Biomediators in Polycystic Ovary Syndrome and Cardiovascular Risk
Abstract
1. Introduction
2. Atherosclerosis and Cardiovascular Disease
3. Potential Biomediators of Cardiovascular Risk in PCOS
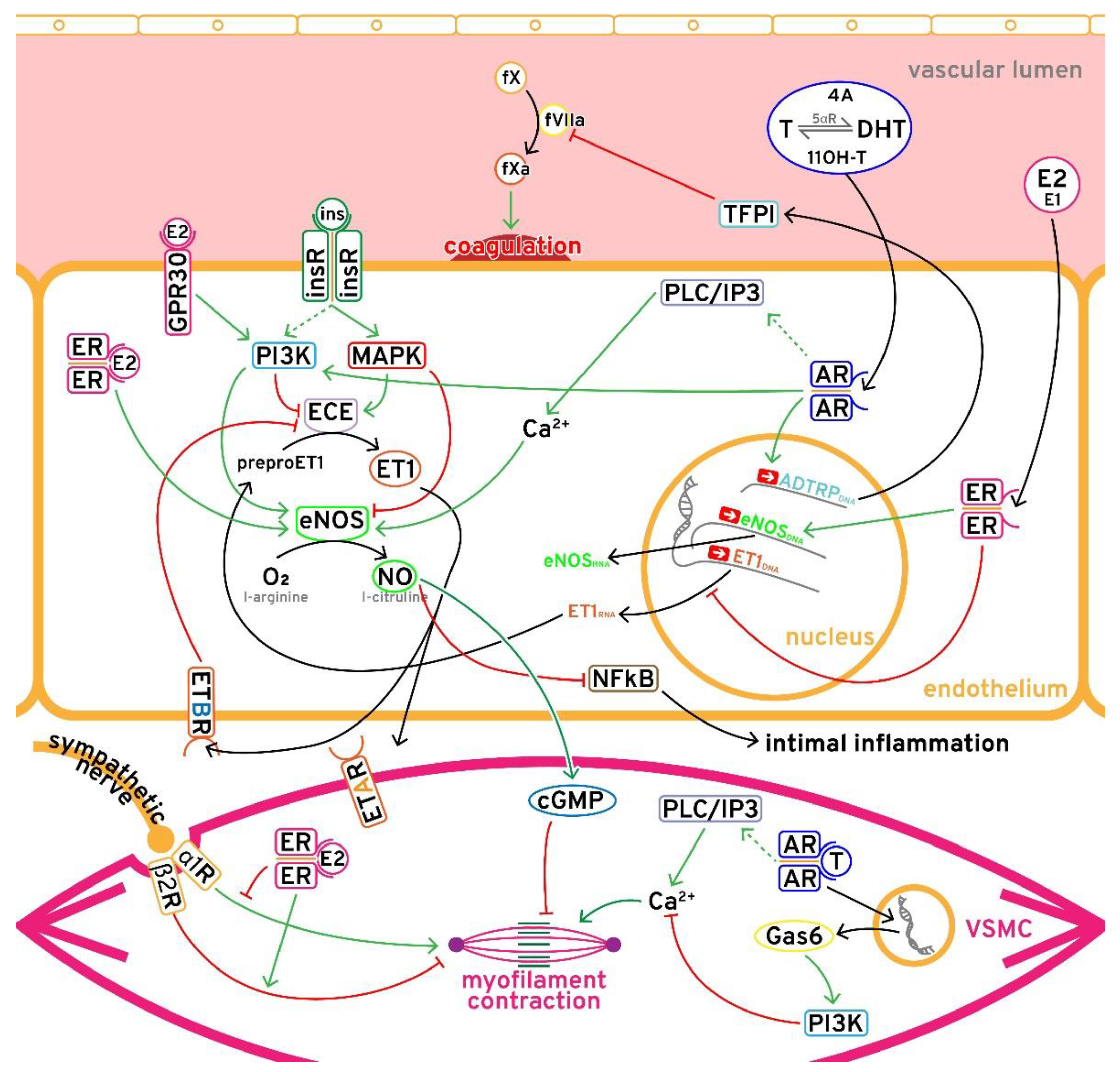
3.1. Hyperandrogenism
3.2. Estrogen-Progesterone Imbalance
3.3. Insulin Resistance
3.4. Low SHBG
4. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. The International PCOS Network Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin. Endocrinol. 2018, 89, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, M. The genetics of the polycystic ovary syndrome. Nat. Clin. Pr. Endocrinol. Metab. 2007, 3, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.G.; Urbanek, M.; Ehrmann, D.A.; Armstrong, L.L.; Lee, J.Y.; Sisk, R.K.; Karaderi, T.; Barber, T.M.; McCarthy, M. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat. Commun. 2015, 6, 7502. [Google Scholar] [CrossRef]
- Day, F.; Karaderi, T.; Jones, M.R.; Meun, C.; He, C.; Drong, A.; Kraft, P.; Lin, N.; Huang, H.; Broer, L.; et al. Large-Scale Genome-Wide Meta Analysis of Polycystic Ovary Syndrome Suggests Shared Genetic Architecture for Different Diagnosis Criteria. bioRxiv 2018, 106179472, 1–20. [Google Scholar] [CrossRef]
- Dapas, M.; Lin, F.T.J.; Nadkarni, G.N.; Sisk, R.; Legro, R.S.; Urbanek, M.; Hayes, M.G.; Dunaif, A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020, 17, e1003132. [Google Scholar] [CrossRef]
- Teede, H.J.; Hutchison, S.; Zoungas, S.; Meyer, C. Insulin Resistance, the Metabolic Syndrome, Diabetes, and Cardiovascular Disease Risk in Women with PCOS. Endocrine 2006, 30, 45–54. [Google Scholar] [CrossRef]
- Carmina, E. Obesity, Adipokines and Metabolic Syndrome in Polycystic Ovary Syndrome. Cardiovasc. Issues Endocrinol. 2012, 40, 40–50. [Google Scholar] [CrossRef]
- Deswal, R.; Yadav, A.; Dang, A. Sex hormone binding globulin-an important biomarker for predicting PCOS risk: A systematic review and meta-analysis. Syst. Biol. Reprod. Med. 2018, 64, 12–24. [Google Scholar] [CrossRef]
- Mendis, S.; Puska, P.; Norrving, B. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization: Geneva, Switzerland, 2011; pp. 2–14. [Google Scholar]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Sun, N.; Torii, R.; Wood, N.B.; Hughes, A.; Thom, S.A.M.; Xu, X.Y. Computational Modeling of LDL and Albumin Transport in an In Vivo CT Image-Based Human Right Coronary Artery. J. Biomech. Eng. 2008, 131, 021003. [Google Scholar] [CrossRef] [PubMed]
- Almirall, J.; Hedenstierna, G. Vascular Response to Hypoxia. Update Intensive Care Emerg. Med. 1991, 87–102. [Google Scholar] [CrossRef]
- Hung, K.T.; Berisha, S.Z.; Ritchey, B.M.; Santore, J.; Smith, J.D. Red Blood Cells Play a Role in Reverse Cholesterol Transport. Arter. Thromb. Vasc. Biol. 2012, 32, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Williams, K.J. The central role of arterial retention of cholesterolrich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 2016, 27, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Hunt, B.J.; Jurd, K.M. Endothelial cell activation. Br Med. J. 1998, 316, 1328–1329. [Google Scholar] [CrossRef] [PubMed]
- Nithianandarajah-Jones, G.N.; Wilm, B.; Goldring, C.E.; Müller, J.; Cross, M.J. ERK5: Structure, regulation and function. Cell. Signal. 2012, 24, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Dekker, R.J.; Van Soest, S.; Fontijn, R.D.; Salamanca, S.; De Groot, P.G.; van Bavel, E.; Pannekoek, H.; Horrevoets, A.J.G. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 2002, 100, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.-H.; Shishido, T.; McClain, C.; Lim, J.H.; Li, J.-D.; Yang, J.; Yan, C.; Abe, J.-I. Extracellular Signal-Regulated Kinase 5 SUMOylation Antagonizes Shear Stress–Induced Antiinflammatory Response and Endothelial Nitric Oxide Synthase Expression in Endothelial Cells. Circ. Res. 2008, 102, 538–545. [Google Scholar] [CrossRef]
- Ckless, K.; Van Der Vliet, A.; Janssen-Heininger, Y. Oxidative-nitrosative stress and post-translational protein modifications: Implications to lung structure-function relations: Arginase modulates NF-κB activity via a nitric oxide-dependent mechanism. Am. J. Respir. Cell Mol. Biol. 2007, 36, 645–653. [Google Scholar] [CrossRef]
- Noor, R.; Mittal, S.; Iqbal, J. Superoxide dismutase--applications and relevance to human diseases. Med. Sci. Monit. 2002, 8, 210–216. [Google Scholar]
- Kim, J.-W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Oladipupo, S.; Hu, S.; Kovalski, J.; Yao, J.; Santeford, A.; Sohn, R.E.; Shohet, R.; Maslov, K.; Wang, L.; Arbeit, J.M. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proc. Natl. Acad. Sci. USA 2011, 108, 13264–13269. [Google Scholar] [CrossRef]
- Matsuura, E.; Hughes, G.R.; A Khamashta, M. Oxidation of LDL and its clinical implication. Autoimmun. Rev. 2008, 7, 558–566. [Google Scholar] [CrossRef]
- Liao, J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Investig. 2013, 123, 540–541. [Google Scholar] [CrossRef]
- Lillis, A.P.; Muratoglu, S.; Au, D.; Migliorini, M.; Lee, M.-J.; Fried, S.K.; Mikhailenko, I.; Strickland, D.K. LDL Receptor-Related Protein-1 (LRP1) Regulates Cholesterol Accumulation in Macrophages. PLoS ONE 2015, 10, e0128903. [Google Scholar] [CrossRef]
- Repaci, A.; Gambineri, A.; Pasquali, R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol. Cell. Endocrinol. 2011, 335, 30–41. [Google Scholar] [CrossRef]
- Chan, S.Y.; Mancini, G.; Kuramoto, L.; Schulzer, M.; Frohlich, J.; Ignaszewski, A. The prognostic importance of endothelial dysfunction and carotid atheromaburden in patients with coronary artery disease. J. Am. Coll. Cardiol. 2003, 42, 1037–1043. [Google Scholar] [CrossRef]
- Macut, D.; Damjanović, S.; Panidis, D.; Spanos, N.; Glisic, B.; Petakov, M.; Rousso, D.; Kourtis, A.; Bjekić, J.; Milić, N. Oxidised low-density lipoprotein concentration–early marker of an altered lipid metabolism in young women with PCOS. Eur. J. Endocrinol. 2006, 155, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Macut, D.; Simic, T.; Lissounov, A.; Pljesa-Ercegovac, M.; Bozic, I.; Djukic, T.; Bjekic-Macut, J.; Matic, M.; Petakov, M.; Suvakov, S.; et al. Insulin Resistance in Non-Obese Women with Polycystic Ovary Syndrome: Relation to Byproducts of Oxidative Stress. Exp. Clin. Endocrinol. Diabetes 2011, 119, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Torry, D.S.; Leavenworth, J.; Chang, M.; Maheshwari, V.; Groesch, K.; Ball, E.R.; Torry, R. Angiogenesis in implantation. J. Assist. Reprod. Genet. 2007, 24, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Qu, Q.; Dai, H.; Liu, Y.; Jiang, L.; Huang, X.; Hao, C. Effects of hypoxia-inducible factor-1α on endometrial receptivity of women with polycystic ovary syndrome. Mol. Med. Rep. 2018, 17, 414–421. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Paterakis, T.; Alexandraki, K.; Piperi, C.; Aessopos, A.; Katsikis, I.; Katsilambros, N.; Kreatsas, G.; Panidis, D. Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum. Reprod. 2006, 21, 1426–1431. [Google Scholar] [CrossRef]
- Tehrani, F.R.; Amiri, M.; Behboudi-Gandevani, S.; Bidhendi-Yarandi, R.; Carmina, E. Cardiovascular events among reproductive and menopausal age women with polycystic ovary syndrome: A systematic review and meta-analysis. Gynecol. Endocrinol. 2020, 36, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.L.; Malek, A.M.; Wild, R.A.; Korytkowski, M.T.; Talbott, E.O. Carotid artery intima-media thickness in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Updat. 2011, 18, 112–126. [Google Scholar] [CrossRef]
- Gambineri, A.; Patton, L.; Altieri, P.; Pagotto, U.; Pizzi, C.; Manzoli, L.; Pasquali, R. Polycystic Ovary Syndrome Is a Risk Factor for Type 2 Diabetes: Results From a Long-Term Prospective Study. Diabetes 2012, 61, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Pierson, R.A.; Lujan, M.E.; Chilibeck, P.D.; McBreairty, L.E.; Gordon, J.J.; Serrao, S.B.; Zello, G.A.; Chizen, D.R. Comprehensive Evaluation of Type 2 Diabetes and Cardiovascular Disease Risk Profiles in Reproductive-Age Women with Polycystic Ovary Syndrome: A Large Canadian Cohort. J. Obstet. Gynaecol. Can. 2019, 41, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Dokras, A. Cardiovascular disease risk in women with PCOS. Steroids 2013, 78, 773–776. [Google Scholar] [CrossRef]
- Meun, C.; Franco, O.; Dhana, K.; Jaspers, L.; Muka, T.; Louwers, Y.; Ikram, M.A.; Fauser, B.C.J.M.; Kavousi, M.; E Laven, J.S. High Androgens in Postmenopausal Women and the Risk for Atherosclerosis and Cardiovascular Disease: The Rotterdam Study. J. Clin. Endocrinol. Metab. 2018, 103, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S. Polycystic ovary syndrome and cardiovascular disease: A premature association? Endocr Rev. 2003, 24, 302–312. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Kempegowda, P.; Jenkinson, C.; Taylor, A.E.; Quanson, J.L.; Storbeck, K.-H.; Arlt, W. 11-Oxygenated C19 Steroids Are the Predominant Androgens in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 840–848. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Zhao, J.; Jin, C.W.; Li, Y.H.; Tang, M.X.; Wang, Z.H.; Zhang, W.; Zhang, Y.; Li, L.; Zhong, M. Testosterone delays vascular smooth muscle cell senescence and inhibits collagen synthesis via the Gas6/Axl signaling pathway. Age (Omaha) 2016, 38, 60. [Google Scholar] [CrossRef]
- Luo, C.; Pook, E.; Tang, B.; Zhang, W.; Li, S.; Leineweber, K.; Cheung, S.-H.; Chen, Q.; Bechem, M.; Hu, J.-S.; et al. Androgen inhibits key atherosclerotic processes by directly activating ADTRP transcription. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 2319–2332. [Google Scholar] [CrossRef] [PubMed]
- Lupu, C.; Zhu, H.; Popescu, N.I.; Wren, J.D.; Lupu, F. Novel protein ADTRP regulates TFPI expression and function in human endothelial cells in normal conditions and in response to androgen. Blood 2011, 118, 4463–4471. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Akishita, M.; Eto, M.; Ogawa, S.; Son, B.-K.; Kato, S.; Ouchi, Y.; Okabe, T. Androgen Receptor-Dependent Activation of Endothelial Nitric Oxide Synthase in Vascular Endothelial Cells: Role of Phosphatidylinositol 3-Kinase/Akt Pathway. Endocrinology 2010, 151, 1822–1828. [Google Scholar] [CrossRef]
- Lieberherr, M.; Grosse, B. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin-sensitive G-protein. J. Biol. Chem. 1994, 269, 7217–7223. [Google Scholar] [CrossRef]
- Foradori, C.; Weiser, M.; Handa, R. Non-genomic actions of androgens. Front. Neuroendocr. 2008, 29, 169–181. [Google Scholar] [CrossRef]
- Morris, P.D.; Malkin, C.J.; Channer, K.S.; Jones, T.H. A mathematical comparison of techniques to predict biologically available testosterone in a cohort of 1072 men. Eur. J. Endocrinol. 2004, 151, 241–249. [Google Scholar] [CrossRef][Green Version]
- Lerchbaum, E.; Schwetz, V.; Rabe, T.; Giuliani, A.; Obermayer-Pietsch, B. Hyperandrogenemia in Polycystic Ovary Syndrome: Exploration of the Role of Free Testosterone and Androstenedione in Metabolic Phenotype. PLoS ONE 2014, 9, e108263. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Zanotti, L.; Fanelli, F.; Mezzullo, M.; Fazzini, A.; Labate, A.M.M.; Repaci, A.; Ribichini, D.; Gambineri, A. Defining Hyperandrogenism in Women With Polycystic Ovary Syndrome: A Challenging Perspective. J. Clin. Endocrinol. Metab. 2016, 101, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Aragaki, A.K.; Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Chlebowski, R.T.; Howard, B.V.; Thomson, C.A.; Margolis, K.L.; et al. Menopausal Hormone Therapy and Long-Term All-Cause and Cause-Specific Mortality: The Women’s Health Initiative Randomized Trials. Obstet. Gynecol. Surv. 2018, 73, 22–24. [Google Scholar] [CrossRef]
- Miller, V.M.; Naftolin, F.; Asthana, S.; Black, D.M.; Brinton, E.A.; Budoff, M.J.; Cedars, M.I.; Dowling, N.M.; Gleason, C.E.; Hodis, H.N.; et al. The Kronos Early Estrogen Prevention Study (KEEPS): What have we learned? Menopause 2019, 26, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Hogg, M.E.; Vavra, A.K.; Banerjee, M.N.; Martinez, J.; Jiang, Q.; Keefer, L.K.; Chambon, P.; Kibbe, M.R. The Role of Estrogen Receptor α and β in Regulating Vascular Smooth Muscle Cell Proliferation is Based on Sex. J. Surg. Res. 2012, 173, e1–e10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weil, B.R.; Manukyan, M.C.; Herrmann, J.; Wang, Y.; Abarbanell, A.; Poynter, J.A.; Meldrum, D.R. Signaling via GPR30 protects the myocardium from ischemia/reperfusion injury. Surgery 2010, 148, 436–443. [Google Scholar] [CrossRef]
- Deschamps, A.; Murphy, E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am. J. Physiol. Circ. Physiol. 2009, 297, H1806–H1813. [Google Scholar] [CrossRef]
- Gilligan, D.M.; Badar, D.M.; A Panza, J.; A Quyyumi, A.; O Cannon, R. Acute vascular effects of estrogen in postmenopausal women. Circulation 1994, 90, 786–791. [Google Scholar] [CrossRef]
- Gavin, K.M.; Seals, D.R.; Silver, A.E.; Moreau, K.L. Vascular Endothelial Estrogen Receptor α Is Modulated by Estrogen Status and Related to Endothelial Function and Endothelial Nitric Oxide Synthase in Healthy Women. J. Clin. Endocrinol. Metab. 2009, 94, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Jakimiuk, A.J.; Weitsman, S.R.; Yen, H.W.; Bogusiewicz, M.; Magoffin, D.A. Estrogen receptor α and β expression in theca and granulosa cells from women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2002, 87, 5532–5538. [Google Scholar] [CrossRef]
- Gregory, C.W.; Wilson, E.M.; Apparao, K.B.C.; Lininger, R.A.; Meyer, W.R.; Kowalik, A.; Fritz, M.A.; Lessey, B.A. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. J. Clin. Endocrinol. Metab. 2002, 87, 2960–2966. [Google Scholar] [CrossRef]
- Wang, A.; Ji, L.; Shang, W.; Li, M.; Chen, L.; White, R.E.; Han, G. Expression of GPR30, ERα and ERβ in endometrium during window of implantation in patients with polycystic ovary syndrome: A pilot study. Gynecol. Endocrinol. 2010, 27, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.C.; Martin, U.S.; Eyster, K.M. Vascular ECE-1 mRNA expression decreases in response to estrogens. Life Sci. 2003, 73, 2973–2983. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Wang, T.-H.; Yang, D.; Fu, X.-D.; Pan, J.-Y. Mechanisms of 17β-estradiol on the production of ET-1 in ovariectomized rats. Life Sci. 2003, 73, 2665–2674. [Google Scholar] [CrossRef]
- Tostes, R.C.; Fortes, Z.B.; Callera, G.E.; Montezano, A.; Touyz, R.M.; Webb, R.C.; Carvalho, M.H.C. Endothelin, sex and hypertension. Clin. Sci. 2007, 114, 85–97. [Google Scholar] [CrossRef]
- Polderman, K.H.; Stehouwer, C.D.; van Kamp, G.J.; Schalkwijk, C.G.; Gooren, L.J. Modulation of plasma endothelin levels by the menstrual cycle. Metabolism 2000, 49, 648–650. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Spina, G.; Kouli, C.; Migdalis, I. Increased Endothelin-1 Levels in Women with Polycystic Ovary Syndrome and the Beneficial Effect of Metformin Therapy. J. Clin. Endocrinol. Metab. 2001, 86, 4666–4673. Available online: https://academic.oup.com/jcem/article/86/10/4666/2848950 (accessed on 6 June 2020). [CrossRef] [PubMed]
- Rao, C.V.; Lei, Z.M. The past, present and future of nongonadal LH/hCG actions in reproductive biology and medicine. Mol. Cell Endocrinol. 2007, 269, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Ziecik, A.J.; Kaczmarek, M.M.; Blitek, A.; Kowalczyk, A.E.; Li, X.; Rahman, N.A. Novel biological and possible applicable roles of LH/hCG receptor. Mol. Cell. Endocrinol. 2007, 269, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ezimokhai, M.; Aloamaka, C.P.; Cherian, T.; Agarwal, M.; Morrison, J. Plasma from normal pregnant women alters the reactivity of rabbit aortic smooth muscle with functional endothelium. Clin. Exp. Pharmacol. Physiol. 1993, 20, 435–442. [Google Scholar] [CrossRef]
- Ezimokhai, M.; Osman, N.; Agarwal, M. Human chorionic gonadotrophin is an endothelium-independent inhibitor of rat aortic smooth muscle contractility. Am. J. Hypertens. 2000, 13, 66–73. [Google Scholar] [CrossRef]
- Tollan, A.; Oian, P.; Kjeldsen, S.E.; Holst, N.; Eide, I. Effects of ovarian stimulation on blood pressure and plasma catecholamine levels. Scand. J. Clin. Lab. Invest 1993, 53, 353–358. [Google Scholar] [CrossRef]
- Banaszewska, B.; Spaczyński, R.Z.; Pelesz, M.; Pawelczyk, L. Incidence of elevated LH/FSH ratio in polycystic ovary syndrome women with normo- and hyperinsulinemia. Rocz. Akad. Med. Bialymst. 2003, 48, 131–134. [Google Scholar] [PubMed]
- Gunning, M.N.; Fauser, B.C.J.M. Are women with polycystic ovary syndrome at increased cardiovascular disease risk later in life? Climacteric 2017, 20, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Williams, C.; Vassard, D.; Pinborg, A.; Schmidt, L. Risk of cardiovascular disease for women with polycystic ovary syndrome: Results from a national Danish registry cohort study. Eur. J. Prev. Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Kashiwagi, A.; Masada, M.; Okamura, T. Molecular mechanisms of impaired endothelial function associated with insulin resistance. Curr. Drug Target -Cardiovasc. Hematol. Disord. 2004, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Potter van Loon, B.J.; Kluft, C.; Radder, J.K.; Blankenstein, M.A.; Meinders, A.E. The cardiovascular risk factor plasminogen activator inhibitor type 1 is related to insulin resistance. Metabolism 1993, 42, 945–949. [Google Scholar] [CrossRef]
- Bastard, J.-P.; Hainque, B. Relationship between plasma plasminogen activator inhibitor 1 and insulin resistance. Diabetes/Metabolism Res. Rev. 2000, 16, 192–201. [Google Scholar] [CrossRef]
- Muniyappa, R.; Sowers, J.R. Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef]
- Muntzel, M.S.; Anderson, E.A.; Johnson, A.K.; Mark, A.L. Mechanisms of Insulin Action on Sympathetic Nerve Activity. Clin. Exp. Hypertens. 1995, 17, 39–50. [Google Scholar] [CrossRef]
- Shinozaki, K.; Ayajiki, K.; Nishio, Y.; Sugaya, T.; Kashiwagi, A.; Okamura, T. Evidence for a Causal Role of the Renin-Angiotensin System in Vascular Dysfunction Associated with Insulin Resistance. Hypertens. 2004, 43, 255–262. [Google Scholar] [CrossRef]
- DeUgarte, C.M.; A Bartolucci, A.; Azziz, R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil. Steril. 2005, 83, 1454–1460. [Google Scholar] [CrossRef]
- Mathur, R.; Alexander, C.J.; Yano, J.; Trivax, B.; Azziz, R. Use of metformin in polycystic ovary syndrome. Am. J. Obstet. Gynecol. 2008, 199, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Burghen, G.A.; Givens, J.R.; Kitabchi, A.E. Correlation of hyperandrogenism with hyperinsulinism in poly cystic ovarian disease. J. Clin. Endocrinol. Metab. 1980, 50, 113–116. [Google Scholar] [CrossRef]
- Zeng, X.; Xie, Y.-J.; Liu, Y.-T.; Long, S.-L.; Mo, Z.-C. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin. Chim. Acta 2020, 502, 214–221. [Google Scholar] [CrossRef]
- Blouin, K.; Boivin, A.; Tchernof, A. Androgens and body fat distribution. J. Steroid Biochem. Mol. Biol. 2008, 108, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Munir, I.; Yen, H.W.; Geller, D.H.; Torbati, D.; Bierden, R.M.; Weitsman, S.R.; Agarwal, S.K.; Magoffin, D.A. Insulin Augmentation of 17α-Hydroxylase Activity Is Mediated by Phosphatidyl Inositol 3-Kinase but Not Extracellular Signal-Regulated Kinase-1/2 in Human Ovarian Theca Cells. Endocrinology 2004, 145, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Gerli, S.; Papaleo, E.; Ferrari, A.; Di Renzo, G.C. Randomized, double blind placebo-controlled trial: Effects of myo-inositol on ovarian function and metabolic factors in women with PCOS. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 347–354. [Google Scholar] [PubMed]
- Unfer, V.; Facchinetti, F.; Orrù, B.; Giordani, B.; Nestler, J. Myo-inositol effects in women with PCOS: A meta-analysis of randomized controlled trials. Endocr. Connect. 2017, 6, 647–658. [Google Scholar] [CrossRef]
- Laganà, A.S.; Rossetti, P.; Buscema, M.; La Vignera, S.; Condorelli, R.A.; Gullo, G.; Granese, R.; Triolo, O. Metabolism and Ovarian Function in PCOS Women: A Therapeutic Approach with Inositols. Int. J. Endocrinol. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Paul, C.; Laganà, A.S.; Maniglio, P.; Triolo, O.; Brady, D.M. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: State-of-the-art and future perspectives. Gynecol. Endocrinol. 2016, 32, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef]
- Chae, S.J.; Kim, J.J.; Choi, Y.M.; Kim, J.M.; Cho, Y.M.; Moon, S.Y. Peroxisome proliferator-activated receptor-γ and its coactivator-1α gene polymorphisms in korean women with polycystic ovary syndrome. Gynecol. Obstet Investig. 2010, 70, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, G.; Steinberg, H.O.; Shepard, M.K.; Hook, G.; Baron, A.D. Troglitazone Therapy Improves Endothelial Function to Near Normal Levels in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 576–580. [Google Scholar] [CrossRef]
- Corton, M.; Botella-Carretero, J.I.; Benguria, A.; Villuendas, G.; Zaballos, Á.; Millán, J.L.S.; Escobar-Morreale, H.; Peral, B. Differential Gene Expression Profile in Omental Adipose Tissue in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Monzillo, L.U.; Hamdy, O. Evaluation of Insulin Sensitivity in Clinical Practice and in Research Settings. Nutr. Rev. 2003, 61, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Van de Voorde, J.; Pauwels, B.; Boydens, C.; Decaluwé, K. Adipocytokines in relation to cardiovascular disease. Metabolism 2013, 62, 1513–1521. [Google Scholar] [CrossRef]
- Lau, W.B.; Ohashi, K.; Wang, Y.; Ogawa, H.; Murohara, T.; Ma, X.-L.; Ouchi, N. Role of Adipokines in Cardiovascular Disease. Circ. J. 2017, 81, 920–928. [Google Scholar] [CrossRef]
- Jaganathan, R.; Ravindran, R.; Dhanasekaran, S. Emerging Role of Adipocytokines in Type 2 Diabetes as Mediators of Insulin Resistance and Cardiovascular Disease. Can. J. Diabetes 2018, 42, 446–456.e1. [Google Scholar] [CrossRef]
- Delitala, A.; Capobianco, G.; Delitala, G.; Cherchi, P.L.; Dessole, S. Polycystic ovary syndrome, adipose tissue and metabolic syndrome. Arch. Gynecol. Obstet. 2017, 296, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.L. Diverse Roles for Sex Hormone-Binding Globulin in Reproduction. Biol. Reprod. 2011, 85, 431–441. [Google Scholar] [CrossRef]
- Mean, F.; Pellaton, M.; Magrini, G. Study on the binding of dihydrotestosterone, testosterone and oestradiol with sex hormone binding globulin. Clin. Chim. Acta 1977, 80, 171–180. [Google Scholar] [CrossRef]
- Del Mar Grasa, M.; Gulfo, J.; Camps, N.; Alcalá, R.; Monserrat, L.; Moreno-Navarrete, J.M.; Ortega, F.J.; Esteve, M.; Remesar, X.; Fernández-López, J.A.; et al. Modulation of SHBG binding to testosterone and estradiol by sex and morbid obesity. Eur. J. Endocrinol. 2017, 176, 393–404. [Google Scholar] [CrossRef]
- Fortunati, N. Sex Hormone-Binding Globulin: Not only a transport protein. What news is around the corner? J. Endocrinol. Investig. 1999, 22, 223–234. [Google Scholar] [CrossRef]
- Hammes, A.; Andreassen, T.K.; Spoelgen, R.; Raila, J.; Hubner, N.; Schulz, H.; Metzger, J.; Schweigert, F.; Luppa, P.B.; Nykjaer, A.; et al. Role of Endocytosis in Cellular Uptake of Sex Steroids. Cell 2005, 122, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Rosner, W.; Hryb, D.J.; Kahn, S.M.; Nakhla, A.M.; Romas, N.A. Interactions of sex hormone-binding globulin with target cells. Mol. Cell. Endocrinol. 2010, 316, 79–85. [Google Scholar] [CrossRef]
- Balogh, A.; Karpati, E.; Schneider, A.E.; Hetey, S.; Szilagyi, A.; Juhasz, K.; Laszlo, G.; Hupuczi, P.; Zavodszky, P.; Papp, Z.; et al. Sex hormone-binding globulin provides a novel entry pathway for estradiol and influences subsequent signaling in lymphocytes via membrane receptor. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Marina, L.; Sojat, A.S.; Maseroli, E.; Spaggiari, G.; Pandurevic, S.; Santi, D. Hormonal profile of menopausal women receiving androgen replacement therapy: A meta-analysis. J. Endocrinol. Investig. 2020, 43, 717–735. [Google Scholar] [CrossRef]
- Hogeveen, K.N.; Talikka, M.; Hammond, G. Human Sex Hormone-binding Globulin Promoter Activity Is Influenced by a (TAAAA) Repeat Element within an Alu Sequence. J. Biol. Chem. 2001, 276, 36383–36390. [Google Scholar] [CrossRef] [PubMed]
- Xita, N.; Tsatsoulis, A. Genetic variants of sex hormone-binding globulin and their biological consequences. Mol. Cell. Endocrinol. 2010, 316, 60–65. [Google Scholar] [CrossRef][Green Version]
- Saltiki, K.; Stamatelopoulos, K.; Voidonikola, P.; Lazaros, L.; Mantzou, E.; Georgiou, I.; Anastasiou, E.; Papamichael, C.; Alevizaki, M. Association of the SHBG gene promoter polymorphism with early markers of atherosclerosis in apparently healthy women. Atherosclerosis 2011, 219, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, G.; Lundberg, P.-A.; Lapidus, L.; Lundgren, H.; Bengtsson, C.; Björntorp, P. Low Sex-Hormone-Binding Globulin Concentration as Independent Risk Factor for Development of NIDDM: 12-Yr Follow-Up of Population Study of Women in Gothenburg, Sweden. Diabetes 1991, 40, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Sutton-Tyrrell, K.; Wildman, R.P.; Matthews, K.A.; Chae, C.; Lasley, B.L.; Brockwell, S.; Pasternak, R.C.; Lloyd-Jones, D.; Sowers, M.F.; Torréns, J.I. Sex Hormone–Binding Globulin and the Free Androgen Index Are Related to Cardiovascular Risk Factors in Multiethnic Premenopausal and Perimenopausal Women Enrolled in the Study of Women Across the Nation (SWAN). Circ 2005, 111, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Katz, M.S.; Stern, M.P.; Dunn, J.F. Association of decreased sex hormone binding globulin and cardiovascular risk factors. Arter. Off. J. Am. Hear. Assoc. Inc. 1989, 9, 136–143. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandurevic, S.; Macut, D.; Fanelli, F.; Pagotto, U.; Gambineri, A. Biomediators in Polycystic Ovary Syndrome and Cardiovascular Risk. Biomolecules 2021, 11, 1350. https://doi.org/10.3390/biom11091350
Pandurevic S, Macut D, Fanelli F, Pagotto U, Gambineri A. Biomediators in Polycystic Ovary Syndrome and Cardiovascular Risk. Biomolecules. 2021; 11(9):1350. https://doi.org/10.3390/biom11091350
Chicago/Turabian StylePandurevic, Srdan, Djuro Macut, Flaminia Fanelli, Uberto Pagotto, and Alessandra Gambineri. 2021. "Biomediators in Polycystic Ovary Syndrome and Cardiovascular Risk" Biomolecules 11, no. 9: 1350. https://doi.org/10.3390/biom11091350
APA StylePandurevic, S., Macut, D., Fanelli, F., Pagotto, U., & Gambineri, A. (2021). Biomediators in Polycystic Ovary Syndrome and Cardiovascular Risk. Biomolecules, 11(9), 1350. https://doi.org/10.3390/biom11091350




