Screening for Fatal Traumatic Brain Injuries in Cerebrospinal Fluid Using Blood-Validated CK and CK–MB Immunoassays
Abstract
:1. Introduction
2. Materials and Methods
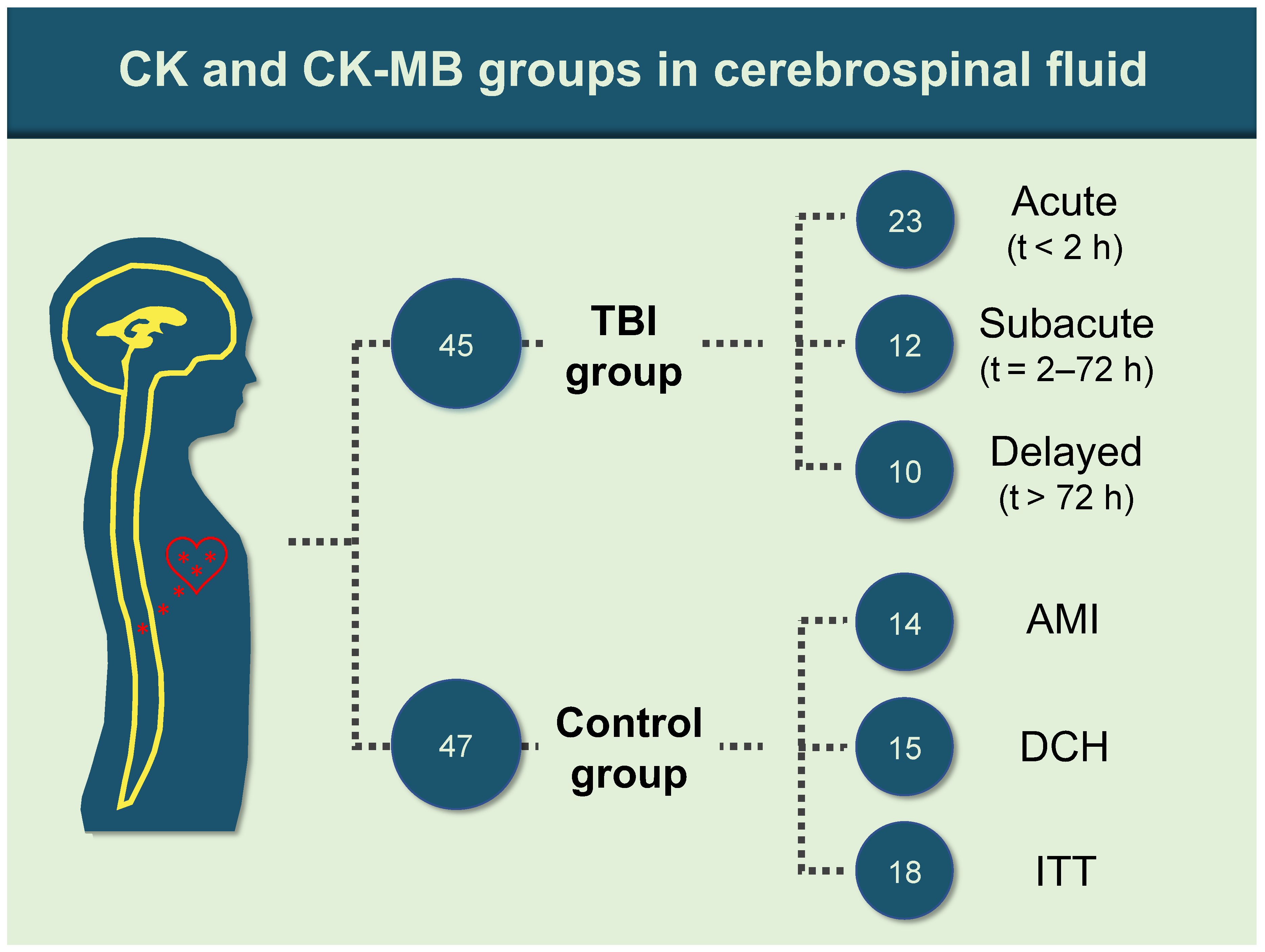
2.1. Sample Retrieval
2.2. Laboratory Procedures
2.3. Statistical Analysis
3. Results
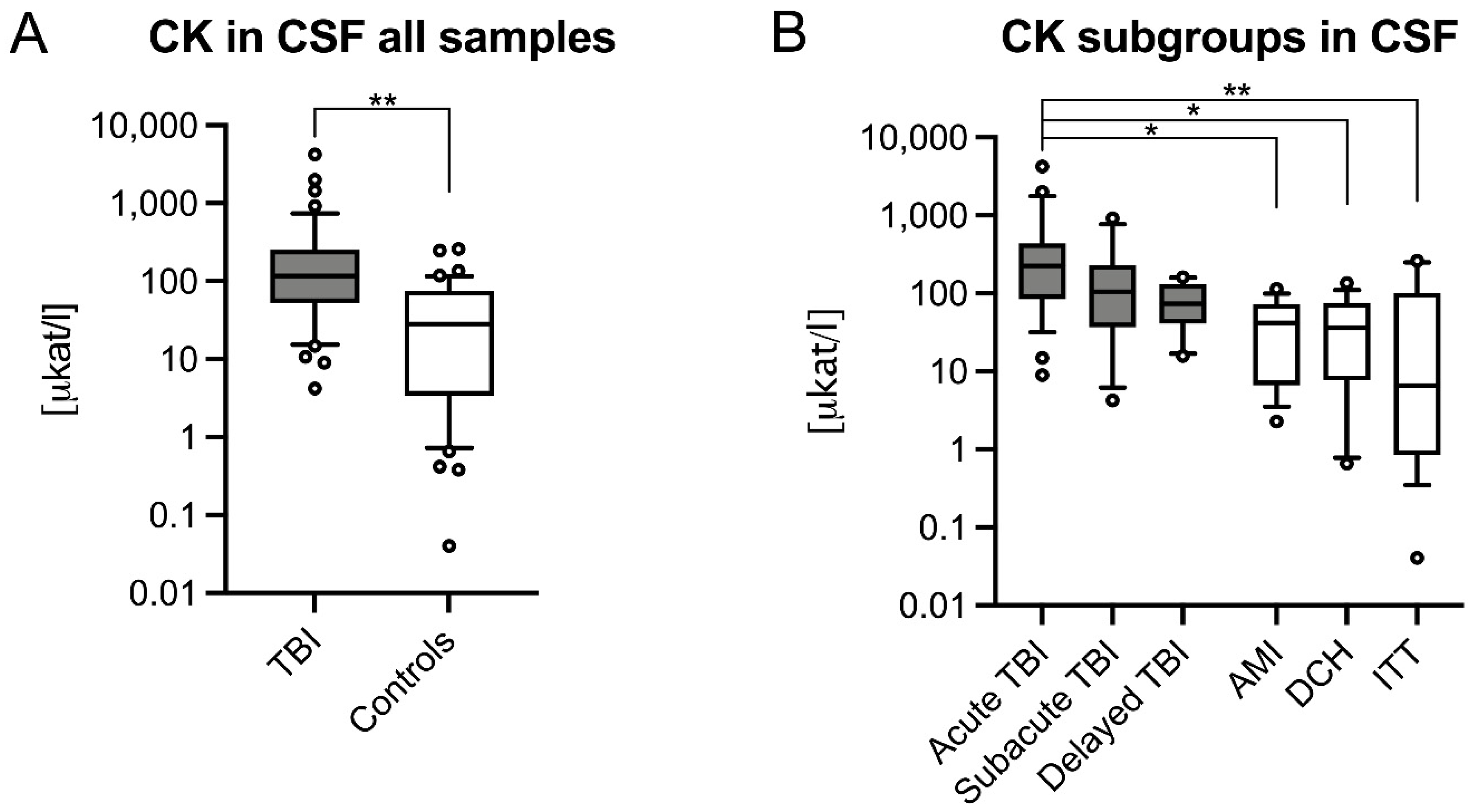
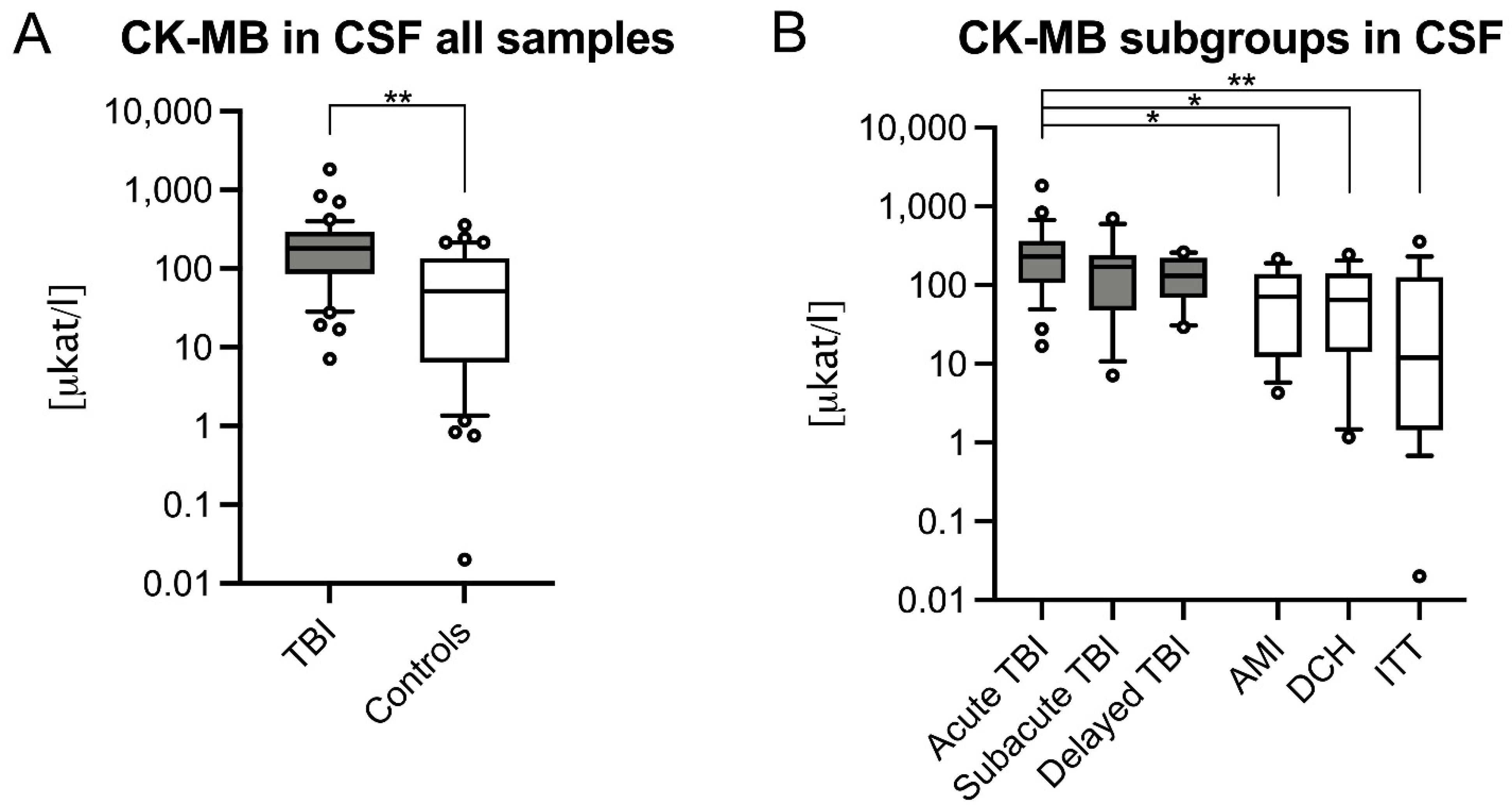
3.1. CSF CK and CK–MB Levels Were Significantly Higher in Acute TBIs
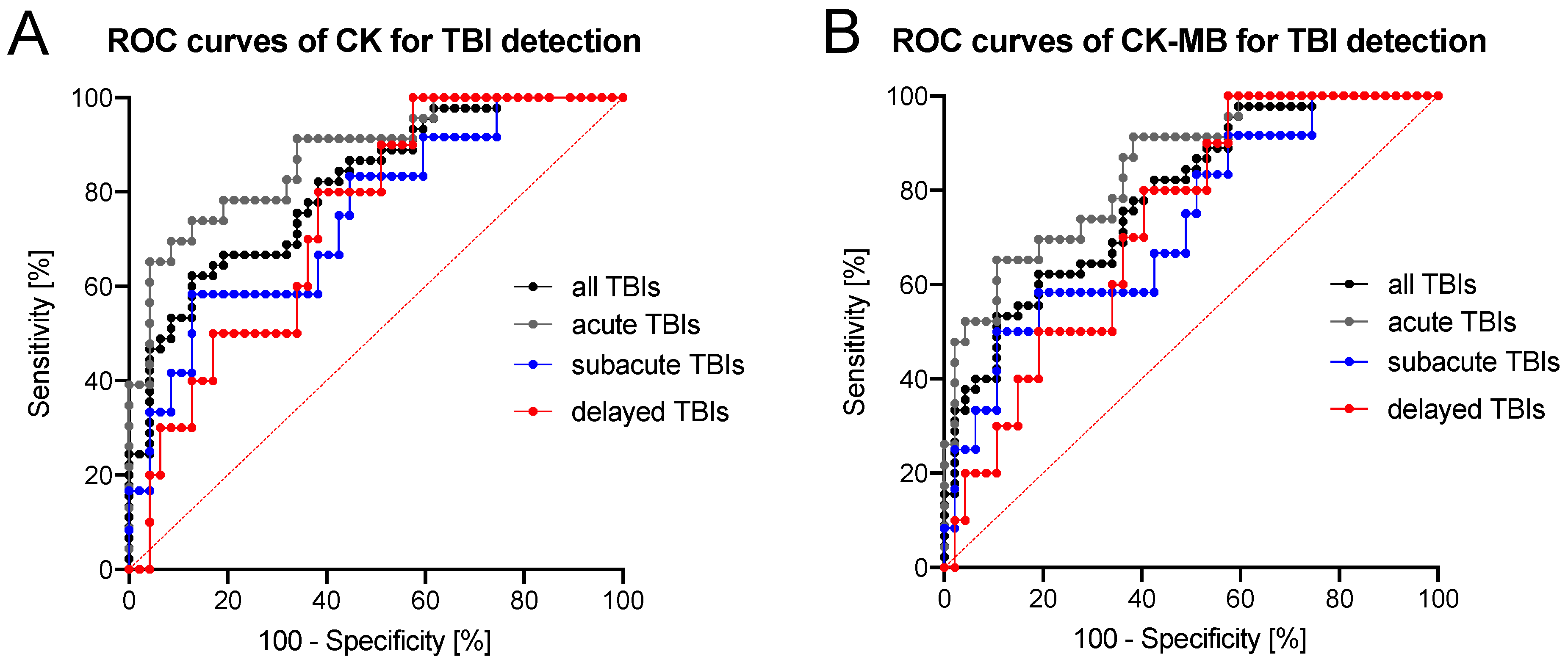
3.2. Diagnostic Accuracy of CK/CK–MB Levels in CSF Is Moderate to High
3.3. Quality Control of Samples and Subgroups with Bivariate Analysis Results
4. Discussion
4.1. Effects of Increased CK Influx into the CSF from Outside the CNS Following Severe TBI
4.2. Increase of CK Is Likely Caused by CK–BB Isoenzyme
4.3. CSF Test Results Are Independent of the Presence of Blood or Contamination
4.4. For TBI Cases, CK Levels in CSF Are Useful for Cause of Death Determinations and Survival Time Estimations but Not for Time since Death Estimations
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Maeda, H.; Zhu, B.-L.; Ishikawa, T.; Quan, L.; Michiue, T. Significance of postmortem biochemistry in determining the cause of death. Leg. Med. 2009, 11, S46–S49. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ishida, K.; Li, Q.; Taniguchi, M.; Oritani, S.; Li, D.R.; Fujita, M.Q.; Maeda, H. Postmortem serum uric acid and creatinine levels in relation to the causes of death. J. Forensic Sci. 2002, 125, 59–66. [Google Scholar] [CrossRef]
- Ondruschka, B.; Pohlers, D.; Sommer, G.; Schober, K.; Teupser, D.; Franke, H.; Dressler, J. S100B and NSE as Useful Postmortem Biochemical Markers of Traumatic Brain Injury in Autopsy Cases. J. Neurotrauma 2013, 30, 1862–1871. [Google Scholar] [CrossRef]
- Ondruschka, B.; Schuch, S.; Pohlers, D.; Franke, H.; Dreßler, J. Acute phase response after fatal traumatic brain injury. Int. J. Leg. Med. 2018, 132, 531–539. [Google Scholar] [CrossRef]
- Ondruschka, B.; Sieber, M.; Kirsten, H.; Franke, H.; Dreßler, J.; Dressler, J. Measurement of Cerebral Biomarkers Proving Traumatic Brain Injuries in Post-Mortem Body Fluids. J. Neurotrauma 2018, 35, 2044–2055. [Google Scholar] [CrossRef]
- Peyron, P.-A.; Lehmann, S.; Delaby, C.; Baccino, E.; Hirtz, C. Biochemical markers of time since death in cerebrospinal fluid: A first step towards “Forensomics”. Crit. Rev. Clin. Lab. Sci. 2019, 56, 274–286. [Google Scholar] [CrossRef]
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef] [Green Version]
- Woodcock, T.; Morganti-Kossmann, M.C. The Role of Markers of Inflammation in Traumatic Brain Injury. Front. Neurol. 2013, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zetterberg, H.; Blennow, K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat. Rev. Neurol. 2016, 12, 563–574. [Google Scholar] [CrossRef]
- Ferreira, L.C.B.; Regner, A.; Miotto, K.D.L.; De Moura, S.; Ikuta, N.; Vargas-Seymour, A.; Chies, J.; Simon, D. Increased levels of interleukin-6, -8 and -10 are associated with fatal outcome following severe traumatic brain injury. Brain Inj. 2014, 28, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Lustenberger, T.; Kern, M.; Relja, B.; Wutzler, S.; Störmann, P.; Marzi, I. The effect of brain injury on the inflammatory response following severe trauma. Immunobiology 2016, 221, 427–431. [Google Scholar] [CrossRef]
- Case, M.E. Inflicted Traumatic Brain Injury in Infants and Young Children. Brain Pathol. 2008, 18, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Hellewell, S.C.; Ziebell, J.; Lifshitz, J.; Morganti-Kossmann, C. Impact Acceleration Model of Diffuse Traumatic Brain Injury. Methods Mol. Biol. 2016, 1462, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.T.; Freeman, J.R.; Broshek, N.K.; Varney, R.N. Acceleration-Deceleration Sport-Related Concussion: The Gravity of It All. J. Athl. Train. 2001, 36, 253–256. [Google Scholar] [PubMed]
- Zwirner, J.; Bohnert, S.; Franke, H.; Garland, J.; Hammer, N.; Möbius, D.; Tse, R.; Ondruschka, B. IL-6 and GFAP as a compelling biomarker combination to detect lethal acute traumatic brain injuries in cerebrospinal fluid. In submission.
- Aydin, S.; Ugur, K.; Aydin, S.; Sahin, I.; Yardim, M. Biomarkers in acute myocardial infarction: Current perspectives. Vasc. Health Risk Manag. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabow, L.; DeSalles, A.A.F.; Becker, D.P.; Yang, M.; Kontos, H.A.; Ward, J.D.; Moulton, R.J.; Clifton, G.; Gruemer, H.D.; Muizelaar, J.P.; et al. CSF brain creatine kinase levels and lactic acidosis in severe head injury. J. Neurosurg. 1986, 65, 625–629. [Google Scholar] [CrossRef]
- Vazquez, M.D.; Sánchez-Rodríguez, F.; Osuna, E.; Diaz, J.; Cox, D.E.; Pérez-Cárceles, M.D.; Martinez, P.; Luna, A.; Pounder, D.J. Creatine Kinase BB and Neuron-Specific Enolase in Cerebrospinal Fluid in the Diagnosis of Brain Insult. Am. J. Forensic Med. Pathol. 1995, 16, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Kaste, M.; Hernesniemi, J.; Somer, H.; Hillbom, M.; Konttinen, A. Creatine kinase isoenzymes in acute brain injury. J. Neurosurg. 1981, 55, 511–515. [Google Scholar] [CrossRef]
- Coplin, W.M.; Longstreth, W.T.; Lam, A.M.; Chandler, W.L.; Mayberg, T.S.; Fine, J.S.; Winn, H.R. Cerebrospinal fluid creatine kinase-BB isoenzyme activity and outcome after subarachnoid hemorrhage. Arch. Neurol. 1999, 56, 1348–1352. [Google Scholar] [CrossRef] [Green Version]
- Tirschwell, D.L.; Longstreth, W.T.; Rauch-Matthews, M.E.; Chandler, W.L.; Rothstein, T.; Wray, L.; Eng, L.J.; Fine, J.; Copass, M.K. Cerebrospinal fluid creatine kinase BB isoenzyme activity and neurologic prognosis after cardiac arrest. Neurology 1997, 48, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Osuna, E.; Pérez-Cárceles, M.; Luna, A.; Pounder, D. Efficacy of cerebro-spinal fluid biochemistry in the diagnosis of brain insult. Forensic Sci. Int. 1992, 52, 193–198. [Google Scholar] [CrossRef]
- Hackenberry, L.; Miner, M.; Rea, G.L.; Woo, J.; Graham, S.H.; Hackenberry, L.; Miner, M.; Rea, G.L.; Woo, J.; Graham, S.H. Biochemical evidence of myocardial injury after severe head trauma. Crit. Care Med. 1982, 10, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Woydt, L.; Bernhard, M.; Kirsten, H.; Burkhardt, R.; Hammer, N.; Gries, A.; Dreßler, J.; Ondruschka, B. Intra-individual alterations of serum markers routinely used in forensic pathology depending on increasing post-mortem interval. Sci. Rep. 2018, 8, 12811. [Google Scholar] [CrossRef] [PubMed]
- Palmiere, C.; Mangin, P. Postmortem chemistry update part II. Int. J. Leg. Med. 2012, 126, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.-L.; Ishikawa, T.; Michiue, T.; Li, D.-R.; Zhao, N.; Bessho, Y.; Kamikodai, Y.; Tsuda, K.; Okazaki, S.; Maeda, H. Postmortem cardiac troponin I and creatine kinase MB levels in the blood and pericardial fluid as markers of myocardial damage in medicolegal autopsy. Leg. Med. 2007, 9, 241–250. [Google Scholar] [CrossRef]
- Bañón, R.; Hernández-Romero, D.; Navarro, E.; Pérez-Cárceles, M.D.; Noguera-Velasco, J.A.; Osuna, E. Combined determination of B-type natriuretic peptide and high-sensitivity troponin I in the postmortem diagnosis of cardiac disease. Forensic Sci. Med. Pathol. 2019, 15, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Inamori-Kawamoto, O.; Michiue, T.; Ikeda, S.; Ishikawa, T.; Maeda, H. Cardiac biomarkers in blood, and pericardial and cerebrospinal fluids of forensic autopsy cases: A reassessment with special regard to postmortem interval. Leg. Med. 2015, 17, 343–350. [Google Scholar] [CrossRef]
- Roche Diagnostics; CK–MB: Mannheim, Germany, 2009.
- Wang, Q.; Michiue, T.; Ishikawa, T.; Zhu, B.-L.; Maeda, H. Combined analyses of creatine kinase MB, cardiac troponin I and myoglobin in pericardial and cerebrospinal fluids to investigate myocardial and skeletal muscle injury in medicolegal autopsy cases. Leg. Med. 2011, 13, 226–232. [Google Scholar] [CrossRef]
- Chandler, W.L.; Clayson, K.J.; Longstreth, W.T.; Fine, J.S. Creatine kinase isoenzymes in human cerebrospinal fluid and brain. Clin. Chem. 1984, 30, 1804–1806. [Google Scholar] [CrossRef]
- Chandler, W.L.; Clayson, K.J.; Longstreth, W.T.; Fine, J.S. Mitochondrial and MB Isoenzymes of Creatine Kinase in Cerebrospinal Fluid from Patients with Hypoxic–Ischemic Brain Damage. Am. J. Clin. Pathol. 1986, 86, 533–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chodobski, A.; Zink, B.J.; Szmydynger-Chodobska, J. Blood–Brain Barrier Pathophysiology in Traumatic Brain Injury. Transl. Stroke Res. 2011, 2, 492–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindblad, C.; Nelson, D.W.; Zeiler, F.; Ercole, A.; Ghatan, P.H.; Von Horn, H.; Risling, M.; Svensson, M.; Agoston, D.V.; Bellander, B.-M.; et al. Influence of Blood–Brain Barrier Integrity on Brain Protein Biomarker Clearance in Severe Traumatic Brain Injury: A Longitudinal Prospective Study. J. Neurotrauma 2020, 37, 1381–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, A.; Sukheeja, D.; Trikha, V.; Pandey, A.K.; Albert, V.; Pandey, R.M. Evaluation of Serum Creatine Kinase and Urinary Myoglobin as Markers in Detecting Development of Acute Renal Failure in Severely Injured Trauma Patients. ISRN Emerg. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cabaniss, C.D. Creatine Kinase. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Thompson, R.; Kynoch, P.A.; Sarjant, J. Immunohistochemical localization of creatine kinase-BB isoenzyme to astrocytes in human brain. Brain Res. 1980, 201, 423–426. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.I.; Dontas, I.A.; Kotsarelis, D.V.; Julien, G.; Karayannacos, P.E.; Diakolios, C.E.; Skalkeas, G.D. Creatine Kinase (CK–BB) Determination in Cerebrospinal Fluid After Acute Experimental Head Injury. Br. J. Neurosurg. 1988, 2, 169–172. [Google Scholar] [CrossRef]
- Hans, P.; Born, J.D.; Chapelle, J.-P.; Milbouw, G. Creatine kinase isoenzymes in severe head injury. J. Neurosurg. 1983, 58, 689–692. [Google Scholar] [CrossRef]
- Zarghami, N.; Yu, H.; Diamandis, E.; Sutherland, D.J. Quantification of creatine kinase BB isoenzyme in tumor cytosols and serum with an ultrasensitive time-resolved immunofluorometric technique. Clin. Biochem. 1995, 28, 243–253. [Google Scholar] [CrossRef]
- McDonald, S.; Sharkey, J.M.; Sun, M.; Kaukas, L.M.; Shultz, S.R.; Turner, R.J.; Leonard, A.V.; Brady, R.D.; Corrigan, F. Beyond the Brain: Peripheral Interactions after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Karkela, J.T. Critical evaluation of postmortem changes in human autopsy cisternal fluid. Enzymes, electrolytes, acid-base balance, glucose and glycolysis, free amino acids and ammonia: Correlation to total brain ischemia. J. Forensic Sci. 1993, 38, 603–616. [Google Scholar] [CrossRef]
| TBI (All) | TBI (<2 h) | TBI (2–72 h) | TBI (>72 h) | Control (All) | AMI | DCH | ITT | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 60 (32.5) | 56 (34) | 63 (21.5) | 52.5 (48.25) | 59 (37) | 75 (25.5) | 53 (30) | 51 (49.5) |
| mbrain (g) | 1440 (310) | 1440 (240) | 1423 (259) | 1558 (407) | 1350 (225) | 1340 (193) | 1400 (190) | 1340 (260) |
| PMI (hours) | 57 (52.5) | 49 (39) | 68.5 (75.8) | 88.5 (88.3) | 52 (39) | 57 (19) | 53 (33) | 21.5 (55.25) |
| Males: Females | 39:6 | 19:4 | 10:2 | 10:0 | 30:17 | 10:4 | 10:5 | 10:8 |
| Group | AUC | 95% Confidence Interval | Threshold Value (μkat/l) | Sensitivity: Specificity Ratio (%) | Positive Likelihood Ratio |
| CK in Cerebrospinal Fluid | |||||
| All TBIs | 0.811 | 0.726–0.897 | 137.2 | 46.7:95.7 | 10.97 |
| Acute TBIs | 0.876 | 0.789–0.963 | 137.2 | 65.2:95.7 | 15.33 |
| Subacute TBIs | 0.748 | 0.593–0.904 | 171.0 | 33.3:95.7 | 7.83 |
| Delayed TBIs | 0.738 | 0.592–0.884 | 123.6 | 30.0:93.6 | 4.70 |
| CK–MB in Cerebrospinal Fluid | |||||
| All TBIs | 0.788 | 0.698–0.878 | 246.4 | 33.3:97.9 | 15.67 |
| Acute TBIs | 0.846 | 0.751–0.940 | 261.8 | 47.8:97.9 | 22.48 |
| Subacute TBIs | 0.729 | 0.569–0.888 | 246.4 | 25.0:97.9 | 11.75 |
| Delayed TBIs | 0.728 | 0.581–0.875 | 229.0 | 20.0:95.7 | 4.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwirner, J.; Anders, S.; Bohnert, S.; Burkhardt, R.; Da Broi, U.; Hammer, N.; Pohlers, D.; Tse, R.; Ondruschka, B. Screening for Fatal Traumatic Brain Injuries in Cerebrospinal Fluid Using Blood-Validated CK and CK–MB Immunoassays. Biomolecules 2021, 11, 1061. https://doi.org/10.3390/biom11071061
Zwirner J, Anders S, Bohnert S, Burkhardt R, Da Broi U, Hammer N, Pohlers D, Tse R, Ondruschka B. Screening for Fatal Traumatic Brain Injuries in Cerebrospinal Fluid Using Blood-Validated CK and CK–MB Immunoassays. Biomolecules. 2021; 11(7):1061. https://doi.org/10.3390/biom11071061
Chicago/Turabian StyleZwirner, Johann, Sven Anders, Simone Bohnert, Ralph Burkhardt, Ugo Da Broi, Niels Hammer, Dirk Pohlers, Rexson Tse, and Benjamin Ondruschka. 2021. "Screening for Fatal Traumatic Brain Injuries in Cerebrospinal Fluid Using Blood-Validated CK and CK–MB Immunoassays" Biomolecules 11, no. 7: 1061. https://doi.org/10.3390/biom11071061
APA StyleZwirner, J., Anders, S., Bohnert, S., Burkhardt, R., Da Broi, U., Hammer, N., Pohlers, D., Tse, R., & Ondruschka, B. (2021). Screening for Fatal Traumatic Brain Injuries in Cerebrospinal Fluid Using Blood-Validated CK and CK–MB Immunoassays. Biomolecules, 11(7), 1061. https://doi.org/10.3390/biom11071061






