Biological Treatments for Temporomandibular Joint Disc Disorders: Strategies in Tissue Engineering
Abstract
:1. Introduction
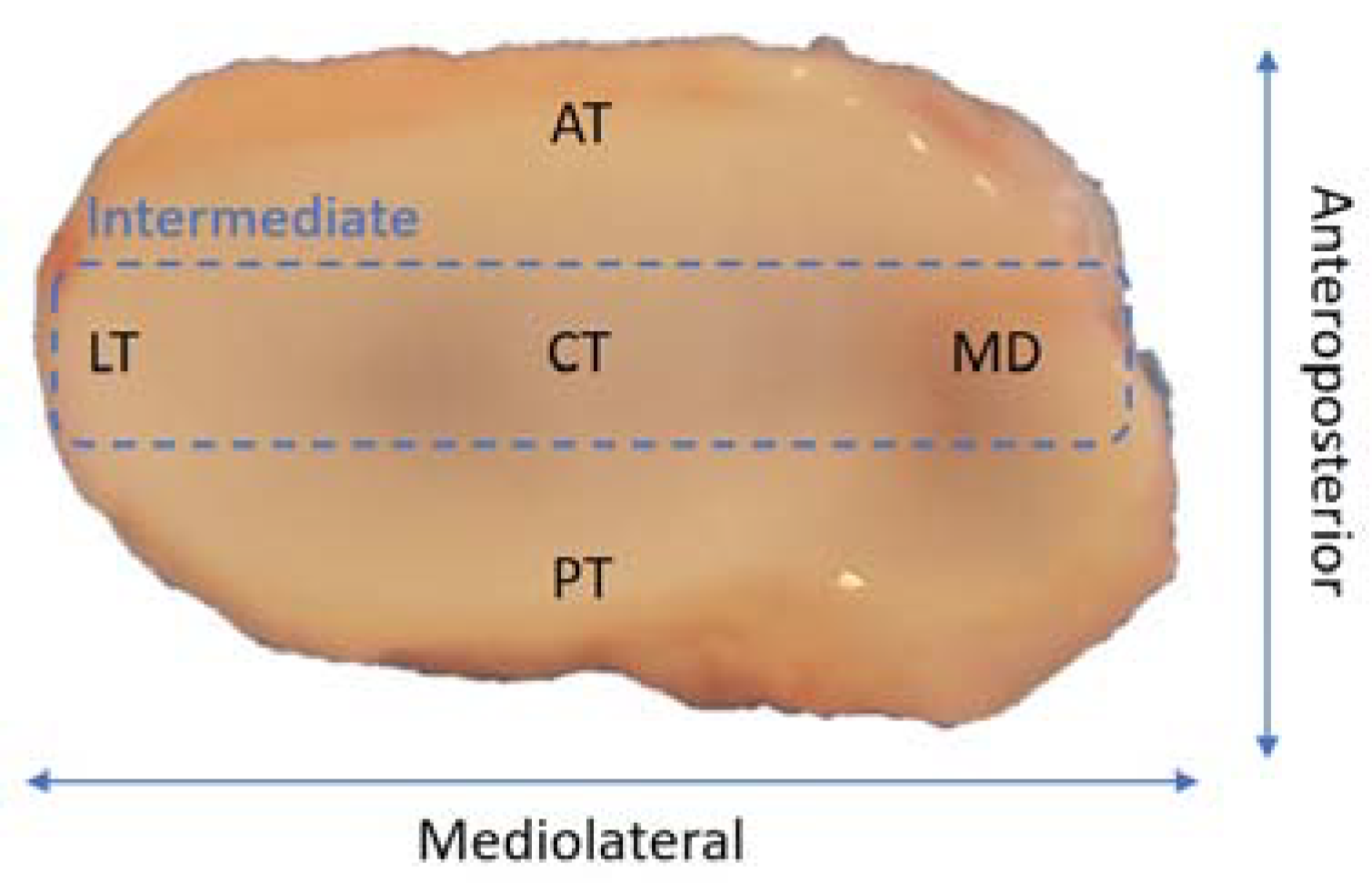
2. Temporomandibular Joint Disc Characterization
3. Temporomandibular Joint Disc Disorders
3.1. Disc Displacement
3.2. Disc Structural Changes
4. Temporomandibular Joint Disc Replacement Approaches
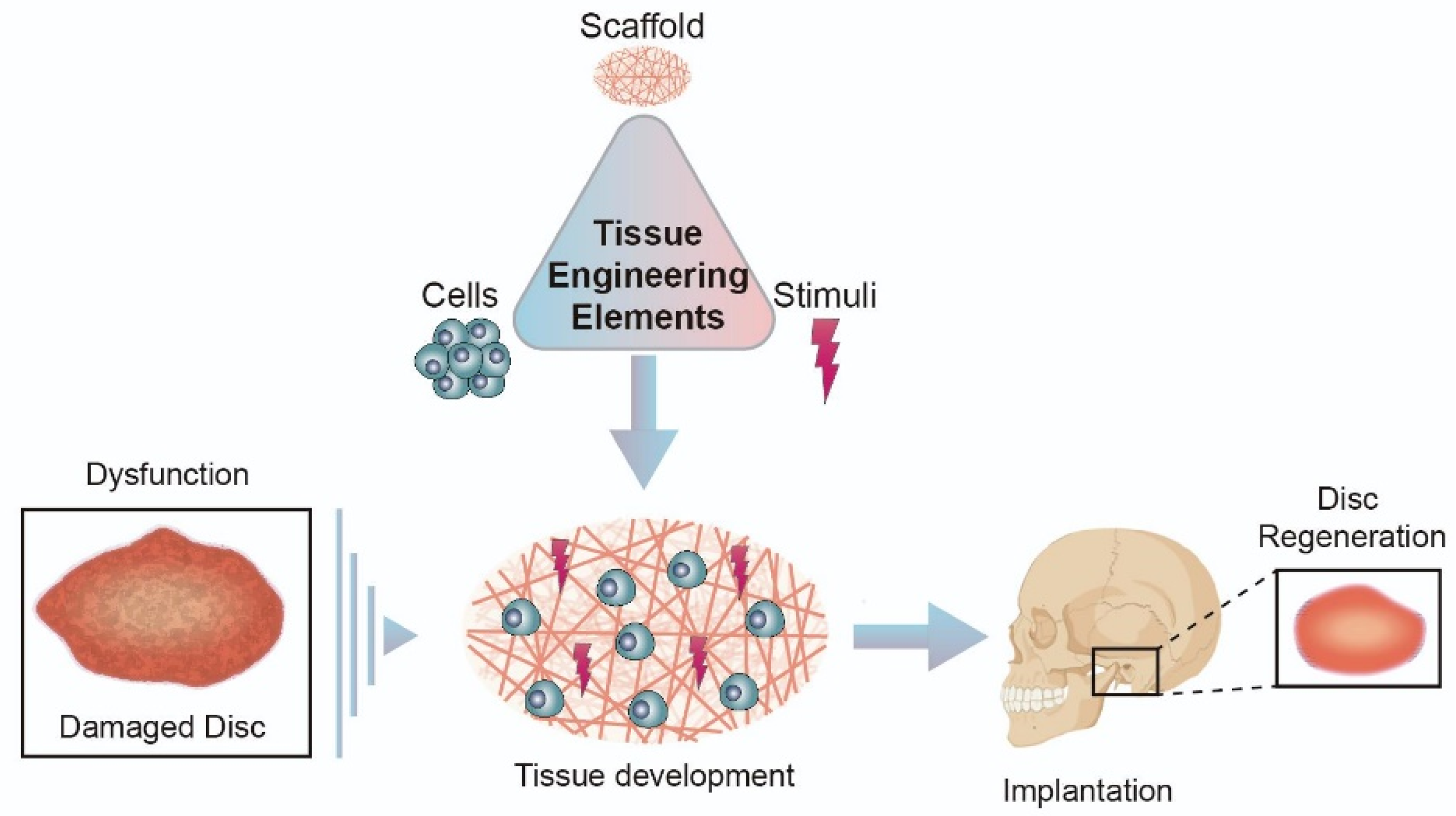
5. Approaches for Temporomandibular Joint Disc Substitution and Repair: Tissue Engineered Implants
5.1. Biomaterials in Disc Regeneration
5.1.1. Natural Biomaterials
5.1.2. Synthetic Biomaterials
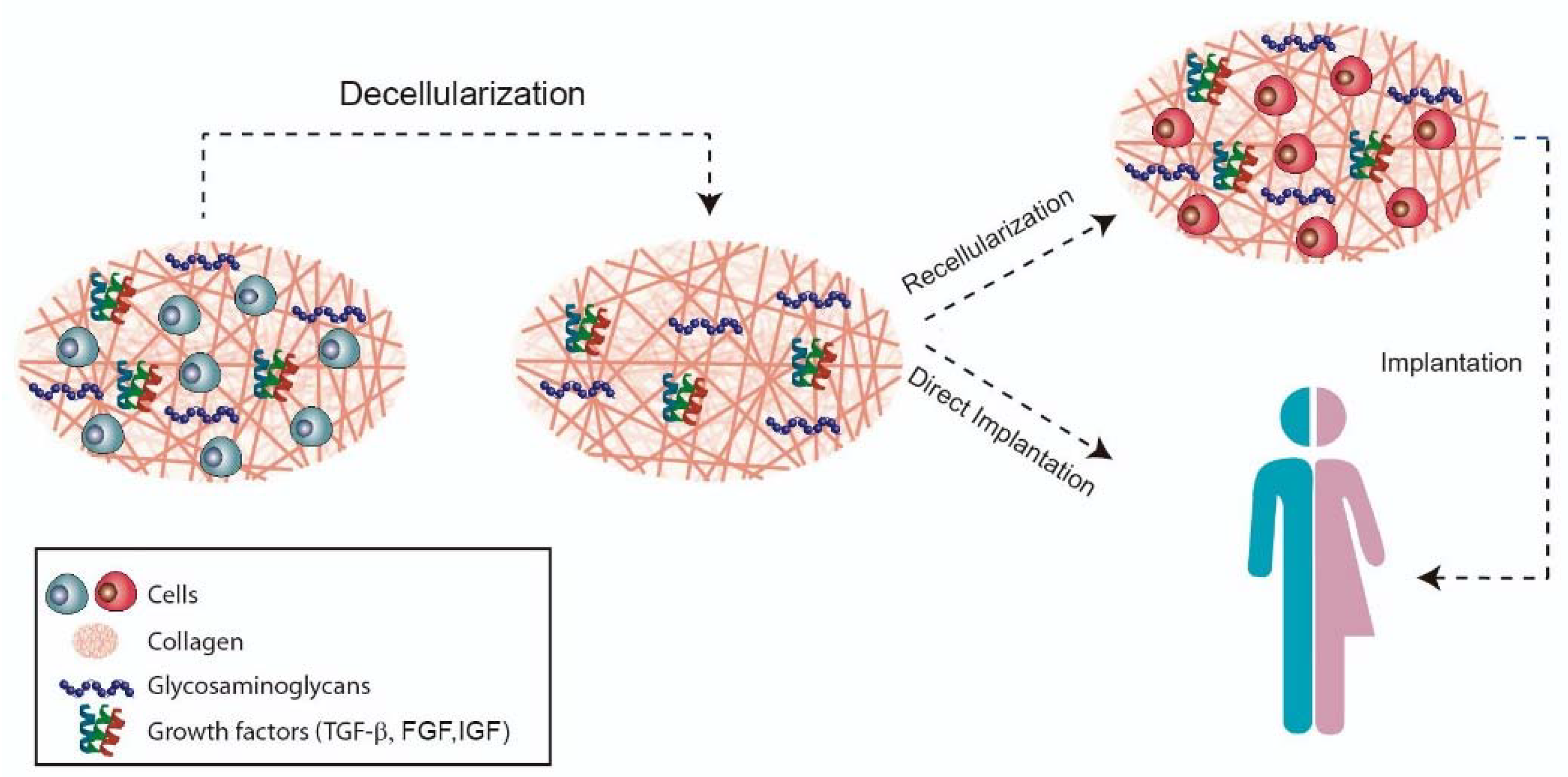
6. Forefront Approaches for Temporomandibular Disc Replacement: Native Decellularized Extracellular Matrices
7. Conclusion and Future Strategies
Author Contributions
Funding
Conflicts of Interest
References
- Riera-Punet, N.; Martinez-Gomis, J.; Willaert, E.; Povedano, M.; Peraire, M. Functional limitation of the masticatory system in patients with bulbar involvement in amyotrophic lateral sclerosis. J. Oral. Rehabil. 2017, 45, 204–210. [Google Scholar] [CrossRef]
- Hargitai, I.A.; Hawkins, J.M.; Ehrlich, A.D. The Temporomandibular Joint. In Temporomandibular Disorders; Gremillion, H.A., Klasse, G.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 91–107. [Google Scholar]
- Willard, V.P.; Zhang, L.G.; Athanasiou, K.A. Tissue Engineering of the Temporomandibular Joint. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 221–235. [Google Scholar]
- Demerjian, G.G.; Barkhordarian, A.; Chiappelli, F. Neuroanatomy of the Trigeminal Nerve and Proximal Innervation of the TMJ. In Temporomandibular Joint and Airway Disorders; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–15. [Google Scholar]
- Donahue, R.P.; Hu, J.C.; Athanasiou, K.A. Remaining Hurdles for Tissue-Engineering the Temporomandibular Joint Disc. Trends Mol. Med. 2019, 25, 241–256. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, G.; Gonçalves, D.; Conti, P. Musculoskeletal Disorders. Dent. Clin. North Am. 2018, 62, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.; Moseley, G.L.; Schiltenwolf, M.; Cashin, A.; Davies, M.; Hübscher, M. Exercise for chronic musculoskeletal pain: A biopsychosocial approach. Musculoskelet. Care 2017, 15, 413–421. [Google Scholar] [CrossRef]
- Cimmino, M.A.; Ferrone, C.; Cutolo, M. Epidemiology of chronic musculoskeletal pain. Best Pr. Res. Clin. Rheumatol. 2011, 25, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Sims, A.B.; Demerjian, G.G. Temporomandibular Joint Dysfunction, Trigeminal Nerve Inflammation, and Biomechanical Dental Treatments for the Suppression of Neurological and Neuropsychiatric Symptoms. In Temporomandibular Joint and Airway Disorders; Springer: Berlin/Heidelberg, Germany, 2018; pp. 95–123. [Google Scholar]
- Manfredini, D.; Guarda-Nardini, L.; Winocur, E.; Piccotti, F.; Ahlberg, J.; Lobbezoo, F. Research diagnostic criteria for temporomandibular disorders: A systematic review of axis I epidemiologic findings. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 112, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Armijo-Olivo, S.L.; Gadotti, I.C. Temporomandibular Disorders. In Pathology and Intervention in Musculoskeletal Rehabilitation; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015; Volume 3, pp. 119–156. [Google Scholar]
- National Institute of Dental and Craniofacial Research. Facial Pain; National Institute of Dental and Craniofacial Research: Bethesda, MD, USA, 2018.
- Alshaban, K.K.; Waheed, Z.G.A. Prevalence of TMJ Disorders among the Patients Attending the Dental Clinic of Ajman University of Science and Technology–Fujairah Campus, UAE. Int. J. Dent. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Van Bellinghen, X.; Idoux-Gillet, Y.; Pugliano, M.; Strub, M.; Bornert, F.; Clauss, F.; Schwinté, P.; Keller, L.; Benkirane-Jessel, N.; Kuchler-Bopp, S.; et al. Temporomandibular Joint Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 446. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Markiewicz, M.; Miloro, M.; Wolford, L.; Pogrel, M.A. Surgery of the Temporomandibular Joint: Discectomy and Arthroplasty. In Contemporary Management of Temporomandibular Disorders; Springer: Berlin/Heidelberg, Germany, 2019; pp. 107–127. [Google Scholar]
- Aryaei, A.; Vapniarsky, N.; Hu, J.C.; Athanasiou, K.A. Recent Tissue Engineering Advances for the Treatment of Temporomandibular Joint Disorders. Curr. Osteoporos. Rep. 2016, 14, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Ingawalé, S.; Goswami, T. Temporomandibular Joint: Disorders, Treatments, and Biomechanics. Ann. Biomed. Eng. 2009, 37, 976–996. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.K.; MacBarb, R.F.; Wong, M.E.; Athanasiou, K.A. Temporomandibular Joint Disorders: A Review of Etiology, Clinical Management, and Tissue Engineering Strategies. Int. J. Oral Maxillofac. Implant. 2013, 28, e393. [Google Scholar] [CrossRef] [Green Version]
- Ângelo, D.F. A Letter to the Editor on “Root of helix inter tragus notch incision (RHITNI) for temporomandibular open surgery. ” Int. J. Surg. 2020, 83, 233–234. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, Z.; He, D.; Hu, N.; Cheng, Z. The effect of TMJ disk repositioning by suturing through open incision on adolescent mandibular asymmetry with and without a functional orthodontic appliance. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 405–414. [Google Scholar] [CrossRef]
- Vapniarsky, N.; Huwe, L.W.; Arzi, B.; Houghton, M.K.; Wong, M.E.; Wilson, J.W.; Hatcher, D.C.; Hu, J.C.; Athanasiou, K.A. Tissue engineering toward temporomandibular joint disc regeneration. Sci. Transl. Med. 2018, 10, eaaq1802. [Google Scholar] [CrossRef] [Green Version]
- Dimitroulis, G. A critical review of interpositional grafts following temporomandibular joint discectomy with an overview of the dermis-fat graft. Int. J. Oral Maxillofac. Surg. 2011, 40, 561–568. [Google Scholar] [CrossRef]
- Wolford, L.; Pitta, M.; Reiche-Fischel, O.; Franco, P. TMJ Concepts/Techmedica custom-made TMJ total joint prosthesis: 5-year follow-up study. Int. J. Oral Maxillofac. Surg. 2003, 32, 268–274. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Ramamoorthi, M.; Wu, D.; Zhang, Z.; Tran, S.D. Scaffolds for temporomandibular joint disc engineering. In Handbook of Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 437–455. [Google Scholar]
- Gupta, S.K.; Mishra, N.C.; Dhasmana, A. Decellularization Methods for Scaffold Fabrication. In Breast Cancer; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–10. [Google Scholar]
- Palla, S. Anatomy and Pathophysiology of the Temporomandibular Joint. In Functional Occlusion in Restorative Dentistry and Prosthodontics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 67–85. [Google Scholar]
- Sakul, B.U.; Bilecenoglu, B.; Ocak, M. Anatomy of the Temporomandibular Joint. In Imaging of the Temporomandibular Joint; Springer: Berlin/Heidelberg, Germany, 2018; pp. 9–41. [Google Scholar]
- Scarr, G.; Harrison, H. Resolving the problems and controversies surrounding temporo-mandibular mechanics. J. Appl. Biomed. 2016, 14, 177–185. [Google Scholar] [CrossRef]
- Lowe, J.; Almarza, A.J. A review of in-vitro fibrocartilage tissue engineered therapies with a focus on the temporomandibular joint. Arch. Oral Biol. 2017, 83, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Karadede, B.; Karadede, B.; Karadede, M.I. Growth, Development, and Ossification of Mandible and Temporomandibular Joint. In Imaging of the Temporomandibular Joint; Springer: Berlin/Heidelberg, Germany, 2018; pp. 43–57. [Google Scholar]
- Asadi, H.; Budenz, A. Anatomy of the Masticatory System. In Temporomandibular Disorders; Springer: Berlin/Heidelberg, Germany, 2017; pp. 17–33. [Google Scholar]
- Trindade, D.; Moura, C.; Ângelo, D.; Alves, N. Functional Organisation and Associated Dysfunctions of the Temporomandibular Joint. In The Temporomandibular Joint: Structure, Function and Clinical Significance; Conrad, J., Ed.; Nova Medicine and Health: New York, NY, USA, 2020; pp. 1–42. [Google Scholar]
- Coombs, M.; Petersen, J.; Wright, G.; Lu, S.; Damon, B.; Yao, H. Structure-Function Relationships of Temporomandibular Retrodiscal Tissue. J. Dent. Res. 2017, 96, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Okeson, J.P. Functional Anatomy and Biomechanics of the Masticatory System. In Management of Temporo-Mandibular Disorders and Occlusion; Elsevier: Amsterdam, The Netherlands, 2019; pp. 2–20. [Google Scholar]
- von Arx, T.; Lozanoff, S. Temporomandibular Joint. In Clinical Oral Anatomy; Springer: Berlin/Heidelberg, Germany, 2017; pp. 525–534. [Google Scholar]
- Stocum, D.L.; Roberts, W.E. Part I: Development and Physiology of the Temporomandibular Joint. Curr. Osteoporos. Rep. 2018, 16, 360–368. [Google Scholar] [CrossRef]
- Kuo, J.; Zhang, L.; Bacro, T.; Yao, H. The region-dependent biphasic viscoelastic properties of human temporomandibular joint discs under confined compression. J. Biomech. 2010, 43, 1316–1321. [Google Scholar] [CrossRef] [Green Version]
- Shu, W.; Liu, L.; Bao, G.; Kang, H. Tissue Engineering of the Temporomandibular Joint Disc: Current Status and Future Trends. Int. J. Artif. Organs 2015, 38, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, S.; Ghazanfari, S.; Mirahmadi, F.; Everts, V.; Smit, T.H.; Koolstra, J.H. The dynamic mechanical viscoelastic properties of the temporomandibular joint disc: The role of collagen and elastin fibers from a perspective of polymer dynamics. J. Mech. Behav. Biomed. Mater. 2019, 100, 103406. [Google Scholar] [CrossRef] [PubMed]
- Acri, T.M.; Shin, K.; Seol, D.; Laird, N.Z.; Song, I.; Geary, S.M.; Chakka, J.L.; Martin, J.A.; Salem, A.K. Tissue Engineering for the Temporomandibular Joint. Adv. Healthc. Mater. 2019, 8, e1801236. [Google Scholar] [CrossRef]
- Gutman, S.; Kim, D.; Tarafder, S.; Velez, S.; Jeong, J.; Lee, C.H. Regionally variant collagen alignment correlates with viscoelastic properties of the disc of the human temporomandibular joint. Arch. Oral Biol. 2018, 86, 1–6. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Almarza, A.J.; Detamore, M.S.; Kalpakci, K.N. Tissue Engineering of Temporomandibular Joint Cartilage. Synth. Lect. Tissue Eng. 2009, 1, 1–122. [Google Scholar] [CrossRef]
- Tanaka, E.; Shibaguchi, T.; Tanaka, M.; Tanne, K. Viscoelastic properties of the human temporomandibular joint disc in patients with internal derangement. J. Oral Maxillofac. Surg. 2000, 58, 997–1002. [Google Scholar] [CrossRef]
- Cuccia, A.M.; Caradonna, C.; Caradonna, D. Manual Therapy of the Mandibular Accessory Ligaments for the Management of Temporomandibular Joint Disorders. J. Am. Osteopath. Assoc. 2011, 111, 102–112. [Google Scholar]
- Rabelo, K.A.; Melo, S.L.S.; Torres, M.G.G.; Peixoto, L.R.; Campos, P.S.F.; Rebello, I.M.C.R.; de Melo, D.P. Assessment of Condyle Position, Fossa Morphology, and Disk Displacement in Symptomatic Patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 199–207. [Google Scholar] [CrossRef]
- Hu, Y.K.; Abdelrehem, A.; Yang, C.; Cai, X.Y.; Xie, Q.Y.; Sah, M.K. Changes in temporomandibular joint spaces after arthroscopic disc repositioning: A self-control study. Sci. Rep. 2017, 7, srep45513. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Schiffman, E.L. Temporomandibular Joint Disorders and Orofacial Pain. Dent. Clin. N. Am. 2016, 60, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R. Executive summary of the Diagnostic Criteria for Temporomandibular Disorders for clinical and research applications. J. Am. Dent. Assoc. 2016, 147, 438–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.-S.; Jeon, D.-M.; Jung, W.-S.; Yang, I.-H.; Lim, W.H.; Ahn, S.-J. Influence of temporomandibular joint disc displacement on craniocervical posture and hyoid bone position. Am. J. Orthod. Dentofac. Orthop. 2015, 147, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.; Brown, Z.L.; Amarista, F.J.; Pham, M. Treatment of malocclusion after TMJ disc repositioning with Mitek anchors. A retrospective review. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Scrivani, S.J.; Keith, D.A.; Kaban, L.B. Temporomandibular Disorders. N. Engl. J. Med. 2008, 359, 2693–2705. [Google Scholar] [CrossRef]
- Bueno, C.H.; Pereira, D.D.; Pattussi, M.P.; Grossi, P.K.; Grossi, M.L. Gender differences in temporomandibular disorders in adult populational studies: A systematic review and meta-analysis. J. Oral Rehabil. 2018, 45, 720–729. [Google Scholar] [CrossRef]
- Incesu, L.; Taşkaya-Yılmaz, N.; Öğütcen-Toller, M.; Uzun, E. Relationship of condylar position to disc position and morphology. Eur. J. Radiol. 2004, 51, 269–273. [Google Scholar] [CrossRef]
- Katzberg, R.W.; Tallents, R.H. Normal and Abnormal Temporomandibular Joint Disc and Posterior Attachment as Depicted by Magnetic Resonance Imaging in Symptomatic and Asymptomatic Subjects. J. Oral Maxillofac. Surg. 2005, 63, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Orhan, K.; Nishiyama, H.; Tadashi, S.; Murakami, S.; Furukawa, S. Comparison of altered signal intensity, position, and morphology of the TMJ disc in MR images corrected for variations in surface coil sensitivity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.M.A.; Abdelrahman, T.E.F. MRI evaluation of TMJ internal derangement: Degree of anterior disc displacement correlated with other TMJ soft tissue and osseous abnormalities. Egypt. J. Radiol. Nucl. Med. 2014, 45, 735–744. [Google Scholar] [CrossRef]
- Taşkaya-Yılmaz, N.; Ögütcen-Toller, M. Magnetic resonance imaging evaluation of temporomandibular joint disc deformities in relation to type of disc displacement. J. Oral Maxillofac. Surg. 2001, 59, 860–865. [Google Scholar] [CrossRef]
- Bae, W.C.; Biswas, R.; Statum, S.; Sah, R.L.; Chung, C.B. Sensitivity of quantitative UTE MRI to the biomechanical property of the temporomandibular joint disc. Skelet. Radiol. 2014, 43, 1217–1223. [Google Scholar] [CrossRef] [Green Version]
- Ivkovic, N.; Racic, M. Structural and Functional Disorders of the Temporomandibular Joint (Internal Disorders). In Maxillofacial Surgery and Craniofacial Deformity–Practices and Updates; IntechOpen: London, UK, 2020. [Google Scholar]
- Machoň, V.; Šedý, J.; Klíma, K.; Hirjak, D.; Foltán, R. Arthroscopic lysis and lavage in patients with temporomandibular anterior disc displacement without reduction. Int. J. Oral Maxillofac. Surg. 2012, 41, 109–113. [Google Scholar] [CrossRef]
- Embree, M.C.; Iwaoka, G.M.; Kong, D.; Martin, B.N.; Patel, R.K.; Lee, A.; Nathan, J.M.; Eisig, S.B.; Safarov, A.; Koslovsky, D.; et al. Soft tissue ossification and condylar cartilage degeneration following TMJ disc perforation in a rabbit pilot study. Osteoarthr. Cartil. 2015, 23, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Guerra, M.F.; Rodríguez-Campo, F.J.; Hernandez, V.E.; Sánchez-Acedo, C.; Usandizaga, J.L.G.-D. Temporomandibular Joint Disc Perforation: Long-Term Results After Operative Arthroscopy. J. Oral Maxillofac. Surg. 2013, 71, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Zhang, S.Y.; Yang, C.; Chen, M.J.; Cai, X.Y.; Haddad, M.S.; Yun, B.; Chen, Z.Z. Correlation between disc displacements and locations of disc perforation in the temporomandibular joint. Dentomaxillofacial Radiol. 2010, 39, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Jeon, K.-J.; Kim, M.-G.; Park, K.-H.; Huh, J.-K. A nomogram for classification of temporomandibular joint disk perforation based on magnetic resonance imaging. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 682–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolwick, M.; Aufdemorte, T.B. Silicone-induced foreign body reaction and lymphadenopathy after temporomandibular joint arthroplasty. Oral Surg. Oral Med. Oral Pathol. 1985, 59, 449–452. [Google Scholar] [CrossRef]
- Kaplan, P.; Ruskin, J.; Tu, H.; Knibbe, M. Erosive arthritis of the temporomandibular joint caused by Teflon-Proplast implants: Plain film features. Am. J. Roentgenol. 1988, 151, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Hartman, L.C.; Bessette, R.W.; Baier, R.E.; Meyer, A.E.; Wirth, J. Silicone rubber temporomandibular joint (TMJ) meniscal replacements: Postimplant histopathologic and material evaluation. J. Biomed. Mater. Res. 1988, 22, 475–484. [Google Scholar] [CrossRef]
- Milam, S.B. Failed implants and multiple operations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1997, 83, 156–162. [Google Scholar] [CrossRef]
- Timmis, D.P.; Aragon, S.B.; van Sickels, J.E.; Aufdemorte, T.B. Comparative study of alloplastic materials for temporomandibular joint disc replacement in rabbits. J. Oral Maxillofac. Surg. 1986, 44, 541–554. [Google Scholar] [CrossRef]
- Tucker, M.R.; Burkes, E. Temporary silastic implantation following discectomy in the primate temporomandibular joint. J. Oral Maxillofac. Surg. 1989, 47, 1290–1295. [Google Scholar] [CrossRef]
- Heffez, L.; Mafee, M.F.; Rosenberg, H.; Langer, B. CT evaluation of TMJ disc replacement with a proplast-teflon laminate. J. Oral Maxillofac. Surg. 1987, 45, 657–665. [Google Scholar] [CrossRef]
- Salash, J.R.; Hossameldin, R.H.; Almarza, A.J.; Chou, J.C.; McCain, J.P.; Mercuri, L.G.; Wolford, L.M.; Detamore, M.S. Potential Indications for Tissue Engineering in Temporomandibular Joint Surgery. J. Oral Maxillofac. Surg. 2016, 74, 705–711. [Google Scholar] [CrossRef]
- Trumpy, I.G.; Roald, B.; Lyberg, T. Morphologic and immunohistochemical observation of explanted Proplast-Teflon temporomandibular joint interpositional implants. J. Oral Maxillofac. Surg. 1996, 54, 63–68. [Google Scholar] [CrossRef]
- Henry, C.; Wolford, L.M. Treatment outcomes for temporomandibular joint reconstruction after Proplast-Teflon implant failure. J. Oral Maxillofac. Surg. 1993, 51, 352–358. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Goodger, N.; Wang, J.; Smagalski, G.; Hepworth, B. Methylmethacrylate as a Space Maintainer in Mandibular Reconstruction. J. Oral Maxillofac. Surg. 2005, 63, 1048–1051. [Google Scholar] [CrossRef]
- Goode, R.L.; Reynolds, B.N. Tobramycin-Impregnated Methylmethacrylate for Mandible Reconstruction. Arch. Otolaryngol. Head Neck Surg. 1992, 118, 201–204. [Google Scholar] [CrossRef] [PubMed]
- De La Peña, A.; de La Peña-Brambila, J.; La Torre, J.P.-D.; Ochoa, M.; Gallardo, G.J. Low-cost customized cranioplasty using a 3D digital printing model: A case report. 3D Print. Med. 2018, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, A.; Mesnard, M. Christensen vs Biomet Microfixation alloplastic TMJ implant: Are there improvements? A numerical study. J. Cranio-Maxillofac. Surg. 2015, 43, 1398–1403. [Google Scholar] [CrossRef]
- Chase, D.C.; Hudson, J.-W.; Gerard, D.A.; Russell, R.; Chambers, K.; Curry, J.R.; Latta, J.E.; Christensen, R.W. The Christensen prosthesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1995, 80, 273–278. [Google Scholar] [CrossRef]
- Ramos, A.; Mesnard, M. Load transfer in Christensen® TMJ in alloplastic total joint replacement for two different mouth apertures. J. Cranio-Maxillofac. Surg. 2014, 42, 1442–1449. [Google Scholar] [CrossRef]
- Quinn, P.; Granquist, E.J. Stock Prostheses for Total Reconstruction of the Temporomandibular Joint. In Temporomandibular Joint Total Joint Replacement—TMJ TJR; Springer: Berlin/Heidelberg, Germany, 2016; pp. 69–90. [Google Scholar]
- Angelo, D.; Morouço, P.; Alves, N.; Viana, T.; Santos, F.; González, R.; Monje, F.; Macias, D.; Carrapiço, B.; Sousa, R.; et al. Choosing sheep (Ovis aries) as animal model for temporomandibular joint research: Morphological, histological and biomechanical characterization of the joint disc. Morphologie 2016, 100, 223–233. [Google Scholar] [CrossRef]
- Smith, J.A.; Sandler, N.; Ozaki, W.H.; Braun, T.W. Subjective and objective assessment of the temporalis myofascial flap in previously operated temporomandibular joints. J. Oral Maxillofac. Surg. 1999, 57, 1058–1065. [Google Scholar] [CrossRef]
- Matukas, V.J.; Lachner, J. The use of autologous auricular cartilage for temporomandibular joint disc replacement: A preliminary report. J. Oral Maxillofac. Surg. 1990, 48, 348–353. [Google Scholar] [CrossRef]
- Dimitroulis, G.; Slavin, J. Histological Evaluation of Full Thickness Skin as an Interpositional Graft in the Rabbit Craniomandibular Joint. J. Oral Maxillofac. Surg. 2006, 64, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A. The autogenous dermal graft in temporomandibular joint disc surgery. J. Oral Maxillofac. Surg. 1988, 46, 948–954. [Google Scholar] [CrossRef]
- Dimitroulis, G. The interpositional dermis-fat graft in the management of temporomandibular joint ankylosis. Int. J. Oral Maxillofac. Surg. 2004, 33, 755–760. [Google Scholar] [CrossRef]
- Thomas, M.; Grande, D.; Haug, R.H. Development of an in vitro temporomandibular joint cartilage analog. J. Oral Maxillofac. Surg. 1991, 49, 854–856. [Google Scholar] [CrossRef]
- Puelacher, W.C.; Wisser, J.; Vacanti, C.A.; Ferraro, N.F.; Jaramillo, D.; Vacanti, J.P. Temporomandibular joint disc replacement made by tissue-engineered growth of cartilage. J. Oral Maxillofac. Surg. 1994, 52, 1172–1177. [Google Scholar] [CrossRef]
- Juran, C.M.; Dolwick, M.F.; McFetridge, P.S. Engineered Microporosity: Enhancing the Early Regenerative Potential of Decellularized Temporomandibular Joint Discs. Tissue Eng. Part A 2015, 21, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Allen, K.D.; Athanasiou, K.A. Tissue Engineering of the TMJ Disc: A Review. Tissue Eng. 2006, 12, 1183–1196. [Google Scholar] [CrossRef]
- Chen, F.M.; Liu, X.H. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Armiento, A.; Stoddart, M.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Nasution, A.K.; Hermawan, H. Degradable Biomaterials for Temporary Medical Implants. In Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2016; pp. 127–160. [Google Scholar]
- Ramdan, R.D.; Sunendar, B.; Hermawan, H. Naturally Derived Biomaterials and Its Processing. In Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Manoukian, O.S.; Sardashti, N.; Stedman, T.; Gailiunas, K.; Ojha, A.; Penalosa, A.; Mancuso, C.; Hobert, M.; Kumbar, S.G. Biomaterials for Tissue Engineering and Regenerative Medicine. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 462–482. [Google Scholar]
- Cao, Z.; Dou, C.; Dong, S. Scaffolding Biomaterials for Cartilage Regeneration. J. Nanomater. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bousnaki, M.; Bakopoulou, A.; Papadogianni, D.; Barkoula, N.-M.; Alpantaki, K.; Kritis, A.; Chatzinikolaidou, M.; Koidis, P. Fibro/chondrogenic differentiation of dental stem cells into chitosan/alginate scaffolds towards temporomandibular joint disc regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 97. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Z.; Li, J.; Meng, Q.; Fang, W.; Long, X. The Pilot Study of Fibrin with Temporomandibular Joint Derived Synovial Stem Cells in Repairing TMJ Disc Perforation. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Kobayashi, E.; Nakahara, T.; Inoue, M.; Shigeno, K.; Tanaka, A.; Nakamura, T. Experimental Study on In Situ Tissue Engineering of the Temporomandibular Joint Disc using Autologous Bone Marrow and Collagen Sponge Scaffold. J. Hard Tissue Biol. 2015, 24, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Almarza, A.J.; Athanasiou, K.A. Seeding Techniques and Scaffolding Choice for Tissue Engineering of the Temporomandibular Joint Disk. Tissue Eng. 2004, 10, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Radke, D.; Jia, W.; Tahtinen, M.; Wang, G.; Zhao, F. Bioengineering Scaffolds for Regenerative Engineering. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 444–461. [Google Scholar]
- Grigore, M.E. Biomaterials for Cartilage Tissue Engineering. J. Tissue Sci. Eng. 2017, 8, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Mäenpää, K.; Ellä, V.; Mauno, J.; Kellomäki, M.; Suuronen, R.; Ylikomi, T.; Miettinen, S. Use of adipose stem cells and polylactide discs for tissue engineering of the temporomandibular joint disc. J. R. Soc. Interface 2009, 7, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Springer, I.N.; Fleiner, B.; Jepsen, S.; Açil, Y. Culture of cells gained from temporomandibular joint cartilage on non-absorbable scaffolds. Biomaterials 2001, 22, 2569–2577. [Google Scholar] [CrossRef]
- Almarza, A.J.; Athanasiou, K.A. Effects of Initial Cell Seeding Density for the Tissue Engineering of the Temporomandibular Joint Disc. Ann. Biomed. Eng. 2005, 33, 943–950. [Google Scholar] [CrossRef]
- Allen, K.D.; Athanasiou, K.A. Scaffold and Growth Factor Selection in Temporomandibular Joint Disc Engineering. J. Dent. Res. 2008, 87, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hagandora, C.K.; Gao, J.; Wang, Y.; Almarza, A.J. Poly (Glycerol Sebacate): A Novel Scaffold Material for Temporomandibular Joint Disc Engineering. Tissue Eng. Part A 2013, 19, 729–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legemate, K.; Tarafder, S.; Jun, Y.; Lee, C. Engineering Human TMJ Discs with Protein-Releasing 3D-Printed Scaffolds. J. Dent. Res. 2016, 95, 800–807. [Google Scholar] [CrossRef]
- Moura, C.; Trindade, D.; Vieira, M.; Francisco, L.; Ângelo, D.F.; Alves, N. Multi-Material Implants for Temporomandibular Joint Disc Repair: Tailored Additive Manufacturing Production. Front. Bioeng. Biotechnol. 2020, 8, 342. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, S.; Koerber, L.; Elsaesser, A.F.; Goldberg-Bockhorn, E.; Seitz, A.M.; Dürselen, L.; Ignatius, A.; Walther, P.; Breiter, R.; Rotter, N. Decellularized Cartilage Matrix as a Novel Biomatrix for Cartilage Tissue-Engineering Applications. Tissue Eng. Part A 2012, 18, 2195–2209. [Google Scholar] [CrossRef]
- Benders, K.E.; van Weeren, P.R.; Badylak, S.F.; Saris, D.B.; Dhert, W.; Malda, J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013, 31, 169–176. [Google Scholar] [CrossRef]
- Lumpkins, S.B.; Pierre, N.; McFetridge, P.S. A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater. 2008, 4, 808–816. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, B.N.; Chung, W.L.; Almarza, A.J.; Pavlick, M.D.; Reppas, S.N.; Ochs, M.W.; Russell, A.J.; Badylak, S.F. Inductive, Scaffold-Based, Regenerative Medicine Approach to Reconstruction of the Temporomandibular Joint Disk. J. Oral Maxillofac. Surg. 2012, 70, 2656–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, B.N.; Chung, W.L.; Pavlick, M.; Reppas, S.; Ochs, M.W.; Russell, A.J.; Badylak, S.F. Extracellular Matrix as an Inductive Template for Temporomandibular Joint Meniscus Reconstruction: A Pilot Study. J. Oral Maxillofac. Surg. 2011, 69, e488–e505. [Google Scholar] [CrossRef] [PubMed]
- Matuska, A.M.; Dolwick, M.F.; McFetridge, P.S. Approaches to improve integration and regeneration of an ex vivo derived temporomandibular joint disc scaffold with variable matrix composition. J. Mater. Sci. Mater. Med. 2018, 29, 152. [Google Scholar] [CrossRef] [PubMed]
- Matuska, A.M.; McFetridge, P.S. Laser micro-ablation of fibrocartilage tissue: Effects of tissue processing on porosity modification and mechanics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 1858–1868. [Google Scholar] [CrossRef]
- Liang, J.; Yi, P.; Wang, X.; Huang, F.; Luan, X.; Zhao, Z.; Liu, C. Acellular matrix hydrogel for repair of the temporomandibular joint disc. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2020, 108, 2995–3007. [Google Scholar] [CrossRef]
| Disc Displacement Types | Characterization | |
|---|---|---|
| Disc Displacement with Reduction (DDwR) | The articular disc is dislocated but able to return to its initial position with condyle translation | |
| Disc Displacement without Reduction (DDwoR) | With limited opening (DDwoRwLO) | The articular disc is locked, not being able to return to its initial position. Presents a restricted mouth opening |
| Without limited opening (DDwoRwoLO) | The articular disc is locked, not being able to return to its initial position. Does not present a restricted mouth opening | |
| Author | Material/Tissue | Fabrication/Decellularization Method | Cells/Growth Factors | Benefits | Limitations |
|---|---|---|---|---|---|
| Tissue Engineering: Natural Materials | |||||
| Thomas et al. [89] | Collagen | Photopolymerisation | Rabbit TMJ disc cells | Growth of a tissue analog in vitro | No fibrous matrix formation |
| Almarza & Athanasiou [103] | Alginate | Crosslinking with CaCl2 | Hogs TMJ disc cells | Cell migration into nodules in the first weeks of culture | No collagen or GAG formation; decrease in the cell population |
| Wu et al. [101] | Combination of fibrin and chitosan | Freeze-drying | Synovium derived MSCs | Fibrin improved cell seeding efficiency; ECM synthesis | Number of cells started to decrease after day 7 of cell seeding |
| Kobayashi et al. [102] | Collagen | Freeze-drying and thermal crosslinking | Bone marrow MSCs | Connective tissue formation | In vivo implantation in rabbits, that only present TMJ rotation movements |
| Bousnaki et al. [100] | Combination of chitosan and alginate | Crosslinking with CaCl2 | Dental pulp stem cells | Fibrocartilage markers expression; adequate mechanical properties | TMJ disc shape and biochemical components were not evaluated |
| Tissue Engineering: Synthetic Materials | |||||
| Puelacher et al. [90] | Combination of PLA and PGA fibers | Spraying fixing technique | Calves chondrocytes | Adequate mechanical properties | Hyaline cartilage formation |
| Springer et al. [107] | PA, ePTFE and PGA monofilaments | Plaiting | Human and porcine TMJ disc and articular eminence cells | Cell attachment to all scaffolds, independently of the cells | Collagen type II production |
| Almarza & Athanasiou [108] | PGA mesh | Not specified (purchased) | Hogs TMJ disc cells | Higher seeding cells results in increased matrix production | Decrease in cell population over the culture period; scaffolds decrease 50% in volume |
| Allen & Athanasiou [109] | PLLA mesh | Not specified (purchased) | Hogs TMJ disc cells and TGF- β1 | Scaffold volume maintained for 6 weeks; Growth factor incorporation yielded cells, collagen and GAG | Low mechanical properties |
| Mäenpää et al. [106] | PLA | Melt-spun | Adipose MSCs | Expression of aggrecan and collagen type I and II | Low degree of cells differentiation |
| Hagandora et al. [110] | PGS sheets | Particulate leaching | Goat TMJ disc cells | High ECM production | Non-homogeneous distribution of cells and matrix |
| Legemate et al. [111] | PCL | Fused deposition modelling | Bone marrow MSCs, CTGF and TGF- β3 | MSCs differentiation and viscoelastic properties are region-dependent | To validate this proposal in vivo and long-term scaffold degradation studies are required |
| Moura et al. [112] | Combination of PCL and PEGDA | Combination of fused deposition modelling and photopolymerisation | ___ | PEGDA as a hydrogel core presents adequate mechanical properties | To validate this proposal in vitro and in vivo studies are required |
| Decellularization | |||||
| Brown et al. [118,119] | Urinary bladder matrix (turned into powder) | 0.1% peracetic acid/4% ethanol | ___ | In vivo test led to tissue formation | Rapid degradation; Lacks histological analysis of the bony structures |
| Lumpkins et al. [116] | Porcine TMJ disc | 1% (w/v) SDS | ___ | Maintained mechanical properties; Cell removal | Collagen fiber compaction; no biochemical quantification |
| Juran et al. [91] | Porcine TMJ disc | 1% (w/v) SDS, lyophilization, rehydration and micropatterning | Umbilical cord MSCs | Cell removal; cell integration and remodelling | no biochemical quantification; Low mechanical properties |
| Matuska et al. [120] | Porcine TMJ disc | 0.1% (w/v) SDS and 2:1 solution of chloroform/methanol | Umbilical cord MSCs | Cell and lipid removal; No citotoxicity | Mechanical properties of the reagents were only assessed separately; no biochemical quantification |
| Matuska & McFetridge [121] | Porcine TMJ disc | 0.1% (w/v) SDS and micropatterning | ___ | Cell removal; Minimal collagen lost | No GAG quantification; in vitro studies were not performed to evaluate the micropatterning |
| Liang et al. [122] | Porcine TMJ disc (solubilised and processed into hydrogel) | Freeze-thaw, 1% Triton X-100, Tris–HCL, trypsin and nucleases | Rabbit chondrocytes | Cell removal; Good injectability and degrability; hydrogel with nanofibrous structure | Sulfated GAG reduction; in vivo inflammation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trindade, D.; Cordeiro, R.; José, H.C.; Ângelo, D.F.; Alves, N.; Moura, C. Biological Treatments for Temporomandibular Joint Disc Disorders: Strategies in Tissue Engineering. Biomolecules 2021, 11, 933. https://doi.org/10.3390/biom11070933
Trindade D, Cordeiro R, José HC, Ângelo DF, Alves N, Moura C. Biological Treatments for Temporomandibular Joint Disc Disorders: Strategies in Tissue Engineering. Biomolecules. 2021; 11(7):933. https://doi.org/10.3390/biom11070933
Chicago/Turabian StyleTrindade, Daniela, Rachel Cordeiro, Henrique Cardoso José, David Faustino Ângelo, Nuno Alves, and Carla Moura. 2021. "Biological Treatments for Temporomandibular Joint Disc Disorders: Strategies in Tissue Engineering" Biomolecules 11, no. 7: 933. https://doi.org/10.3390/biom11070933
APA StyleTrindade, D., Cordeiro, R., José, H. C., Ângelo, D. F., Alves, N., & Moura, C. (2021). Biological Treatments for Temporomandibular Joint Disc Disorders: Strategies in Tissue Engineering. Biomolecules, 11(7), 933. https://doi.org/10.3390/biom11070933








