1063. [Google Scholar] [CrossRef] [PubMed]
- Ojima, Y.; Mizuno, M.; Kuboki, Y.; Komori, T. In vitro effect of platelet-derived growth factor-BB on collagen synthesis and proliferation of human periodontal ligament cells. Oral Dis. 2003, 9, 144–151. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, S.H.; Min, H.K.; Lee, J.H. Dual growth factor-immobilized asymmetrically porous membrane for bone-to-tendon interface regeneration on rat patellar tendon avulsion model. J. Biomed. Mater. Res. A 2018, 106, 115–125. [Google Scholar] [CrossRef]
- Yang, X.; Zhan, P.; Wang, X.; Zhang, Q.; Zhang, Y.; Fan, H.; Li, R.; Zhang, M. Polydopamine-assisted PDGF-BB immobilization on PLGA fibrous substrate enhances wound healing via regulating anti-inflammatory and cytokine secretion. PLoS ONE 2020, 15, e0239366. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Das, R.; Silva, M.J.; Sakiyama-Elbert, S.; Harwood, F.L.; Zampiakis, E.; Kim, H.M.; Amiel, D.; Gelberman, R.H. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J. Orthop. Res. 2009, 27, 1209–1215. [Google Scholar] [CrossRef]

| Study | Animal Type | Models Established | Dosage | Time Post Operation | Outcome | Conclusion |
|---|---|---|---|---|---|---|
| [9] | Rabbits | Achilles tendon of rabbit | PDGF-BB: 8 μg | 3 weeks | Tensile strength↑ | The tendon tensile strength was increased by a factor of 2 and there was no additional pro-fibrotic effect. |
| [20] | New Zealand White rabbits | MCL rupture | PDGF-BB: 400 ng, 20 mg | 6 weeks | Ultimate load↑ Ultimate elongation values↑ | The application of PDGF-BB significantly improved the ultimate load, failure absorption energy and ultimate elongation values of the femoral–MCL–tibial complex. In addition, the high-dose group improved more structural properties of the complex compared to the low-dose PDGF-BB group. |
| [19] | Horses aged 2–5 years | Superficial digital flexor tendon | rhPDGF-BB: 1, 10, 50, 100 ng/mL | 24 h, 48 h, 6 days | Type I collagen↑ Type III collagen↓ | The use of rhPDGF-BB may be beneficial for equine tendon repair, particularly through the induction of type I collagen Mrna. |
| [18] | Dogs | The flexor digitorum profundus tendons. | PDGF-BB: 10 ng/mL | 2 h, 24 h | Cell proliferation↑ Collagen synthesis↑ | PDGF-BB can stimulate tendon cell proliferation and collagen synthesis. |
| [8] | Sprague Dawley rats | The supraspinatus tendon | rhPDGF-BB: 30, 100, 300 μg/mL | 5 days | Cell proliferation↑ Angiogenesis↑ | The rhPDGF-BB enhanced cell proliferation and angiogenesis in the early stages of healing. |
| [21] | White Leghorn chickens | Flexor tendon repair | PDGF-BB: 10 μg/mL | 12 weeks | Cell proliferation↑ Angiogenesis↑ | The results showed that the combination of heparinized ELAS with PDGF-BB improved biomechanics and vascularity during tendon healing 12 weeks after primary repair. |
| [22] | Dogs | Deep toe flexor tendon | PDGF-BB: 5 ng/mL | 1, 4 weeks | α5β1↑ αvβ3↑ Cell proliferation↑ | PDGF-BB promotes tendon healing by upregulating integrins. |
| [17] | Swedish Loop rabbits | Deep flexor tendons and extrasynovial peroneal tendons | PDGF-BB: 0.1, 0.3, 1.0, 3.0, 10.0, 30.0, 100.0 ng/mL | 4 days | Proteoglycan synthesis rate↑ Collagen synthesis↑ DNA synthesis↑ | The effects of PDGF-BB on proteoglycan synthesis rate, collagen synthesis and DNA synthesis were dose-dependent for both medial and proximal synovial internal flexor tendon segments and peroneal extrasynovial tendon segments, and the doses were between 0.1–30 and 0.1–100 ng/mL, 0.1–30 ng/mL; 0.1–30 and 0.1–0.3 ng/mL, between 0.1−30 ng/mL; between 0.1–30 and 0.1–100 ng/mL, between 0.1–10 ng/mL. |
| [23] | New Zealand White rabbits | MCL rupture and repair | PDGF-BB: 400 ng, 20 mg | 1 week | Ultimate load↑ Ultimate elongation↑ Energy of failure absorption↑ | PDGF-BB improves the quality of the healing medial collateral ligament, and it may have similar potential to promote healing in other ligaments as well. |
| [24] | Chicken | Flexor tendons | PDGF-BB: 10, 50, 100 picomolar | 8, 24 h | DNA synthesis↑ | PDGF-BB stimulates DNA synthesis to promote tendon cell matrix repair. |
| [25] | New Zealand White rabbits | Deep flexor tendons | PDGF-BB: 10, 50, 100, 150 ng/mL | 2, 4, 6 days | Cell proliferation↑ Collagen synthesis↑ | Tendons from rabbits showed a significant dose-dependent response to PDGF in the concentration range from 10 ng/mL to 150 ng/mL. |
| [26] | In vitro | Rat flexor tendon fibroblasts | PDGF-BB: 1, 5, 10, 20, 50, 100, 150, 200, 250 μg/L | 12, 24, 36, 48, 60, 72 h | Cell proliferation↑ (Except for 250 μg/L) | PDGF-BB can promote the proliferation of tendon cells in a definite range of concentration and time. |
| [27] | Rats | MCL rupture and repair | PDGF-BB: 5 μg | 2 weeks | Cell proliferation↑ Collagen synthesis↑ | PDGF-BB significantly increased the proliferation of fibroblasts and promoted collagen synthesis. |
| [28] | In vitro | Rabbit ACL and MCL cells | PDGF-BB: 1, 100 ng/mL | 12 h | Cell migration↑ (Except for 100 ng/mL) | Low concentrations of PDGF-BB can stimulate cell motility. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Jiang, L.; Lyu, K.; Lu, J.; Long, L.; Wang, X.; Liu, T.; Li, S. A Promising Candidate in Tendon Healing Events—PDGF-BB. Biomolecules 2022, 12, 1518. https://doi.org/10.3390/biom12101518
Chen Y, Jiang L, Lyu K, Lu J, Long L, Wang X, Liu T, Li S. A Promising Candidate in Tendon Healing Events—PDGF-BB. Biomolecules. 2022; 12(10):1518. https://doi.org/10.3390/biom12101518
Chicago/Turabian StyleChen, Yixuan, Li Jiang, Kexin Lyu, Jingwei Lu, Longhai Long, Xiaoqiang Wang, Tianzhu Liu, and Sen Li. 2022. "A Promising Candidate in Tendon Healing Events—PDGF-BB" Biomolecules 12, no. 10: 1518. https://doi.org/10.3390/biom12101518
APA StyleChen, Y., Jiang, L., Lyu, K., Lu, J., Long, L., Wang, X., Liu, T., & Li, S. (2022). A Promising Candidate in Tendon Healing Events—PDGF-BB. Biomolecules, 12(10), 1518. https://doi.org/10.3390/biom12101518





