Surface Bioactivation of Polyether Ether Ketone (PEEK) by Sulfuric Acid and Piranha Solution: Influence of the Modification Route in Capacity for Inducing Cell Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Functional Surface PEEK
2.3. Surface Characterizations
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Contact Angle Analysis
2.3.3. Cytotoxicity Assay
2.3.4. Cell Adhesion and Proliferation
2.3.5. Statistical Analysis
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
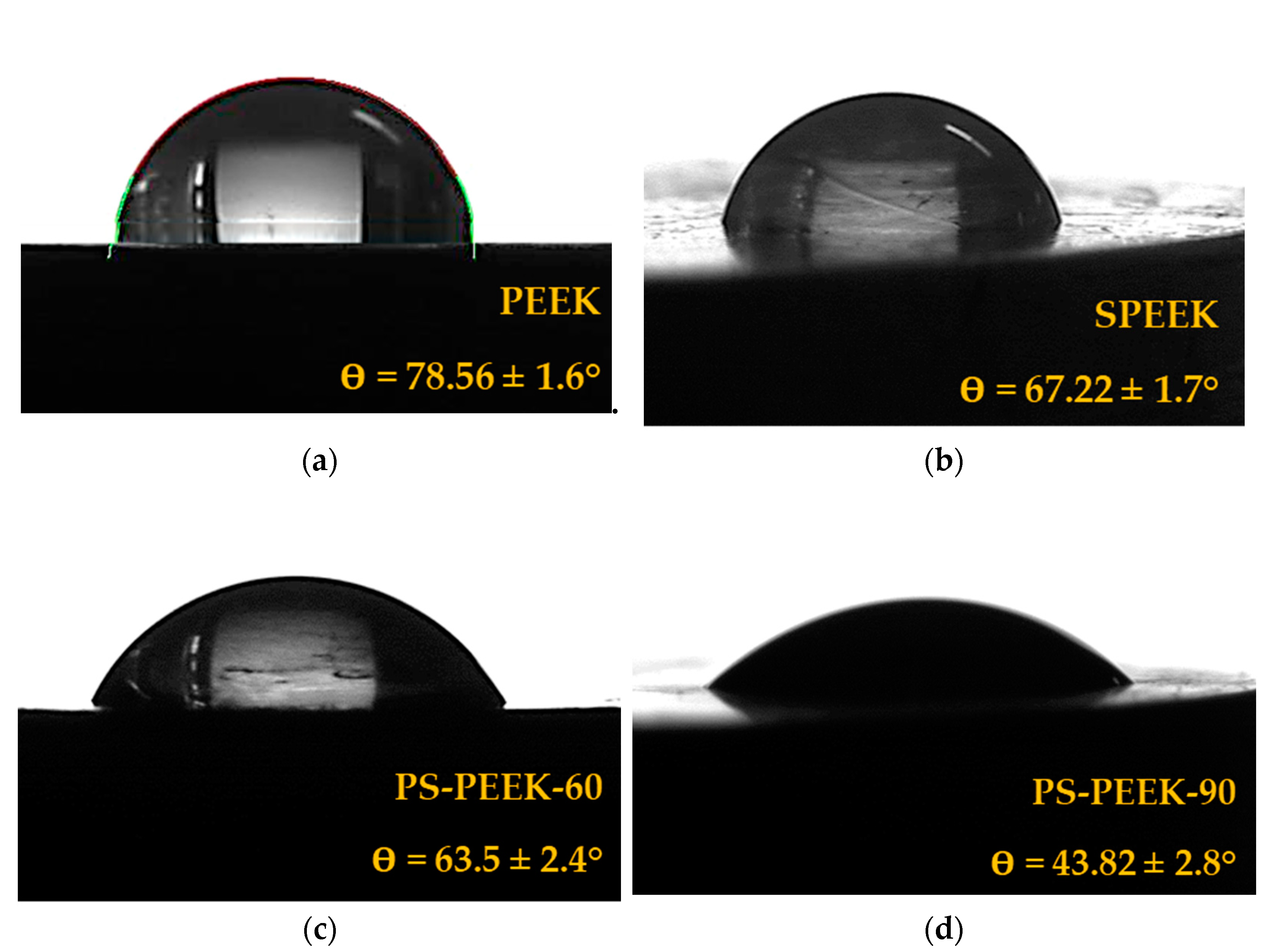
3.2. Contact Angle Analysis
3.3. Cytotoxicity Assay
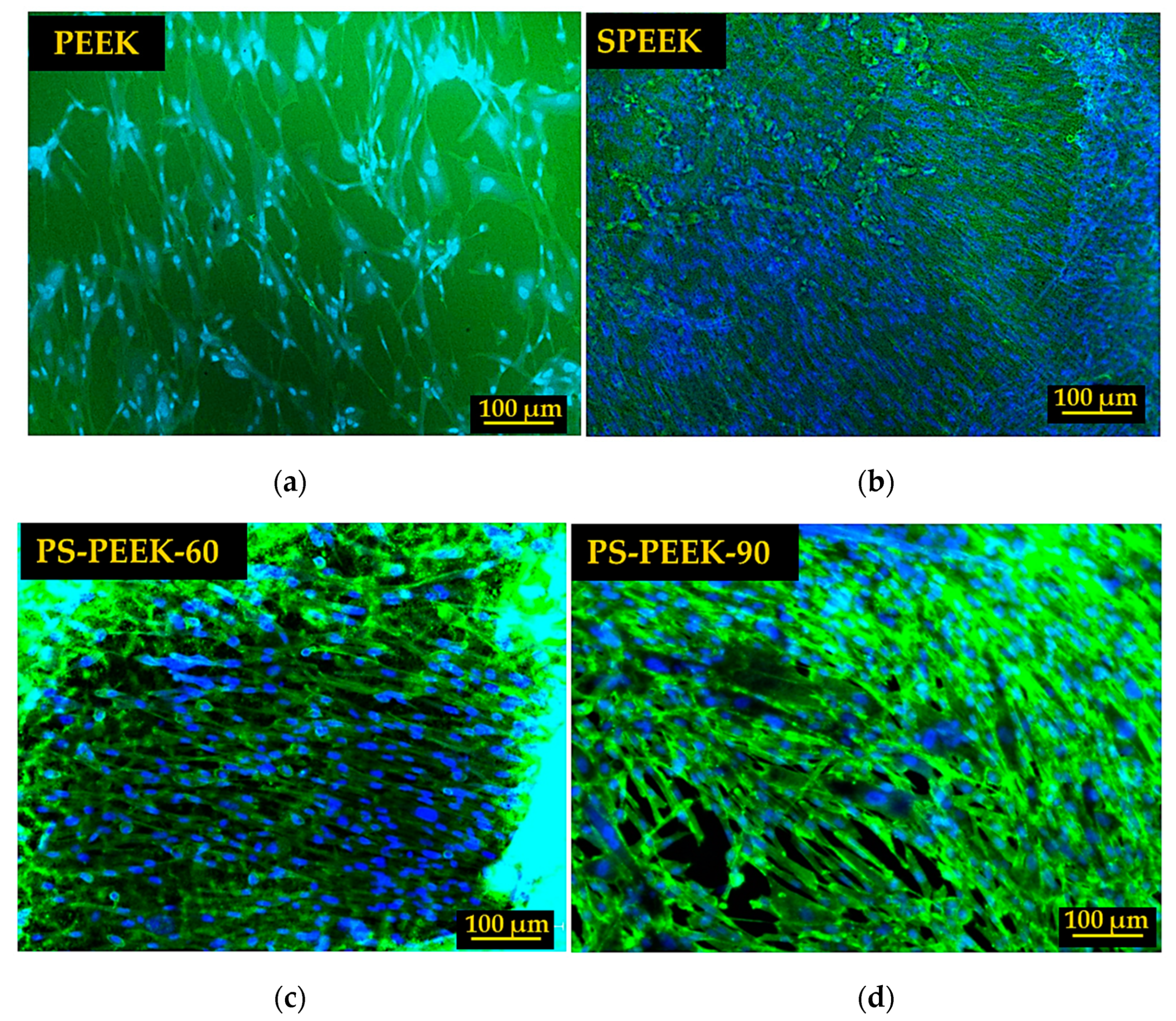
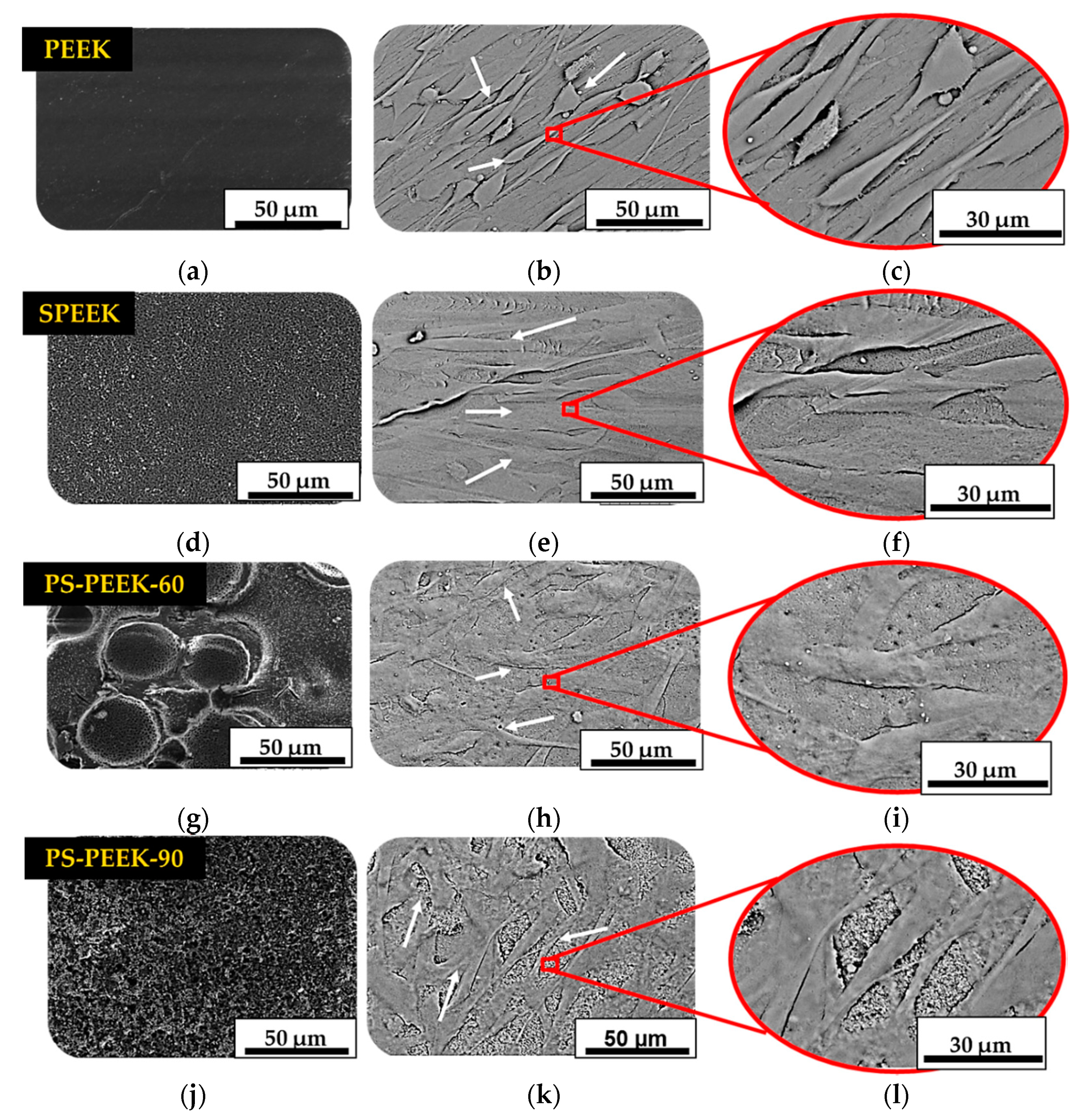
3.4. Cell Adhesion and Proliferation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.M. PEEK Biomaterials Handbook, 1st ed.; Elsevier: Oxford, UK, 2019. [Google Scholar]
- Liu, X.; Gan, K.; Liu, H.; Song, X.; Chen, T.; Liu, C. Antibacterial properties of nano-silver coated PEEK prepared through magnetron sputtering. Dent. Mater. 2017, 33, e348–e360. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.J.; Lorman, B.; Fedder, I.L. Improved response of osteoprogenitor cells to titanium plasma-sprayed PEEK surfaces. Colloids Surf. B Biointerfaces 2019, 175, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Tang, T. Current strategies to improve the bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef]
- Deng, L.; Deng, Y.; Xie, K. AgNPs-decorated 3D printed PEEK implant for infection control and bone repair. Colloids Surf. B Biointerfaces 2017, 160, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of osteointegration property between PEKK and PEEK: Effects of surface structure and chemistry. Biomaterials 2018, 170, 116–126. [Google Scholar] [CrossRef]
- Durham, J.W., 3rd; Rabiei, A. Deposition, Heat Treatment And Characterization of Two Layer Bioactive Coatings on Cylindrical PEEK. Surf. Coat Technol. 2016, 301, 106–113. [Google Scholar] [CrossRef]
- Evans, N.T.; Torstrick, F.B.; Lee, C.S.; Dupont, K.M.; Safranski, D.L.; Chang, W.A.; Macedo, A.E.; Lin, A.S.; Boothby, J.M.; Whittingslow, D.C.; et al. High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater. 2015, 13, 159–167. [Google Scholar] [CrossRef]
- Wiącek, A.E.; Terpiłowski, K.; Jurak, M.; Worzakowska, M. Effect of low-temperature plasma on chitosan-coated PEEK polymer characteristics. Eur. Polym. J. 2016, 78, 1–13. [Google Scholar] [CrossRef]
- Hallmann, L.; Mehl, A.; Sereno, N.; Hämmerle, C.H.F. The improvement of adhesive properties of PEEK through different pre-treatments. Appl. Surf. Sci. 2012, 258, 7213–7218. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.J. Polymer sulfonation-A versatile route to prepare proton-conducting membrane material for advanced technologies. Arab. J. Sci. Eng. 2003, 28, 183–194. [Google Scholar]
- Jin, X.; Bishop, M.T.; Ellis, T.S.; Karasz, F.E. A sulphonated poly (aryl ether ketone). Br. Polym. J. 1985, 17, 4–10. [Google Scholar] [CrossRef]
- Bailly, C.; Williams, D.J.; Karasz, F.E.; MacKnight, W.J. The sodium salts of sulphonated poly (aryl-ether-ether-ketone)(PEEK): Preparation and characterization. Polymer 1987, 28, 1009–1016. [Google Scholar] [CrossRef]
- Almasi, D.; Izman, S.; Assadian, M.; Ghanbari, M.; Abdul Kadir, M.R. Crystalline ha coating on peek via chemical deposition. Appl. Surf. Sci. 2014, 314, 1034–1040. [Google Scholar] [CrossRef]
- Wang, W.; Luo, C.J.; Huang, J.; Edirisinghe, M. PEEK surface modification by fast ambient-temperature sulfonation for bone implant applications. J. R. Soc. Interface 2019, 16, 20180955. [Google Scholar] [CrossRef]
- Montero, J.F.; Tajiri, H.A.; Barra, G.M.; Fredel, M.C.; Benfatti, C.A.; Magini, R.S.; Pimenta, A.L.; Souza, J.C. Biofilm behavior on sulfonated poly(ether-ether-ketone) (sPEEK). Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70 Pt 1, 456–460. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, H.M.; Wang, W.; Li, P.; Xu, Z.; Chong, E.Y.; Yan, C.H.; Yeung, K.W.; Chu, P.K. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterials 2013, 34, 9264–9277. [Google Scholar] [CrossRef]
- Othman, M.; Ismail, A.; Mustafa, A.J.M.P.J. Physico-chemical study of sulfonated poly (ether ether ketone) membranes for direct methanol fuel cell application. Malays. Polym. J. 2007, 2, 10–28. [Google Scholar]
- Hallmann, L.; Ulmer, P.; Lehmann, F.; Wille, S.; Polonskyi, O.; Johannes, M.; Kobel, S.; Trottenberg, T.; Bornholdt, S.; Haase, F.; et al. Effect of surface modifications on the bond strength of zirconia ceramic with resin cement resin. Dent Mater. 2016, 32, 631–639. [Google Scholar] [CrossRef]
- Melo, R.M.; Souza, R.O.; Dursun, E.; Monteiro, E.B.; Valandro, L.F.; Bottino, M.A. Surface Treatments of Zirconia to Enhance Bonding Durability. Oper. Dent. 2015, 40, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Jordan, P.; Schmidlin, P.R.; Roos, M.; Eichberger, M.; Gernet, W.; Keul, C. PEEK surface treatment effects on tensile bond strength to veneering resins. J. Prosthet. Dent. 2014, 112, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Uhrenbacher, J.; Schmidlin, P.R.; Keul, C.; Eichberger, M.; Roos, M.; Gernet, W.; Stawarczyk, B. The effect of surface modification on the retention strength of polyetheretherketone crowns adhesively bonded to dentin abutments. J. Prosthet. Dent. 2014, 112, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U.; Zipperle, M.; Rischka, K.; Petschelt, A.; Muller, F.A. Hydroxylation of dental zirconia surfaces: Characterization and bonding potential. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 461–467. [Google Scholar] [CrossRef]
- Zandparsa, R.; Talua, N.A.; Finkelman, M.D.; Schaus, S.E. An In Vitro Comparison of Shear Bond Strength of Zirconia to Enamel Using Different Surface Treatments. J. Prosthodont. 2014, 23, 117–123. [Google Scholar] [CrossRef]
- ASTM International. AMERICAN SOCIETY FOR TESTING AND MATERIALS. D7334: Standard Practice for Surface Wettability of Coatings, Substrates and Pigments by Advancing Contact Angle 91 Measurement, 8th ed.; ASTM International: West Conshohocken, PA, USA, 2013; p. 3. [Google Scholar]
- Gatti, M.C.A.; da Silva, R.V.; Tarpani, J.R. Análise térmica do laminado PEEK/carbono submetido a diferentes rotas de processamento. Matér. Rio Jan. 2006, 11, 332–339. [Google Scholar] [CrossRef][Green Version]
- Mazur, R.L.; Botelho, E.C.; Costa, M.L.; Rezende, M.C. Avaliações térmica e reológica da matriz termoplástica PEKK utilizada em compósitos aeronáuticos. Polímeros 2008, 18, 237–243. [Google Scholar] [CrossRef]
- Nakamura, H.; Nakamura, T.; Noguchi, T.; Imagawa, K. Photodegradation of PEEK sheets under tensile stress. Polym. Degrad. Stab. 2006, 91, 740–746. [Google Scholar] [CrossRef]
- Doğan, H.; Inan, T.Y.; Koral, M.; Kaya, M. Organo-montmorillonites and sulfonated PEEK nanocomposite membranes for fuel cell applications. Appl. Clay Sci. 2011, 52, 285–294. [Google Scholar] [CrossRef]
- Dutra, J.C.; Santos, T.R.; Gomes, A.S. Nanostructured Polyelectrolytes Based on SPEEK/TiO2 for Direct Ethanol Fuel Cells (DEFCs). Polim. Cienc. E Tecnol. 2014, 24, 43–48. [Google Scholar] [CrossRef][Green Version]
- Silthampitag, P.; Chaijareenont, P.; Tattakorn, K.; Banjongprasert, C.; Takahashi, H.; Arksornnukit, M. Effect of surface pretreatments on resin composite bonding to PEEK. Dent. Mater. J. 2016, 35, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Al-Gharabli, S.; Kujawa, J.; Mavukkandy, M.O.; Arafat, H.A. Functional groups docking on PVDF membranes: Novel Piranha approach. Eur. Polym. J. 2017, 96, 414–428. [Google Scholar] [CrossRef]
- Chu, M.M.; Chou, J.-H. Fluid-kinetics enhanced selective etching process of NiPt film by piranha chemistry in silicide formation for complementary metal oxide semiconductor fabrication. Thin. Solid Film. 2012, 520, 5482–5488. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Hieda, A.; Uemura, N.; Hashimoto, Y.; Toda, I.; Baba, S. In vivo bioactivity of porous polyetheretherketone with a foamed surface. Dent. Mater. J. 2017, 36, 222–229. [Google Scholar] [CrossRef]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, Z.; Peng, Y.; Hu, L.; Guney, T.; Tang, B. Facile Surface Modification Method for Synergistically Enhancing the Biocompatibility and Bioactivity of Poly(ether ether ketone) That Induced Osteodifferentiation. Acs Appl. Mater. Interfaces 2019, 11, 27503–27511. [Google Scholar] [CrossRef]
- Gan, K.; Liu, H.; Jiang, L.; Liu, X.; Song, X.; Niu, D.; Chen, T.; Liu, C. Bioactivity and antibacterial effect of nitrogen plasma immersion ion implantation on polyetheretherketone. Dent. Mater. 2016, 32, e263–e274. [Google Scholar] [CrossRef]



| Treatment | Immersion Time(s) | Code Names |
|---|---|---|
| Sulfuric Acid H2SO4 (98%) | 90 | SPEEK |
| Piranha Solution 2:1 w/v H2SO4 (98%):H2O2 (35%) | 60 | PS-PEEK-60 |
| Piranha Solution 2:1 w/v H2SO4 (98%):H2O2 (35%) | 90 | PS-PEEK-90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, F.S.F.; Vieira, M.; da Silva, H.N.; Tomás, H.; Fook, M.V.L. Surface Bioactivation of Polyether Ether Ketone (PEEK) by Sulfuric Acid and Piranha Solution: Influence of the Modification Route in Capacity for Inducing Cell Growth. Biomolecules 2021, 11, 1260. https://doi.org/10.3390/biom11091260
dos Santos FSF, Vieira M, da Silva HN, Tomás H, Fook MVL. Surface Bioactivation of Polyether Ether Ketone (PEEK) by Sulfuric Acid and Piranha Solution: Influence of the Modification Route in Capacity for Inducing Cell Growth. Biomolecules. 2021; 11(9):1260. https://doi.org/10.3390/biom11091260
Chicago/Turabian Styledos Santos, Flavia Suzany Ferreira, Mariana Vieira, Henrique Nunes da Silva, Helena Tomás, and Marcus Vinícius Lia Fook. 2021. "Surface Bioactivation of Polyether Ether Ketone (PEEK) by Sulfuric Acid and Piranha Solution: Influence of the Modification Route in Capacity for Inducing Cell Growth" Biomolecules 11, no. 9: 1260. https://doi.org/10.3390/biom11091260
APA Styledos Santos, F. S. F., Vieira, M., da Silva, H. N., Tomás, H., & Fook, M. V. L. (2021). Surface Bioactivation of Polyether Ether Ketone (PEEK) by Sulfuric Acid and Piranha Solution: Influence of the Modification Route in Capacity for Inducing Cell Growth. Biomolecules, 11(9), 1260. https://doi.org/10.3390/biom11091260








