Obtaining a Reliable Diagnostic Biomarker for Diabetes Mellitus by Standardizing Salivary Glucose Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Participants
2.3. Sample Collection
2.3.1. Procedures of Whether to Rinse
2.3.2. Procedures of Chewing Times
2.3.3. Procedures of Centrifugation Speed
2.3.4. Procedures of Stimulus Conditions
2.3.5. Procedures of Storage Conditions
2.3.6. Procedures of Freeze/Thaw Cycles
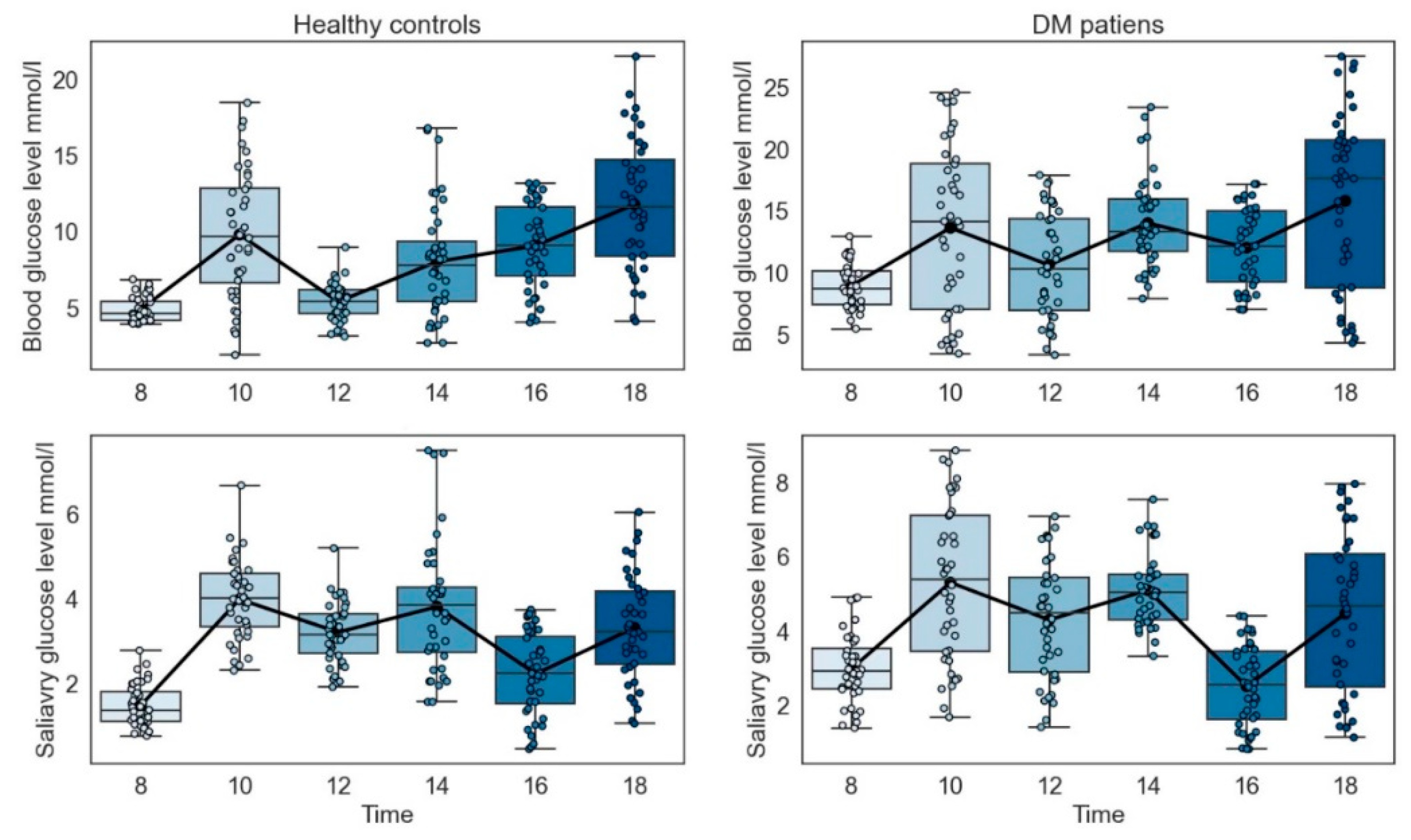
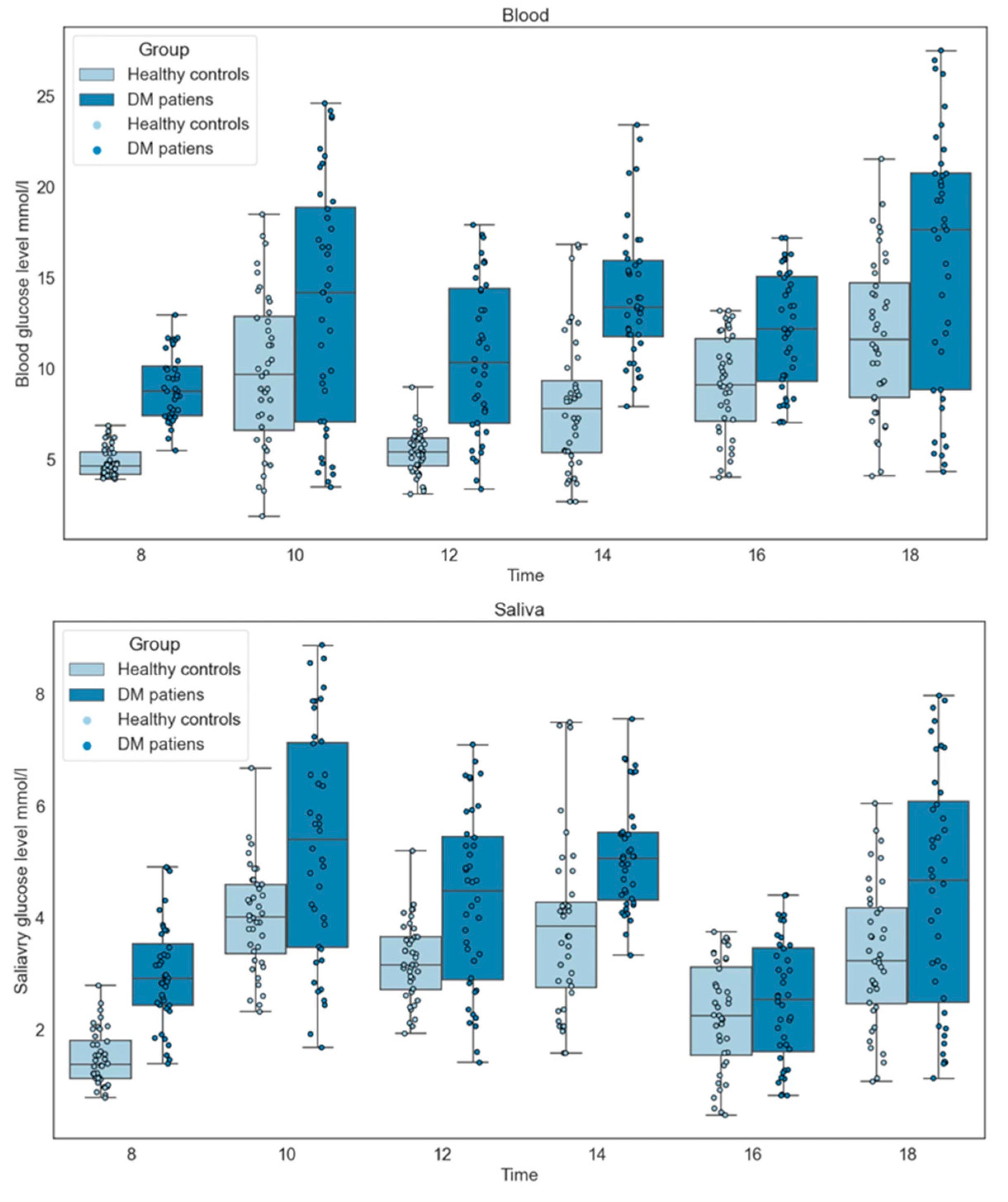
2.3.7. Procedures for Collection Time
2.3.8. Procedures of Pathological Factors
2.4. Estimation of Blood and Salivary Glucose
2.5. Statistics
3. Results
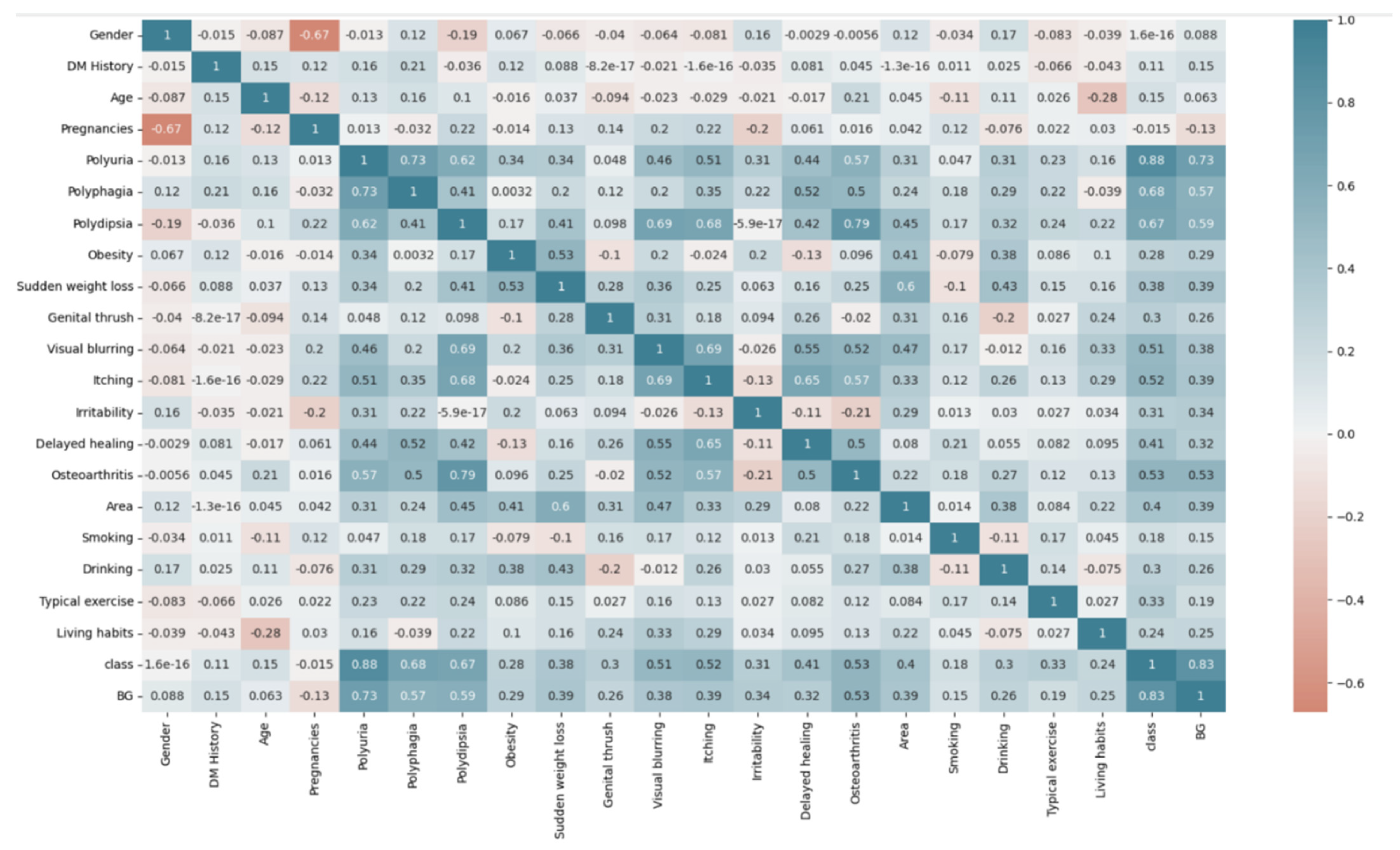
3.1. Questionnaire Survey
3.2. Whether to Rinse
3.3. Chewing Times
3.4. Centrifugation Speed
3.5. Stimulus Conditions
3.6. Storage Conditions
3.7. Freeze/Thaw Cycles
3.8. Collection Time
3.9. Pathological Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, M.H.; Eres, R.; Vasan, S. Understanding loneliness in the twenty-first century: An update on correlates, risk factors, and potential solutions. Soc. Psychiatry Psychiatr. Epidemiol. 2020, 55, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Salehidoost, R.; Mansouri, A.; Amini, M.; Aminorroaya Yamini, S.; Aminorroaya, A. Diabetes and all-cause mortality, a 18-year follow-up study. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cheng, Y.; Tang, Z.; Chen, J.; Xia, Y.; Xu, C.; Cao, X. Potential Mechanisms of Methylglyoxal-Induced Human Embryonic Kidney Cells Damage: Regulation of Oxidative Stress, DNA Damage, and Apoptosis. Chem. Biodivers. 2022, 19, e202100829. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Sylvia, H.L.; Frank, B.H. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef]
- Zadeh, R.S.; Eshelman, P.; Setla, J.; Kennedy, L.; Hon, E.; Basara, A. Environmental design for end-of-life care: An integrative review on improving the quality of life and managing symptoms for patients in institutional settings. J. Pain Symptom Manag. 2018, 55, 1018–1034. [Google Scholar] [CrossRef]
- Sim, R.; Lee, S.W.H. Patient preference and satisfaction with the use of telemedicine for glycemic control in patients with type 2 diabetes: A review. Patient Prefer. Adherence 2021, 15, 283. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D. Enzyme-based glucose sensor: From invasive to wearable device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef]
- Martino, G.; Caputo, A.; Bellone, F.; Quattropani, M.C.; Vicario, C.M. Going beyond the visible in type 2 diabetes mellitus: Defense mechanisms and their associations with depression and health-related quality of life. Front. Psychol. 2020, 11, 267. [Google Scholar] [CrossRef]
- Bartolome, F.; Orive, G.; Carro, E. Standardizing salivary lactoferrin measurements to obtain a robust diagnostic biomarker for Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12173. [Google Scholar] [CrossRef]
- Klausberger, M.; Duerkop, M.; Haslacher, H.; Wozniak-Knopp, G.; Cserjan-Puschmann, M.; Perkmann, T.; Grabherr, R. A comprehensive antigen production and characterisation study for easy-to-implement, specific and quantitative SARS-CoV-2 serotests. EBioMedicine 2021, 67, 103348. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, H.; Zhu, J.; Liao, Z.; Wang, S.; Liu, W. Correlations of Salivary and Blood Glucose Levels among Six Saliva Collection Methods. Int. J. Environ. Res. Public Health 2022, 19, 4122. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, H.; Zhu, J.; Peng, L.; Duan, Z.; Liu, T.; Liu, W. Unstimulated Parotid Saliva Is a Better Method for Blood Glucose Prediction. Appl. Sci. 2021, 11, 11367. [Google Scholar] [CrossRef]
- Khurshid, Z.; Asiri, F.Y.I.; Al Wadaani, H. Human saliva: Non-invasive fluid for detecting novel coronavirus (2019-nCoV). Int. J. Environ. Res. Public Health 2020, 17, 2225. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Paderni, C.; Saccone, R.; Fede, O.D.; Wolff, A.; Giannola, L.I. Human buccal mucosa as an innovative site of drug delivery. Curr. Pharm. Des. 2010, 16, 641. [Google Scholar] [CrossRef] [PubMed]
- González-Muñoz, B.; Garrido-Vargas, F.; Pavez, C.; Osorio, F.; Chen, J.; Bordeu, E.; Brossard, N. Wine astringency: More than just tannin–protein interactions. J. Sci. Food Agric. 2022, 102, 1771–1781. [Google Scholar] [CrossRef]
- Brito, A.C.M.; Bezerra, I.M.; Borges, M.H.D.S.; Cavalcanti, Y.W.; Almeida, L.D.F.D.D. Effect of different salivary glucose concentrations on dual-species biofilms of Candida albicans and Streptococcus mutans. Biofouling 2021, 37, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Seraphim, A.P.C.G.; Chiba, F.Y.; Pereira, R.F.; Mattera, M.S.D.L.C.; Moimaz, S.A.S.; Sumida, D.H. Relationship among periodontal disease, insulin resistance, salivary cortisol, and stress levels during pregnancy. Braz. Dent. J. 2016, 27, 123–127. [Google Scholar] [CrossRef]
- Aps, J.K.M.; Martens, L.C. The physiology of saliva and transfer of drugs into saliva. Forensic Sci. Int. 2005, 150, 119–131. [Google Scholar] [CrossRef]
- Amone, L.; Matzeu, G.; Omenetto, F.G. Stabilization of salivary biomarkers. ACS Biomater. Sci. Eng. 2021, 7, 5451–5473. [Google Scholar]
- Mani, V.; Beduk, T.; Khushaim, W.; Ceylan, A.E.; Timur, S.; Wolfbeis, O.S.; Salama, K.N. Electrochemical sensors targeting salivary biomarkers: A comprehensive review. TrAC Trends Anal. Chem. 2021, 135, 116164. [Google Scholar] [CrossRef]
- Mohamed, R.; Campbell, J.L.; Cooper-White, J.; Dimeski, G.; Punyadeera, C. The impact of saliva collection and processing methods on CRP, IgE, and Myoglobin immunoassays. Clin. Transl. Med. 2012, 1, 19. [Google Scholar] [CrossRef]
- Chibly, A.M.; Aure, M.H.; Patel, V.N.; Hoffman, M.P. Salivary gland function, development, and regeneration. Physiol. Rev. 2022, 102, 1495–1552. [Google Scholar] [CrossRef] [PubMed]
- Gazal, G. Management of an emergency tooth extraction in diabetic patients on the dental chair. Saudi Dent. J. 2020, 32, 1–6. [Google Scholar] [CrossRef]
- Puttaswamy, K.A.; Puttabudhi, J.H.; Raju, S. Correlation between salivary glucose and blood glucose and the implications of salivary factors on the oral health status in type 2 diabetes mellitus patients. J. Int. Soc. Prev. Community Dent. 2017, 7, 28. [Google Scholar] [CrossRef]
- Rohleder, N.; Nater, U.M. Determinants of salivary α-amylase in humans and methodological considerations. Psychoneuroendocrinology 2009, 34, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Bapat, S.; Nagarajappa, R.; Ramesh, G.; Bapat, K. Effect of propolis mouth rinse on oral microorganisms—A randomized controlled trial. Clin. Oral Investig. 2021, 25, 6139–6146. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, H.; Zhu, J.; Liao, Z.; Wang, S.; Liu, W. Investigation of Whole and Glandular Saliva as a Biomarker for Alzheimer’s Disease Diagnosis. Brain Sci. 2022, 12, 595. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, H.; Wang, S. Stimulated Parotid Saliva Is a Better Method for Depression Prediction. Biomedicines 2022, 10, 2220. [Google Scholar] [CrossRef]
- Ornelas-González, A.; Ortiz-Martínez, M.; González-González, M. Enzymatic Methods for Salivary Biomarkers Detection: Overview and Current Challenges. Molecules 2021, 26, 7026. [Google Scholar] [CrossRef]
- Dhanya, M.; Hegde, S. Salivary glucose as a diagnostic tool in Type II diabetes mellitus: A case-control study. Niger. J. Clin. Pract. 2016, 19, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Navazesh, M.; Kumar, S.K.S. Measuring salivary flow: Challenges and opportunities. J. Am. Dent. Assoc. 2008, 139, 35S–40S. [Google Scholar] [CrossRef] [PubMed]
- Norton, V.; Lignou, S.; Bull, S.P.; Gosney, M.A.; Methven, L. An investigation of the influence of age and saliva flow on the oral retention of whey protein and its potential effect on the perception and acceptance of whey protein beverages. Nutrients 2020, 12, 2506. [Google Scholar] [CrossRef] [PubMed]
- Uygun, H.D.E.; Uygun, Z.O.; Canbay, E.; Sağın, F.G.; Sezer, E. Non-invasive cortisol detection in saliva by using molecularly cortisol imprinted fullerene-acrylamide modified screen printed electrodes. Talanta 2020, 206, 120225. [Google Scholar] [CrossRef]
- Mészáros, K.; Karvaly, G.; Márta, Z.; Magda, B.; Tőke, J.; Szücs, N.; Patócs, A. Diagnostic performance of a newly developed salivary cortisol and cortisone measurement using an LC–MS/MS method with simple and rapid sample preparation. J. Endocrinol. Investig. 2018, 41, 315–323. [Google Scholar] [CrossRef]
- Ciurli, A.; Liebl, M.; Derks, R.J.; Neefjes, J.J.; Giera, M. Spatially resolved sampling for untargeted metabolomics: A new tool for salivomics. Iscience 2021, 24, 102768. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, T.; Bhargava, M.; Raj, R.; Vaibhav, V.; Kishore, J. Salivary and Serum Glucose Levels in Diabetes Mellitus Patients versus Control–A Randomised Control Trial. J. Med. Life 2020, 13, 235. [Google Scholar]
- Mozaffari, H.R.; Sharifi, R.; Raygani, A.V.; Sadeghi, M.; Nikray, S.; Naseri, R. Salivary Profile in Adult Type 2 Diabetes Mellitus Patients: A Case-control Study. JPMA. J. Pak. Med. Assoc. 2019, 69, 190–194. [Google Scholar]
- Ramenzoni, L.L.; Lehner, M.P.; Kaufmann, M.E.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Oral diagnostic methods for the detection of periodontal disease. Diagnostics 2021, 11, 571. [Google Scholar] [CrossRef]
- Takeda, I.; Stretch, C.; Barnaby, P.; Bhatnager, K.; Rankin, K.; Fu, H.; Slupsky, C. Understanding the human salivary metabolome. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. Vivo 2009, 22, 577–584. [Google Scholar] [CrossRef]
- Jha, N.; Seikaly, H.; Harris, J.; Williams, D.; Liu, R.; McGaw, T.; Barnaby, P. Prevention of radiation induced xerostomia by surgical transfer of submandibular salivary gland into the submental space. Radiother. Oncol. 2003, 66, 283–289. [Google Scholar] [CrossRef]
- Newbrun, E. Observations on the amylase content and flow rate of human saliva following gustatory stimulation. J. Dent. Res. 1962, 41, 459–465. [Google Scholar] [CrossRef]
- Kawamura, Y.; Funakoshi, M.; Nishiyama, T.; Majima, T.; Kamada, A. Relations between taste qualities and parotid gland secretion rate. Nihon seirigaku zasshi. J. Physiol. Soc. Jpn. 1964, 26, 495–502. [Google Scholar]
- Schipper, R.G.; Silletti, E.; Vingerhoeds, M.H. Saliva as research material: Biochemical, physicochemical and practical aspects. Arch. Oral Biol. 2007, 52, 1114–1135. [Google Scholar] [CrossRef]
- Casals, G.; Hanzu, F.A. Cortisol measurements in Cushing’s syndrome: Immunoassay or mass spectrometry. Ann. Lab. Med. 2020, 40, 285–296. [Google Scholar] [CrossRef]
- Authelin, J.R.; Rodrigues, M.A.; Tchessalov, S.; Singh, S.K.; McCoy, T.; Wang, S.; Shalaev, E. Freezing of biologicals revisited: Scale, stability, excipients, and degradation stresses. J. Pharm. Sci. 2020, 109, 44–61. [Google Scholar] [CrossRef]
- Hillebrand, J.J.; Heijboer, A.C.; Endert, E. Effects of repeated freeze–thaw cycles on endocrine parameters in plasma and serum. Ann. Clin. Biochem. 2017, 54, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Emekli-Alturfan, E.; Kasikci, E.; Alturfan, A.A.; Pisiriciler, R.; Yarat, A. Effect of sample storage on stability of salivary glutathione, lipid peroxidation levels, and tissue factor activity. J. Clin. Lab. Anal. 2009, 23, 93–98. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, M.; Zhu, J.; Zhang, H.; Duan, Z.; Wang, S.; Liu, W. Developments in diagnostic applications of saliva in Human Organ Diseases. Med. Nov. Technol. Devices 2022, 13, 100115. [Google Scholar] [CrossRef]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef]
- Katić, A.; Kašuba, V.; Kopjar, N.; Lovaković, B.T.; Čermak, A.M.M.; Mendaš, G.; Želježić, D. Effects of low-level imidacloprid oral exposure on cholinesterase activity, oxidative stress responses, and primary DNA damage in the blood and brain of male Wistar rats. Chem. Biol. Interact. 2021, 338, 109287. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Sherbet, D.P.; Elsbernd, B.L.; Goldstein, J.L.; Brown, M.S.; Zhao, T.J. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J. Biol. Chem. 2012, 287, 17942–17950. [Google Scholar] [CrossRef] [PubMed]
- Ramey, E.R.; Goldstein, M.S. The adrenal cortex and the sympathetic nervous system. Physiol. Rev. 1957, 37, 155–195. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.; Hashemi, S.; Boostani, N.; Zokaee, H. A New Method to Evaluate Fasting Plasma Glucose by Salivary Glucose Measurement. Available online: www.sid.ir (accessed on 21 August 2022).
- Ivanovski, K.; Naumovski, V.; Kostadinova, M.; Pesevska, S.; Drijanska, K.; Filipce, V. Xerostomia and Salivary Levels of Glucose and Urea in Patients with Diabetes; Macedonian Academy of Sciences and Arts: Skopje, Macedonia, 2012; Volume 33. [Google Scholar]
- Abikshyeet, P.; Ramesh, V.; Oza, N. Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 149. [Google Scholar]
- Arakawa, T.; Tomoto, K.; Nitta, H.; Toma, K.; Takeuchi, S.; Sekita, T.; Mitsubayashi, K. A wearable cellulose acetate-coated mouthguard biosensor for in vivo salivary glucose measurement. Anal. Chem. 2020, 92, 12201–12207. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef]
- Verhulst, M.J.; Loos, B.G.; Gerdes, V.E.; Teeuw, W.J. Evaluating all potential oral complications of diabetes mellitus. Front. Endocrinol. 2019, 10, 56. [Google Scholar] [CrossRef]
- Jurysta, C.; Bulur, N.; Oguzhan, B.; Satman, I.; Yilmaz, T.M.; Malaisse, W.J.; Sener, A. Salivary glucose concentration and excretion in normal and diabetic subjects. J. Biomed. Biotechnol. 2009, 2009, 430426. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable sensors for monitoring the physiological and biochemical profile of the athlete. NPJ Digit. Med. 2019, 2, 72. [Google Scholar] [CrossRef]
- Xue, J.; Min, F.; Ma, F. Research on diabetes prediction method based on machine learning. J. Phys. Conf. Ser. IOP Publ. 2020, 1684, 012062. [Google Scholar] [CrossRef]
- Yadan, Z.; Jian, W.; Yifu, L.; Haiying, L.; Jie, L.; Hairui, L. Solving the inverse problem based on UPEMD for electrocardiographic imaging. Biomed. Signal Processing Control 2022, 76, 103665. [Google Scholar] [CrossRef]
- Harrison, T.A.; Hindorff, L.A.; Kim, H.; Wines, R.C.; Bowen, D.J.; McGrath, B.B.; Edwards, K.L. Family history of diabetes as a potential public health tool. Am. J. Prev. Med. 2003, 24, 152–159. [Google Scholar] [CrossRef]
- Islam, M.M.; Ferdousi, R.; Rahman, S.; Bushra, H.Y. Likelihood prediction of diabetes at early stage using data mining techniques. In Computer Vision and Machine Intelligence in Medical Image Analysis; Springer: Singapore, 2020; pp. 113–125. [Google Scholar]
- Jothi, N.; Husain, W. Data mining in healthcare—A review. Procedia Comput. Sci. 2015, 72, 306–313. [Google Scholar] [CrossRef]
| Whether to Rinse | No | Yes | p |
|---|---|---|---|
| 70 times 1 min | 1.63 ± 0.79 | 1.22 ± 0.29 | 0.28 |
| 70 times 3 min | 0.86 ± 0.27 | 0.85 ± 0.11 | 0.946 |
| 5 min with cotton swab | 1.67 ± 1.01 | 1.26 ± 0.74 | 0.288 |
| Measure | Chewing Times | Total | Female | Male | p |
|---|---|---|---|---|---|
| Salivary glucose | 40–50 times/min | 1.54 ± 0.51 | 1.87 ± 0.51 | 1.33 ± 0.38 | 0.087 |
| 50–60 times/min | 1.15 ± 0.34 | 1.03 ± 0.33 | 1.27 ± 0.38 | 0.409 | |
| 60–70 times/min | 1.27 ± 0.41 | 1.18 ± 0.66 | 1.36 ± 0.37 | 0.601 | |
| Saliva volume | 40–50 times/min | 0.84 ± 0.14 | 0.76 ± 0.17 | 0.89 ± 0.03 | 0.174 |
| 50–60 times/min | 1.02 ± 0.43 | 1.09 ± 0.33 | 0.97 ± 0.62 | 0.691 | |
| 60–70 times/min | 1.09 ± 0.40 | 1.35 ± 0.29 | 0.92 ± 0.32 | 0.097 |
| Centrifugation Speed (3 min) | |||||
|---|---|---|---|---|---|
| 1000 r/min | 2000 r/min | 3000 r/min | 4000 r/min | 5000 r/min | p |
| 1.42 ± 0.17 | 1.50 ± 0.23 | 1.53 ± 0.53 * | 1.53 ± 0.64 | 1.53 ± 0.66 | 0.026 |
| Stimulus Level * | ||||||
|---|---|---|---|---|---|---|
| Measure | Stimulus | 0 | 1 | 2 | 3 | p |
| Salivary glucose | Sodium chloride | 1.67 ± 0.23 | 1.63 ± 0.28 | 1.47 ± 0.21 | 1.41 ± 0.14 | 0.812 |
| Citric acid | 1.63 ± 0.53 | 1.58 ± 0.49 | 1.49 ± 0.27 | 1.42 ± 0.15 | 0.079 | |
| Salivary flow rate | Sodium chloride | 0.51 ± 0.11 | 1.77 ± 0.21 | 2.01 ± 0.23 | 1.53 ± 0.17 | 0.054 |
| Citric acid | 0.72 ± 0.07 | 2.26 ± 0.31 | 2.38 ± 0.15 | 1.67 ± 0.14 | 0.068 | |
| 0 min * | Room Temperature ** 2 h | 4 °C 2 h *** | 5 d | 10 d | 15 d | 20 d | 25 d | 30 d | p |
|---|---|---|---|---|---|---|---|---|---|
| 1.53 ± 0.53 | 1.53 ± 0.49 | 1.53 ± 0.54 | 1.53 ± 0.61 | 1.53 ± 0.72 | 1.53 ± 0.53 | 1.51 ± 0.59 | 1.51 ± 0.76 | 1.51 ± 0.89 | 0.037 |
| Freeze/Thaw Cycles | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | p |
| 1.53 ± 0.53 | 1.53 ± 0.61 | 1.53 ± 0.72 | 1.52 ± 0.53 | 1.51 ± 0.59 | 1.49 ± 0.76 | 1.48 ± 0.89 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Zhang, H.; Wang, S.; Lu, J.; He, J.; Liu, L.; Liu, W. Obtaining a Reliable Diagnostic Biomarker for Diabetes Mellitus by Standardizing Salivary Glucose Measurements. Biomolecules 2022, 12, 1335. https://doi.org/10.3390/biom12101335
Cui Y, Zhang H, Wang S, Lu J, He J, Liu L, Liu W. Obtaining a Reliable Diagnostic Biomarker for Diabetes Mellitus by Standardizing Salivary Glucose Measurements. Biomolecules. 2022; 12(10):1335. https://doi.org/10.3390/biom12101335
Chicago/Turabian StyleCui, Yangyang, Hankun Zhang, Song Wang, Junzhe Lu, Jinmei He, Lanlan Liu, and Weiqiang Liu. 2022. "Obtaining a Reliable Diagnostic Biomarker for Diabetes Mellitus by Standardizing Salivary Glucose Measurements" Biomolecules 12, no. 10: 1335. https://doi.org/10.3390/biom12101335
APA StyleCui, Y., Zhang, H., Wang, S., Lu, J., He, J., Liu, L., & Liu, W. (2022). Obtaining a Reliable Diagnostic Biomarker for Diabetes Mellitus by Standardizing Salivary Glucose Measurements. Biomolecules, 12(10), 1335. https://doi.org/10.3390/biom12101335






