Matrix Metalloproteinases and Glaucoma
Abstract
1. Introduction
2. Results and Discussion
2.1. Pathogenesis of Glaucoma concerning Matrix Metalloproteinases
2.1.1. Pathogenesis of Glaucoma Subtypes
2.1.2. Matrix Metalloproteinases and Trabecular Meshwork with Glaucoma
2.1.3. Matrix Metalloproteinases and Neuroretina with Glaucoma
2.2. Genetic Polymorphism in Matrix Metalloproteinases and Glaucoma Subtypes
| Genes | SNP | Risky Glaucoma Type | Protective Glaucoma Type | Systemic Risks Affecting Glaucoma | Clinical Characteristics | References |
|---|---|---|---|---|---|---|
| MMP-1 | rs1799750 | POAG (Multiple ethnicities) XFG (Asian) | - | Hypertension | Earlier onset of POAG Likely to cause optic nerve damage Likely to increase AH outflow resistance Abnormal accumulation of matrix in TM of POAG patients | [91,122,123,124,125,126,127,128,130,153,154] |
| MMP-9 | rs3918249 | POAG (Asian) PACG (Caucasian) | POAG (Caucasian) PACG (Multiple ethnicities) XFG (Caucasian) | - | Likely to decrease axial length of eye in PACG Likely to increase IOP | [126,131,132,133,137,139] |
| rs2250889 | POAG (Caucasian) PACG (Asian) XFG (Caucasian) | - | - | Elevation of plasma MMP-9 level Decreased IOP in POAG | [126,132,137] | |
| rs17576 | POAG (Asian) PACG (Asian, Caucasian) | POAG (Caucasian) | Hypertension Diabetes | Elevation of plasma MMP-9 level Decreased IOP in POAG | [122,126,131,133,137,139,140,141,155] | |
| rs3918254 | PACG (Asian) | - | - | - | [135] | |
| rs2274755 | NTG (Asian) | - | - | Likely to cause weakness in lamina cribrosa | [144] | |
| rs3918242 | POAG (Asian) PACG (Asian) | POAG (Caucasian) | - | Elevation of plasma MMP-9 level | [124,126,145,146] |
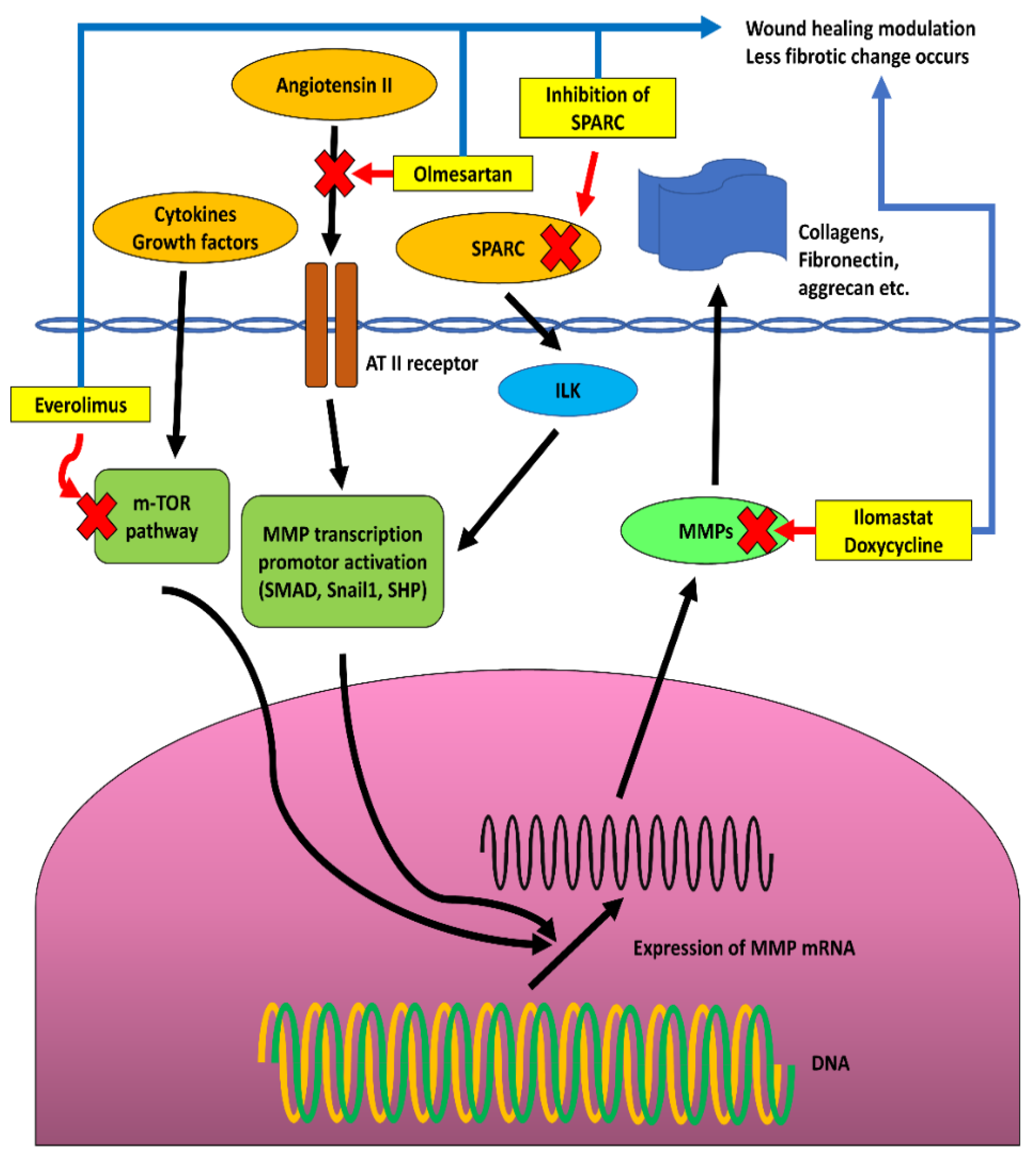
2.3. Matrix Metalloproteinases and Wound Healing in Glaucoma Surgical Treatments
2.4. Ocular Surface Change by Matrix Metalloproteinase Function during Medical Treatment for Glaucoma
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- McCawley, L.J.; Matrisian, L.M. Matrix Metalloproteinases: They’re Not Just for Matrix Anymore! Curr. Opin. Cell Biol. 2001, 13, 534–540. [Google Scholar] [CrossRef]
- Zhang, Y.; Klassen, H.J.; Tucker, B.A.; Perez, M.-T.R.; Young, M.J. CNS Progenitor Cells Promote a Permissive Environment for Neurite Outgrowth via a Matrix Metalloproteinase-2-Dependent Mechanism. J. Neurosci. 2007, 27, 4499–4506. [Google Scholar] [CrossRef]
- Van Lint, P.; Libert, C. Chemokine and Cytokine Processing by Matrix Metalloproteinases and Its Effect on Leukocyte Migration and Inflammation. J. Leukoc. Biol. 2007, 82, 1375–1381. [Google Scholar] [CrossRef]
- McQuibban, G.A.; Gong, J.H.; Tam, E.M.; McCulloch, C.A.; Clark-Lewis, I.; Overall, C.M. Inflammation Dampened by Gelatinase A Cleavage of Monocyte Chemoattractant Protein-3. Science 2000, 289, 1202–1206. [Google Scholar] [CrossRef]
- Mannello, F.; Luchetti, F.; Falcieri, E.; Papa, S. Multiple Roles of Matrix Metalloproteinases during Apoptosis. Apoptosis 2005, 10, 19–24. [Google Scholar] [CrossRef]
- Mott, J.D.; Werb, Z. Regulation of Matrix Biology by Matrix Metalloproteinases. Curr. Opin. Cell Biol. 2004, 16, 558–564. [Google Scholar] [CrossRef]
- Cauwe, B.; Van den Steen, P.E.; Opdenakker, G. The Biochemical, Biological, and Pathological Kaleidoscope of Cell Surface Substrates Processed by Matrix Metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 113–185. [Google Scholar] [CrossRef]
- Tonti, G.A.; Mannello, F.; Cacci, E.; Biagioni, S. Neural Stem Cells at the Crossroads: MMPs May Tell the Way. Int. J. Dev. Biol. 2009, 53, 1–17. [Google Scholar] [CrossRef]
- Chakraborti, S.; Mandal, M.; Das, S.; Mandal, A.; Chakraborti, T. Regulation of Matrix Metalloproteinases: An Overview. Mol. Cell Biochem. 2003, 253, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G. Myocardial Matrix Remodeling and the Matrix Metalloproteinases: Influence on Cardiac Form and Function. Physiol. Rev. 2007, 87, 1285–1342. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Matrix Metalloproteinases and Their Multiple Roles in Neurodegenerative Diseases. Lancet Neurol. 2009, 8, 205–216. [Google Scholar] [CrossRef]
- Miller, J.P.; Holcomb, J.; Al-Ramahi, I.; de Haro, M.; Gafni, J.; Zhang, N.; Kim, E.; Sanhueza, M.; Torcassi, C.; Kwak, S.; et al. Matrix Metalloproteinases Are Modifiers of Huntingtin Proteolysis and Toxicity in Huntington’s Disease. Neuron 2010, 67, 199–212. [Google Scholar] [CrossRef]
- Rivera, S.; Khrestchatisky, M.; Kaczmarek, L.; Rosenberg, G.A.; Jaworski, D.M. Metzincin Proteases and Their Inhibitors: Foes or Friends in Nervous System Physiology? J. Neurosci. 2010, 30, 15337–15357. [Google Scholar] [CrossRef]
- Yong, V.W. Metalloproteinases: Mediators of Pathology and Regeneration in the CNS. Nat. Rev. Neurosci. 2005, 6, 931–944. [Google Scholar] [CrossRef]
- Fujioka, H.; Dairyo, Y.; Yasunaga, K.; Emoto, K. Neural Functions of Matrix Metalloproteinases: Plasticity, Neurogenesis, and Disease. Biochem. Res. Int. 2012, 2012, 789083. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and Pathophysiology of Matrix Metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef]
- Nagase, H.; Woessner, J.F. Matrix Metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef]
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768. [Google Scholar] [CrossRef]
- Ratnikov, B.I.; Cieplak, P.; Gramatikoff, K.; Pierce, J.; Eroshkin, A.; Igarashi, Y.; Kazanov, M.; Sun, Q.; Godzik, A.; Osterman, A.; et al. Basis for Substrate Recognition and Distinction by Matrix Metalloproteinases. Proc. Natl. Acad. Sci. USA 2014, 111, E4148–E4155. [Google Scholar] [CrossRef] [PubMed]
- Alipour, H.; Raz, A.; Zakeri, S.; Dinparast Djadid, N. Therapeutic Applications of Collagenase (Metalloproteases): A Review. Asian Pac. J. Trop. Biomed. 2016, 6, 975–981. [Google Scholar] [CrossRef]
- Hannocks, M.-J.; Zhang, X.; Gerwien, H.; Chashchina, A.; Burmeister, M.; Korpos, E.; Song, J.; Sorokin, L. The Gelatinases, MMP-2 and MMP-9, as Fine Tuners of Neuroinflammatory Processes. Matrix Biol. 2019, 75–76, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, A.; Popovski, N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics 2021, 11, 480. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Coban, M.; Mahajan, S.; Hockla, A.; Sankaran, B.; Downey, G.P.; Radisky, D.C.; Radisky, E.S. Engineering of Tissue Inhibitor of Metalloproteinases TIMP-1 for Fine Discrimination between Closely Related Stromelysins MMP-3 and MMP-10. J. Biol. Chem. 2022, 298, 101654. [Google Scholar] [CrossRef]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of Matrix Metalloproteinase in Wound Healing. Am. J. Transl. Res. 2022, 14, 4391–4405. [Google Scholar]
- Fogarasi, M.; Dima, S. The Catalytic Domain Mediates Homomultimerization of MT1-MMP and the Prodomain Interferes with MT1-MMP Oligomeric Complex Assembly. Biomolecules 2022, 12, 1145. [Google Scholar] [CrossRef]
- Chuliá-Peris, L.; Carreres-Rey, C.; Gabasa, M.; Alcaraz, J.; Carretero, J.; Pereda, J. Matrix Metalloproteinases and Their Inhibitors in Pulmonary Fibrosis: EMMPRIN/CD147 Comes into Play. Int. J. Mol. Sci. 2022, 23, 6894. [Google Scholar] [CrossRef]
- Loreto, C.; Polizzi, A.; Filetti, V.; Pannone, G.; Dos Santos, J.N.; Venezia, P.; Leonardi, R.; Isola, G. Expression of Matrix Metalloproteinases 7 and 9, Desmin, Alpha-Smooth Muscle Actin and Caldesmon, in Odontogenic Keratocyst Associated with NBCCS, Recurrent and Sporadic Keratocysts. Biomolecules 2022, 12, 775. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Robinson, M.R.; Dibas, M.; Stamer, W.D. Matrix Metalloproteinases and Glaucoma Treatment. J. Ocul. Pharmacol. Ther. 2020, 36, 208–228. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Fingert, J.H.; Kuehn, M.H.; Alward, W.L.M. Primary Open-Angle Glaucoma. N. Engl. J. Med. 2009, 360, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Khaw, P.T. Primary Open-Angle Glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Quigley, H.A. Number of People with Glaucoma Worldwide. Br. J. Ophthalmol. 1996, 80, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A. Reduction of Intraocular Pressure and Glaucoma Progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268. [Google Scholar] [CrossRef]
- Iwase, A.; Suzuki, Y.; Araie, M.; Yamamoto, T.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.; Shimizu, H.; Tomita, G. The Prevalence of Primary Open-Angle Glaucoma in Japanese: The Tajimi Study. Ophthalmology 2004, 111, 1641–1648. [Google Scholar] [CrossRef]
- Bouhenni, R.A.; Dunmire, J.; Sewell, A.; Edward, D.P. Animal Models of Glaucoma. J. Biomed. Biotechnol. 2012, 2012, 692609. [Google Scholar] [CrossRef]
- Johnson, T.V.; Tomarev, S.I. Rodent Models of Glaucoma. Brain Res. Bull. 2010, 81, 349–358. [Google Scholar] [CrossRef]
- Guo, Y.; Cepurna, W.O.; Dyck, J.A.; Doser, T.A.; Johnson, E.C.; Morrison, J.C. Retinal Cell Responses to Elevated Intraocular Pressure: A Gene Array Comparison between the Whole Retina and Retinal Ganglion Cell Layer. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3003. [Google Scholar] [CrossRef][Green Version]
- Myers, J.S. Retinal Ganglion Cell Apoptosis in Glaucoma Is Related to Intraocular Pressure and IOP-Induced Effects on Extracellular Matrix. Yearb. Ophthalmol. 2006, 2006, 83–85. [Google Scholar] [CrossRef]
- De Groef, L.; Van Hove, I.; Dekeyster, E.; Stalmans, I.; Moons, L. MMPs in the Trabecular Meshwork: Promising Targets for Future Glaucoma Therapies? Investig. Ophthalmol. Vis. Sci. 2013, 54, 7756–7763. [Google Scholar] [CrossRef]
- De Groef, L.; Van Hove, I.; Dekeyster, E.; Stalmans, I.; Moons, L. MMPs in the Neuroretina and Optic Nerve: Modulators of Glaucoma Pathogenesis and Repair? Investig. Ophthalmol. Vis. Sci. 2014, 55, 1953. [Google Scholar] [CrossRef] [PubMed]
- Shima, I.; Katsuda, S.; Ueda, Y.; Takahashi, N.; Sasaki, H. Expression of Matrix Metalloproteinases in Wound Healing after Glaucoma Filtration Surgery in Rabbits. Ophthalmic Res. 2007, 39, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Zaleska-Żmijewska, A.; Strzemecka, E.; Wawrzyniak, Z.M.; Szaflik, J.P. Extracellular MMP-9-Based Assessment of Ocular Surface Inflammation in Patients with Primary Open-Angle Glaucoma. J. Ophthalmol. 2019, 2019, 1240537. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.A.; Lee, Y.; Zhang, J.-J.; Marshall, J. Characterization of the Gelatinase System of the Laminar Human Optic Nerve, and Surrounding Annulus of Bruch’s Membrane, Choroid, and Sclera. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2358. [Google Scholar] [CrossRef][Green Version]
- Foster, P.J.; Buhrmann, R.; Quigley, H.A.; Johnson, G.J. The Definition and Classification of Glaucoma in Prevalence Surveys. Br. J. Ophthalmol. 2002, 86, 238–242. [Google Scholar] [CrossRef]
- Dandona, L.; Dandona, R.; Mandal, P.; Srinivas, M.; John, R.K.; McCarty, C.A.; Rao, G.N. Angle-Closure Glaucoma in an Urban Population in Southern India. The Andhra Pradesh Eye Disease Study. Ophthalmology 2000, 107, 1710–1716. [Google Scholar] [CrossRef]
- Foster, P.J.; Oen, F.T.; Machin, D.; Ng, T.P.; Devereux, J.G.; Johnson, G.J.; Khaw, P.T.; Seah, S.K. The Prevalence of Glaucoma in Chinese Residents of Singapore: A Cross-Sectional Population Survey of the Tanjong Pagar District. Arch. Ophthalmol. 2000, 118, 1105–1111. [Google Scholar] [CrossRef]
- Vajaranant, T.S.; Nayak, S.; Wilensky, J.T.; Joslin, C.E. Gender and Glaucoma: What We Know and What We Need to Know. Curr. Opin. Ophthalmol. 2010, 21, 91–99. [Google Scholar] [CrossRef]
- Foster, P.J.; Alsbirk, P.H.; Baasanhu, J.; Munkhbayar, D.; Uranchimeg, D.; Johnson, G.J. Anterior Chamber Depth in Mongolians: Variation with Age, Sex, and Method of Measurement. Am. J. Ophthalmol. 1997, 124, 53–60. [Google Scholar] [CrossRef]
- Alsbirk, P.H. Anterior Chamber Depth and Primary Angle-Closure Glaucoma. I. An Epidemiologic Study in Greenland Eskimos. Acta Ophthalmol. 1975, 53, 89–104. [Google Scholar] [CrossRef]
- He, M.; Wang, D.; Zheng, Y.; Zhang, J.; Yin, Q.; Huang, W.; Mackey, D.A.; Foster, P.J. Heritability of Anterior Chamber Depth as an Intermediate Phenotype of Angle-Closure in Chinese: The Guangzhou Twin Eye Study. Investig. Ophthalmol. Vis. Sci. 2008, 49, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ritch, R. Exfoliation Syndrome-the Most Common Identifiable Cause of Open-Angle Glaucoma. J. Glaucoma 1994, 3, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Zenkel, M.; Schlötzer-Schrehardt, U. The Composition of Exfoliation Material and the Cells Involved in Its Production. J. Glaucoma 2014, 23, S12–S14. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U.; Naumann, G.O. Ocular and Systemic Pseudoexfoliation Syndrome. Am. J. Ophthalmol. 2006, 141, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Gottfredsdottir, M.S.; Sverrisson, T.; Musch, D.C.; Stefansson, E. Chronic Open-Angle Glaucoma and Associated Ophthalmic Findings in Monozygotic Twins and Their Spouses in Iceland. J. Glaucoma 1999, 8, 134–139. [Google Scholar] [CrossRef]
- Aasved, H. Study of Relatives of Persons with Fibrillopathia Epitheliocapsularis (Pseudoexfoliation of the Lens Capsule). Acta Ophthalmol. 1975, 53, 879–886. [Google Scholar] [CrossRef]
- Kozobolis, V.P.; Detorakis, E.T.; Sourvinos, G.; Pallikaris, I.G.; Spandidos, D.A. Loss of Heterozygosity in Pseudoexfoliation Syndrome. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1255–1260. [Google Scholar]
- Challa, P. Genetics of Pseudoexfoliation Syndrome. Curr. Opin. Ophthalmol. 2009, 20, 88–91. [Google Scholar] [CrossRef]
- Turalba, A.V.; Chen, T.C. Clinical and Genetic Characteristics of Primary Juvenile-Onset Open-Angle Glaucoma (JOAG). Semin. Ophthalmol. 2008, 23, 19–25. [Google Scholar] [CrossRef]
- Fingert, J.H.; Stone, E.M.; Sheffield, V.C.; Alward, W.L.M. Myocilin Glaucoma. Surv. Ophthalmol. 2002, 47, 547–561. [Google Scholar] [CrossRef]
- Ashworth Briggs, E.L.; Toh, T.; Eri, R.; Hewitt, A.W.; Cook, A.L. TIMP1, TIMP2, and TIMP4 Are Increased in Aqueous Humor from Primary Open Angle Glaucoma Patients. Mol. Vis. 2015, 21, 1162–1172. [Google Scholar] [PubMed]
- Peters, J.C.; Bhattacharya, S.; Clark, A.F.; Zode, G.S. Increased Endoplasmic Reticulum Stress in Human Glaucomatous Trabecular Meshwork Cells and Tissues. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3860–3868. [Google Scholar] [CrossRef] [PubMed]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular Matrix in the Trabecular Meshwork: Intraocular Pressure Regulation and Dysregulation in Glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Gabelt, B.T.; Geiger, B.; Kaufman, P.L. The Role of the Actomyosin System in Regulating Trabecular Fluid Outflow. Exp. Eye Res. 2009, 88, 713–717. [Google Scholar] [CrossRef]
- Tane, N.; Dhar, S.; Roy, S.; Pinheiro, A.; Ohira, A.; Roy, S. Effect of Excess Synthesis of Extracellular Matrix Components by Trabecular Meshwork Cells: Possible Consequence on Aqueous Outflow. Exp. Eye Res. 2007, 84, 832–842. [Google Scholar] [CrossRef]
- Oh, D.-J.; Martin, J.L.; Williams, A.J.; Russell, P.; Birk, D.E.; Rhee, D.J. Effect of Latanoprost on the Expression of Matrix Metalloproteinases and Their Tissue Inhibitors in Human Trabecular Meshwork Cells. Invest. Ophthalmol. Vis. Sci. 2006, 47, 3887–3895. [Google Scholar] [CrossRef]
- Keller, K.E.; Acott, T.S. The Juxtacanalicular Region of Ocular Trabecular Meshwork: A Tissue with a Unique Extracellular Matrix and Specialized Function. J. Ocul. Biol. 2013, 1, 3. [Google Scholar]
- Tamm, E.R. The Trabecular Meshwork Outflow Pathways: Structural and Functional Aspects. Exp. Eye Res. 2009, 88, 648–655. [Google Scholar] [CrossRef]
- Alexander, J.P.; Samples, J.R.; Van Buskirk, E.M.; Acott, T.S. Expression of Matrix Metalloproteinases and Inhibitor by Human Trabecular Meshwork. Investig. Ophthalmol. Vis. Sci. 1991, 32, 172–180. [Google Scholar]
- Kelley, M.J.; Rose, A.Y.; Song, K.; Chen, Y.; Bradley, J.M.; Rookhuizen, D.; Acott, T.S. Synergism of TNF and IL-1 in the Induction of Matrix Metalloproteinase-3 in Trabecular Meshwork. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2634–2643. [Google Scholar] [CrossRef]
- Bradley, J.; Vranka, J.; Colvis, C.; Conger, D.; Alexander, J.; Fisk, A.; Samples, J.; Acott, T. Effect of Matrix Metalloproteinases Activity on Outflow in Perfused Human Organ Culture. Investig. Ophthalmol. Vis. Sci. 1999, 39, 2649–2658. [Google Scholar]
- Snider, E.J.; Hardie, B.A.; Li, Y.; Gao, K.; Splaine, F.; Kim, R.K.; Vannatta, R.T.; Read, A.T.; Ethier, C.R. A Porcine Organ-Culture Glaucoma Model Mimicking Trabecular Meshwork Damage Using Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2021, 62, 18. [Google Scholar] [CrossRef]
- Acott, T.S.; Kelley, M.J. Extracellular Matrix in the Trabecular Meshwork. Exp. Eye Res. 2008, 86, 543–561. [Google Scholar] [CrossRef]
- Bradley, J.M.B.; Kelley, M.J.; Rose, A.; Acott, T.S. Signaling Pathways Used in Trabecular Matrix Metalloproteinase Response to Mechanical Stretch. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5174–5181. [Google Scholar] [CrossRef]
- Bradley, J.M.B.; Kelley, M.J.; Zhu, X.; Anderssohn, A.M.; Alexander, J.P.; Acott, T.S. Effects of Mechanical Stretching on Trabecular Matrix Metalloproteinases. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1505–1513. [Google Scholar]
- Luna, C.; Li, G.; Liton, P.B.; Epstein, D.L.; Gonzalez, P. Alterations in Gene Expression Induced by Cyclic Mechanical Stress in Trabecular Meshwork Cells. Mol. Vis. 2009, 15, 534–544. [Google Scholar]
- WuDunn, D. The Effect of Mechanical Strain on Matrix Metalloproteinase Production by Bovine Trabecular Meshwork Cells. Curr. Eye Res. 2001, 22, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Fleenor, D.L.; Pang, I.-H.; Clark, A.F. Involvement of AP-1 in Interleukin-1α–Stimulated MMP-3 Expression in Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Aga, M.; Bradley, J.M.; Keller, K.E.; Kelley, M.J.; Acott, T.S. Specialized Podosome- or Invadopodia-like Structures (PILS) for Focal Trabecular Meshwork Extracellular Matrix Turnover. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5353–5365. [Google Scholar] [CrossRef]
- Guo, M.-S.; Wu, Y.-Y.; Liang, Z.-B. Hyaluronic Acid Increases MMP-2 and MMP-9 Expressions in Cultured Trabecular Meshwork Cells from Patients with Primary Open-Angle Glaucoma. Mol. Vis. 2012, 18, 1175–1181. [Google Scholar]
- Knepper, P.A.; Goossens, W.; Hvizd, M.; Palmberg, P.F. Glycosaminoglycans of the Human Trabecular Meshwork in Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1360–1367. [Google Scholar]
- Schlötzer-Schrehardt, U.; Lommatzsch, J.; Küchle, M.; Konstas, A.G.P.; Naumann, G.O.H. Matrix Metalloproteinases and Their Inhibitors in Aqueous Humor of Patients with Pseudoexfoliation Syndrome/Glaucoma and Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Määttä, M.; Tervahartiala, T.; Harju, M.; Airaksinen, J.; Autio-Harmainen, H.; Sorsa, T. Matrix Metalloproteinases and Their Tissue Inhibitors in Aqueous Humor of Patients with Primary Open-Angle Glaucoma, Exfoliation Syndrome, and Exfoliation Glaucoma. J. Glaucoma 2005, 14, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in Ophthalmology: Drug Delivery Innovations, Pharmacology, Clinical Applications, and Future Perspectives. Drug Deliv. Transl. Res. 2021, 11, 866–893. [Google Scholar] [CrossRef] [PubMed]
- Phulke, S.; Kaushik, S.; Kaur, S.; Pandav, S. Steroid-Induced Glaucoma: An Avoidable Irreversible Blindness. J. Curr. Glaucoma Pract. 2017, 11, 67–72. [Google Scholar] [CrossRef]
- Mohd Nasir, N.A.; Agarwal, R.; Krasilnikova, A.; Sheikh Abdul Kadir, S.H.; Iezhitsa, I. Effect of Dexamethasone on the Expression of MMPs, Adenosine A1 Receptors and NFKB by Human Trabecular Meshwork Cells. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190373. [Google Scholar] [CrossRef]
- Saadat, F.; Raji, A.; Eslami, K.Z.M.B.; Pezeshki, M.; Aalizadeh, M.R.K.N. Alteration in Matrix Metalloproteinases (MMPS) Activity in Fibroblast Cell Line by Dexamethasone: A Possible Mechanism in Corticosteroid-Induced Glaucoma. Iran. J. Allergy Asthma Immunol. 2003, 2, 145–148. [Google Scholar]
- Hofmaier, F.; Hauck, S.M.; Amann, B.; Degroote, R.L.; Deeg, C.A. Changes in Matrix Metalloproteinase Network in a Spontaneous Autoimmune Uveitis Model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2314–2320. [Google Scholar] [CrossRef]
- Agapova, O.A.; Kaufman, P.L.; Lucarelli, M.J.; Gabelt, B.T.; Hernandez, M.R. Differential Expression of Matrix Metalloproteinases in Monkey Eyes with Experimental Glaucoma or Optic Nerve Transection. Brain Res. 2003, 967, 132–143. [Google Scholar] [CrossRef]
- Sun, M.-H.; Chen, K.-J.; Tsao, Y.-P.; Kao, L.-Y.; Han, W.-H.; Lin, K.-K.; Pang, J.-H.S. Down-Regulation of Matrix Metalloproteinase-9 by Pyrrolidine Dithiocarbamate Prevented Retinal Ganglion Cell Death after Transection of Optic Nerve in Rats. Curr. Eye Res. 2011, 36, 1053–1063. [Google Scholar] [CrossRef]
- Agapova, O.A.; Ricard, C.S.; Salvador-Silva, M.; Hernandez, M.R. Expression of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Human Optic Nerve Head Astrocytes. Glia 2001, 33, 205–216. [Google Scholar] [CrossRef]
- Yan, X.; Tezel, G.; Wax, M.B.; Edward, D.P. Matrix Metalloproteinases and Tumor Necrosis Factor Alpha in Glaucomatous Optic Nerve Head. Arch. Ophthalmol. 2000, 118, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.; Chignell, A.H.; Limb, G.A. Predominance of MMP-1 and MMP-2 in Epiretinal and Subretinal Membranes of Proliferative Vitreoretinopathy. Exp. Eye Res. 1999, 68, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, C.F.; Downs, J.C. Premise and Prediction—How Optic Nerve Head Biomechanics Underlies the Susceptibility and Clinical Behavior of the Aged Optic Nerve Head. J. Glaucoma 2008, 17, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Emery, J.M.; Landis, D.; Paton, D.; Boniuk, M.; Craig, J.M. The Lamina Cribrosa in Normal and Glaucomatous Human Eyes. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1974, 78, 290–297. [Google Scholar]
- Downs, J.C.; Girkin, C.A. Lamina Cribrosa in Glaucoma. Curr. Opin. Ophthalmol. 2017, 28, 113–119. [Google Scholar] [CrossRef]
- Quill, B.; Docherty, N.G.; Clark, A.F.; O’Brien, C.J. The Effect of Graded Cyclic Stretching on Extracellular Matrix–Related Gene Expression Profiles in Cultured Primary Human Lamina Cribrosa Cells. Invesigt. Ophthalmol. Vis. Sci. 2011, 52, 1908. [Google Scholar] [CrossRef]
- Kirwan, R.P.; Crean, J.K.; Fenerty, C.H.; Clark, A.F.; O’Brien, C.J. Effect of Cyclical Mechanical Stretch and Exogenous Transforming Growth Factor-beta1 on Matrix Metalloproteinase-2 Activity in Lamina Cribrosa Cells from the Human Optic Nerve Head. J. Glaucoma 2004, 13, 327–334. [Google Scholar] [CrossRef]
- Morrison, J.C. Integrins in the Optic Nerve Head: Potential Roles in Glaucomatous Optic Neuropathy (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 453–477. [Google Scholar]
- Akhter, N.; Nix, M.; Abdul, Y.; Singh, S.; Husain, S. Delta-Opioid Receptors Attenuate TNF-α–Induced MMP-2 Secretion from Human ONH Astrocytes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6605–6611. [Google Scholar] [CrossRef][Green Version]
- Albon, J.; Karwatowski, W.S.; Avery, N.; Easty, D.L.; Duance, V.C. Changes in the Collagenous Matrix of the Aging Human Lamina Cribrosa. Br. J. Ophthalmol. 1995, 79, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.C.; Jerdan, J.A.; Dorman, M.E.; Quigley, H.A. Structural Proteins of the Neonatal and Adult Lamina Cribrosa. Arch. Ophthalmol. 1989, 107, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Addicks, E.M.; Green, W.R.; Maumenee, A.E. Optic Nerve Damage in Human Glaucoma. II. The Site of Injury and Susceptibility to Damage. Arch. Ophthalmol. 1981, 99, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, C.F.; Downs, J.C.; Bellezza, A.J.; Hart, R.T. Three-Dimensional Reconstruction of Normal and Early Glaucoma Monkey Optic Nerve Head Connective Tissues. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4388–4399. [Google Scholar] [CrossRef]
- Yang, H.; Downs, J.C.; Girkin, C.; Sakata, L.; Bellezza, A.; Thompson, H.; Burgoyne, C.F. 3-D Histomorphometry of the Normal and Early Glaucomatous Monkey Optic Nerve Head: Lamina Cribrosa and Peripapillary Scleral Position and Thickness. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4597–4607. [Google Scholar] [CrossRef] [PubMed]
- Halfter, W.; Willem, M.; Mayer, U. Basement Membrane-Dependent Survival of Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, M.; Chintala, S.K. Optic Nerve Ligation Leads to Astrocyte-Associated Matrix Metalloproteinase-9 Induction in the Mouse Retina. Neurosci. Lett. 2004, 356, 140–144. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, M.; Chintala, S.K. Kainic Acid-Mediated Upregulation of Matrix Metalloproteinase-9 Promotes Retinal Degeneration. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2374–2383. [Google Scholar] [CrossRef]
- Zhang, X.; Chintala, S.K. Influence of Interleukin-1 Beta Induction and Mitogen-Activated Protein Kinase Phosphorylation on Optic Nerve Ligation-Induced Matrix Metalloproteinase-9 Activation in the Retina. Exp. Eye Res. 2004, 78, 849–860. [Google Scholar] [CrossRef]
- Manabe, S.-I.; Gu, Z.; Lipton, S.A. Activation of Matrix Metalloproteinase-9 via Neuronal Nitric Oxide Synthase Contributes to NMDA-Induced Retinal Ganglion Cell Death. Invest. Ophthalmol. Vis. Sci. 2005, 46, 4747–4753. [Google Scholar] [CrossRef]
- Chintala, S.K.; Zhang, X.; Austin, J.S.; Fini, M.E. Deficiency in Matrix Metalloproteinase Gelatinase B (MMP-9) Protects against Retinal Ganglion Cell Death after Optic Nerve Ligation. J. Biol. Chem. 2002, 277, 47461–47468. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.C.; Corredor, R.G.; Obeso, B.A.; Trakhtenberg, E.F.; Wang, Y.; Ponmattam, J.; Dvoriantchikova, G.; Ivanov, D.; Shestopalov, V.I.; Goldberg, J.L.; et al. Β1 Integrin-Focal Adhesion Kinase (FAK) Signaling Modulates Retinal Ganglion Cell (RGC) Survival. PLoS ONE 2012, 7, e48332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sakamoto, T.; Hata, Y.; Kubota, T.; Hisatomi, T.; Murata, T.; Ishibashi, T.; Inomata, H. Expression of Matrix Metalloproteinases and Their Inhibitors in Experimental Retinal Ischemia-Reperfusion Injury in Rats. Exp. Eye Res. 2002, 74, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Kaul, M.; Yan, B.; Kridel, S.J.; Cui, J.; Strongin, A.; Smith, J.W.; Liddington, R.C.; Lipton, S.A. S-Nitrosylation of Matrix Metalloproteinases: Signaling Pathway to Neuronal Cell Death. Science 2002, 297, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Kido, N.; Inatani, M.; Honjo, M.; Yoneda, S.; Hara, H.; Miyawaki, N.; Honda, Y.; Tanihara, H. Dual Effects of Interleukin-1beta on N-Methyl-D-Aspartate-Induced Retinal Neuronal Death in Rat Eyes. Brain Res. 2001, 910, 153–162. [Google Scholar] [CrossRef]
- Friedman, D.S.; Wolfs, R.C.W.; O’Colmain, B.J.; Klein, B.E.; Taylor, H.R.; West, S.; Leske, M.C.; Mitchell, P.; Congdon, N.; Kempen, J.; et al. Prevalence of Open-Angle Glaucoma among Adults in the United States. Arch. Ophthalmol. 2004, 122, 532–538. [Google Scholar] [CrossRef]
- Abu-Amero, K.; Kondkar, A.A.; Chalam, K.V. An Updated Review on the Genetics of Primary Open Angle Glaucoma. Int. J. Mol. Sci. 2015, 16, 28886–28911. [Google Scholar] [CrossRef]
- Hampe, J.; Franke, A.; Rosenstiel, P.; Till, A.; Teuber, M.; Huse, K.; Albrecht, M.; Mayr, G.; De La Vega, F.M.; Briggs, J.; et al. A Genome-Wide Association Scan of Nonsynonymous SNPs Identifies a Susceptibility Variant for Crohn Disease in ATG16L1. Nat. Genet. 2007, 39, 207–211. [Google Scholar] [CrossRef]
- Shastry, B.S. SNP Alleles in Human Disease and Evolution. J. Hum. Genet. 2002, 47, 0561–0566. [Google Scholar] [CrossRef]
- Tower, G.B.; Coon, C.I.; Brinckerhoff, C.E. The 2G Single Nucleotide Polymorphism (SNP) in the MMP-1 Promoter Contributes to High Levels of MMP-1 Transcription in MCF-7/ADR Breast Cancer Cells. Breast Cancer Res. Treat. 2003, 82, 75–82. [Google Scholar] [CrossRef]
- Markiewicz, L.; Pytel, D.; Mucha, B.; Szymanek, K.; Szaflik, J.; Szaflik, J.P.; Majsterek, I. Altered Expression Levels of MMP1, MMP9, MMP12, TIMP1, and IL-1β as a Risk Factor for the Elevated IOP and Optic Nerve Head Damage in the Primary Open-Angle Glaucoma Patients. Biomed. Res. Int. 2015, 2015, 812503. [Google Scholar] [CrossRef]
- Micheal, S.; Yousaf, S.; Khan, M.I.; Akhtar, F.; Islam, F.; Khan, W.A. Polymorphisms in Matrix Metalloproteinases MMP1 and MMP9 Are Associated with Primary Open-Angle and Angle Closure Glaucoma in a Pakistani Population. Mol. Vis. 2013, 9, 441–447. [Google Scholar]
- He, M.; Wang, W.; Han, X.; Huang, W. Matrix Metalloproteinase-1 Rs1799750 Polymorphism and Glaucoma: A Meta-Analysis. Ophthalmic Genet. 2017, 38, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, L.; Majsterek, I.; Przybylowska, K.; Dziki, L.; Waszczyk, M.; Gacek, M.; Kaminska, A.; Szaflik, J.; Szaflik, J.P. Gene Polymorphisms of the MMP1, MMP9, MMP12, IL-1β and TIMP1 and the Risk of Primary Open-Angle Glaucoma. Acta Ophthalmol. 2013, 91, e516–e523. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, X.; Xu, J.; Ma, J.; Chen, X.; Chen, B.; Gu, Y.; Wang, K. Association of Gene Polymorphisms With Primary Open Angle Glaucoma: A Systematic Review and Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1105. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. Functionally Significant Polymorphisms of the MMP9 Gene Are Associated with Primary Open-Angle Glaucoma in the Population of Russia. Eur. J. Ophthalmol. 2022, 11206721221083722. [Google Scholar] [CrossRef]
- Mossböck, G.; Weger, M.; Faschinger, C.; Zimmermann, C.; Schmut, O.; Renner, W.; El-Shabrawi, Y. Role of Functional Single Nucleotide Polymorphisms of MMP1, MMP2, and MMP9 in Open Angle Glaucomas. Mol. Vis. 2010, 16, 1764–1770. [Google Scholar]
- Tsironi, E.E.; Pefkianaki, M.; Tsezou, A.; Kotoula, M.G.; Dardiotis, E.; Almpanidou, P.; Papathanasiou, A.A.; Rodopoulou, P.; Chatzoulis, D.Z.; Hadjigeorgiou, G.M. Evaluation of MMP1 and MMP3 Gene Polymorphisms in Exfoliation Syndrome and Exfoliation Glaucoma. Mol. Vis. 2009, 15, 2890–2895. [Google Scholar]
- Nislawati, R.; Zainal, A.T.F.; Ismail, A.; Waspodo, N.; Kasim, F.; Gunawan, A.M.A.K. Role of Hypertension as a Risk Factor for Open-Angle Glaucoma: A Systematic Review and Meta-Analysis. BMJ Open Ophthalmol. 2021, 6, e000798. [Google Scholar] [CrossRef]
- Moskalenko, M.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. Polymorphisms of the Matrix Metalloproteinase Genes Are Associated with Essential Hypertension in a Caucasian Population of Central Russia. Sci. Rep. 2021, 11, 5224. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Zhang, S. Association of MMP-9 Gene Polymorphisms with Glaucoma: A Meta-Analysis. Ophthalmic Res. 2016, 55, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Starikova, D.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. Novel Data about Association of the Functionally Significant Polymorphisms of the MMP9 Gene with Exfoliation Glaucoma in the Caucasian Population of Central Russia. Ophthalmic Res. 2021, 64, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, M.S.; Burdon, K.P.; Kuot, A.; Hewitt, A.W.; Craig, J.E. Matrix Metalloproteinase-9 Genetic Variation and Primary Angle Closure Glaucoma in a Caucasian Population. Mol. Vis. 2011, 17, 1420–1424. [Google Scholar] [PubMed]
- Rong, S.S.; Tang, F.Y.; Chu, W.K.; Ma, L.; Yam, J.C.S.; Tang, S.M.; Li, J.; Gu, H.; Young, A.L.; Tham, C.C.; et al. Genetic Associations of Primary Angle-Closure Disease: A Systematic Review and Meta-Analysis. Ophthalmology 2016, 123, 1211–1221. [Google Scholar] [CrossRef]
- Gao, X.-J.; Hou, S.-P.; Li, P.-H. The Association between Matrix Metalloprotease-9 Gene Polymorphisms and Primary Angle-Closure Glaucoma in a Chinese Han Population. Int. J. Ophthalmol. 2014, 7, 397–402. [Google Scholar] [CrossRef]
- Shi, H.; Zhu, R.; Hu, N.; Shi, J.; Zhang, J.; Jiang, L.; Jiang, H.; Guan, H. Association of Frizzled-Related Protein (MFRP) and Heat Shock Protein 70 (HSP70) Single Nucleotide Polymorphisms with Primary Angle Closure in a Han Chinese Population: Jiangsu Eye Study. Mol. Vis. 2013, 19, 128–134. [Google Scholar]
- Zhao, F.; Fan, Z.; Huang, X. Role of Matrix Metalloproteinase-9 Gene Polymorphisms in Glaucoma: A Hospital-Based Study in Chinese Patients. J. Clin. Lab. Anal. 2020, 34, e23105. [Google Scholar] [CrossRef]
- Cong, Y.; Guo, X.; Liu, X.; Cao, D.; Jia, X.; Xiao, X.; Li, S.; Fang, S.; Zhang, Q. Association of the Single Nucleotide Polymorphisms in the Extracellular Matrix Metalloprotease-9 Gene with PACG in Southern China. Mol. Vis. 2009, 15, 1412–1417. [Google Scholar]
- Wu, M.-Y.; Wu, Y.; Zhang, Y.; Liu, C.-Y.; Deng, C.-Y.; Peng, L.; Zhou, L. Associations between Matrix Metalloproteinase Gene Polymorphisms and Glaucoma Susceptibility: A Meta-Analysis. BMC Ophthalmol. 2017, 17, 48. [Google Scholar] [CrossRef]
- Minyaylo, O.; Starikova, D.; Moskalenko, M.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. Dataset of Allele and Genotype Frequencies of the Three Functionally Significant Polymorphisms of the MMP Genes in Russian Patients with Primary Open-Angle Glaucoma, Essential Hypertension and Peptic Ulcer. Data Brief 2020, 31, 106004. [Google Scholar] [CrossRef]
- Saravani, S.; Yari, D.; Saravani, R.; Azadi Ahmadabadi, C. Association of COL4A3 (Rs55703767), MMP-9 (Rs17576) and TIMP-1 (Rs6609533) Gene Polymorphisms with Susceptibility to Type 2 Diabetes. Biomed. Rep. 2017, 6, 329–334. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Chen, X.-W. Diabetes and Risk of Glaucoma: Systematic Review and a Meta-Analysis of Prospective Cohort Studies. Int. J. Ophthalmol. 2017, 10, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Wiggs, J.L.; Pasquale, L.R.; Sun, X.; Fan, B.J. Association of Matrix Metalloproteinase-9 (MMP9) Variants with Primary Angle Closure and Primary Angle Closure Glaucoma. PLoS ONE 2016, 11, e0157093. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.; Won, H.-H.; Kee, C. The Association of Single-Nucleotide Polymorphisms in the MMP-9 Gene with Normal Tension Glaucoma and Primary Open-Angle Glaucoma. Curr. Eye Res. 2018, 43, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Kupani, M.; Pandey, R.K.; Mannan, R.; Pruthi, A.; Mehrotra, S. Genetic Association of -1562C>T Polymorphism in the MMP9 Gene with Primary Glaucoma in a North Indian Population. PLoS ONE 2018, 13, e0192636. [Google Scholar] [CrossRef]
- Saleh, V.M.; Auda, I.G.; Ali, E.N. The Functional Polymorphism -863 C/A in the TNF-α Gene Is Associated with Primary Open-Angle Glaucoma Development in Iraqi Patients. Gene Rep. 2022, 28, 101653. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Leung, K.-S.; Ma, S.L.; So, H.C.; Huang, D.; Tang, N.L.-S.; Wong, M.-H. Genome-Wide Search for SNP Interactions in GWAS Data: Algorithm, Feasibility, Replication Using Schizophrenia Datasets. Front. Genet. 2020, 11, 1003. [Google Scholar] [CrossRef]
- Gerke, J.; Lorenz, K.; Cohen, B. Genetic Interactions between Transcription Factors Cause Natural Variation in Yeast. Science 2009, 323, 498–501. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Amankwah, E.K.; Tseng, T.-S.; Qu, X.; Chen, D.-T.; Park, J.Y. SNP-SNP Interaction Network in Angiogenesis Genes Associated with Prostate Cancer Aggressiveness. PLoS ONE 2013, 8, e59688. [Google Scholar] [CrossRef]
- Dong, S.-S.; Hu, W.-X.; Yang, T.-L.; Chen, X.-F.; Yan, H.; Chen, X.-D.; Tan, L.-J.; Tian, Q.; Deng, H.-W.; Guo, Y. SNP-SNP Interactions between WNT4 and WNT5A Were Associated with Obesity Related Traits in Han Chinese Population. Sci. Rep. 2017, 7, 43939. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Leung, K.-S.; Tang, N.L.S.; Wong, M.-H. Discovering Genetic Factors for Psoriasis through Exhaustively Searching for Significant Second Order SNP-SNP Interactions. Sci. Rep. 2018, 8, 15186. [Google Scholar] [CrossRef]
- Svinareva, D.I. The contribution of gene-gene interactions of polymorphic loci of matrix metalloproteinases to susceptibility to primary open-angle glaucoma in men. Res. Results Biomed. 2020, 6, 63–77. [Google Scholar] [CrossRef]
- Majsterek, I.; Markiewicz, L.; Przybylowska, K.; Gacek, M.; Kurowska, A.K.; Kaminska, A.; Szaflik, J.; Szaflik, J.P. Association of MMP1-1607 1G/2G and TIMP1 372 T/C Gene Polymorphisms with Risk of Primary Open Angle Glaucoma in a Polish Population. Med. Sci. Monit. 2011, 17, CR417–CR421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aga, M.; Bradley, J.M.; Wanchu, R.; Yang, Y.; Acott, T.S.; Keller, K.E. Differential Effects of Caveolin-1 and -2 Knockdown on Aqueous Outflow and Altered Extracellular Matrix Turnover in Caveolin-Silenced Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5497–5509. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-J.; Chiang, T.-H.; Shih, Y.-F.; Lu, S.-C.; Lin, L.L.-K.; Shieh, J.-W.; Wang, T.-H.; Samples, J.R.; Hung, P.-T. The Association of Single Nucleotide Polymorphisms in the MMP-9 Genes with Susceptibility to Acute Primary Angle Closure Glaucoma in Taiwanese Patients. Mol. Vis. 2006, 12, 1223–1232. [Google Scholar] [PubMed]
- Sivak, J.M.; Fini, M.E. MMPs in the Eye: Emerging Roles for Matrix Metalloproteinases in Ocular Physiology. Prog. Retin. Eye Res. 2002, 21, 1–14. [Google Scholar] [CrossRef]
- Terai, N.; Schloetzer-Schrehardt, U.; Lampel, J.; Böhm, A.G.; Rummelt, C.; Schmidt, E.; Pillunat, L.E. Effect of Latanoprost and Timolol on the Histopathology of the Human Conjunctiva. Br. J. Ophthalmol. 2009, 93, 219–224. [Google Scholar] [CrossRef]
- Leonardi, A.; Brun, P.; Di Stefano, A.; Motterle, L.; Abatangelo, G. Matrix Metalloproteases in Vernal Keratoconjunctivitis, Nasal Polyps and Allergic Asthma. Clin. Exp. Allergy 2007, 37, 872–879. [Google Scholar] [CrossRef]
- Fukuda, K.; Fujitsu, Y.; Kumagai, N.; Nishida, T. Inhibition of Matrix Metalloproteinase-3 Synthesis in Human Conjunctival Fibroblasts by Interleukin-4 or Interleukin-13. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2857–2864. [Google Scholar] [CrossRef]
- Mizoue, S.; Iwai, M.; Ide, A.; Suzuki, J.; Horiuchi, M.; Shiraishi, A.; Ohashi, Y. Role of Angiotensin II Receptor Subtypes in Conjunctival Wound Healing. Curr. Eye Res. 2006, 31, 129–136. [Google Scholar] [CrossRef]
- Helin, M.; Rönkkö, S.; Puustjärvi, T.; Teräsvirta, M.; Ollikainen, M.; Uusitalo, H. Conjunctival Inflammatory Cells and Their Predictive Role for Deep Sclerectomy in Primary Open-Angle Glaucoma and Exfoliation Glaucoma. J. Glaucoma 2011, 20, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Broadway, D.C.; Grierson, I.; O’Brien, C.; Hitchings, R.A. Adverse Effects of Topical Antiglaucoma Medication. II. The Outcome of Filtration Surgery. Arch. Ophthalmol. 1994, 112, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.E. Trabeculectomy. Preliminary Report of a New Method. Am. J. Ophthalmol. 1968, 66, 673–679. [Google Scholar] [CrossRef]
- Spaeth, G.L. A Prospective, Controlled Study to Compare the Scheie Procedure with Watson’s Trabeculectomy. Ophthalmic Surg. 1980, 11, 688–694. [Google Scholar]
- Addicks, E.M.; Quigley, H.A.; Green, W.R.; Robin, A.L. Histologic Characteristics of Filtering Blebs in Glaucomatous Eyes. Arch. Ophthalmol. 1983, 101, 795–798. [Google Scholar] [CrossRef]
- Awadein, A.; El Sayed, Y.M. Excision of Tenon Capsule in Pediatric Trabeculectomy: A Controlled Study. J. Glaucoma 2016, 25, 39–44. [Google Scholar] [CrossRef]
- Wong, T.T.L.; Mead, A.L.; Khaw, P.T. Matrix Metalloproteinase Inhibition Modulates Postoperative Scarring after Experimental Glaucoma Filtration Surgery. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1097–1103. [Google Scholar] [CrossRef]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef]
- Xue, H.; McCauley, R.L.; Zhang, W.; Martini, D.K. Altered Interleukin-6 Expression in Fibroblasts from Hypertrophic Burn Scars. J. Burn. Care Rehabil. 2000, 21, 142–146. [Google Scholar] [CrossRef]
- Eren, K.; Turgut, B.; Akin, M.M.; Demir, T. The Suppression of Wound Healing Response with Sirolimus and Sunitinib Following Experimental Trabeculectomy in a Rabbit Model. Curr. Eye Res. 2016, 41, 367–376. [Google Scholar] [CrossRef]
- Nakamura-Shibasaki, M.; Ko, J.-A.; Takenaka, J.; Chikama, T.; Sonoda, K.-H.; Kiuchi, Y. Matrix Metalloproteinase and Cytokine Expression in Tenon Fibroblasts during Scar Formation after Glaucoma Filtration or Implant Surgery in Rats. Cell Biochem. Funct. 2012, 7, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.T.L.; Sethi, C.; Daniels, J.T.; Limb, G.A.; Murphy, G.; Khaw, P.T. Matrix Metalloproteinases in Disease and Repair Processes in the Anterior Segment. Surv. Ophthalmol. 2002, 47, 239–256. [Google Scholar] [CrossRef]
- Chintala, S.K.; Wang, N.; Diskin, S.; Mattox, C.; Kagemann, L.; Fini, M.E.; Schuman, J.S. Matrix Metalloproteinase Gelatinase B (MMP-9) Is Associated with Leaking Glaucoma Filtering Blebs. Exp. Eye Res. 2005, 81, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Liu, C.J.; Chiu, A.W.; Wang, Y.-C.; Huan, S.K.; Lee, F.-L.; Chen, S.-J.; Hsu, W.-M.; Hsieh, S.-L. A Feasible Tool to Detect MRNA Expression of Matrix Metalloproteinases and Their Tissue Inhibitors in Human Tenon’s Capsule. Ophthalmic Res. 2002, 34, 375–379. [Google Scholar] [CrossRef]
- Liu, C.J.-L.; Huang, Y.-L.; Chiu, A.W.; Ju, J.-P. Transcript Expression of Matrix Metalloproteinases in the Conjunctiva Following Glaucoma Filtration Surgery in Rabbits. Ophthalmic Res. 2004, 36, 114–119. [Google Scholar] [CrossRef]
- Cabourne, E.; Clarke, J.C.K.; Schlottmann, P.G.; Evans, J.R. Mitomycin C versus 5-Fluorouracil for Wound Healing in Glaucoma Surgery. Cochrane Database Syst. Rev. 2015, 11, CD006259. [Google Scholar] [CrossRef]
- Bindlish, R.; Condon, G.P.; Schlosser, J.D.; D’Antonio, J.; Lauer, K.B.; Lehrer, R. Efficacy and Safety of Mitomycin-C in Primary Trabeculectomy: Five-Year Follow-Up. Ophthalmology 2002, 109, 1336–1341. [Google Scholar] [CrossRef]
- Casson, R.; Rahman, R.; Salmon, J.F. Long Term Results and Complications of Trabeculectomy Augmented with Low Dose Mitomycin C in Patients at Risk for Filtration Failure. Br. J. Ophthalmol. 2001, 85, 686–688. [Google Scholar] [CrossRef]
- Kawashima, Y.; Saika, S.; Yamanaka, O.; Okada, Y.; Ohkawa, K.; Ohnishi, Y. Immunolocalization of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Human Subconjunctival Tissues. Curr. Eye Res. 1998, 17, 445–451. [Google Scholar] [CrossRef]
- McCluskey, P.; Molteno, A.; Wakefield, D.; Di Girolamo, N. Otago Glaucoma Surgery Outcome Study: The Pattern of Expression of MMPs and TIMPs in Bleb Capsules Surrounding Molteno Implants. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2161–2164. [Google Scholar] [CrossRef]
- Välimäki, J.; Uusitalo, H. Matrix Metalloproteinases (MMP-1, MMP-2, MMP-3 and MMP-9, and TIMP-1, TIMP-2 and TIMP-3) and Markers for Vascularization in Functioning and Non-Functioning Bleb Capsules of Glaucoma Drainage Implants. Acta Ophthalmol. 2015, 93, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Seol, B.R.; Lee, S.Y.; Kim, Y.J.; Kim, Y.K.; Jeoung, J.W.; Park, K.H. Comparison of the Efficacy and Safety of Trabeculectomy with Mitomycin C According to Concentration: A Prospective Randomized Clinical Trial. JCM 2020, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Helin-Toiviainen, M.; Rönkkö, S.; Puustjärvi, T.; Rekonen, P.; Ollikainen, M.; Uusitalo, H. Conjunctival Matrix Metalloproteinases and Their Inhibitors in Glaucoma Patients. Acta Ophthalmol. 2015, 93, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Zuo, C.; Wu, K.; Zhang, S.; Qin, X.; Li, Y.; Gao, X.; Huang, D.; Lin, M. A Comparison of Subconjunctival Wound Healing between Different Methods of Dissecting Subconjunctival Tissues. Ophthalmic Res. 2020, 64, 99–107. [Google Scholar] [CrossRef]
- Hoffman, R.S.; Crandall, A.S.; Crandall, D.A.; Fine, I.H.; Packer, M.; Sims, A.C. Minimally Invasive External Mini-Glaucoma Shunt Implantation without Conjunctival Dissection. J. Glaucoma 2014, 23, 254–257. [Google Scholar] [CrossRef]
- Cioffi, G.A.; Van Buskirk, E.M. Corneal Trabeculectomy without Conjunctival Incision. Extended Follow-up and Histologic Findings. Ophthalmology 1993, 100, 1077–1082. [Google Scholar] [CrossRef]
- Daniels, J.T.; Cambrey, A.D.; Occleston, N.L.; Garrett, Q.; Tarnuzzer, R.W.; Schultz, G.S.; Khaw, P.T. Matrix Metalloproteinase Inhibition Modulates Fibroblast-Mediated Matrix Contraction and Collagen Production In Vitro. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1104. [Google Scholar] [CrossRef]
- Martin-Martin, B.; Tovell, V.; Dahlmann-Noor, A.H.; Khaw, P.T.; Bailly, M. The Effect of MMP Inhibitor GM6001 on Early Fibroblast-Mediated Collagen Matrix Contraction Is Correlated to a Decrease in Cell Protrusive Activity. Eur. J. Cell Biol. 2011, 90, 26–36. [Google Scholar] [CrossRef]
- Wong, T.T.L.; Mead, A.L.; Khaw, P.T. Prolonged Antiscarring Effects of Ilomastat and MMC after Experimental Glaucoma Filtration Surgery. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2018. [Google Scholar] [CrossRef]
- Suh, W.; Han, K.E.; Han, J.R. Safety of Using Matrix Metalloproteinase Inhibitor in Experimental Glaucoma Filtration Surgery. J. Korean Med. Sci. 2017, 32, 666. [Google Scholar] [CrossRef]
- Martorana, G.M.; Schaefer, J.L.; Levine, M.A.; Lukowski, Z.L.; Min, J.; Meyers, C.A.; Schultz, G.S.; Sherwood, M.B. Sequential Therapy with Saratin, Bevacizumab and Ilomastat to Prolong Bleb Function Following Glaucoma Filtration Surgery in a Rabbit Model. PLoS ONE 2015, 10, e0138054. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Ramamurthy, N.S.; McNamara, T.F.; Greenwald, R.A.; Rifkin, B.R. Tetracyclines Inhibit Connective Tissue Breakdown: New Therapeutic Implications for an Old Family of Drugs. Crit. Rev. Oral Biol. Med. 1991, 2, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.A.; Cook, S.D. Doxycycline-a Role in Ocular Surface Repair. Br. J. Ophthalmol. 2004, 88, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ezra, D.G.; Burton, M.J.; Bailly, M. Doxycycline Prevents Matrix Remodeling and Contraction by Trichiasis-Derived Conjunctival Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4675–4682. [Google Scholar] [CrossRef] [PubMed]
- Sen, E.; Balikoglu-Yilmaz, M.; Bozdag-Pehlivan, S.; Sungu, N.; Aksakal, F.N.; Altinok, A.; Tuna, T.; Unlu, N.; Ustun, H.; Koklu, G.; et al. Effect of Doxycycline on Postoperative Scarring After Trabeculectomy in an Experimental Rabbit Model. J. Ocul. Pharmacol. Ther. 2010, 26, 399–406. [Google Scholar] [CrossRef]
- Cinik, R.; Yüksel, N.; Pirhan, D.; Aslan, M.Ş.; Subaşı, C.; Karaöz, E. The Effect of Everolimus on Scar Formation in Glaucoma Filtering Surgery in a Rabbit Model. Curr. Eye Res. 2016, 41, 1438–1446. [Google Scholar] [CrossRef]
- Eisen, H.J.; Tuzcu, E.M.; Dorent, R.; Kobashigawa, J.; Mancini, D.; Valantine-von Kaeppler, H.A.; Starling, R.C.; Sørensen, K.; Hummel, M.; Lind, J.M.; et al. Everolimus for the Prevention of Allograft Rejection and Vasculopathy in Cardiac-Transplant Recipients. N. Engl. J. Med. 2003, 349, 847–858. [Google Scholar] [CrossRef]
- Hasskarl, J. Everolimus. In Small Molecules in Oncology; Martens, U.M., Ed.; Recent Results in Cancer Research; Springer International Publishing: Cham, Switzerland, 2018; pp. 101–123. ISBN 978-3-319-91442-8. [Google Scholar]
- Pascual, J.; Boletis, I.N.; Campistol, J.M. Everolimus (Certican) in Renal Transplantation: A Review of Clinical Trial Data, Current Usage, and Future Directions. Transplant. Rev. 2006, 20, 1–18. [Google Scholar] [CrossRef]
- Tedesco-Silva, H.; Saliba, F.; Barten, M.J.; De Simone, P.; Potena, L.; Gottlieb, J.; Gawai, A.; Bernhardt, P.; Pascual, J. An Overview of the Efficacy and Safety of Everolimus in Adult Solid Organ Transplant Recipients. Transplant. Rev. 2022, 36, 100655. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.-Z.; Yao, L.; Wang, J.-M. Anti-Proliferative Effect of Olmesartan on Tenon’s Capsule Fibroblasts. Int. J. Ophthalmol. 2016, 9, 669–676. [Google Scholar] [CrossRef]
- Ball, K.J.; Williams, P.A.; Stumpe, K.O. Relative Efficacy of an Angiotensin II Antagonist Compared with Other Antihypertensive Agents. Olmesartan Medoxomil versus Antihypertensives. J. Hypertens Suppl. 2001, 19, S49–S56. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Li, Y.; Hu, K.; Dai, C.; Liu, Y. Hepatocyte Growth Factor Receptor Signaling Mediates the Anti-Fibrotic Action of 9-Cis-Retinoic Acid in Glomerular Mesangial Cells. Am. J. Pathol. 2005, 167, 947–957. [Google Scholar] [CrossRef]
- Nezzar, H.; Chiambaretta, F.; Marceau, G.; Blanchon, L.; Faye, B.; Dechelotte, P.; Rigal, D.; Sapin, V. Molecular and Metabolic Retinoid Pathways in the Human Ocular Surface. Mol. Vis. 2007, 13, 1641–1650. [Google Scholar] [PubMed]
- Liu, Y.; Kimura, K.; Orita, T.; Suzuki, K.; Teranishi, S.; Mori, T.; Sonoda, K.-H. Inhibition by a Retinoic Acid Receptor γ Agonist of Extracellular Matrix Remodeling Mediated by Human Tenon Fibroblasts. Mol. Vis. 2015, 21, 1368–1377. [Google Scholar]
- Bornstein, P.; Sage, E.H. Matricellular Proteins: Extracellular Modulators of Cell Function. Curr. Opin. Cell Biol. 2002, 14, 608–616. [Google Scholar] [CrossRef]
- Seet, L.-F.; Su, R.; Toh, L.Z.; Wong, T.T. In Vitro Analyses of the Anti-Fibrotic Effect of SPARC Silencing in Human Tenon’s Fibroblasts: Comparisons with Mitomycin C. J. Cell Mol. Med. 2012, 16, 1245–1259. [Google Scholar] [CrossRef]
- Seet, L.-F.; Su, R.; Barathi, V.A.; Lee, W.S.; Poh, R.; Heng, Y.M.; Manser, E.; Vithana, E.N.; Aung, T.; Weaver, M.; et al. SPARC Deficiency Results in Improved Surgical Survival in a Novel Mouse Model of Glaucoma Filtration Surgery. PLoS ONE 2010, 5, e9415. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Mechanisms of Tubulointerstitial Fibrosis. JASN J. Am. Soc. Nephrol. 2010, 21, 1819–1834. [Google Scholar] [CrossRef]
- Deng, J.; Peng, M.; Wang, Z.; Zhou, S.; Xiao, D.; Deng, J.; Yang, X.; Peng, J.; Yang, X. Novel Application of Metformin Combined with Targeted Drugs on Anticancer Treatment. Cancer Sci. 2019, 110, 23–30. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Chiang, E.-P.I.; Syu, J.-N.; Chao, C.-Y.; Lin, H.-Y.; Lin, C.-C.; Yang, M.-D.; Tsai, S.-Y.; Tang, F.-Y. Treatment of 13-Cis Retinoic Acid and 1,25-Dihydroxyvitamin D3 Inhibits TNF-Alpha-Mediated Expression of MMP-9 Protein and Cell Invasion through the Suppression of JNK Pathway and MicroRNA 221 in Human Pancreatic Adenocarcinoma Cancer Cells. PLoS ONE 2021, 16, e0247550. [Google Scholar] [CrossRef]
- Kaufhold, S.; Bonavida, B. Central Role of Snail1 in the Regulation of EMT and Resistance in Cancer: A Target for Therapeutic Intervention. J. Exp. Clin. Cancer Res. 2014, 33, 62. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Gatwood, J.; Brooks, C.; Meacham, R.; Abou-Rahma, J.; Cernasev, A.; Brown, E.; Kuchtey, R.W. Facilitators and Barriers to Glaucoma Medication Adherence. J. Glaucoma 2022, 31, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y. Racial and Ethnic Disparities in Adherence to Glaucoma Follow-up Visits in a County Hospital Population. Arch. Ophthalmol. 2011, 129, 872. [Google Scholar] [CrossRef]
- Newman-Casey, P.A.; Blachley, T.; Lee, P.P.; Heisler, M.; Farris, K.B.; Stein, J.D. Patterns of Glaucoma Medication Adherence over Four Years of Follow-Up. Ophthalmology 2015, 122, 2010–2021. [Google Scholar] [CrossRef]
- Cvenkel, B.; Štunf, Š.; Srebotnik Kirbiš, I.; Strojan Fležar, M. Symptoms and Signs of Ocular Surface Disease Related to Topical Medication in Patients with Glaucoma. Clin. Ophthalmol. 2015, 9, 625–631. [Google Scholar] [CrossRef]
- Steven, D.W.; Alaghband, P.; Lim, K.S. Preservatives in Glaucoma Medication. Br. J. Ophthalmol. 2018, 102, 1497–1503. [Google Scholar] [CrossRef]
- Wong, J.K.W.; Leung, T.K.; Lai, J.S.; Chan, J.C. Evaluation of Adverse Effects of Topical Glaucoma Medications on Trabeculectomy Outcomes Using the Glaucoma Medications Intensity Index. Ophthalmol. Ther. 2022, 11, 387–401. [Google Scholar] [CrossRef]
- Couture, C.; Zaniolo, K.; Carrier, P.; Lake, J.; Patenaude, J.; Germain, L.; Guérin, S.L. The Tissue-Engineered Human Cornea as a Model to Study Expression of Matrix Metalloproteinases during Corneal Wound Healing. Biomaterials 2016, 78, 86–101. [Google Scholar] [CrossRef]
- Mackiewicz, Z.; Määttä, M.; Stenman, M.; Konttinen, L.; Tervo, T.; Konttinen, Y.T. Collagenolytic Proteinases in Keratoconus. Cornea 2006, 25, 603–610. [Google Scholar] [CrossRef]
- Silva, B.L.; Cardozo, J.B.; Marback, P.; Machado, F.C.; Galvão, V.; Santiago, M.B. Peripheral Ulcerative Keratitis: A Serious Complication of Rheumatoid Arthritis. Rheumatol. Int. 2010, 30, 1267–1268. [Google Scholar] [CrossRef]
- Lobo, A.-M.; Agelidis, A.M.; Shukla, D. Pathogenesis of Herpes Simplex Keratitis: The Host Cell Response and Ocular Surface Sequelae to Infection and Inflammation. Ocul. Surf. 2019, 17, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-L.; Kuo, B.-I.; Wu, J.-H.; Huang, W.-L.; Su, C.-C.; Chen, W.-L. Anti-Glaucoma Agents-Induced Pseudodendritic Keratitis Presumed to Be Herpetic Simplex Keratitis: A Clinical Case Series. Sci. Rep. 2021, 11, 21443. [Google Scholar] [CrossRef] [PubMed]
- Lopilly Park, H.-Y.; Kim, J.H.; Lee, K.M.; Park, C.K. Effect of Prostaglandin Analogues on Tear Proteomics and Expression of Cytokines and Matrix Metalloproteinases in the Conjunctiva and Cornea. Exp. Eye Res. 2012, 94, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.; Miyai, T.; Nejima, R.; Miyata, K.; Mimura, T.; Usui, T.; Aihara, M.; Araie, M.; Amano, S. Effect of Latanoprost on the Expression of Matrix Metalloproteinases and Tissue Inhibitor of Metalloproteinase 1 on the Ocular Surface. Arch. Ophthalmol. 2010, 128, 466–471. [Google Scholar] [CrossRef]
- Kim, D.W.; Seo, J.H.; Lim, S.-H. Evaluation of Ocular Surface Disease in Elderly Patients with Glaucoma: Expression of Matrix Metalloproteinase-9 in Tears. Eye 2021, 35, 892–900. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, C.; Lin, X.; Wu, Y.; Ouyang, W.; Tang, L.; Ye, S.; Wang, Y.; Li, W.; Zhang, X.; et al. 0.005% Preservative-Free Latanoprost Induces Dry Eye-Like Ocular Surface Damage via Promotion of Inflammation in Mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3375–3384. [Google Scholar] [CrossRef]
- Ito, T.; Ohguro, H.; Mamiya, K.; Ohguro, I.; Nakazawa, M. Effects of Antiglaucoma Drops on MMP and TIMP Balance in Conjunctival and Subconjunctival Tissue. Investig. Ophthalmol. Vis. Sci. 2006, 47, 823–830. [Google Scholar] [CrossRef]
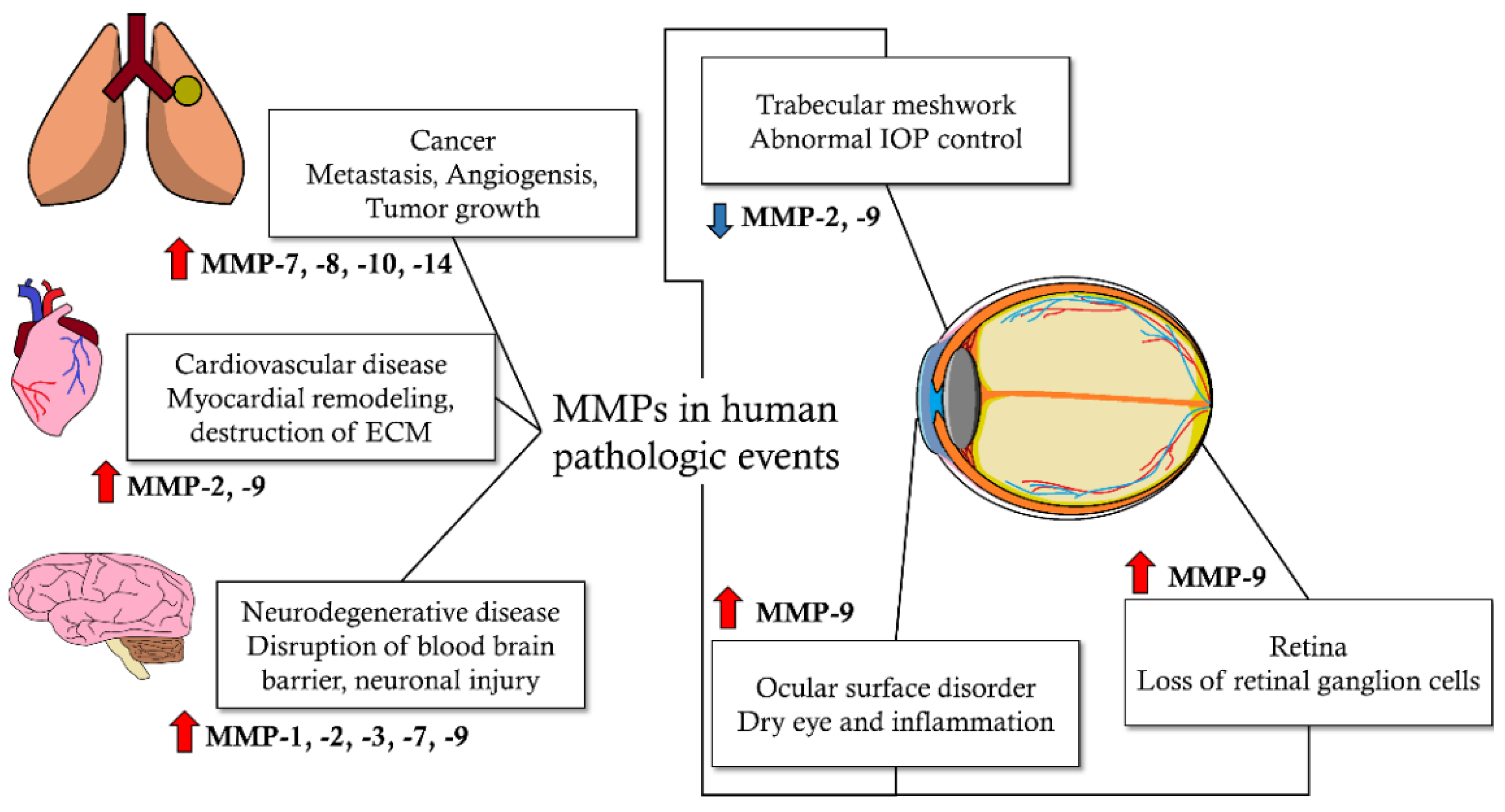
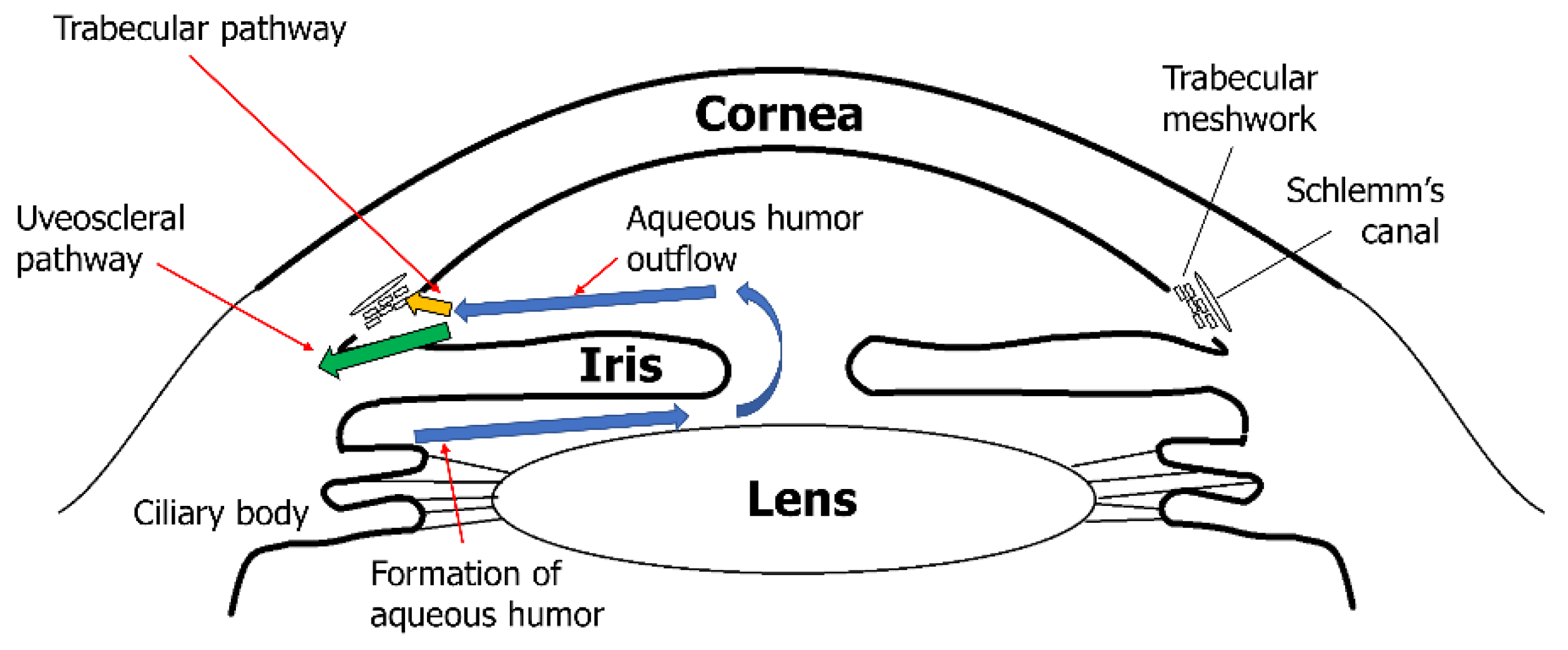
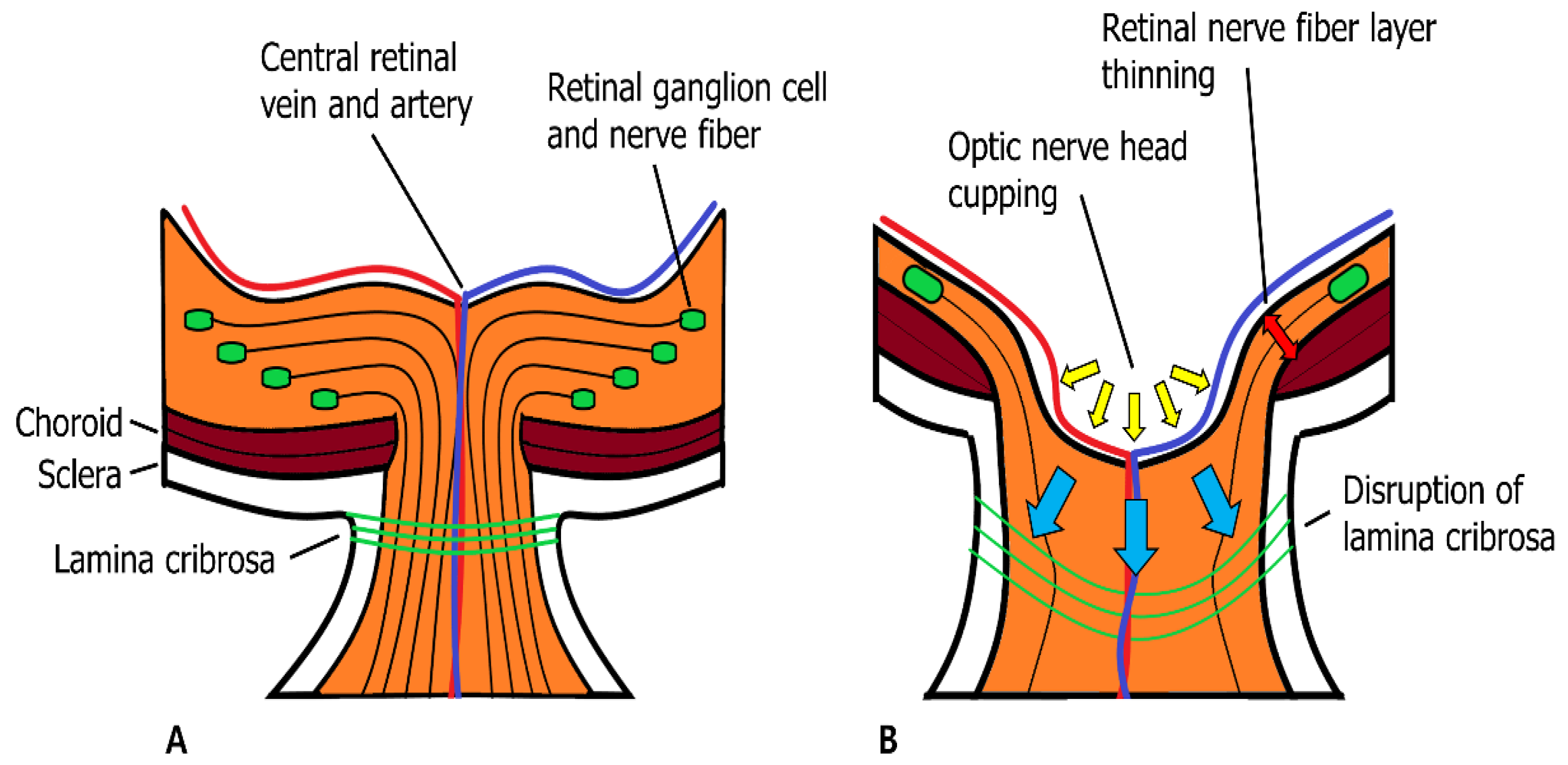
| MMP Subtypes | MMP Number | Substrates |
|---|---|---|
| Collagenases [20,21,22] | MMP-1 | type I, II, III, VI, and X collagens; aggrecan; and entactin |
| MMP-8 | type I, II, and III collagens and aggrecan | |
| MMP-13 | type I, II, and III collagens | |
| Gelatinases [20,23,24] | MMP-2 | type I, IV, V, VII, X, and XI collagens; vitronectin; fibronectin; aggrecan; laminin; elastin; and tenascin C |
| MMP-9 | type IV, V, and XIV collagens; entactin; vitronectin; aggrecan; and elastin | |
| Stormelysins [20,25,26] | MMP-3 | type III, IV, IX, and X collagens; aggrecan; fibronectin; laminin; tenascin C; and vitronectin |
| MMP-10 | type IV collagen; aggrecan; and fibronectin | |
| MMP-11 | type IV collagen; fibronectin; laminin; and aggrecan | |
| Membrane-type MMPs [20,27] | MMP-14 | Activator of pro-MMP-2; type I, II, and III collagens; vitronectin; fibronectin; and laminin-1 |
| MMP-15 | Activator of pro-MMP-2; tenascin; fibronectin; and aggrecan | |
| MMP-16 | Activator of pro-MMP-2; fibronectin; and type III collagen | |
| MMP-17 | Fibrin and cleaves pro-TNF-α | |
| MMP-24 | Activator of pro-MMP-2 | |
| MMP-25 | type IV collagen; gelatin; fibronectin; and fibrin | |
| Metalloelastases [20,26,28] | MMP-12 | type IV collagen; elastin; proteoglycan; fibronectin; laminin; and vitronectin |
| MMP-19 | cartilage oligomeric matrix protein and aggrecan | |
| MMP-20 | enamel matrix proteins | |
| MMP-28 | cartilage oligomeric matrix protein and aggrecan | |
| Other MMPs [20,26,29] | MMP-7 | aggrecan; fibronectin; laminin; and type IV collagen |
| MMP-26 | fibronectin; type IV collagen; fibrinogen; and gelatin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.H.; Lim, S.-H. Matrix Metalloproteinases and Glaucoma. Biomolecules 2022, 12, 1368. https://doi.org/10.3390/biom12101368
Kim MH, Lim S-H. Matrix Metalloproteinases and Glaucoma. Biomolecules. 2022; 12(10):1368. https://doi.org/10.3390/biom12101368
Chicago/Turabian StyleKim, Moo Hyun, and Su-Ho Lim. 2022. "Matrix Metalloproteinases and Glaucoma" Biomolecules 12, no. 10: 1368. https://doi.org/10.3390/biom12101368
APA StyleKim, M. H., & Lim, S.-H. (2022). Matrix Metalloproteinases and Glaucoma. Biomolecules, 12(10), 1368. https://doi.org/10.3390/biom12101368







