A Novel Method for the Determination of Vitamin D Metabolites Assessed at the Blood-Cerebrospinal Fluid Barrier
Abstract
:1. Introduction
2. Material and Methods
2.1. Patient Samples
2.2. Measurement of 25(OH)D3, 25(OH)D2 and 24,25(OH)2D3
2.3. Measurement of Albumin and Estimation of BCB Function
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Performance of the LC-MS/MS Method for Vitamin D Metabolite Measurement in CSF
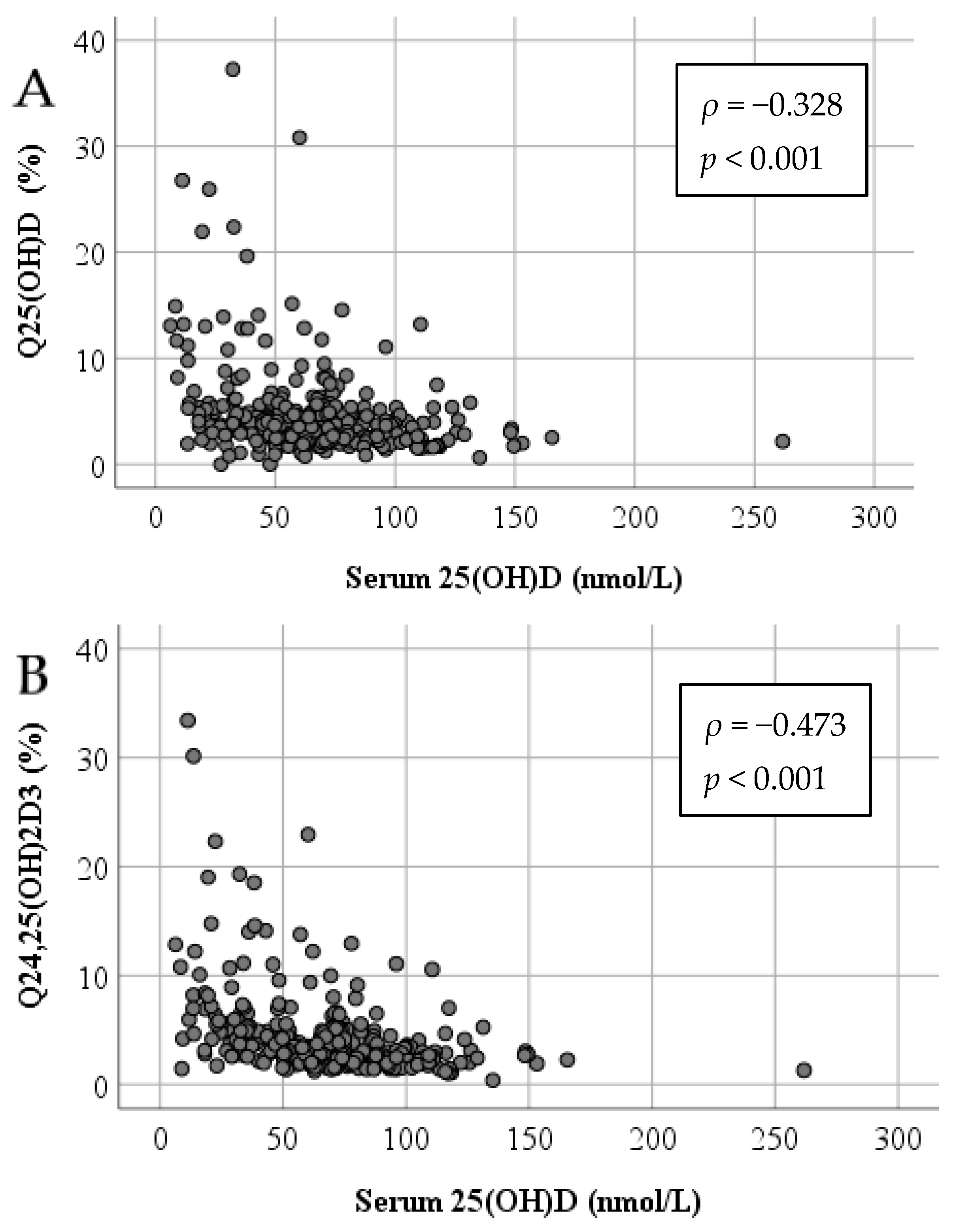
3.3. Impact of Serum 25(OH)D on Vitamin D Metabolites in CSF
3.4. BCB Function and the Relationship of Vitamin D Metabolites in Serum and CSF
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeLuca, G.C.; Kimball, S.M.; Kolasinski, J.; Ramagopalan, S.V.; Ebers, G.C. Review: The role of vitamin D in nervous system health and disease. Neuropathol Appl. Neurobiol. 2013, 39, 458–484. [Google Scholar] [CrossRef] [PubMed]
- Harms, L.R.; Burne, T.H.; Eyles, D.W.; McGrath, J.J. Vitamin D and the brain. Best Pract. Res. Clin. Endocrinol Metab. 2011, 25, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.M.; van der Zwaluw, N.L.; van Wijngaarden, J.P.; Dhonukshe-Rutten, R.A.; in’t Veld, P.H.; Feskens, E.J.; Smeets, P.A.; Kessels, R.P.; van de Rest, O.; de Groot, L.C. Higher Serum 25-Hydroxyvitamin D and Lower Plasma Glucose Are Associated with Larger Gray Matter Volume but Not with White Matter or Total Brain Volume in Dutch Community-Dwelling Older Adults. J. Nutr. 2015, 145, 1817–1823. [Google Scholar] [CrossRef] [Green Version]
- Johansson, P.; Almqvist, E.G.; Johansson, J.O.; Mattsson, N.; Andreasson, U.; Hansson, O.; Wallin, A.; Blennow, K.; Zetterberg, H.; Svensson, J. Cerebrospinal fluid (CSF) 25-hydroxyvitamin D concentration and CSF acetylcholinesterase activity are reduced in patients with Alzheimer’s disease. PLoS ONE 2013, 8, e81989. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakis, I.; Pase, M.P.; Beiser, A.; Booth, S.L.; Jacques, P.F.; Rogers, G.; DeCarli, C.; Vasan, R.S.; Wang, T.J.; Himali, J.J.; et al. Association of Serum Vitamin D with the Risk of Incident Dementia and Subclinical Indices of Brain Aging: The Framingham Heart Study. J. Alzheimers Dis. 2016, 51, 451–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohnke, C.; Herrmann, M.; Berger, K. Associations of major depressive disorder and related clinical characteristics with 25-hydroxyvitamin D levels in middle-aged adults. Nutr. Neurosci. 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Balabanova, S.; Richter, H.P.; Antoniadis, G.; Homoki, J.; Kremmer, N.; Hanle, J.; Teller, W.M. 25-Hydroxyvitamin D, 24, 25-dihydroxyvitamin D and 1,25-dihydroxyvitamin D in human cerebrospinal fluid. Klin. Wochenschr. 1984, 62, 1086–1090. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, J.H.; Jung, M.H.; Cho, M.C. Total 25-hydroxy vitamin D level in cerebrospinal fluid correlates with serum total, bioavailable, and free 25-hydroxy vitamin D levels in Korean population. PLoS ONE 2019, 14, e0213389. [Google Scholar] [CrossRef]
- Holmoy, T.; Lossius, A.; Gundersen, T.E.; Moen, S.M.; Castellazzi, M.; Fainardi, E.; Casetta, I. Intrathecal levels of vitamin D and IgG in multiple sclerosis. Acta Neurol. Scand. 2012, 125, e28–e31. [Google Scholar] [CrossRef] [PubMed]
- Holmøy, T.; Moen, S.M.; Gundersen, T.A.; Holick, M.F.; Fainardi, E.; Castellazzi, M.; Casetta, I. 25-hydroxyvitamin D in cerebrospinal fluid during relapse and remission of multiple sclerosis. Mult. Scler. 2009, 15, 1280–1285. [Google Scholar] [CrossRef]
- Ding, S.; Schoenmakers, I.; Jones, K.; Koulman, A.; Prentice, A.; Volmer, D.A. Quantitative determination of vitamin D metabolites in plasma using UHPLC-MS/MS. Anal. Bioanal. Chem. 2010, 398, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, M.; Gallagher, J.C.; Peacock, M.; Schlingmann, K.P.; Konrad, M.; DeLuca, H.F.; Sigueiro , R.; Lopez, B.; Mourino, A.; Maestro, M.; et al. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J. Clin. Endocrinol Metab. 2014, 99, 2567–2574. [Google Scholar] [CrossRef]
- Farrell, C.J.; Soldo, J.; McWhinney, B.; Bandodkar, S.; Herrmann, M. Impact of assay design on test performance: Lessons learned from 25-hydroxyvitamin D. Clin. Chem. Lab. Med. 2014, 52, 1579–1587. [Google Scholar] [CrossRef]
- Herrmann, M. The measurement of 25-hydroxy vitamin D-an analytical challenge. Clin. Chem. Lab. Med. 2012, 50, 1873–1875. [Google Scholar] [CrossRef] [PubMed]
- Volmer, D.A.; Mendes, L.R.; Stokes, C.S. Analysis of vitamin D metabolic markers by mass spectrometry: Current techniques, limitations of the “gold standard” method, and anticipated future directions. Mass Spectrom. Rev. 2015, 34, 2–23. [Google Scholar] [CrossRef]
- Zelzer, S.; Goessler, W.; Herrmann, M. Measurement of vitamin D metabolites by mass spectrometry, an analytical challenge. J. Lab. Precis. Med. 2018, 3, 99. [Google Scholar] [CrossRef]
- Zelzer, S.; Meinitzer, A.; Enko, D.; Simstich, S.; Le Goff, C.; Cavalier, E.; Herrmann, M.; Goessler, W. Simultaneous determination of 24,25- and 25,26-dihydroxyvitamin D3 in serum samples with liquid-chromatography mass spectrometry-A useful tool for the assessment of vitamin D metabolism. J. Chromatogr. B 2020, 1158, 122394. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Cavalier, E.; Bhattoa, H.P.; Perez-Lopez, F.R.; Lopez-Baena, M.T.; Perez-Roncero, G.R.; Chedraui, P.; Annweiler, C.; Della Casa, S.; Zelzer, S.; et al. Vitamin D testing: Advantages and limits of the current assays. Eur. J. Clin. Nutr. 2020, 74, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H. Proteins in cerebrospinal fluid and blood: Barriers, CSF flow rate and source-related dynamics. Restor. Neurol. Neurosci. 2003, 21, 79–96. [Google Scholar] [PubMed]
- Reiber, H.; Peter, J.B. Cerebrospinal fluid analysis: Disease-related data patterns and evaluation programs. J. Neurol. Sci. 2001, 184, 101–122. [Google Scholar] [CrossRef]
- US-FDA. Bioanalytical Method Validation: Guidance for Industry; CVM: Rockville, MD, USA; CDER: Rockville, MD, USA; FDA: Beltsville, MD, USA, 2018.
- Thompson, M.; Wood, R. Harmonized guidelines for single laboratory validation of methods of analysis. Pure Appl. Chem. 2002, 74, 21. [Google Scholar] [CrossRef]
- Reiber, H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin. Chim. Acta 2001, 310, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol Metab. 2001, 86, 888–894. [Google Scholar] [PubMed] [Green Version]
- Fu, G.K.; Lin, D.; Zhang, M.Y.; Bikle, D.D.; Shackleton, C.H.; Miller, W.L.; Portale, A.A. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol. Endocrinol. 1997, 1, 1961–1970. [Google Scholar]
- Menegaz, D.; Mizwicki, M.T.; Barrientos-Duran, A.; Chen, N.; Henry, H.L.; Norman, A.W. Vitamin D receptor (VDR) regulation of voltage-gated chloride channels by ligands preferring a VDR-alternative pocket (VDR-AP). Mol. Endocrinol. 2011, 25, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Groves, N.J.; McGrath, J.J.; Burne, T.H. Vitamin D as a neurosteroid affecting the developing and adult brain. Annu. Rev. Nutr. 2014, 34, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Ali, P.; Labriffe, M.; Navasiolava, N.; Custaud, M.A.; Dinomais, M.; Annweiler, C.; SAM Group. Vitamin D concentration and focal brain atrophy in older adults: A voxel-based morphometric study. Ann. Clin. Transl. Neurol. 2020, 7, 554–558. [Google Scholar] [CrossRef] [Green Version]
- Tumani, H.; Huss, A.; Bachhuber, F. The cerebrospinal fluid and barriers-anatomic and physiologic considerations. Handb. Clin. Neurol. 2017, 146, 21–32. [Google Scholar]
- Vernetti, L.; Gough, A.; Baetz, N.; Blutt, S.; Broughman, J.R.; Brown, J.A.; Foulke-Abel, J.; Hasan, N.; In, J.; Kelly, E.; et al. Functional Coupling of Human Microphysiology Systems: Intestine, Liver, Kidney Proximal Tubule, Blood-Brain Barrier and Skeletal Muscle. Sci. Rep. 2017, 7, 42296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiber, H.; Zeman, D.; Kušnierová, P.; Mundwiler, E.; Bernasconi, L. Diagnostic relevance of free light chains in cerebrospinal fluid-The hyperbolic reference range for reliable data interpretation in quotient diagrams. Clin. Chim. Acta. 2019, 497, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Slomski, A.T.; Kim, T.K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.K.Y.; Tuckey, R.C. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 2015, 5, 14875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slomski, A.T.; Kim, T.K.; Hobrath, J.V.; Janjetovic, Z.; Oak, A.S.W.; Postlethwaite, A.; Lin, Z.; Li, W.; Takeda, Y.; Jetten, A.M.; et al. Characterization of a new pathway that activates lumisterol in vivo to biologically active hydroxylumisterols. Sci. Rep. 2017, 7, 11434. [Google Scholar] [CrossRef] [Green Version]

| Vitamin D metabolites | 25(OH)D3 | 25(OH)D2 | 24,25(OH)2D3 |
|---|---|---|---|
| m/z ratio | 558.4/298 | 570.2/298 | 574.2/298 |
| Linear range (nmol/L) | 7.8–250 | 1.5–48 | 1.5–48 |
| Correlation coefficient (r2) | 0.999 | 0.997 | 0.998 |
| Intra-day precision (CV%) | 2.2–5.9 | 1.9–8.4 | 3.4–8.7 |
| Inter-day precision (CV%) | 1.5–4.7 | 2.8–4.1 | 4.2–9.6 |
| LoD (nmol/L) | 0.35 | 0.1 | 0.025 |
| LoQ (nmol/L) | 0.7 | 0.7 | 0.05 |
| Recovery (%) | 92.1 | 92.9 | 91.5 |
| Parameter | All Patients | Without BCB Dysfunction | With BCB Dysfunction | p-Values | |||
|---|---|---|---|---|---|---|---|
| (n = 292) | (n = 175) | (n = 117) | P1 | P2 | P3 | ||
| Albumin | Serum (g/dL) | 4.1 (3.7–4.4) | 4.2 (3.9–4.5) | 3.9 (3.6–4.3) | <0.001 | <0.001 | <0.001 |
| CSF (g/L) | 0.27 (0.20–0.38) | 0.21 (0.17–0.25) | 0.40 (0.34–0.58) | ||||
| QALB (× 10−3) | 6.8 (4.9–9.7) | 5.2 (3.9–6.4) | 10.8 (8.9–14.6) | ||||
| 25(OH)D | Serum (nmol/L) | 63.8 (43.4–83.9) | 66.9 (46.6–87.7) | 60.0 (38.2–76.1) | 0.038 | <0.001 | <0.001 |
| CSF (nmol/L) | 2.1 (1.5–3.5) | 1.8 (1.3–2.5) | 3.3 (2.2–4.8) | ||||
| Q (%) | 3.7 (2.4–5.4) | 2.7 (2.1–3.7) | 5.3 (4.1–8.1) | ||||
| 24,25(OH)2D3 | Serum (nmol/L) | 4.2 (2.2–6.2) | 4.4 (2.4–6.4) | 3.6 (2.1–5.9) | 0.102 | <0.001 | <0.001 |
| CSF (nmol/L) | 0.12 (0.08–0.18) | 0.10 (0.08–0.15) | 0.18 (0.10–0.31) | ||||
| Q (%) | 3.3 (2.4–6.2) | 2.6 (2.0–3.5) | 4.9 (3.7–7.4) | ||||
| Parameter | All Patients, n = 292 | Without CSF-Blood Barrier Dysfunction, n = 175 | With CSF-Blood Barrier Dysfunction, n = 117 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <30 nmol/L (n = 38) | 30–50 nmol/L (n = 64) | >50 nmol/L (n = 190) | p-Value | <30 nmol/L (n = 19) | 30–50 nmol/L (n = 36) | >50 nmol/L (n = 120) | p-Value | <30 nmol/L (n = 19) | 30–50 nmol/L (n = 28) | >50 nmol/L (n = 70) | p-Value | |
| QALB | 7.3 | 7.2 | 6.3 | <0.001 | 5.3 | 5.8 | 5.2 | 0.537 | 13.0 | 11.5 | 10.3 | 0.053 |
| (5.1–13.0) | (5.2–11.0) # | (4.7–9.3) + | (3.4–6.8) | (3.8–6.7) | (3.9–6.0) | (9.9–23.1) | (9.5–16.7) | (8.6–13.6) | ||||
| Q25(OH)D | 5.3 | 4.1 | 3.1 | <0.001 | 3.9 | 3.4 | 2.5 | <0.001 | 9.3 | 5.6 | 5.0 | 0.004 |
| (3.6–10.5) | (3.1–5.8) # | (2.3–4.8) + | (2.3–5.3) | (2.4–4.1) # | (2.0–3.4) + | (5.5–13.4) | (4.5–8.9) | (4.0–6.7) + | ||||
| Q24,25(OH)2D3 | 5.9 | 4.2 | 2.8 | <0.001 | 4.2 | 3.5 | 2.4 | <0.001 | 9.5 | 4.9 | 4.2 | <0.001 |
| (3.9–9.5) * | (3.1–5.5) # | (2.1–3.9) + | (2.8–5.6) | (2.6–4.4) # | (1.9–2.9) + | (6.0–13.3) * | (4.1–7.4) # | (3.4–5.6) + | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelzer, S.; Meinitzer, A.; Herrmann, M.; Goessler, W.; Enko, D. A Novel Method for the Determination of Vitamin D Metabolites Assessed at the Blood-Cerebrospinal Fluid Barrier. Biomolecules 2021, 11, 1288. https://doi.org/10.3390/biom11091288
Zelzer S, Meinitzer A, Herrmann M, Goessler W, Enko D. A Novel Method for the Determination of Vitamin D Metabolites Assessed at the Blood-Cerebrospinal Fluid Barrier. Biomolecules. 2021; 11(9):1288. https://doi.org/10.3390/biom11091288
Chicago/Turabian StyleZelzer, Sieglinde, Andreas Meinitzer, Markus Herrmann, Walter Goessler, and Dietmar Enko. 2021. "A Novel Method for the Determination of Vitamin D Metabolites Assessed at the Blood-Cerebrospinal Fluid Barrier" Biomolecules 11, no. 9: 1288. https://doi.org/10.3390/biom11091288
APA StyleZelzer, S., Meinitzer, A., Herrmann, M., Goessler, W., & Enko, D. (2021). A Novel Method for the Determination of Vitamin D Metabolites Assessed at the Blood-Cerebrospinal Fluid Barrier. Biomolecules, 11(9), 1288. https://doi.org/10.3390/biom11091288








