Bioguided Isolation of Cyclopenin Analogues as Potential SARS-CoV-2 Mpro Inhibitors from Penicillium citrinum TDPEF34
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Methods
2.2. Fermentation
2.3. Bio-Guided Isolation
2.4. Bioactivity against SARS-CoV-2 Mpro
2.5. Docking and Molecular Dynamic Simulation
2.6. Drug-Likeness and ADMET Prediction
3. Results and Discussion
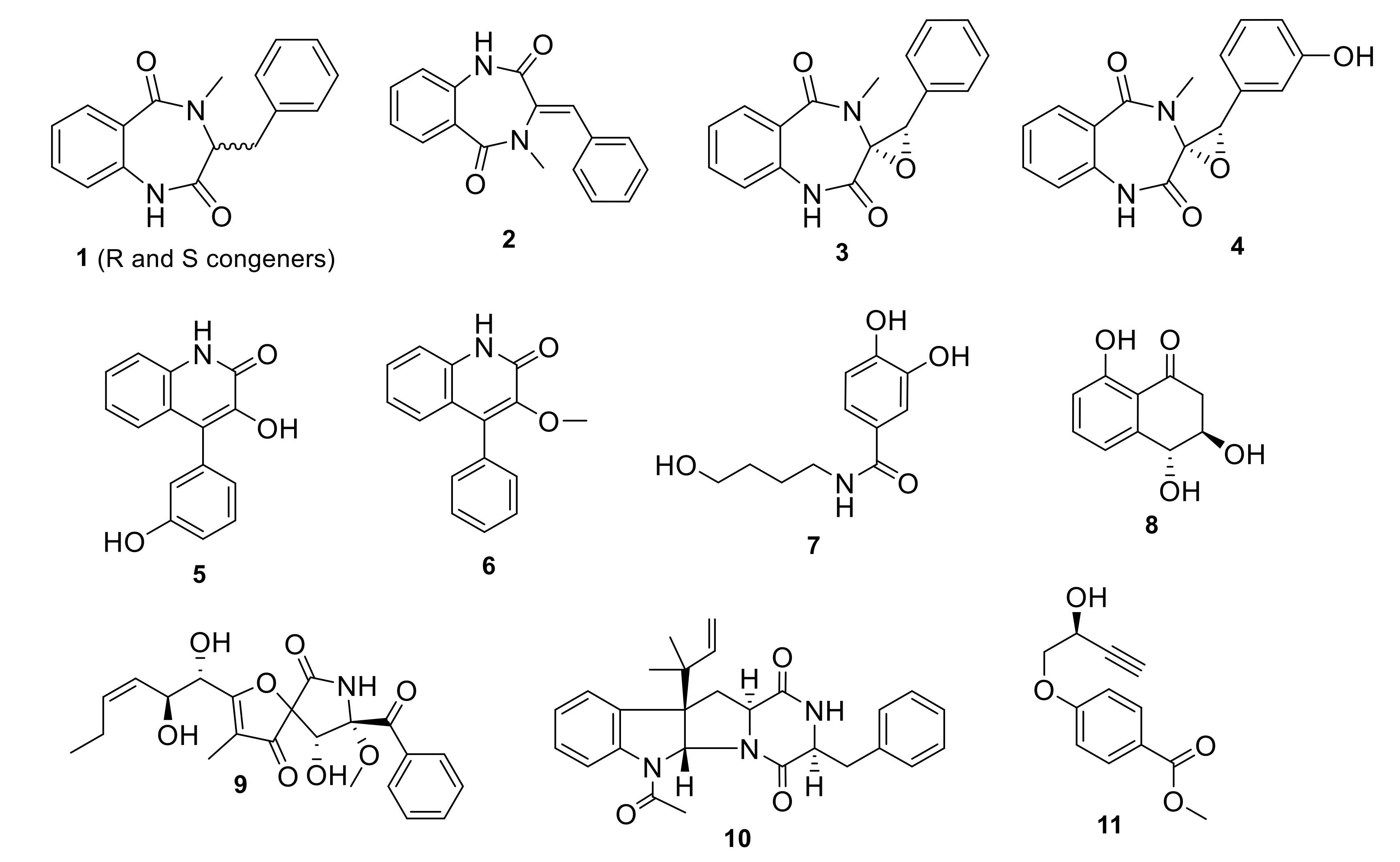
3.1. Fermentation and Metabolites Isolation
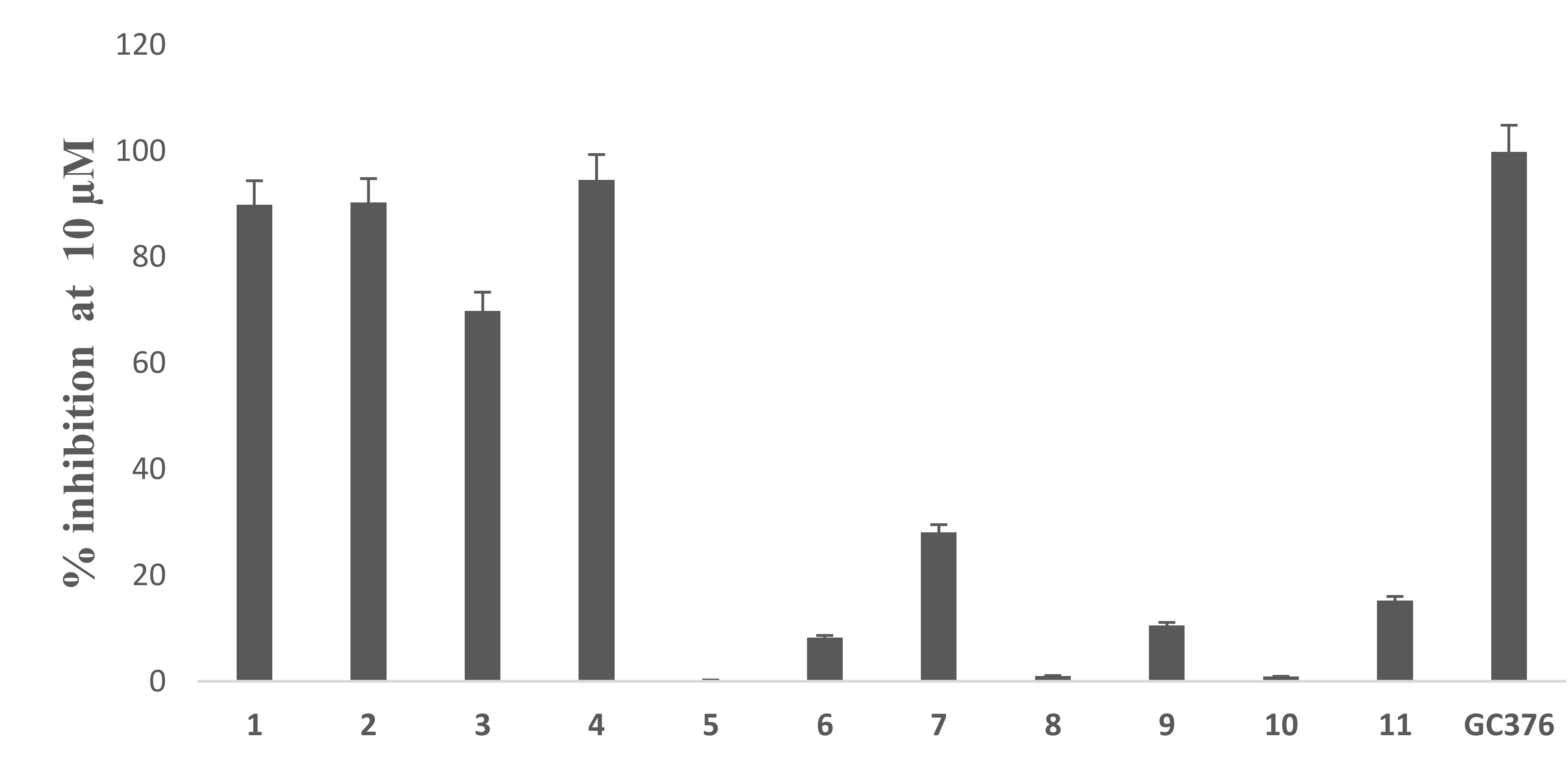
3.2. In Vitro Assay of Mpro Inhibition
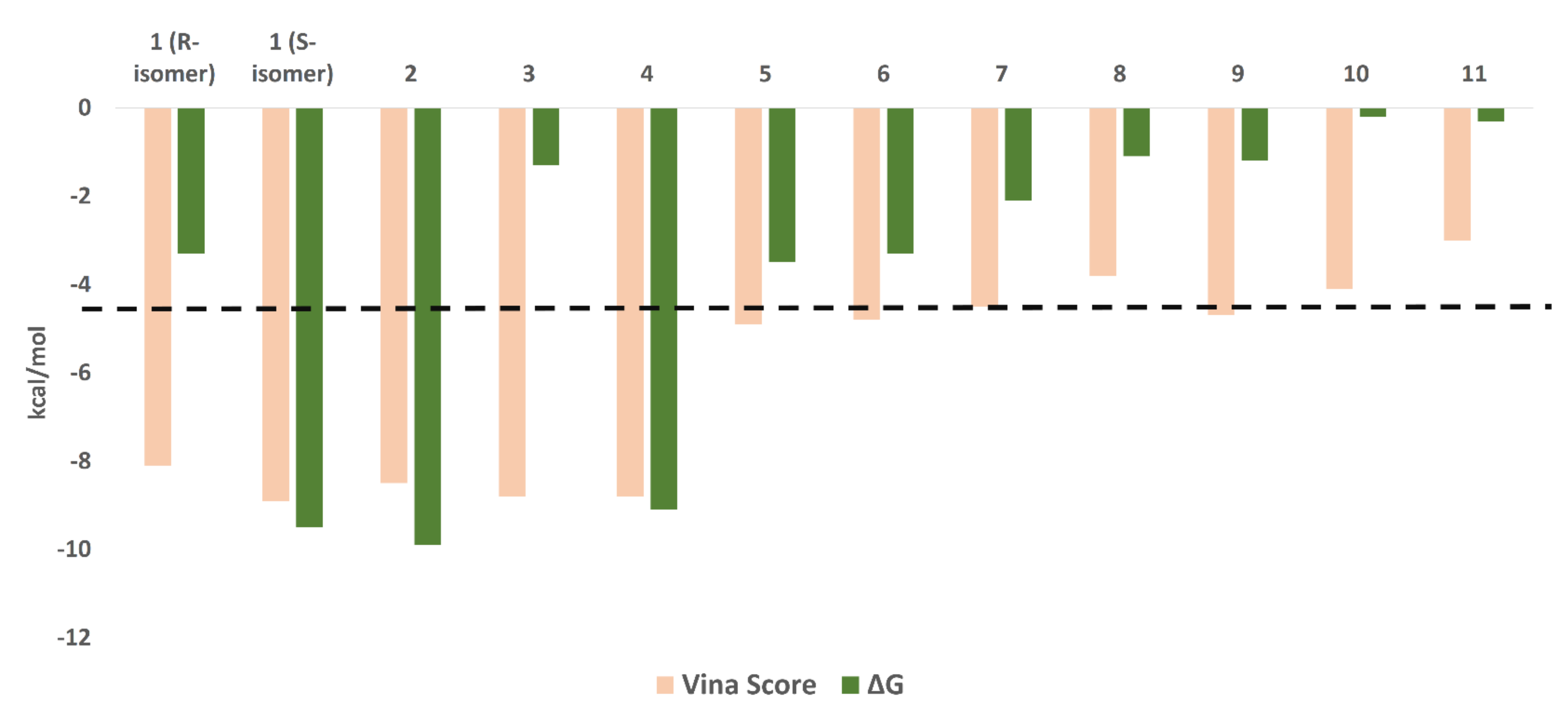
3.3. In Silico Study
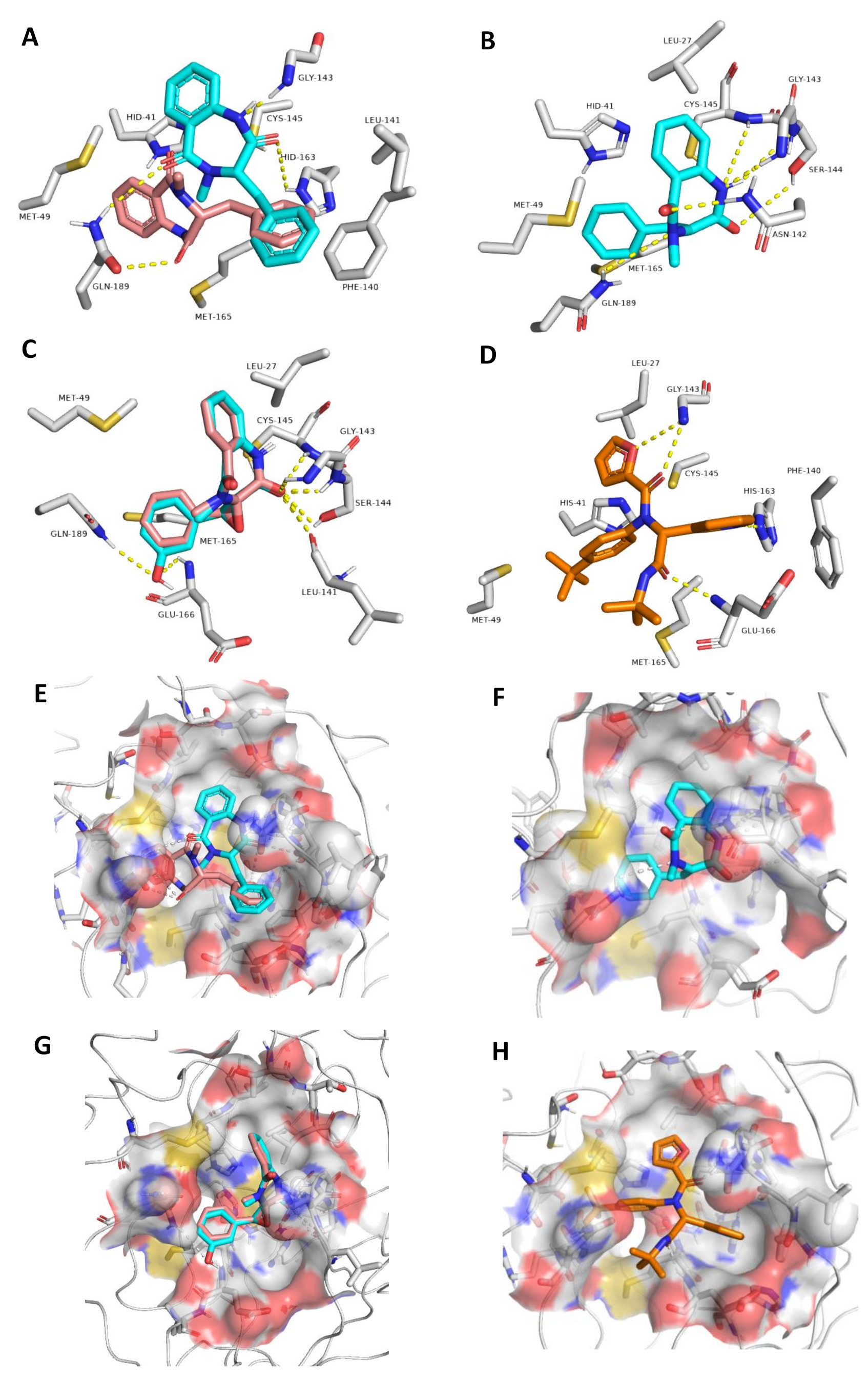
3.3.1. Ensemble Docking
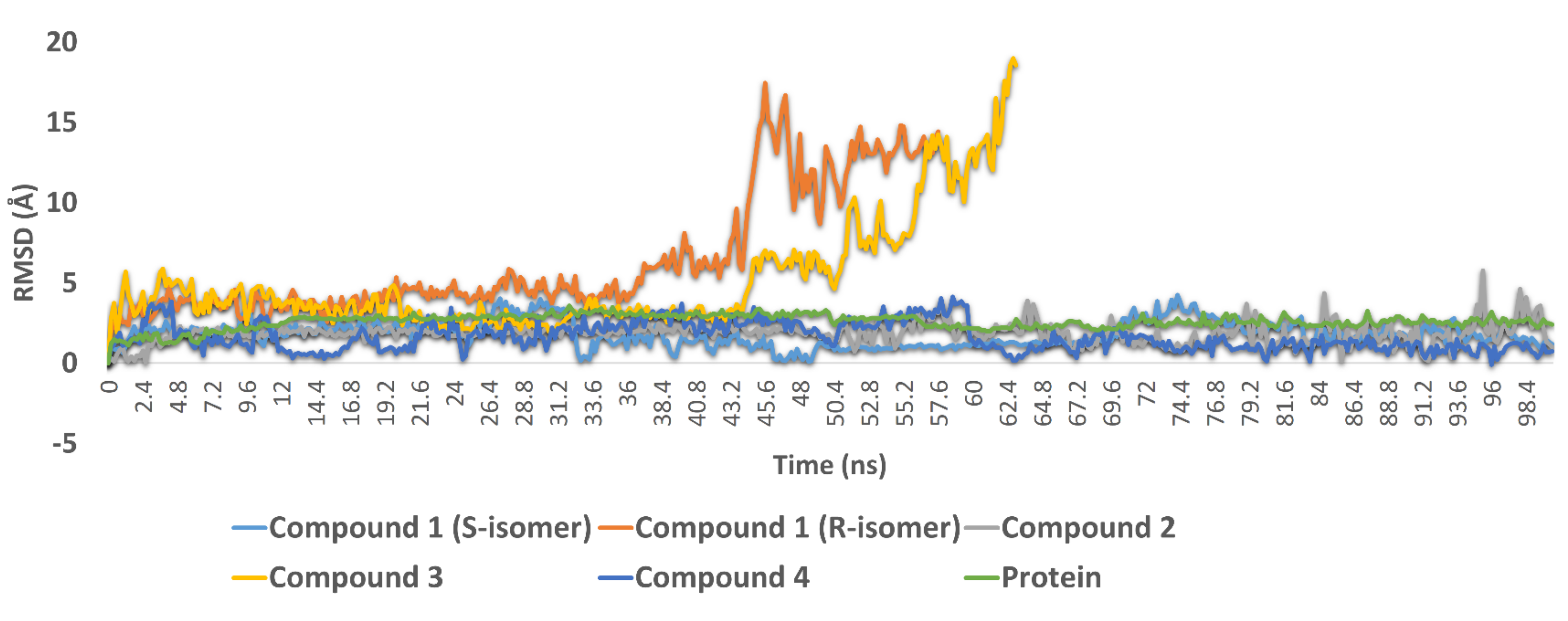
3.3.2. Molecular Dynamic Simulations
3.4. Drug Likeness and ADMET Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sa-Ngiamsuntorn, K.; Suksatu, A.; Pewkliang, Y.; Thongsri, P.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Pitiporn, S.; Chaopreecha, J.; et al. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J. Nat. Prod. 2021, 84, 1261–1270. [Google Scholar] [CrossRef]
- Fu, L.; Ye, F.; Feng, Y.; Yu, F.; Wang, Q.; Wu, Y.; Zhao, C.; Sun, H.; Huang, B.; Niu, P.; et al. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat. Comm. 2020, 11, 4417. [Google Scholar] [CrossRef]
- Orfali, R.; Rateb, M.E.; Hassan, H.M.; Alonazi, M.; Gomaa, M.R.; Mahrous, N.; GabAllah, M.; Kandeil, A.; Perveen, S.; Abdelmohsen, U.R.; et al. Sinapic Acid Suppresses SARS CoV-2 Replication by Targeting Its Envelope Protein. Antibiotics 2021, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V.; Hayashi, Y.; Jung, S.H. An overview of severe acute respiratory syndrome–coronavirus (SARS-CoV) 3CL protease inhibitors: Peptidomimetics and small molecule chemotherapy. J. Med. Chem. 2016, 59, 6595–6628. [Google Scholar] [CrossRef]
- Su, H.X.; Yao, S.; Zhao, W.F.; Li, M.J.; Liu, J.; Shang, W.J.; Xie, H.; Ke, C.Q.; Hu, H.C.; Gao, M.N.; et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol. Sin. 2020, 41, 1167–1177. [Google Scholar] [CrossRef]
- Pavlova, A.; Lynch, D.L.; Daidone, I.; Zanetti-Polzi, L.; Smith, M.D.; Chipot, C.; Kneller, D.W.; Kovalevsky, A.; Coates, L.; Golosov, A.A.; et al. Inhibitor binding influences the protonation states of histidines in SARS-CoV-2 main protease. Chem. Sci. 2020, 12, 1513–1527. [Google Scholar] [CrossRef]
- Wen, C.C.; Kuo, Y.H.; Jan, J.T.; Liang, P.H.; Wang, S.Y.; Liu, H.G.; Lee, C.K.; Chang, S.T.; Kuo, C.J.; Lee, S.S.; et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [Green Version]
- Alhadrami, H.A.; Sayed, A.M.; Sharif, A.M.; Azhar, E.I.; Rateb, M.E. Olive-Derived Triterpenes Suppress SARS COV-2 Main Protease: A Promising Scaffold for Future Therapeutics. Molecules 2021, 26, 2654. [Google Scholar] [CrossRef]
- Mani, J.S.; Johnson, J.B.; Steel, J.C.; Broszczak, D.A.; Neilsen, P.M.; Walsh, K.B.; Naiker, M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020, 284, 197989. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Patel, S.K.; Pathak, M.; Tiwari, R.; Singh, B.R.; Sah, R.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J.; Leblebicioglu, H. Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 23. [Google Scholar] [CrossRef]
- Cao, R.; Hu, H.; Li, Y.; Wang, X.; Xu, M.; Liu, J.; Zhang, H.; Yan, Y.; Zhao, L.; Li, W.; et al. Anti-SARS-CoV-2 potential of artemisinins in vitro. ACS Infect. Dis. 2020, 6, 2524–2531. [Google Scholar] [CrossRef]
- Uckun, F.M.; Saund, S.; Windlass, H.; Trieu, V. Repurposing Anti-Malaria Phytomedicine Artemisinin as a COVID-19 Drug. Front. Pharmacol. 2021, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.M.; Alhadrami, H.A.; El-Gendy, A.O.; Shamikh, Y.I.; Belbahri, L.; Hassan, H.M.; Abdelmohsen, U.R.; Rateb, M.E. Microbial natural products as potential inhibitors of SARS-CoV-2 main protease (Mpro). Microorganisms 2020, 8, 970. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Sayed, A.M.; Al-Khatabi, H.; Alhakamy, N.A.; Rateb, M.E. Scaffold Hopping of α-Rubromycin Enables Direct Access to FDA-Approved Cromoglicic Acid as a SARS-CoV-2 MPro Inhibitor. Pharmaceuticals 2021, 14, 541. [Google Scholar] [CrossRef] [PubMed]
- Alhadrami, H.A.; Sayed, A.M.; Hassan, H.M.; Youssif, K.A.; Gaber, Y.; Moatasim, Y.; Kutkat, O.; Mostafa, A.; Ali, M.A.; Rateb, M.E.; et al. Cnicin as an Anti-SARS-CoV-2: An Integrated In Silico and In Vitro Approach for the Rapid Identification of Potential COVID-19 Therapeutics. Antibiotics 2021, 10, 542. [Google Scholar] [CrossRef]
- Ben Mefteh, F.; Daoud, A.; Chenari Bouket, A.; Thissera, B.; Kadri, Y.; Cherif-Silini, H.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Oszako, T.; et al. Date palm tree root-derived endophytes as fungal cell factories for diverse bioactive metabolites. Int. J. Mol. Sci. 2018, 19, 1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, A.M.; Khalaf, A.M.; Abdelrahim, M.E.; Elgendy, M.O. Repurposing of some anti-infective drugs for COVID-19 treatment: A surveillance study supported by an in silico investigation. Int. J. Clin. Pract. 2021, 75, e13877. [Google Scholar] [CrossRef]
- Tutone, M.; Perricone, U.; Almerico, A.M. Conf-VLKA: A structure-based revisitation of the Virtual Lock-and-key Approach. J. Mol. Graph. Model. 2017, 71, 50–57. [Google Scholar] [CrossRef]
- Lagunin, A.A.; Dubovskaja, V.I.; Rudik, A.V.; Pogodin, P.V.; Druzhilovskiy, D.S.; Gloriozova, T.A.; Filimonov, D.A.; Sastry, N.G.; Poroikov, V.V. CLC-Pred: A freely available web-service for in silico prediction of human cell line cytotoxicity for drug-like compounds. PLoS ONE 2018, 13, e0191838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Fang, P.; Tang, J.; Wu, Z.; Li, X.; Li, S.; Wang, Y.; Liu, G.; He, Z.; Gou, D.; et al. A novel cyclic dipeptide from deep marine-derived fungus Aspergillus sp. SCSIOW2. Nat. Prod. Res. 2016, 30, 52–57. [Google Scholar] [CrossRef]
- Framm, J.; Nover, L.; Azzouny, A.E.; Richter, H.; Winter, K.; Werner, S.; Luckner, M. Cyclopeptin und Dehydrocyclopeptin. Eur. J. Biochem. 1973, 37, 78–85. [Google Scholar] [CrossRef]
- Pan, C.; Shi, Y.; Chen, X.; Chen, C.-T.A.; Tao, X.; Wu, B. New compounds from a hydrothermal vent crab-associated fungus Aspergillus versicolor XZ-4. Org. Biomol. Chem. 2017, 15, 1155–1163. [Google Scholar] [CrossRef]
- Kusano, M.; Koshino, H.; Uzawa, J.; Fujioka, S.; Kawano, T.; Kimura, Y. Nematicidal Alkaloids and Related Compounds Produced by the Fungus Penicillium cf. simplicissimum. Biosci. Biotech. Biochem. 2000, 64, 2559–2568. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Jiang, C.-S.; Li, G.; Guo, Y.-W. (+)-Cyclopenol, a new naturally occurring 7-membered 2,5-dioxopiperazine alkaloid from the fungus Penicillium sclerotiorum endogenous with the Chinese mangrove Bruguiera gymnorrhiza. J. Asian Nat. Prod. Res. 2014, 16, 542–548. [Google Scholar] [CrossRef]
- Fremlin, L.J.; Piggott, A.M.; Lacey, E.; Capon, R.J. Cottoquinazoline A and cotteslosins A and B, metabolites from an Australian marine-derived strain of Aspergillus versicolor. J. Nat. Prod. 2009, 72, 666–670. [Google Scholar] [CrossRef]
- Wei, M.Y.; Yang, R.Y.; Shao, C.L.; Wang, C.Y.; Deng, D.S.; She, Z.G.; Lin, Y.C. Isolation, structure elucidation, crystal structure, and biological activity of a marine natural alkaloid, viridicatol. Chem. Nat. Compd. 2011, 47, 322–325. [Google Scholar] [CrossRef]
- Ma, Y.-m.; Qiao, K.; Kong, Y.; Li, M.-Y.; Guo, L.-X.; Miao, Z.; Fan, C. A new isoquinolone alkaloid from an endophytic fungus R22 of Nerium indicum. Nat. Prod. Res. 2017, 31, 951–958. [Google Scholar] [CrossRef]
- El Euch, I.Z.; Frese, M.; Sewald, N.; Smaoui, S.; Shaaban, M.; Mellouli, L. Bioactive secondary metabolites from new terrestrial Streptomyces sp. TN82 strain: Isolation, structure elucidation and biological activity. Med. Chem. Res. 2018, 27, 1085–1092. [Google Scholar] [CrossRef]
- Guo, W.; Kong, X.; Zhu, T.; Gu, Q.; Li, D. Penipyrols A–B and peniamidones A–D from the mangrove derived Penicillium solitum GWQ-143. Arc. Pharmacal. Res. 2015, 38, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.; Biele, C.; Drogies, K.H.; Steingröver, K.; Aust, H.J.; Draeger, S.; Schulz, B. Fusidilactones, a new group of polycyclic lactones from an endophyte, Fusidium sp. Eur. J. Org. Chem. 2002, 2002, 2331–2336. [Google Scholar] [CrossRef]
- Bloch, P.; Tamm, C.; Bollinger, P.; Petcher, T.J.; Weber, H.P. Pseurotin, a New Metabolite of Pseudeurotium ovalis STOLK Having an Unusual Hetero-Spirocyclic System. Helv. Chim. Acta 1976, 59, 133–137. [Google Scholar] [CrossRef]
- Saraiva, N.N.; Rodrigues, B.S.F.; Jimenez, P.C.; Guimarães, L.A.; Torres, M.C.M.; Rodrigues-Filho, E.; Pfenning, L.H.; Abreu, L.M.; Mafezoli, J.; de Mattos, M.C.; et al. Cytotoxic compounds from the marine-derived fungus Aspergillus sp. recovered from the sediments of the Brazilian coast. Nat. Prod. Res. 2015, 29, 1545–1550. [Google Scholar] [CrossRef]
- Wang, F.-Z.; Li, D.-H.; Zhu, T.-J.; Zhang, M.; Gu, Q.-Q. Pseurotin A1 and A2, two new 1-oxa-7-azaspiro [4.4] non-2-ene-4, 6-diones from the holothurian-derived fungus Aspergillus fumigatus WFZ-25. Can. J. Chem. 2010, 89, 72–76. [Google Scholar] [CrossRef]
- Arai, K.; Kimura, K.; Mushiroda, T.; Yamamoto, Y. Structures of fructigenines A and B, new alkaloids isolated from Penicillium fructigenum Takeuchi. Chem. Pharm. Bull. 1989, 37, 2937–2939. [Google Scholar] [CrossRef] [Green Version]
- Nakahara, S.; Kusano, M.; Fujioka, S.; Shimada, A.; Kimura, Y. Penipratynolene, a novel nematicide from Penicillium bilaiae Chalabuda. Biosci. Biotech. Biochem. 2004, 68, 257–259. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, M.; Lin, Y.; Du, S.; Liu, Z.; Ju, J.; Suzuki, H.; Sawada, M.; Umezawa, K. Inhibition of cellular inflammatory mediator production and amelioration of learning deficit in flies by deep sea Aspergillus derived cyclopenin. J. Antibiot. 2020, 73, 622–629. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Kramer, M.; Essmann, F.; Grond, S. A new acetylenic compound and other bioactive metabolites from a shark gill-derived Penicillium strain. Rec. Nat. Prod. 2017, 11, 31–36. [Google Scholar]
- Mohamed, L.W.; El-Yamany, M.F. Design and synthesis of novel 1,4-benzodiazepine derivatives and their biological evaluation as cholinesterase inhibitors. Arch. Pharmacal Res. 2012, 35, 1369–1377. [Google Scholar] [CrossRef]
- Bin, Y.-L.; He, F.-M.; Li, C.-F.; Liu, S.-Z.; Qiu, Y.-K.; Tang, X.-X.; Xie, B.-Y. Bioactive compounds derived from the marine-derived fungus MCCC3A00951 and their influenza neuraminidase inhibition activity in vitro and in silico. Nat. Prod. Res. 2020, 33, 1–8. [Google Scholar] [CrossRef]
- Kitamura, N.; Sacco, M.D.; Ma, C.; Hu, Y.; Townsend, J.A.; Meng, X.; Wang, J. Expedited Approach toward the Rational Design of Noncovalent SARS-CoV-2 Main Protease Inhibitors. J. Med. Chem. 2021. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]

| Compound | Docking Score | ΔG * | H-Bonding | Water Bridges | Hydrophobic Interactions |
|---|---|---|---|---|---|
| 1 (R-isomer) | −8.1 | −3.3 | GLN189 | HIS41, CYS44, CYS145 | HIS41, MET49, PHE140, |
| 1 (S-isomer) | −8.9 | −9.5 | HIS163, GLN189, GLY143 | HIS163, GLY143, GLN189 | HIS41, PHE140, CYS145, MET165 |
| 2 | −8.5 | −9.9 | LEU141, GLY143, SER144, CYS145, GLN189 | THR25, THR45, SER46, ASN142, GLY143, SER144, HIS163, GLU166, GLN189 | LEU27, HIS41, MET49 |
| 3 | −8.8 | −1.5 | LEU41, GLY143, SER144, CYS145 | - | LEU27 and MET49 |
| 4 | −8.8 | −9.1 | LEU41, GLY143, SER144, CYS145, GLU166, GLN189 | THR26, SER46, GLY143, SER144, GLU166, ARG188, GLN189, THR190 | LEU27, MET49, MET165 |
| Co-crystalized ligand | −9.0 ** | −9.1 | GLY143, HIS166, GLU166 | - | LEU27, HIS41, MET49, PHE140, MET165 |
| Compounds | Lipinski a | Veber b | GIT Absorbtion c | BBB d | CYP2D6 e | Bioavailability Score f |
|---|---|---|---|---|---|---|
| 1 (S & R isomers) | Yes | Yes | High | No | No | 0.55 |
| 2 | Yes | Yes | High | No | No | 0.55 |
| 4 | Yes | Yes | High | No | No | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thissera, B.; Sayed, A.M.; Hassan, M.H.A.; Abdelwahab, S.F.; Amaeze, N.; Semler, V.T.; Alenezi, F.N.; Yaseen, M.; Alhadrami, H.A.; Belbahri, L.; et al. Bioguided Isolation of Cyclopenin Analogues as Potential SARS-CoV-2 Mpro Inhibitors from Penicillium citrinum TDPEF34. Biomolecules 2021, 11, 1366. https://doi.org/10.3390/biom11091366
Thissera B, Sayed AM, Hassan MHA, Abdelwahab SF, Amaeze N, Semler VT, Alenezi FN, Yaseen M, Alhadrami HA, Belbahri L, et al. Bioguided Isolation of Cyclopenin Analogues as Potential SARS-CoV-2 Mpro Inhibitors from Penicillium citrinum TDPEF34. Biomolecules. 2021; 11(9):1366. https://doi.org/10.3390/biom11091366
Chicago/Turabian StyleThissera, Bathini, Ahmed M. Sayed, Marwa H. A. Hassan, Sayed F. Abdelwahab, Ngozi Amaeze, Valeria T. Semler, Faizah N. Alenezi, Mohammed Yaseen, Hani A. Alhadrami, Lassaad Belbahri, and et al. 2021. "Bioguided Isolation of Cyclopenin Analogues as Potential SARS-CoV-2 Mpro Inhibitors from Penicillium citrinum TDPEF34" Biomolecules 11, no. 9: 1366. https://doi.org/10.3390/biom11091366
APA StyleThissera, B., Sayed, A. M., Hassan, M. H. A., Abdelwahab, S. F., Amaeze, N., Semler, V. T., Alenezi, F. N., Yaseen, M., Alhadrami, H. A., Belbahri, L., & Rateb, M. E. (2021). Bioguided Isolation of Cyclopenin Analogues as Potential SARS-CoV-2 Mpro Inhibitors from Penicillium citrinum TDPEF34. Biomolecules, 11(9), 1366. https://doi.org/10.3390/biom11091366










