Abstract
Calcium (Ca2+) homeostasis is vital for insect development and metabolism, and the endoplasmic reticulum (ER) is a major intracellular reservoir for Ca2+. The inositol 1,4,5- triphosphate receptor (IP3R) and ryanodine receptor (RyR) are large homotetrameric channels associated with the ER and serve as two major actors in ER-derived Ca2+ supply. Most of the knowledge on these receptors derives from mammalian systems that possess three genes for each receptor. These studies have inspired work on synonymous receptors in insects, which encode a single IP3R and RyR. In the current review, we focus on a fundamental, common question: “why do insect cells possess two Ca2+ channel receptors in the ER?”. Through a comparative approach, this review covers the discovery of RyRs and IP3Rs, examines their structures/functions, the pathways that they interact with, and their potential as target sites in pest control. Although insects RyRs and IP3Rs share structural similarities, they are phylogenetically distinct, have their own structural organization, regulatory mechanisms, and expression patterns, which explains their functional distinction. Nevertheless, both have great potential as target sites in pest control, with RyRs currently being targeted by commercial insecticide, the diamides.
1. Introduction
Calcium (Ca2+) is a key second messenger that plays important roles in numerous cellular and physiological processes, including cell motility, membrane transport processes, gene expression and regulation, nuclear pore regulation, vesicle fusion, neurotransmission, muscle contraction, hormone biosynthesis, and apoptosis [1]. Similar to other animals, Ca2+ is also essential for insects [2] where it is involved in development and metamorphosis [3], reproduction [4], sex pheromone synthesis [5], cold sensing [6], neurotransmitter release [7], olfactory responses [8], carbohydrate [9] and lipid metabolism [10], and diapause [11]. Due to these essential roles, it is critical to maintain cellular Ca2+ homeostasis [12].
In animal cells, Ca2+ homeostasis is coordinated through channels, transporters and pumps located in the plasma membrane, the endoplasmic reticulum (ER) [13], as well as other organelles, such as the Golgi apparatus [14], mitochondria [15], and lysosomes [16]. Calcium binding proteins in the cytosol or organelles are also involved in the maintenance of Ca2+ levels by functioning as calcium buffers [10,11]. Extracellular Ca2+ concentrations are relatively high (1–2 mM), while the cytoplasm of most cells contains much lower resting Ca2+ concentrations (in the 100 nM range) [17]. Calcium entry via the plasma membrane is a major route to supply Ca2+ needed for the cell; however, cellular organelles, in particular the ER (sarcoplasmic reticulum—SR for muscle cells) (100–500 μM), supply Ca2+ and trigger Ca2+ signals rapidly when the intracellular levels of Ca2+ are low [17]. This occurs through the activation of intracellular Ca2+ channels associated with the ER. The two major Ca2+ release channels are the inositol 1,4,5-trisphosphate receptor (IP3R), activated by the secondary messenger inositol 1,4,5-trisphosphate (IP3), Ca2+, and the ryanodine receptor (RyR), named after its high affinity for the plant alkaloid ryanodine, which is mainly activated by Ca2+ and possibly by other secondary messengers [18,19,20,21,22]. The IP3R and RyR are both members of a family of tetrameric intracellular Ca2+-release channels and are encoded by single genes in insects, whereas humans possess three IP3R (IP3R1–3) and RyR (RyR1–3) genes with distinct tissue expression patterns and subcellular localization. Both receptors activate Ca2+ release from the ER/SR to the cytosol or other organelles; therefore, they serve as major links between extra- and intracellular stimuli, leading to regulation of various cellular processes [13,21]. It is noteworthy that they can also be associated with mitochondria [23,24,25] or membrane contact sites [26,27].
It is an ongoing question as to why animals possess two similar biochemical tools (RyR and IP3R) associated with the ER for the coordination of intracellular Ca2+ homeostasis [28]. Studies on the structure and localization of these channels together with expression, mutation, recombination, and functional genomic studies have provided important clues in distinguishing the functional attributes of RyR or IP3R channels in mammalian models. The two receptors also share structural and functional features in insects. Studies on insect IP3Rs and RyRs have been limited but have increased significantly in the last decade. Cloning of the genes encoding these receptors together with structural and functional analyses have provided important insights into our understanding of the role of these receptors in intracellular Ca2+ homeostasis, lipid metabolism, muscle function, neuronal signaling in relation to photoreceptors, olfaction, locomotor activities, and development in insects. The discovery of the diamide group of insecticides, which selectively target insect RyRs and affect Ca2+ homeostasis, has focused attention on these receptors and IP3Rs. In the current review, we first introduce the RyRs and IP3Rs from mammalian models that inspired the discovery of their insect counterparts (Section 2). We then present insect IP3Rs and RyRs from a comparative perspective according to their structure (Section 3), their involvement in the Ca2+ metabolic pathways (Section 4), functions (Section 5), and their potential as targets in pest control (Section 6).
2. Discovery of RyRs and IP3Rs
The first RyR gene (RyR1) was first isolated from rabbit skeletal muscle [29], followed by isolation of the rabbit cardiac muscle isoform (RyR2) [30]. A third isoform (RyR3), distinct from both the skeletal and cardiac muscle isoforms, was isolated from rabbit brain [31]. In contrast to mammals, insect genomes encode only one RyR. The first insect RyR was identified from Drosophila melanogaster (Diptera: Drosophilidae) [32,33]. The D. melanogaster RyR shows approximately 45%–47% amino acid identity with the three mammalian RyRs. RyRs have since been identified from the lepidopterans Heliothis virescens (Noctuidae) [34,35], Bombyx mori (Bombycidae) [36], Cnaphalocrocis medinalis (Crambidae) [37], Plutella xylostella (Plutellidae) [38,39], Ostrinia furnacalis (Crambidae) [40], Helicoverpa armigera (Noctuidae) [41], Pieris rapae (Pieridae) [42], Chilo suppressalis (Crambidae) [43,44], Spodoptera exigua (Noctuidae) [45], Grapholita molesta (Tortricidae) [46], Tuta absoluta (Gelechiidae) [47], and S. frugiperda [48], the dipteran Bactrocera dorsalis (Tephritidae) [49], the coleopterans Tribolium castaneum (Tenebrionidae) [50] and Leptinotarsa decemlineata (Chrysomelidae) [51], and the hemipterans Laodelphax striatellus (Delphacidae) [43], Bemisia tabaci (Aleyrodidae) [43], Nilaparvata lugens (Delphacidae) [52], Sogatella furcifera (Delphacidae) [53], Myzus persicae (Aphididae) [54], Toxoptera citricida (Aphididae) [55], Dialeurodes citri (Aleyrodidae) [56] (Table 1).
The IP3R was first purified from rat cerebellum [57] and the gene encoding the first isoform (IP3R1) cloned from mouse cerebellum tissues [58]. This was followed by cloning of the IP3R2 isoform from rat brain [59] and IP3R3 from a rat insulinoma cell line [60]. Not surprisingly, the first insect IP3R was also identified from D. melanogaster [32,61]. The D. melanogaster IP3R has approximately 60% amino acid identity with the three mammalian IP3Rs, indicating a closer relatedness between mammalian and insect IP3Rs than to RyRs [32,61]. Compared to insect RyRs, an only limited number of studies on the identification of insect IP3Rs are available. IP3Rs have been identified from the coleopterans T. castaneum [50] and L. decemlineata [Doğan and Toprak, unpublished], from the hemipterans B. tabaci [62] and M. persicae [63] and the hymeopteran Bombus terrestris (Apidae) [63] (Table 1).

Table 1.
Insect ryanodine receptors (RyRs) and inositol triphosphate receptors (IP3Rs) identified to date.
Table 1.
Insect ryanodine receptors (RyRs) and inositol triphosphate receptors (IP3Rs) identified to date.
| Receptor | Species | Amino Acid (residue) | cDNA Size (bp) | Molecular Weight (kDa) | Reference |
|---|---|---|---|---|---|
| RyRs | Lepidoptera | ||||
| Bombyx mori (Bombycidae) | 5084 | 15,255 * | 575 | [36] | |
| Cnaphalocrocis medinalis (Crambidae) | 5087 | 15,773 | 574 | [37] | |
| Plutella xylostella (Plutellidae) | 5123 | 15,748 | 579 | [38] | |
| 5164 | 16,113 | 584 | [39] | ||
| Ostrinia furnacalis (Crambidae) | 5108 | 16,211 | 577 | [40] | |
| Helicoverpa armigera (Noctuidae) | 5142 | 16,083 | 581 | [41] | |
| Pieris rapae (Pieridae) | 5107 | 15,540 | 578 | [42] | |
| Chilo suppressalis (Crambidae) | 5133 | 16,392 | 581 | [43] | |
| 5133 | 16,102 | 581 | [44] | ||
| 5128 | 15,402 | 580 | [64] | ||
| Spodoptera exigua (Noctuidae) | 5118 | 15,748 | 579 | [45] | |
| Grapholita molesta (Tortricidae) | 5133 | 16,299 | 580 | [46] | |
| Tuta absoluta (Gelechiidae) | 5121 | 16,431 | 579 | [47] | |
| Spodoptera frugiperda | 5109 | 15,330 | 578 | [48] | |
| Diptera | |||||
| Drosophila melanogaster (Drosophilidae) | 5134 | 15,405 * | 581 | [65] | |
| Bactrocera dorsalis (Tephritidae) | 5140 | 15,750 | 582 | [49] | |
| Coleoptera | |||||
| Tribolium castaneum (Tenebrionidae) | 5094 | 15,308 | 577 | [50] | |
| Leptinotarsa decemlineata (Chrysomelidae) | 5128 | 15,792 | 582 | [51] | |
| Hemiptera | |||||
| Laodelphax striatellus (Delphacidae) | 5115 | 15,910 | 579 | [43] | |
| Bemisia tabaci (Aleyrodidae) | 5139 | 15,763 | 581 | [43] | |
| Nilaparvata lugens (Delphacidae) | 5140 | 15,735 | 581 | [52] | |
| Sogatella furcifera (Delphacidae) | 5128 | 15,985 | 579 | [53] | |
| Myzus persicae (Aphididae) | 5101 | 15,306 * | 580 | [54] | |
| Toxoptera citricida (Aphididae) | 5101 | 15,639 | 580 | [55] | |
| Dialeurodes citri (Aleyrodidae) | 5126 | 15,538 | 579 | [56] | |
| IP3Rs | Diptera | ||||
| Drosophila melanogaster (Drosophilidae) | 2833 | 9558 | 319 | [61] | |
| Coleoptera | |||||
| Tribolium castaneum (Tenebrionidae) | 2724 | 8175 * | 309 | [50] | |
| Leptinotarsa decemlineata (Chrysomelidae) | 2736 | 8211 * | 312 | Doğan and Toprak, unpublished | |
| Hemiptera | |||||
| Bemisia tabaci (Aleyrodidae) | 2733 | 8202 * | 311 | [62] | |
| Myzus persicae (Aphididae) | 3790 | 11,373 * | - | [63] | |
| Hymenoptera | |||||
| Bombus terrestris (Apidae) | 2727 | 10,966 | 309 | [63] | |
* Translated region.
3. Structure of RyRs and IP3Rs
Both RyRs and IP3Rs are members of the voltage-sensitive ion channel (VIC) superfamily and form homomeric tetramers resembling a square mushroom. In mammalian RyRs, each monomer (~5000 amino acids) has a molecular weight of around 550–580 kDa, while each IP3R monomer (~2700 amino acids) has a molecular weight of around 260 kDa [22,66,67]. Several high-resolution structures of mammalian RyR [68,69,70,71,72,73] and IP3R domains [28,74,75,76,77,78] have been determined by X-ray crystallography, NMR, and cryogenic electron microscopy. RyRs and IP3Rs share 30–35% homology at the amino acid level and primarily consist of a large, N-terminal, hydrophilic domain (a.k.a. the “foot structure”), a dissimilar central modulatory domain, and a small, conserved, C-terminal domain with 6 transmembrane regions forming the Ca2+ conducting channel pore [73,79,80] (Table 2). Notably, the large N-terminal hydrophilic domain and the small C-terminal hydrophilic domains both face the cytoplasm. The N-terminal domain of IP3R forms the binding pocket for the native ligand IP3 and includes three subdomains, the IP3-binding core β (IBC-β) and α (IBC-α) which interact with IP3, and the suppressor (inhibitory) domain (SD) which reduces the affinity for IP3 [81,82,83,84,85]. Notably, IP3Rs without an SD bind IP3 with high affinity, but do not release Ca2+, suggesting the SD is essential for IP3-induced channel gating [82,84,86]. RyRs, although N-terminal domain does not bind IP3, have a similar arrangement as the N-terminal domain of IP3R and includes three subdomains termed A, B and C corresponding to the SD, IBC-β and IBC-α, respectively [28,87]. These lead to modulation of the gating of the Ca2+ pore that occurs between the fifth and sixth transmembrane segments in the carboxy-terminal domain [81,88]. The structural domains common to both RyRs and IP3Rs in mammalians are the MIR (Mannosyltransferase, IP3R and RyR, pfam02815), RIH (RyR and IP3R Homology, pfam01365), and RIH-associated (pfam08454) domains [89] (Table 2). However, repeats termed the “SPRY domain (pfam00622)”, originally identified from Dictyostelium discoideum tyrosine kinase spore lysis A and the mammalian RyRs, and the “RyR domain (pfam02026)” are unique to RyRs [71,90,91,92]. The MIR domain is proposed to have a ligand transferase function [93], while the RIH domain might form the IP3 binding site together with the MIR domain in IP3Rs [94]. On the other hand, SPRY domains are typically known to mediate protein-protein interactions [95,96], while the function of RyR domain is unknown. The ryanodine-binding site is also localized to the carboxy terminus of both proteins within or close to the pore region [97]. Notably, the primary Ca2+ binding protein, calmodulin, interacts with RyRs in lipid bilayers [98] and binds to the RyR channel cytoplasmic assembly around 10 nm from the putative entrance to the transmembrane pore [99,100,101]. The N-terminal ligand-binding region of IP3R1 contains a calmodulin-binding domain that binds calmodulin independently of Ca2+ and mediates the inhibition of IP3 binding to IP3R1 [102].

Table 2.
Comparison of structural and functional features of mammalian and insect RyR and IP3Rs.
Insect RyRs are commonly composed of 5084–5164 residues with a molecular weight of 574–582 kDa. Crystal structures of the P. xylostella RyR N-terminal domain [103], Repeat34 domain [104] and SPRY2 domain [105], and the N-terminal domain of Apis mellifera RyR [106] are the only ones available. Therefore, the entire structural domain organization and key regions of insect RyRs are based on limited X-ray crystallography predictions and comparative modeling studies using the mammalian counterparts [107]. These studies revealed that the basic structure of insect RyRs is similar to their mammalian counterparts (Table 2). Insect RyRs are commonly composed of a large amino-terminal region including a MIR domain, two RIH domains, three SPRY domains, four RyR repeat domains, one RIH-associated domain, and a carboxy-terminal region including six transmembrane domains and two calcium-binding EF-hand domains [49,50,53,55,56] (Figure 1). Recently, Lin et al. [107] generated multiple structural models of P. xylostella RyR based on the rabbit RyR1 cryo-EM structure. This revealed that PxRyR is highly modular and consists of 20 individual domains, including 3 N-terminal domains, 3 SPRY domains, 3 insect divergent regions (IDR), 2 RYR repeat domains, 3 solenoid [SOL] domains, a shell-core linker peptide (SCLP) domain, an EF-hand domain (EF1&2), a thumb and forefinger (TaF) domain, a pseudo voltage-sensor domain (pVSD), a pore-forming (PF) domain and a C-terminal domain (CTD) with six transmembrane helices. There is evidence indicating the N-terminal cytoplasmic domain modulates the gating of the channel pore located in the C-terminus similar to that in mammalian RyRs [49,53,56,103,106]. The proposed pore (loop), including the characteristic “GXRXGGGXGD” motif [108], is located between the C-terminal helices 5 and 6 [37,39,41,109]. Notably, the loop is proposed to act as a selectivity filter for ions in both mammalian RyRs and IP3Rs, suggesting it also likely to enable the channels to discriminate between ions in insects. It is also worth noting that mutagenesis of residues in this region of both RyR and IP3R impairs channel conductance in mammalians [108,110,111]. Residues I5023, R5039, and D5043 (numbering based on P. xylostella RyR- GenBank accession number AET09964) [39] between TM5 and TM6 are conserved in insect RyRs [46,49,50,55,56] and the corresponding residues (I4897, R4913, and D4917) in rabbit RyR1 play role in the activity and conductance of the Ca2+ release channel [30,112]. A glutamate residue proposed to be involved in Ca2+ sensitivity in rabbit RyR1 (E4032) [113] and RyR3 (E3885) [114] is also conserved in insect RyRs (E4201 in PxRyR) [46,50]. The lepidopteran RyRs show sequence divergence from other insect RyRs in the carboxy-terminal region, especially in the region proximal to the pore-forming segment [37]. Lepidopterans differ from the non-lepidopteran RyRs at 9 conserved positions: Q4594, I4790, N4999, N5001, N5012, L5027, L5058, N5090, and T5141 (numbering based on P. xylostella RyR) [37,39,41,115,116]. Four of these (N4999, N5001, N5012, L5027) are clustered near the pore-forming segment, while L5058 is located in transmembrane helix 6 [37,39,41] and corresponds to I4862 in the mouse RyR2, which plays a crucial role in RyR channel activation and gating [117]. Additionally, 8 of the 9 conserved residues (except Q4594 corresponding to K4536 in DmRyR, GenBank accession number NP_476991) corresponding to M4748, D4957, K4959, H4970, I4985, I5016, G5048 and Q5099, respectively, in D. melanogaster RyR are also conserved amongst non-lepidopteran or invertebrate RyRs [37]. Notably, Q4594 is located in the insect divergent region (IDR) with several different amino acids being found at this position, but mostly lysine in Coleoptera, Hymenoptera, and some Diptera [63]. These residues might be involved in differences in channel properties between lepidopteran and non-lepidopteran insect RyRs and in the species with selective toxicity of diamide insecticides [37,41,116]; for further discussion see Section 6. However, the divergence is similar to the two mammalian divergent regions, DR1 and DR2 [118]. The two regions in insect RyRs also exhibit lower similarities to each other and have been defined as insect divergent region 1 (IDR1, amino acids located at 1299–1522 in L. decemlineata RyR) and 2 (IDR2, amino acids located at 4395–4721) [41,51,52]. These regions might also be involved in the distinct channel properties of insect RyR isoforms [51]. In contrast, the two EF-hand Ca2+ binding motifs originally reported in the lobster RyR [119] are conserved in the carboxy-terminus of insect RyRs (4250–4261 and 4285–4296 in P. xylostella RyR) [39]. However, the structural model of PxRyR by Lin et al. [107] revealed that the Ca2+ is coordinated by the negatively charged side chains of E4062 and E4136 in the RIH-associated domain, and the backbone carbonyl of T5127 in the C-terminal domain. A relatively recent study on mammalian cardiac RyR2 revealed that the EF-hand domain was not necessary for cytosolic Ca2+ activation but required for ER Ca2+ [120]. Nevertheless, EF-hand motifs are required for regulation of RyRs by calmodulin [121]. Although this topic requires investigation in insects, binding sites of calmodulin in rabbit RyR1 have already been detected (amino acid positions 3614–3643) [122], and putative corresponding sites have been proposed for insect RyRs (e.g., amino acid positions 3756–3785 in LdRyR) [51].
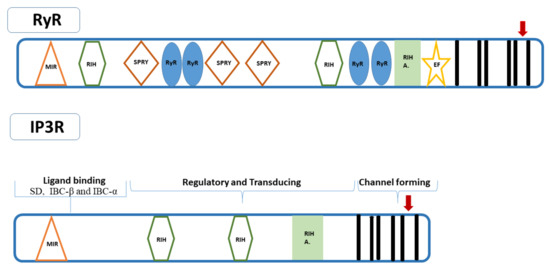
Figure 1.
The conserved domains for RyR are listed as following MIR (Mannosyltransferase, IP3R, and RyR, pfam02815), RIH (RyR and IP3R Homology, pfam01365), the SPRY (spIA and RyR domains, pfam00622), RyR domain (pfam02026) [71,90,91,92], RIH A domains (RIH-associated, pfam08454) [89], EF-hands, and putative transmembrane domain (TM1-TM6). IP3R has three putative functional regions: ligand binding, central regulatory, and channel forming sites. Ligand binding region includes three subdomains, the IP3-binding core β (IBC-β) and α (IBC-α) that interact with IP3; and the suppressor domain (SD) reducing the affinity for IP3 [81,82,83,84,85]. The conserved domains for IP3R are listed as following MIR RIH, RIH A, and TM1-TM6. Arrow corresponding to TM5 and TM6 including the suppressor domain and ligand binding, which leads to modulation of the gating of the Ca2+ pore in both channels.
Insect IP3Rs are commonly composed of 2724–2833 residues with a molecular weight of 309–319 kDa (Table 1). No study has examined the crystal structures of insect IP3Rs yet. Therefore, the entire structural domain organization and key regions of insect RyRs are based on the predictions of sequence features and comparisons with their mammalian counterparts. Nevertheless, predictions on the structural domain organization of IP3Rs reveal differences and are limited to the IP3Rs from D. melanogaster [61,83], T. castaneum [50], and B. tabaci [62] (Figure 1). The D. melanogaster IP3R is composed of a middle-coupling domain (N651-W2359), a putative Ca2+-sensor region (G1986-S2354), and a carboxy-terminal channel-forming domain (S2360-Q2829) with six transmembrane domains (TM1-TM6) and a pore-forming region [83]. The B. tabaci IP3R contains an inositol 1,4,5-trisphosphate/ryanodine receptor domain (residues 6–229), three MIR domains (residues 116–168, 298–333 and 237–420), two RIH domains (residues 460–664 and 1185–1366), a RIH-associated domain (residues 1918–2037), an oligosaccharide repeat unit polymerase domain (residues 2234–2450), an identity helices domain (residues 4925–5060), and a Sec2p domain (residues 2669–2708) [62]. Troczka et al. [63] conducted a pfam search of conserved domains from insect IP3Rs which revealed the presence of six domains, including an IP3 binding region, a MIR domain, two RIH domains, a RIH-associated domain, and the transmembrane ion transport domain. The MIR, RIH, RIH-associated regulatory domains at the amino terminus, together with the six transmembrane helices including the GXRXGGGXGD selectivity motif between TM5 and TM6 in the carboxy terminal region, appear to be common to both insect IP3Rs and RyRs [50], similar to the mammalian RyRs and IP3Rs [91] (Figure 1, Table 2). Notably, there are also functionally orthologous regions, such as the N-terminal regions including the suppressor and ligand binding domains, which lead to modulation of the gating of the Ca2+ pore at the carboxy terminus. The 11 residues in the IBC core recognizing IP3 in mouse IP3R1 [67] are conserved in T. castaneum IP3R (R267, T268, T269, G270, R271, R496, K500, R503, Y560, R561, K562) [50]. Additionally, seven residues in the amino-terminal suppression domain of the mouse IP3R1 that were shown to be critical for inhibition of IP3 binding [74], were also present in TcIP3R (L31, L33, V34, D35, R37, R55, K128). It is noteworthy that aphid IP3Rs appear to create relatively larger channels (around 1000 residues with a molecular weight of 100 kDa) compared to other insect IP3Rs (Table 1) [63]. Nevertheless, the overall structural domain organization of M. persicae IP3R does not change other than the additional amino acids scattered across the entire length of the protein, including within the functionally important domains [63]. Larger IP3R-like channels are also present in various protozoan species [123,124]. This raises the question whether such divergence is present in other families, which will require identification of more insect IP3Rs.
Alternative splicing of RyR mRNA [125,126,127,128] and IP3Rs [129] is common in mammalians, leading to differences in Ca2+ releasing patterns. The expression of splicing variants of RyRs and IP3Rs is regulated both in a tissue-specific and developmental manner. Alternative mRNA splicing was also detected for both insect RyR and IP3Rs in many species, with several variants being specific to different tissues and/or developmental stages [33,37,39,41,49,50,51,52,55,56,130], suggesting a functional diversity for RyRs and IP3Rs in insect physiology. For example, B. dorsalis RyR mRNA possesses four alternative splice variants (ASI-ASIV) [49], while G. molesta [46], D. citri [56], and T. citricida [55] RyRs were found to have five, three, and one alternative splicing variant, respectively. Amongst these sites, the splicing site located within the second SPRY domain in the N-terminal part of the channel (amino acids 1135–1167 of the M. persicae RyR) appears to be quite common in insects [37,40,52,54]. As the second SPRY domain is considered to be a protein–protein interaction domain involved in various biological functions [95,131], splicing variants generated at this location might have different protein–protein interactions [37,63]. Toxoptera citricida RyR alternate splicing has been shown to occur by intron retention, a rare splicing event in animals [55]. In contrast, M. persicae RyR mRNA lacks an alternative splicing variant [54]. On the other hand, at least one alternative splicing site was detected in D. melanogaster [91] and T. castaneum (located between amino acid residues 922–929) [50] RyR mRNA. This alternative splice site is also conserved in the human IP3R1 [132]. The functional implications of alternative splicing in insect Rys and IP3R mRNA has not been studied and requires further investigation.
Phylogenetic analysis of RyRs and IP3Rs from a variety of vertebrate and invertebrate species (Table S1) reveals two major clades, the RyR clade and the IP3R clade (Figure 2). In each clade, invertebrate and vertebrate RyRs or IP3Rs are clustered separately. In invertebrate isoforms of each clade, spider RyR or IP3R forms a subclade, while the insect RyRs or IP3Rs form another subclade. In the vertebrate isoforms of RyRs, RyR1, and RyR3 isoforms are clustered in one subclade, while RyR2 isoforms are clustered in another subclade. In the vertebrate isoforms of IP3Rs, IP3R2, and IP3R3 isoforms are clustered in one subclade, while IP3R1 isoforms are clustered in another subclade. Overall, one could say that each receptor is formed through a gene duplication in invertebrates, which leads to generation of vertebrate RyRs and IP3Rs. The three isoforms of each receptor in vertebrates appear to derive via distinct gene duplication events.
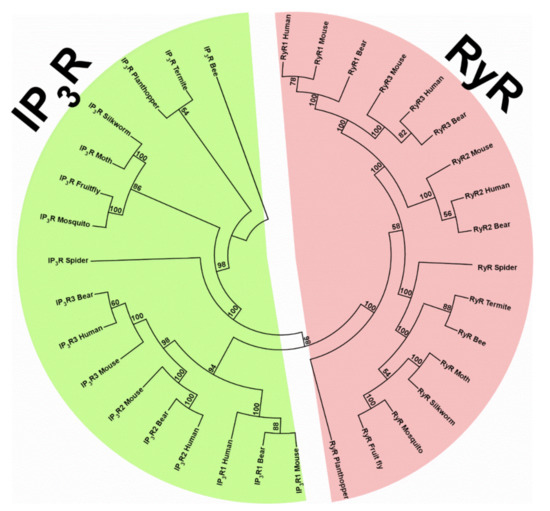
Figure 2.
Phylogenetic analysis tree of IP3R and RyR, constructed by aligning amino acid sequences from representative species of animal phyla using the MUSCLE algorithm of MEGA-X software, version 10.0 (www.megasoftware.net) (accessed on 21 March 2021) [133]. Phylogenetic trees were constructed by using the maximum likelihood method and Le Gascuel model [134]. The bootstrap consensus tree inferred from 1000 replicates is taken to represent the evolutionary history of the taxa analyzed [135]. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed. Representative proteins and their accession numbers are given in Supplementary Table S1.
4. Pathway
Although RyRs and IP3Rs are closely related Ca2+ release channels, their regulatory pathways are different [136]. Regardless, reduction in intracellular levels of Ca2+ leads to activation of both channels and is primarily coordinated by a process called “Store-Operated Calcium Entry (SOCE)”. Both IP3R and RyR are the major biochemical components of the SOCE process and mediate release of Ca2+ from the ER into the cytosol or other organelles, such as mitochondria [124,137,138], lysosomes [139,140,141], and the Golgi apparatus [142]. The other major component of this process is the Sarco/endoplasmic reticulum Ca2+-ATPase [SERCA], which pumps Ca2+ from the cytosol into the ER lumen. There are other players involved in SOCE, for example, the stromal interaction molecule (STIM)-Orai1 complex. STIM is normally located in the ER transmembrane and senses luminal Ca2+ depletion, which leads to its translocation to junctions between the ER and plasma membrane where it couples with the plasma membrane Ca2+ channel protein Orai1 [143]. This coupling activates Ca2+ release-activated Ca2+ (CRAC) channels in the plasma membrane, allowing Ca2+ influx from the extracellular pools to the cytosol and then from the cytosol to the ER through SERCA [144]. Notably, SERCA might associate with IP3R upon depletion of ER Ca2+ resulting in enhanced SOCE activity [145,146,147,148]; however, this has not been shown in insect models. Elevation of cytosolic Ca2+ to certain levels inactivates CRAC channels thereby terminating Ca2+ influx into the cell, a process known as Ca2+-dependent inactivation (CDI) [149]. It is noteworthy that the primary Ca2+-binding protein, calmodulin, is involved in CDI by binding to STIM, leading to disruption of the STIM-Orai1 complex [150]. The activation of either RyR or IP3R is initiated by various external (e.g., light, pheromones, allelochemicals, insecticides) or internal (e.g., neurotransmitters, hormones, growth factors, feeding status, developmental stage, flight) signals that are adjusted based on the biology of insects and associated physiological processes. Activation of the channels might be specific to an organ or cell requiring either the RyR or the IP3R.
IP3Rs are expressed in most cells, in particular in the ER of neurons [151], fat body adipocytes [Doğan et al., unpublished], and oocytes [152] (Table 2). IP3R signaling pathway is integrated with several other signaling pathways, such as the insulin/target of rapamycin (TOR) pathway [153,154]. Low concentrations of cytoplasmic Ca2+ activate IP3R, while high concentrations (above 300 nM) inhibit channel activity [21,153]. Various receptors in the plasma membrane of the cell, such as G-protein-coupled receptors (GPCRs), stimulate phospholipase C (PLC) that hydrolyzes the phosphorylated plasma membrane glycolipid, phosphatidylinositol 4,5-bisphosphate (PIP2), into secondary messengers diacylglycerol (DAG) and IP3. IP3 binds to IP3-binding sites in the N-terminus of the tetrameric IP3R to initiate conformational changes that are transmitted down to the transmembrane region leading to opening of the Ca2+-permeable pore ~7 nm away from the IBC to release the Ca2+ from the ER [155,156]. The IBC form a clam-shaped structure and residues in the IBC required for IP3 binding are conserved in IP3Rs, but not in RyRs [28,81]. Notably, studies on mammalian IP3Rs revealed that IP3 binding alone is not sufficient to activate IP3Rs [153]. Indeed, IP3 binding primes IP3Rs to bind Ca2+ and Ca2+ binding triggers channel opening [157,158]. Insect IP3Rs might also require binding of both IP3 and Ca2+ to open the channel; however, this has not been demonstrated. It is also noteworthy that IP3 must bind to each of the four subunits of IP3R; the 4- and 5-phosphates of IP3 moiety are essential for binding, while the 1-phosphate enhances affinity [159]. Activation of IP3R propagates regenerative Ca2+ signals by Ca2+-induced Ca2+ release (CICR) leading to generation of cell-wide Ca2+ spikes, oscillations or localized Ca2+ “puffs” arising from simultaneous opening of a small cluster of IP3Rs [160,161,162]. Calcium spikes through IP3R are the main event leading to differential gene expression [153,163]; however, oscillations are also quite common and have been described in many insect cells, including those from salivary glands [164], neurons [165,166], and oocytes [152]. Activity of the IP3Rs is also regulated through post-translational modifications, primarily by phosphorylation and dephosphorylation via protein kinases and phosphatases, respectively [167]. For example, the 3′,5′-cyclic monophosphate (cyclic AMP:cAMP)-dependent protein kinase (PKA) phosphorylates IP3R resulting in an increase in Ca2+ release in mammals [168]. However, D. melanogaster IP3R lacks PKA sites indicating that it is not regulated by PKA [61]. Other phosphorylation agents, such as the AKT kinase (PKB), protein kinase C (PKC), or Ca2+/calmodulin-dependent protein kinase II (CaMKII), might be involved in the phosphorylation of insect IP3Rs similar to that in mammalians [83,167,169,170]. IP3 is deactivated by phosphorylation to IP4 or dephosphorylation to IP2 thereby terminating the IP3R signaling pathway [171]. Proteins that have EF-hand Ca2+-binding motifs, such as calmodulin, can also regulate the activity of the IP3Rs. Calmodulin has been shown to inhibit the binding of IP3 to IP3Rs in mammals in a dose-dependent manner [102,172]. Endogenous calmodulin is essential for the proper activation of the IP3R [173]. The direct effect of calmodulin has not been experimentally shown for insect IP3Rs; however, in D. melanogaster, IP3R and calmodulin compete for binding to transient receptor potential (TRP) proteins, which are known to form plasma membrane channels [174].
RyRs have a more restricted distribution compared to IP3Rs and are predominantly found in the SR of muscle cells and the ER of neurons (Table 2). RyR activation occurs through binding of Ca2+ to high affinity binding sites [142,175]. RyR is normally closed at low cytosolic Ca2+ (100–200 nM); submicromolar levels of Ca2+ act on the RyR channel by increasing open channel probability [92,176,177,178]. A small amount of Ca2+ in the cytosol near the receptor causes it to release even more Ca2+; however, as the concentration of intracellular Ca2+ rises to millimolar concentrations, RyR channel activation becomes inhibited, preventing the total depletion of SR Ca2+ [35,179,180,181]. Like cytosolic Ca2+, adenine nucleotides also have a biphasic effect on (3H)ryanodine binding [182]; however, this has not been demonstrated for insect RyRs yet. Mammalian RyR activity is regulated by PKA, in particular via the residues in the Repeat34 domain of the channel [69,183]. This phosphorylation has been shown to increase the channel activity [184]. In P. xylostella RyR, PKA phosphorylation sites have been detected in the Repeat34 domain, which might regulate the interaction with the neighboring SPRY3 domain [104]. The phosphorylation pattern is temperature-dependent with a lower thermal stability compared to the analogous Repeat34 domain in mammalian RyR isoforms [104]. Notably, mammalian RyR function is known to be modulated also by CaMKII; however, this topic requires investigation in insects (Table 2). On the other hand, the primary Ca2+ binding protein, calmodulin has different effects depending on the Ca2+ levels and the type of the RyR in mammalians. Calmodulin activates (at low Ca2+ levels) or inhibits (at high Ca2+ levels) the RyR1 and RyR3 channels, while only inhibitory effects were reported for RyR2 [98,99,185,186]. Although potential calmodulin binding sites have been detected in insect RyRs [33,51], the direct effect of calmodulin on RyR activity in insects has not been demonstrated; however, limited findings provide a hint to calmodulin–channel interaction. Drosophila melanogaster calmodulin mutants with a single amino acid change (V91G) were found to possess abnormal Ca2+ release in response to depolarization of muscles, which was linked to failed regulation of the RyR [187]. Inhibition of calmodulin has been also shown to enhance the light-induced Ca2+ release from internal stores in photoreceptor neurons, indicating calmodulin is involved in the termination of the light response [188,189,190]. Calmodulin rescued the inactivated photoresponse in the presence of ryanodine, suggesting a link between RyR activation and calmodulin action [188,189]. As the activation of the D. melanogaster visual cascade also includes the cation influx channels transient receptor potential (TRP) protein, which also requires IP3R signaling [191], the interaction of calmodulin with both channels in insects requires further investigation.
5. Functions
RyRs mediate many cellular and physiological activities, such as muscle contraction, neurotransmitter release, and hormone secretion [17] (Table 2). In accordance with these roles, RyRs are associated with the SR of muscles and the ER of neurons and many other cell types. The mammalian RyR1 and RyR2 are predominately found in skeletal and cardiac muscles, respectively, while RyR3 is relatively abundant in brain and certain skeletal tissues but is also expressed at low levels in multiple tissues [192,193,194]. Neuronal expression of RyR varies, but RyR2 is most abundant. Notably, RYR2 is the major cellular mediator of CICR in animal cells. In contrast to mammalians, there is only one isoform of RyR in insects. The initial studies on insect RyRs have been conducted on D. melanogaster. These studies revealed RyR is expressed in muscles of the body wall, visceral muscles around the gut, central nervous systems, and optic lobe and retina in the embryonic, larval, and adult stages [32,33,195]. In D. melanogaster adults, RyR mRNA was detected in tubular muscles and at a lower level in neuronal tissues [32,188] but not ovaries [196,197]. Among head, eyes, antennae and legs, the highest expression was detected in legs [32]. Subsequent studies have examined the site-specific and developmental expression of insect RyR genes in insects other than D. melanogaster. For example, the highest expression level of RyR was detected in the thorax compared with the head and abdomen in adult B. dorsalis [49] and P. rapae [42], suggesting RyR is involved in the modulation of intracellular Ca2+ levels for locomotory activities. Similarly, RyR expression was higher in the adult thorax compared to the abdomen; however, the highest expression was detected in the head in D. citri [56]. Similar results were also found in H. armigera larvae [41], P. rapae adults [42], L. decemlineata larvae [51], S. furcifera nymphs [53] and T. citricida adults [55] with higher expression in the head and/or thorax than the abdomen. In contrast, no significant difference in RyR expression levels between the head, thorax, and abdomen were detected in the fourth instar larva of P. xylostella [39]. A more specific analysis of different tissues in the third instar L. decemlineata larvae indicated that RyR expression level was highest in foregut, at moderate levels in the hindgut and epidermis, and to a lower extent in the fat body, midgut, ventral ganglia, and Malpighian tubules [51]. In the the fourth instar larvae of P. rapae, RyR was primarily expressed in the epidermis, at moderate levels in nerve cords, hemocytes, the midgut, and least in the fat body and Malpighian tubules [42]. In the fifth instar larvae of C. suppressalis, RyR was primarily expressed in the head (including brain and muscle), at moderate levels in the integument and the haemolymph, and least in the fat body, Malpighian tubules, the midgut, and the silk gland [64]. Such distribution of RyR mRNAs is not unexpected considering that more muscles are distributed around the foregut, the hindgut, and attached to the epidermis [51]. Nevertheless, the commonly reported higher expression in the thorax and the head are in accordance with the lowest expression in eggs and highest expression in juvenile or adult stages, considering that the mobile stages, such as larvae or adults, require muscle activity. Thus, RyR expression was highest in larval or adult stages and lowest in eggs in O. furnacalis [40], B. dorsalis [49], H. armigera [41], L. decemlineata [51], and T. castaneum [50]. Similarly, RyR expression was lowest in eggs; however, it was higher in nymphs than adults in D. citri [56]. In another hemipteran, S. furcifera, RyR expression in the fifth instar nymph was significantly higher than in the eggs or female adults; however, no significant difference was detected between the eggs and female adults [53]. This trend is similar to that in C. suppressalis with the highest expression in the third instar larvae, but with similar expression in eggs, pupae, and adults [64]. In N. lugens, RyR transcript levels in female adults were significantly higher than in first to fifth instar nymphs; however, the lowest expression was still in eggs [52]. The expression level of RyR in T. citricida adults were also found to be significantly higher than those in nymphs [55], while no significant difference in the expression levels of RyR was found between nymphs and adults [54]. In contrast to most studies, RyR expression levels in eggs, larvae, and adults were all found to be similar in the lepidopteran P. xylostella [39]. In brief, these studies, except that by Wang et al. [39], indicate that the expression of RyR is higher in adult or juvenile stages (larva or nymph) than in eggs, suggesting involvement of RyRs in locomotory activities. Notably, the immobile pupal stages can also have high expression of RyR [40,41,46]. Although most larval muscles are histolyzed during the early-mid phase of pupal development, new muscles are formed at the late pupal stage [198], suggesting that RyR expression might fluctuate during pupal transition and be elevated depending on the timing of sampling [51]. It is noteworthy that upregulation of RyR expression in pupae might be related to factors other than muscle formation. Notably, RyR expression patterns might also be different between sexes. For example, RyR expression was found to be significantly higher in males in S. furcifera [53], N. lugens [52], and G. molesta [46]. However, the reason for this sex-dependent variation in insect RyR genes is not currently known. Nevertheless, the higher RyR expression in the thorax compared to the abdomen is in accordance with the primary function of RyRs in the mediation of excitation-contraction coupling in muscles, which is primarily located in the thorax for mobility [198]. On the other hand, higher expression of RyR in the head is in accordance with the involvement of this body part in nerve conduction, hormone secretion and sensory activities, processes that are regulated by RyR activity. It is noteworthy that expression levels of different RyR mRNA splicing variants vary between different developmental stages and tissues [33,37,39,40,41,46,49,52,55,65]. In contrast, M. persicae RyR mRNA lacks an alternative splicing event, which might be related to its asexual reproduction phase [54]. Alternative splicing of RyR mRNAs is common in mammalians with more than 12 distinct splice variants identified to date, leading to important differences in their channel functioning [125,126,199,200]. Some splice variants suppress Ca2+ release, while some contribute to distinct Ca2+ release patterns [126,127,128]. Interestingly, T. citricida RyR mRNA splicing occur by intron retention [55]. Such a splicing event is rare in animals, leading to generation of an optional exon. However, the inclusion of this exon was shown to induce a premature stop codon in T. citricida RyR mRNA, encoding a truncated protein [55]. Nevertheless, alternative splicing might be critical in generating a diversity of RyRs, leading to subsequent phenotypic changes, in particular for insects which have a single RyR gene.
IP3Rs are involved in the key events related to the gene expression, development, learning, memory, neuronal signaling, and sensory transduction [129,136] (Table 2). In accordance with these roles, genes encoding IP3R are expressed in many cell types, but primarily associated with the ER of neurons. IP3R1 is the predominant neuronal isoform and present in endothelial cells, while IP3R2 is the predominant isoform in contractile myocardial cells and the sinoatrial node and IP3R3 in the intestinal crypt, ovary cells, villus epithelial cells, and the microvillous cells in the olfactory system [201,202,203,204]. Insect genomes possess a single IP3R gene. The first D. melanogaster IP3R gene was reported by Yoshikawa et al. [61] and is expressed mainly in the central nervous system [151], but also other tissues, such as fat body [205] and ovaries [196,197]. A confocal microscopic investigation revealed that IP3R is present in all tissues of adult D. melanogaster and at more homogeneous in levels than RyR [195]. However, the level of transcription in the appendages, containing mainly legs, antennae, wings, and seta, was the highest among all the parts of adult flies [61]. IP3R mRNA was also abundant in the thorax. Among the head, eyes, antennae and legs, the highest expression was detected at antennae [32]. Developmental expression of IP3R revealed that the gene is expressed at the highest levels in adults, at moderate levels in eggs, followed by early and mid stage pupa, and least in larvae [61]. Although many studies have been conducted on insect RyRs, the studies on non-Drosophila IP3Rs are restricted to only a few insects. Liu et al. [50] reported that the highest and lowest expression levels of IP3R were detected in 1-day-old larvae and 3-day-old eggs, respectively, in T. castaneum. In B. tabaci, IP3R was primarily expressed in larvae, unlike D. melanogaster, while expression was moderate in pseudopupa and female adults, and least in eggs [62]. Nevertheless, the higher expression in adults or larvae compared to eggs is similar to those reported for insect RyR genes and is in accordance with the possible involvement of IP3R in locomotor activities [61], sensory transduction [32] and muscle development [206]. Sex-dependent differential expression of IP3R genes was reported from a single insect species. The trend was in favor of females, contrasting to those reported for RyR genes [62]; however, further studies are necessary to make a conclusion. As was reported for RyR mRNA, alternative splicing of IP3R mRNA is also common in mammalians [129]. At least one of these splice sites appears to be conserved in D. melanogaster [91].
As we already introduced the site-specific and developmental expression patterns of both RyR and IP3R genes, their involvement in insect life processes highlighting lipid metabolism, muscle excitation and contraction in locomotor activities, visualization and olfactory responses, and development are summarized below.
5.1. Lipid Metabolism
Various studies in mammals revealed the involvement of Ca2+ in lipid metabolism [143,207,208,209,210,211,212,213]. These studies inspired those in insects, which confirmed the involvement of Ca2+ in lipid metabolism in insects [214]. The center of the insect lipid metabolism is the fat body, which is primarily composed of the adipocytes that are able to store tremendous amounts of lipids in their cytosolic lipid droplets [214,215,216]. The data on the involvement of Ca2+ in insect lipid metabolism is limited and derives mostly from the model insect D. melanogaster where increased levels of cytosolic Ca2+ in adipocytes lead to fat reduction, whereas decreased cytosolic Ca2+ levels induce fat accumulation [217,218,219,220,221,222,223]. Several other studies on non-Drosophila insects also demonstrated the involvement of Ca2+ in lipid metabolism, which occurs via the primary Ca2+ signaling molecules calmodulin, calcineurin and regucalcin [10]. These studies together indicate that cytosolic Ca2+ levels correspond with the levels of triglycerides in lipid droplets. This raises the question as to where RyRs and IP3Rs stand in this interaction as the two major intracellular Ca2+ suppliers residing in the ER.
Most of the data on the involvement of insect ER Ca2+ channels in lipid metabolism are related to IP3Rs, which induce lipolysis in insect adipocytes. The loss of IP3R leads to elevated levels of triglycerides with enlarged lipid droplets in the fat body and hyperphagia in D. melanogaster adults [218]. In line with this, fat body-specific knockdown of IP3R leads to an increase in lipid droplet size and triglyceride accumulation in adult flies [222]. The lipolysis is primarily under the control of the adipokinetic hormone (AKH) which binds to AKH-receptor in adipocytes, leading to generation of the secondary messenger cAMP and the PLC [224]. The cAMP induces PKA, leading to activation of the lipolytic transcription factor foxO acting on lipase genes [219]. In parallel, PLC hydrolyzes PIP2 to IP3, which binds to IP3R, leading to activation of the channel and an elevation in cytosolic Ca2+ levels [214]. Therefore, AKH activity leads to lipolysis in parallel to the increase in cytosolic levels of Ca2+ in adipocytes [214]. While the increase in cytosolic levels of Ca2+ transmits the AKH signal, the exact mechanism is not known [219,220,225]. Subramanian et al. [218] reported that reduced insulin signaling in IP3R-mutants might be one of the reasons for IP3R deficiency-related obesity. It is also noteworthy that knockdown of IP3R, either in all neurons or in peptidergic neurons alone, mimics the IP3R mutant phenotype with elevated lipid stores and hyperphagia [217]. IP3R-mediated Ca2+ release in neurons is significantly reduced in these mutants, while the level of short neuropeptide F (sNPF), which is involved in hyperphagia, is elevated [219,220,223] suggesting that IP3R-mediated Ca2+ signals modulate neural circuits for feeding [218,226,227] and that sNPF is likely to be involved in the activation of IP3Rs in neurons [228]. In brief, impaired lipid metabolism derives primarily from peptidergic neurons. These neurons are also associated with the stomatogastric nervous system. On the other hand, AKH-induced lipolysis has been reported only in adults of D. melanogaster as manipulation of cytosolic Ca2+ levels in the larval fat body does not have a significant effect on larval fat stores [219,229]. In contrast, insects, such as L. decemlineata, accumulate greater amounts of lipid at the larval stage, which show impaired lipid metabolism upon silencing Ca2+-signaling genes [10,216]. Therefore, the dynamics of lipid metabolism in relation to Ca2+ might be different depending on the species.
Knowledge on the involvement of RyRs in insect lipid metabolism is restricted to a single study. In D. melanogaster adults, fat body-specific knockdown of RyR leads to an increase in lipid droplet size and triglyceride levels, suggesting a lipolytic role for RyRs [222]. On the other hand, loss of the fat body seipin gene in D. melanogaster adults leads to reduction in triglyceride storage and lipid droplet size, which is linked to impaired SERCA activity, suggesting seipin and SERCA function together to promote fat storage in adipose tissue [222,230]. Interestingly, adipose tissue-specific knockdown of RyR partially restores fat storage in seipin mutants, while IP3R silencing did not rescue this phenotype [222]. These findings indicate a complex interaction between the receptors with other molecules involved in Ca2+ homeostasis in fat body adipocytes. It is noteworthy that opposite effects were reported on the levels and cellular sites of Ca2+ on fat storage in hepatocytes compared to adipocytes in mammals. Increased cytosolic and reduced ER calcium levels induce triglyceride accumulation leading to lipogenesis, whereas reduced cytosolic and increased ER calcium levels reduce triglyceride accumulation leading to lipolysis in hepatocytes and their orthologous cells in the insect fat body, oenocytes [214,222,231]. This suggests that IP3R acts as an obesity gene in hepatocytes or oenocytes [222]. However, the data is restricted to D. melanogaster and, therefore, this topic requires further investigation in other insect species.
5.2. Muscle Excitation and Contraction in Locomotor Activities
Calcium is an essential element in the excitation and contraction of muscles [232,233]. ER-released Ca2+ is a major source for the stimulation of muscle cells in invertebrates from nematodes towards insects [234,235,236,237]. Insect muscle contraction is similar to that in vertebrate skeletal muscles as in both SR release Ca2+ that binds to troponin, a regulatory protein on the thin filament. Troponin activate another regulatory protein, tropomyosin, which causes muscle contraction [238,239]. In contrast, relaxation occurs as the Ca2+ pump on the SR membrane transports Ca2+ ions back into the SR lumen. This raises the question as to whether RyR or IP3R or both are involved in Ca2+-related muscle excitation and contraction in insects.
RyRs play a central role in the excitation/contraction (EC) coupling of cardiac and skeletal muscles in mammals [17,240,241]. Studies in D. melanogaster indicated that RyR is mainly expressed in the muscles of the body wall, visceral muscles around the alimentary canal, as well as the central nervous system [33,65,242]. Similarly, high levels of RyR expression in muscles have been also reported from non-Drosophila insects, such as H. virescens [35] and L. decemlineata [51]. Partial loss of RyR led to impairment of hypodermal, visceral, and circulatory muscles, indicating RyR is essential for proper muscle function and EC coupling in larval body wall muscles [33,242]. Drosophila melanogaster RyR mutants also have a severe defect in the ingestion and passage of food into the gut, confirming that the head and visceral muscles are impaired [242]. On the other hand, mutation calmodulin leads to specific impairment in muscle Ca2+ flux, which was found to be related to failed regulation of RyR [187]. RyR activity is also necessary for the spontaneous rhythmic contractions of the lateral oviduct muscles in the cricket, Gryllus bimaculatus (Orthoptera: Gryllidae) [237]. Similarly, proctolin induced Ca2+ release from the SR, via RyR, plays a major role in hyperneural muscle contractions in Periplaneta americana (Blattodea: Blattidae), while IP3R-induced Ca2+ release has little impact [243].
IP3Rs also play a role in the EC and regulation of skeletal, cardiac, and smooth muscle cell functions in mammals [153,244]. Involvement of IP3R in insect muscle activity has not been studied in detail. IP3R is expressed in D. melanogaster adult muscles, particularly in legs which contain tubular muscles, but to a lesser extent in the thorax, which contains the fibrillary muscles [32,61]. However, it is not known whether IP3R has a possible role in tubular or fibrillar muscle function regulation in D. melanogaster. In G. bimaculatus, IP3R regulates the amplitude of rhythmic contractions of lateral oviduct muscles; however, the effect was considered minimal [237]. Notably, the inhibitor used in that study, 2-aminoethoxydiphenyl borate, might also inhibit other SOCE molecules, such as SERCA [245], or other volume-regulated anion channels independently from intracellular Ca2+ signaling modulation [246]. Further investigation, possibly with other select IP3R inhibitors, is required. The involvement of Ca2+ in EC of lateral oviduct muscles via the action of several neurohormones was also reported in other studies. For example, octopamine, via the intracellular messenger cAMP, inhibits contraction of the oviducts, while proctolin, via the PLC/IP3R, stimulates contraction [247,248,249,250,251]. In Schistocerca gregaria (Orthoptera: Acrididae), ryanodine had no effect on proctolin-stimulated foregut muscle contraction, instead, gut muscle contraction was dependent on proctolin receptor-specific activation of the PLC signaling cascade leading to generation of IP3 [252]. The authors proposed that the potentiation of contractions by proctolin is mediated by activation of IP3-induced Ca2+ release from the SR, in contrast to the model of proctolin action on tonic muscle contractions of P. americana [243]. These findings support the notion that neurohormones act on the muscles, therefore, their activity is indeed controlled by neuronal signaling pathways [253]. There are various studies on the involvement of neuronal Ca2+ levels leading to muscle action, in particular related to locomotor activities such as flight, walking or climbing. For example, the mutations in IP3R resulted in strong flight deficits in D. melanogaster [226,254]. Furthermore, pan-neuronal knockdown of the IP3R leads to significant defects in wing posture in Drosophila, indicating IP3R in neurons is necessary during pupal development for flight [227,255]. Examination of Ca2+ signals in cultured pupal neurons in D. melanogaster IP3R mutants also revealed high spontaneous Ca2+ influx and reduced SOCE, which might lead to loss of flight [256]. These defects and deficits were indeed found to be related to impairment of the IP3R signaling induced by neurohormones, primarily the amine-type, and their G-protein coupled receptors in the neurons (e.g., aminergic neurons) [227,254,255,257,258,259]. IP3R in neurons can also be induced by other signaling molecules, such as neurotransmitters [256,259], nevertheless, IP3R-dependent Ca2+ release is essential for neuronal activity. Thus, expression of IP3R in aminergic neurons during pupal development was found to rescue the adult flight deficit in D. melanogaster IP3R mutants, suggesting the involvement of IP3R in flight is related to its role in development [227,254,256]. Other SOCE components, such as STIM-ORAI involved in the extracellular Ca2+ influx, are also necessary for normal flight activity [226]. Insect leg muscles are also innervated by neuromodulatory octopaminergic DUM (dorsal unpaired median) neurons or motor neurons [166,260,261,262,263]. In S. gregaria, the Ca2+ signal in such neurons is dependent on IP3R and PLC activation, but not on RyR [264]. In brief, intracellular Ca2+ stores in neurons are required for insect rhythmic motor functions which leads to muscle activity and IP3R signaling plays a central role in this supply.
The contradictory results on RyR-induced muscle EC [237,243,265] or IP3R- [248,252] still raises questions. The absence of functional genomic studies, such as RNAi, or sophisticated visualization techniques makes it difficult to make conclusive statements on this topic. Nevertheless, the maintenance of intracellular Ca2+ levels in muscle cells is a requirement for muscle EC; this probably requires RyR and IP3R acting on neuronal pathways.
5.3. Visual and Olfactory Sensory Transduction
Visualization and olfactory responses play a crucial role in insect survival as they are involved in accessing food sources, protecting insects from threats, and finding mates to reproduce [266]. This occurs primarily via sensory systems in the eye and antennae; each possesses a small region of tissue, called receptor cells, that are sensitive to a specific stimulus [267,268]. Receptor cells are neurons or other specialized cells and convert odor or light signals into an electrical response that is transmitted to the brain for the processing, a mechanism commonly known as signal transduction [268]. This might be named as “phototransduction” for visualization, and “olfactory sensory transduction” for odor recognition.
Phototransduction starts in ommatidia, units of the insect compound eye that contain sensory neurons known as retinal (visual) cells. The rhabdomere is the central photoreceptive region in each retinal cell and contains photopigment molecules, called rhodopsins [269,270]. Absorption of a photon by rhodopsin leads to activation of the heterotrimeric Gq protein complex, which in turn stimulates PLC to hyrolyzes PIP2 to a proton, and the secondary messengers hyrophilic IP3 and hyrophobic DAG [267]. The released proton and the mechanical forces caused by PIP2 hydrolysis results in opening of light-sensitive, relatively Ca2+-selective, “transient receptor potential” (TRP) channels and TRP-like (TRPL) channels which mediate an ionic current responsible for generation of a quantum bump, known as the bump current [271,272,273,274,275]. Calcium is involved in phototransduction; however, studies on the involvement of IP3R and RyR are limited. High expression of IP3R in retina of adult D. melanogaster suggested a potential role for IP3R in visual transduction [32,61]. However, studies on D. melanogaster IP3R mutants revealed that Ca2+ release via IP3R does not contribute to phototransduction [276,277], instead, PLC activation leads to the opening of light-sensitive Ca2+ channels in photoreceptors [278]. A subsequent study in D. melanogaster proposed that Ca2+ release via IP3R might have a critical role in light excitation. Silencing of IP3R specifically in adult photoreceptor cells significantly reduced light-response amplitude in adult photoreceptor cells [279]. Kohn et al. [279] also reported that IP3R silencing leads to a reduction in PLC catalytic activity, while elevation of intracellular Ca2+ rescued the suppressed light responsiveness phenotype. These findings suggest that Ca2+ release from internal stores is necessary to increase PLC activity required for bump current, and that functional cooperation between IP3R and PLC is necessary for light responsiveness [279]. This study also posits that the reason for lack of connection between IP3R and phototransduction in previous studies [276,277] was due to leakage of trace amounts of Ca2+ from patchclamp recording electrodes, effectively replacing the Ca2+ that would have been released from IP3-sensitive stores. However, a more recent study using RNAi or IP3R-null mutants [280] challenged the work by Kohn et al. [279] supports the the previous findings indicating that IP3R does not have a role in phototransduction. Bollepalli et al. [280] argues that phototransduction in D. melanogaster is compromised by the Gal4 transcription factor used to regulate dsRNA in these experiments, which is not the case for the IP3R knockdown or mutation in the study by Kohn et al. [279]. These contradictory findings demand further examination on the possible role of IP3R in phototransduction. The role of RyR in Ca2+ regulation photoreceptor via RyR is equally ambiguous [188,189]. Localization of RyR close to the light-sensitive microvilli in compound eyes of D. melanogaster suggested a possible role for RyR in Ca2+ dependent-phototransduction [281]. However, analysis of mutants in which RyR expression was selectively eliminated in the adult eye demonstrated that this channel does not play a role in phototransduction [242].
Calcium is also involved in olfactory sensory transduction [282,283,284,285]. Insects perceive odorants with sensory organs called sensilla which are mainly on their antennae. Olfactory sensilla possess tiny pores that project towards olfactory receptor neurons (ORNs) [268]. The dendrites of these bipolar cells extend into a sensillar lumen, while their axons lie in the second (antennal) lobe in the brain. Upon adsorption of an odorant molecule, such as a volatile or an insoluble odorant like a pheromone, in the sensilla, it diffuses into the sensillum via pores, binds to a specific odorant binding protein (OBP) or pheromone binding protein (PBP) in the sensillar lymph and is transferred to olfactory receptors (ORs) on the dendrites of OSNs [286,287,288]. ORs are both ligand-gated and cyclic-nucleotide-activated ion channels and function as heterodimers consisting of a variable odor-specific ligand binding receptor protein that defines their specificity, and a constant highly conserved co-receptor protein, Orco [289,290,291,292]. Orco itself can also act as a non-specific, spontaneously-opening ion channel permeable to Ca2+. Other types of receptors are located in different types of sensilla (e.g., ionotropic glutamate-like receptors, gustatory receptors) [268,293,294]. Therefore, both metabotropic and ionotropic signaling mediates odor transduction at ORNs and binding of the odor molecules into ORs leads to cell depolarization and generation of action potentials, which transmit the olfactory signal to the antennal lobe [295]. The transduction mechanism in OSNs is mediated by cAMP relies on PKC instead of PKA, and/or the PLC-linked IP3-signaling pathways [290,291,294,296,297,298,299,300,301,302,303,304]. Intracellular Ca2+ stores were found to contribute to the ORN responses [285,303,305], raising the question whether IP3R and/or RyR are involved in odor transduction pathways. High expression of IP3R in antennae in adult D. melanogaster suggests a potential role for IP3R in olfactory transduction [32,61]. Additionally, the IP3R is present in the olfactory sensory neurons of a variety of species [306,307,308]. However, olfactory responses to a number of different odorants were found to be normal in hypomorphic combinations of D. melanogaster IP3R mutant alleles [257,309]. On the other hand, a subset of these IP3R alleles, including a null allele, were found to exhibit a faster recovery after a strong odor pulse, suggesting that IP3R might be required for maintenance of olfactory adaptation in antennae [309]. In a subsequent study, the magnitude and duration of the odor-induced Ca2+ response in ORNs was decreased upon targeting IP3R and RyR by RNAi, as well as by specific blockers, such as thapsigargin or ryanodine [285]. Furthermore, flies expressing IP3R or RyR dsRNA were defective in odor-adaptation [285,303,305]. The magnitude and duration of the Ca2+-response was also found to be decreased in cAMP-defective flies based on silencing of the adenyl cyclase gene “rutabaga” and the phosphodiesterase gene “dunce” [303], in accordance with previous reports that demonstrated involvement of cAMP in olfactory reception [310,311,312]. Furthermore, simultaneous knock-down of RyR or IP3R in combination with knock-down of rutabaga and/or dunce generated even stronger effects with smaller amplitudes and a shorter duration of Ca2+ response to various odors [303]. It is worth noting that when only IP3R or RyR expression is perturbed, perception of odorants (odor-acuity) is not affected, but adaptation to odorants is defective [285]. When cAMP-level is disturbed, odor-perception is affected and the amplitude of the second phase (adaptation to odorants) is completely abolished [303]. Furthermore, in double mutant flies, simultaneous perturbation of both cAMP and IP3-signaling severely affects both the first and the second phase and they are unable to detect or adapt to odorants [303]. Therefore, the first phase of olfactory response appears to be mediated by cAMP, which is important for olfactory perception, while the second phase mediated by the intracellular Ca2+-signaling pathway is important for odor-adaptation. Due to the limited number of studies, the mechanisms of insect odor transduction are still controversial [298,304,313]. It is also noteworthy that induction of either secondary messenger (cAMP or IP3) may be odor-specific [303,311,312,314].
In conclusion, evidence as to the role of IP3R and RyR in phototransduction or olfactory responses is limited, and further research is required.
5.4. Development
Both RyR and IP3R have essential roles in development. This is in accordance with the fact that expression of either RyR [39,40,41,49,50,51,53,56] or IP3R [50,62] is up-regulated during development in many insect species. Studies in D. melanogaster indicated that both genes are also necessary for embryonal development, in particular for development of nervous system and muscles [32,188,189,206].
Loss of IP3R in D. melanogaster leads to lethality in the second instar larvae accompanied by delays in molting from the first to the second instar and lower 20-hydroxyecdysone (20E) levels [205,276,315]. A lethal phenotype with a delayed molting is also observed in PKA mutants [205,316]. Disruption of either the IP3R or cAMP pathway also delays second to third larval instar, third larval instar to pupal, and pupal to adult transitions [205]. Furthermore, PKA and IP3R mutant alleles have a synergistic negative effect on larval molting, suggesting IP3R signaling acts in parallel with the cAMP pathway to regulate molting [205]. Exogenous 20E rescues the molting delays caused by disruption of either pathway, suggesting both pathways control 20E levels during molting [205,315]. Indeed, 20E was shown to induce both extracellular and intracellular Ca2+ release, leading to activation of PKC and CaMKII that are both involved in 20E-directed gene expression [317,318,319,320]. Similar to that in D. melanogaster, silencing of IP3R led to failures in molting and larval-pupal and pupal-adult metamorphosis in the beetle T. castaneum [50]. A relatively recent study investigated the larval to pupal switch under nutrient stress in D. melanogaster, which revealed that the larval-pupal transition requires IP3R/Ca2+ signaling in glutamatergic interneurons of the mid-ventral ganglion [321]. The nutrient stress sensed by multidendritic cholinergic sensory neurons is conveyed first to glutamatergic interneurons via the acetylcholine receptor, then to medial neurosecretory cells, and finally to the ring gland, leading to stimulation of neuropeptides that induce ecdysteroid biosynthetic genes in the ring gland via IP3R signaling to allow pupariation on a protein-deficient diet [321]. The authors suggested that activity in this circuit is an adaptation that provides a layer of regulation to help overcome nutritional stress upon protein deprivation during development. Other studies on neurodevelopment in D. melanogaster larvae indicated that IP3R is essential in particular in aminergic cells for development and survival, and IP3R-mediated Ca2+ release is required to facilitate release of amine type hormones from aminergic cells or serotonergic and dopaminergic neurons [254,257,258,259,322,323]. Thus, expression of IP3R in aminergic neurons during pupal development rescues the onset adult flight deficit in IP3R- D. melanogaster mutants [227,254]. As IP3R is also expressed in ovaries in contrast to RyR [196,197] and is likely to be involved in Ca2+ oscillations in ovaries [152], it may also be necessary for egg activation and ovary development. On the other hand, IP3R-mediated Ca2+ oscillations also occur in wing imaginal discs that give rise to wings in adults, conferring another role of IP3R signaling in development [324].
Several studies have examined the role of RyR in insect development. Mutation of D. melanogaster RyR leads to formation of normal embryos that give rise to larvae with growth defects that die four–seven days during their first instar [242]. Heterozygous individuals containing one copy of the RyR mutant allele rescue the calmodulin-lethal phenotypes, further indicating the vital role of RyR [187]. In T. castaneum, silencing of RyR does not cause any failure in molting or larval-pupal and pupal-adult metamorphosis, in contrast to IP3R silencing in the same beetle; however, abnormalities in the folding of the hind wings and crawling behavior in adults occur, which might be related to impairment of muscle EC-coupling [50].
Developmental physiology also includes topics such as autophagy and the autophagic programmed cell death that play key roles in development, morphogenesis, and regeneration [325,326]. Intracellular Ca2+ levels are critical in this respect as lower Ca2+ concentrations induce autophagy, while higher Ca2+ concentrations switch autophagy to apoptosis [327]. The role of RyR and IP3R in these processes is a topic for future investigation.
6. Potential of RyR and IP3R as Target Sites in Pest Control
Due to their essential roles, insect Ca2+ channels have great potential as target sites for the development of insecticides [328,329,330,331]. As the divergence between mammalian and insect RyRs are greater compared to IP3Rs, RyRs might be considered safer targets for insecticidal molecules [332]. While the discovery of diamide insecticides has prompted studies on insect RyRs, no insecticidal compounds targeting IP3Rs have been developed to date. The idea of targeting RyRs goes back to the discovery of the plant alkaloid ryanodine from the tropical American shrub, Ryania speciosa (Flacourtiaceae), which has high affinity to RyR and interferes with Ca2+ signaling in muscles; there receptors are aptly named RyR [333]. Ryanodine keeps the RyR channel partially open leading to Ca2+ depletion. The insecticidal activity of R. speciosa extracts were first described by Rogers and co-workers in 1946 on a range of lepidopteran and hemipteran pests [334,335]. High toxicity of ryanodine on mammalians was an obstacle to its use as an insecticide; however, it inspired the development of more selective and safer insecticides targeting the operation of RyRs, currently comprised of ryanodine receptor modulators in the Insecticide Resistance Action Committee (IRAC) Group 28 [336]. Based on their common chemistry, these insecticides are generally referred to as diamides.
Diamides are derivatives of benzenedicarboxamide or phthalic acid (flubendiamide, Class I) and anthranilic acid (chlorantraniliprole, cyantraniliprole, and cyclaniliprole, Class II], and selectively activate insect RyRs in the SR and ER in neuromuscular tissues. This causes Ca2+ channels to remain partially open leading to an excessive and uncontrollable release of stored Ca2+ ions from the ER into the cytosol of muscle cells [337,338,339] resulting in feeding cessation, uncoordinated muscle contraction, paralysis, and death [330,339]. The first diamide registered, flubendiamide, was co-developed by Nihon Nohyaku Co. Ltd. (NNC) and Bayer CropScience [181,332,340,341] and registered in 2006 [340,342]. This was followed by the introduction of chlorantraniliprole [177] developed by DuPont USA in 2007 and cyantraniliprole [343,344] that were co-developed by DuPont and Syngenta in 2008. A fourth chemical, the cyclaniliprole developed by ISK [336], was registered and introduced into the market in 2017, while the most recent one, tetraniliprole developed by Bayer was approved in 2020 [345]. Both benzenedicarboxamide and anthranilic acid derivatives are active against a broad range of lepidopteran pests. The anthranilic acid derivatives are also active sucking hemipterans and coleopterans. Clorantraniliprole has contact, systemic and translaminar activity and exhibits extremely high efficacy against lepidopterans and leaf beetles, as well several dipterans, such as leafminers (Liriomyza spp.), isopterans, such as sugar cane termites (Microtermes obesi, and Odontotermes obesus), and hemipterans, such as whiteflies (Bemisia spp.) [343,344,346]. Cyantraniliprole is mainly active against sucking and piercing insects, such as aphids, whiteflies, leafhoppers, psyllids, and thrip due to its systemic properties [344,347,348,349,350]. Cyclaniliprole, is labeled for use against aphids, leaf-feeding caterpillars, mealybugs, thrips, and whiteflies and has contact and translaminar activity [336], while tetraniliprole is labeled for use against white grubs, annual bluegrass weevils, caterpillars, and billbugs (https://www.environmentalscience.bayer.us/turf-and-ornamentals-management/golf-course-management/portfolios-and-solutions/new-bayer-insecticide) (accessed on 4 April 2021).
Diamide insecticides have low mammalian toxicity and are considered safe for beneficial insects and mites, which make them environmentally friendly [343,344]. These features, together with their efficacy, has led to extensive use. A survey on the global insecticide market in 2013 revealed that diamides accounted around 1.2 billion U.S. dollars of global insecticide sales, representing approx. 8% of the insecticide market [336]. The current annual market value is predicted to be around $2.3 billion [351]. This ranks diamides third in the market, accounting for 12% of the global market after neonicotinoids (Group 4A) and synthetic pyrethroids (Group 3A) which account for 24 and 15%, respectively [351]. Additionally, at least three more diamide insecticides (cyhalodiamide, and tetrachlorantraniliprole and unnamed); as well as a third class of diamides, “pyrrole-2 carboxamides” are currently under development, suggesting the use of diamides will continue to increase [345,351,352,353]. However, intensive and repetitive use of the diamides has led to the development of high levels of insecticide resistance in the field, which requires a better understanding of the mode of action of this class of insecticides.
Diamides act on RyR and induce Ca2+ release from intracellular Ca2+ stores in insect muscle cells [36,42,338], but also elicit intracellular Ca2+ release in isolated insect neurons [177,181,340,354]. Silencing RyR in S. furcifera [53] or L. decemlineata [51] greatly decreases chlorantraniliprole-induced mortality indicating that RyRs are targets of diamides. On the other hand, flubendiamide stimulates SERCA activity, which is attributed to a decrease in ER Ca2+ levels [341,355]. Efforts have focused on the binding sites of diamides on RyR. Diamides are incorporated directly into the transmembrane domain of the RyR; however, RyR activation also requires the N-terminus for flubendiamide sensitivity [36]. Deletion experiments on the carboxy-terminal region of the B. mori RyR revealed that the binding region of flubendiamide is located in the transmembrane domain of the RyR comprising amino acid residues 4111–5084, while the region in the N-terminal cytoplasmic domain correspond to residues at 183–290 [36]. HEK cells expressing either Δ183–290 mutants or a chimeric RyR in which amino acids 4111–5084 were replaced with the counterpart sequence in rabbit RyR2, exhibit failure in Ca2+ mobilization in response to flubendiamide, but not to caffeine [36]. A similar study based on the replacement of a 46 amino acid segment (S4610-A4655) in D. melanogaster RyR (GenBank accession number: D17389) C-terminal domain with that of a nematode RyR led to insensitivity to diamides [356]. Notably, this shorter region corresponds to A4659-A4703 in PxRyR, which is within the larger region examined by Kato et al. [36]. However, this region does not overlap with the the highly conserved pore region in D. melanogaster RyR (aa 4973–4982), where ryanodine binds, or the TM10, which plays a crucial role in human RyR channel activation and gating [97,117,356,357]. A computational modeling approach based on rabbit RyR1 also indicated that I4790 and G4946 (in P. xylostella RyR) are likely to be involved in forming the diamide binding site [358]. On the other hand, radioligand displacement experiments using microsomal membrane preparations of H. virescens and P. americana muscles indicate that flubendiamide and chlorantraniliprole interact with a binding site that is distinct from the ryanodine binding site [177,178,181,338,359]. Furthermore, radioligand binding studies with house fly muscle membranes provided evidence that flubendiamide and chlorantraniliprole bind at different, allosterically-coupled RyR sites [360]. Recently, a high resolution (3.2 Å) cryo-electron microscopy structure of the rabbit RyR1 in complex with chlorantraniliprole, together with mutagenesis studies revealed that twelve amino acid residues (Y4697, K4700, Y4701, L4704, I4790, Y4918, S4919, Y4922, D4942, V4943, G4946, and F4947 based on P. xylostella RyR) comprise the putative chlorantraniliprole binding pocket [361]. Furthermore, a radioligand binding study also suggested that the anthranilic diamides share a common binding site with the pyrrole-2 carboxamides [345]. In brief, despite extensive structural and functional studies, there is not a consensus on the the exact binding site of diamide insecticides. It is also possible that the amino acids in the diamide binding sites of RyRs vary amongst species [56,107,115,116,360,362].
The main goal of identifying diamide binding sites in insect RyRs is related to the development of insecticide resistance and whether there are mutations in these regions that inhibit binding of diamides leading to resistance. Diamide resistance appears to have developed very rapidly as a result of their extensive use due to the lack of alternatives with similar efficacy [363,364]. The initial reports on the development of resistance from field-collected populations have come from Adoxophyes honmai (Lepidoptera: Tortricidae) against flubendiamide in Japan [365], Choristoneura rosaceana (Lepidoptera: Tortricidae) against chlorantraniliprole in the U.S.A. [366], and Aphis gossypii (Hemiptera: Aphididae) against cyantraniliprole in Italy [347], all collected from the field in 2007. This was followed by reports of resistance developed by P. xylostella [367], S. litura [368], and S. exigua against clorantraniliprole in China [369,370], as well as by B. tabaci against both clorantraniliprole and cyantraniliprole in the U.S.A. [371], with field collection in 2008 and 2009 for all. In 2010, field-collected samples showed further cases of resistance by P. xylostella against flubendiamide and/or clorantraniliprole in Thailand [372] and China [373,374]. In the same year, resistance against clorantraniliprole was found in A. honmai in Japan [365] and C. suppressalis in China [375]. Field populations of at least six lepidopteran species (P. xylostella, C. suppressalis, T. absoluta, A. honmai, S. exigua, and S. frugiperda) and two hemipterans (A. gossypii and B. tabaci) from 11 countries including Brazil, China, Greece, Italy, Japan, Korea, Mexico, Phillippines, Puerto Rico, Spain, and Thailand have developed moderate to significant levels of resistance (relative ratio ≥10) to diamides (Table 3) [44,47,130,347,358,365,368,369,370,372,373,374,375,376,377,378,379,380,381,382,383,384,385,386,387,388,389,390,391,392,393,394,395,396]. The highest resistance ratios (RRs) 519,157-fold for flubendiamide [387], 288,995-fold for clorantraniliprole [385], 18,423-fold for cyantraniliprole [385], and 11,250-fold for cyclaniliprole [390] (Table 3). The highest resistance levels against flubendiamide were recorded for P. xylostella populations in Brazil [387] and that against cyclaniliprole for S. exigua in Korea [390]. Resistance against clorantraniliprole and cyantraniliprole developed in T. absoluta in Brazil [385] (Table 3). On the other hand, lower levels of resistance (Relative Ratio ≤10) have also been reported from various pests, such as C. medinalis against chlorantraniliprole [397], Chrysodeixis includens against flubendiamide and chlorantraniliprole [398], or by non-lepidopteran species, such as B. dorsalis [399] or the aphids A. gossypii, and M. persicae [347] against cyantraniliprole or whitefly B. tabaci against chlorantraniliprole and cyantraniliprole [371]. It is noteworthy that cross-resistance within or between each class of diamides have been also reported [384,400,401,402,403]. This is problematic for new diamides. An investigation on a new diamide, tetraniliprole, in China, which has not been registered yet, revealed that RRs in Chinese field populations of S. exigua compared to a susceptible strain were found to be 8.6–128.1, in parallel to the RRs obtained for chlorantraniliprole [394]. This suggests that chlorantraniliprole has cross-resistance with tetraniliprole, as tetraniliprole has not been used in China. Overall, inseciticide resistance management plans should avoid of rotation of anthranilic and phthalic acid diamides [336,404].

Table 3.
Resistance developed by field-populations against diamides to date.
Detailed examination of RyRs from field-collected or lab-selected resistant strains revealed mutations that affected residues located in the C-terminal transmembrane spanning domains [358,362,373,376], in accordance with this region being a binding site for diamides. Most of these studies were conducted in P. xylostella, but to a lesser extent in T. absoluta and C. suppressalis, S. exigua, and S. frugiperda. Four mutations in insect RyRs are associated with diamide resistance; 1) G4946E/V located at the interface between transmemrane domain 4 (TM4) and the TM4-TM5 linker (numbering is based on PxRyR), 2) I4790M/T within the upper TM2 or TM3, 3) E1338D at the N-terminus, and 4) Q4594L in a flexible loop located in DR1 before the pseudo voltage-sensor domain [47,48,107,109,358,362,373,376,378,381,389,393,405]. Ligand binding assays showed that the binding affinity of chlorantraniliprole to native microsomal membranes from field-resistant populations with the G4946E mutation was significantly lower than that in the susceptible strains [358,362]. In another study, binding and efficacy of both flubendiamide and chlorantraniliprole were dramatically impaired in recombinant P. xylostella RyR with the G4946E mutation, while affinity to other ligands, such as caffeine or ryanodine, did not change [109]. In a relatively recent study, CRISPR/Cas9 genome-modified S. exigua larvae with the G4946E mutation exhibited 223-, 336-, and >1000-fold increase in resistance to chlorantraniliprole, cyantraniliprole and flubendiamide, respectively [402]. Similarly, CRISPR/Cas9 modified D. melanogaster flies with the G4946V mutation were also found to exhibit high levels of resistance against flubendiamide (RR: 91.3) and chlorantraniliprole (RR:195), but less so against cyantraniliprole (RR:5.4) [405], further indicating the importance of this mutation for diamide resistance. Studies using a recombinant D. melanogaster RyR with G4946E mutation expressed in Sf9 cells revealed that this mutation confers a high degree of resistance also against pyrrole-2-carboxamides [345]. It is noteworthy that the glycine at position 4946 is conserved amongst insect species, except in the dipteran midge Belgica antarctica, the mite Tetranychus urticae and the hemipteran mealybug Ferrisia virgata [63]. The replacement of glycine with a glutamic acid or valine in the resistant strains is likely to have a major impact on the movement of the S5 and S6 helices, which control opening and closing of the RyR channel pore, leading to an inhibition or decrease in the binding of diamide insecticides to the channel [109,331]. On the other hand, D. melanogaster flies naturally wild-type for the I4790M mutation exhibit low to moderate resistance to diamides, while the M4790I mutation leads to higher levels of susceptibility to flubendiamide (RR: −15.3 fold), but less to chlorantraniliprole (RR: −7.5) and cyantraniliprole (RR: −2.3) [405]. As mentioned in Section 3. Structure, the isoleucine residue at position 4790 is specific to lepidopterans (in contrast to commonly conserved G4946 in insects) as is a methionine in D. melanogaster and all other insects and arachnids, suggesting I4790 might be responsible for the differential sensitivities of the P. xylostella, T. absoluta, and possibly beetles and other insects to diamides [63,115,116,358,363,373,405]. Homology models of the PxRyR based on rabbit RyR1 indicated that the I4790M mutation in TM2 is located directly opposite to the G4946E mutation (the distance between the two residues is only ~15 Å) in the pseudo voltage sensor domain, suggesting that these two regions might define the diamide binding sites on the receptor [107,109,331,358,362]. The model of PxRyR by Lin et al. [107] further indicated that G4946 is near the entrance to the pocket and that the mutation to glutamatic acid narrows the entrance to the pocket, whereas I4790 is located deep in the pocket and the mutation to methionine makes the pocket shallower. The study by Douris et al. [405] also indicates that G4946V mutations confers very high levels of resistance as the RR of the G4946V mutants to M4790I susceptible mutants is 1400 and 1465 for flubendiamide and chlorantraniliprole, respectively, suggesting both mutations may contribute synergistically to the overall resistance phenotype [406]. Regarding the Q4594L mutation, Q4594 is conserved amongst lepidopterans, while I4790 is lysine in D. melanogaster and coleopterans, hymentopterans and some other Dipterans; however, its involvement in diamide binding is not currently known, other than it being mutated in resistant populations [63,373]. The same is valid for E1338, which is located in the insect divergent region 2 (IDR2) between SPRY2 and SPRY3 domains and appears not to be conserved in insects [63,107]. A recent study on a Chinese field population of C. suppressalis resistant to chlorantraniliprole revealed a new mutation Y4667D/C (corresponding to Y4701 in PxRyR), which might confer to high levels of resistance [44]. However, the functional importance of the Y4667D/C, the E1338D and the Q4594L mutations has not been demonstrated to date.
Other mechanisms might also confer to diamide resistance; this includes metabolic resistance and down-regulation of RyR. Metabolic resistance against inseciticides develops through elevated levels of detoxification enzymes, such as cytochrome P450 monooxygenases (P450), glutathione S-transferases (GST) and esterases. The synergistics, piperonyl butoxide (PBO) an inhibitor of P450, diethyl maleate (DEM) a depletor of glutathione, S,S,S-tributylphosphorothioate (DEF) an esterase inhibitor, and triphenyl phosphate (TPP) a carboxylesterase inhibitor, lowered the LC50/LD50 values of chlorantraniliprole in L. decemlineata [407], P. xylostella [130], C. suppressalis [44] and S. frugiperda [48]. Additionally, higher levels of cytochrome P450 enzyme and esterases were reported from laboratory strains selected with chlorantraniliprole [44,408,409]. Similarly, transcriptomic profile of chlorantraniliprole-resistant field populations of P. xylostella revealed that most of the metabolic detoxification enzyme genes were slightly up-regulated [410]. Up-regulation of cytochrome P450 genes by chlorantraniliprole or an increase in the chlorantraniliprole-linked mortality upon silencing of a cytochrome P450 gene have been also reported [411,412,413]. In contrast, synergism tests and biochemical assays showed no obvious correlations between diamide resistance and three detoxifying enzymes in C. suppressalis [389] and S. exigua [369]. It is noteworthy indicating that a detoxification mechanism via the ATP-binding cassette (ABC) transporters is also possible [345,414,415]. Down-regulation of RyR might also be a possible resistance mechanism to diamide insecticides, which was demonstrated via RNAi in S. furcifera [53] and L. decemlineata [51]. Down-regulation of RyR led to a decrease in the diamide efficacy. In another study, RyR was found to be slightly down-regulated in P. xylostella populations with lower to moderate levels of resistance (RR: 6–35 fold) against chlorantraniliprole, while the gene was significantly down-regulated in a population with high levels of resistance (RR:1750-fold) [410]. Similarly, RyR was down-regulated in C. suppressalis upon treatment with chlorantraniliprole [44]. Down-regulation of RyR might slow the release and depletion of intracellular Ca2+ stores from the SR in muscles and the ER of many cell types when induced by RyR activators, and consequently enhances resistance to diamide insecticides [53]. It is noteworthy that there are cases reporting over-expression of RyR genes in chlorantraniliprole-resistant populations or up-regulation induced by diamides [38,64,416].
As mentioned before, studies on IP3Rs as targets in pest control are limited due to their higher similarity with their mammalian counterparts. Nevertheless, a single study has examined the role of IP3R in diamide resistance. Interestingly, silencing IP3R in B. tabaci adults dramatically decreased susceptibility to cyantraniliprole [62], similar to the decreased chlorantraniliprole-induced mortality upon RyR silencing in S. furcifera [53] and L. decemlineata [51]. It is interesting that continuous administration of cyantraniliprole down-regulates IP3R expression during the entire period of the treatment in B. tabaci, which might be a strategy to adjust the RyR-linked increase in intracellular Ca2+ and decreased ER Ca2+ levels [62]. However, this topic requires further investigation.
There might be other pest control tools targeting cellular Ca2+ homeostasis and interfering with IP3R and RyR. Botanicals, entomopathogens, repellents, toxins, Ca2+ inhibitors or biomolecules such as dsRNA or miRNAs or peptide agonists or antagonists are promising in this regard. For example, Ma et al. [417] examined the effect of wilforine, a novel botanical insecticide from the root bark of thunder duke vine, Tripterygium wilfordii (Celastraceae) [418] on Mythimna separate (Lepidoptera: Noctuidae). This investigation revealed that wilforine acts on myocytes leading to an increase in cytosolic Ca2+ levels when applied at nanomolar levels and activates both RyR and IP3R based on use of specific inhibitors of both channel proteins [417]. Similarly, both IP3R and RyR in neurons are activated by the botanical insecticide Celangulin I, extracted from Chinese bittersweet Celastrus angulatus, another species from Celastraceae [419]. Other biological agents, such as entomopathogenic viruses, or repellents, such as DEET, or bacterial toxins, such as Bacillus thuringiensis Cry toxins might also interfere directly or indirectly with Ca2+ signaling and intracellular Ca2+ levels [420,421,422,423,424,425,426,427,428,429]. Development of dsRNA-based insecticides interfering with cellular Ca2+ homeostasis also has great potential in this manner [10,430,431,432]. Co-application of the agents above with diamides might also have a potential within a combined tactic, which also requires further investigation.
7. Conclusions
In conclusion, Ca2+ homeostasis is vital for insects, and the ER is one of the major intracellular sources for Ca2+. The RyR and IP3R are the two channel proteins associated with the ER and are involved in the intracellular Ca2+ supply. Insects possess a single RyR and IP3R gene, in contrast to mammalians which possess three for each. Both RyR and IP3Rs cluster separately in phylogenetic analyses; however, they share common domains, such as the MIR, RIH, RIH-associated regulatory domains at the amino-terminus, and transmembrane helices at the carboxy-terminus. Alternative splicing, which is regulated in a tissue-specific and developmental manner, occurs for both genes and each receptor has its own, distinct, regulatory mechanism. IP3R genes are expressed in most cells, in particular in the ER of neurons, adipocytes, and oocytes, while RyR gene expression has a more restricted distribution and is predominantly found in the SR of muscle cells and the ER of neurons. Both receptors have essential roles in insect physiology and development. RyRs mediate many cellular and physiological activities related to muscle contraction and hormone secretion, while IP3Rs are involved in key events related to learning, memory, neuronal signaling, lipid metabolism, and sensory transduction. Efforts have concentrated on the development of pest control strategies targeting the operation of RyRs and IP3Rs; however, RyRs appear to be safer targets due to their lower similarity with mammalian counterparts compared to IP3Rs. Diamides are the best examples of a pest control chemistry targeting RyRs, although resistance developed by pests against diamides has become an increasing issue. Various pest control tactics based on use of botanicals, microbials and toxins, as well as biomolecules such as dsRNA and miRNAs, targeting cellular Ca2+ homeostasis and affecting the operation of RyRs and/or IP3Rs directly or indirectly might be also promising.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biom11071031/s1, Table S1: Proteins used in the phylogenetic analysis and alignments in the current review.
Author Contributions
Conceptualization, U.T.; investigation, U.T. and C.D.; writing—original draft preparation, U.T. and C.D.; writing—review and editing, U.T., C.D. and D.H.; visualization, U.T. and C.D.; supervision, U.T.; project administration, U.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to acknowledge Oyak Biyoteknoloji [Oyak Biotech Co.], Turkey for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Taylor, W.C. Calcium regulation in insects. Adv. Insect Physiol. 1987, 19, 155–186. [Google Scholar] [CrossRef]
- Gu, S.H.; Chow, Y.S.; O’Reilly, D.R. Role of calcium in the stimulation of ecdysteroidogenesis by recombinant prothoracicotropic hormone in the prothoracic glands of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 1998, 28, 861–867. [Google Scholar] [CrossRef]
- Takeo, S.; Tsuda, M.; Akahori, S.; Matsuo, T.; Aigaki, T. The calcineurin regulator sra plays an essential role in female meiosis in Drosophila. Curr. Biol. 2006, 16, 1435–1440. [Google Scholar] [CrossRef]
- Yoshiga, T.; Yokoyama, N.; Imai, N.; Ohnishi, A.; Moto, K.; Matsumoto, S. cDNA cloning of calcineurin heterosubunits from the pheromone gland of the silkmoth, Bombyx mori. Insect Biochem. Mol. Biol. 2002, 32, 477–486. [Google Scholar] [CrossRef]
- Teets, N.M.; Yi, S.X.; Lee, R.E.; Denlinger, D.L., Jr. Calcium signaling mediates cold sensing in insect tissues. Proc. Natl. Acad. Sci. USA 2013, 110, 9154–9159. [Google Scholar] [CrossRef] [PubMed]
- Bronk, P.; Kuklin, E.A.; Gorur-Shandilya, S.; Liu, C.; Wiggin, T.D.; Reed, M.L.; Marder, E.; Griffith, L.C. Regulation of eag by Ca2+/calmodulin controls presynaptic excitability in Drosophila. J. Neurophysiol. 2018, 119, 1665–1680. [Google Scholar] [CrossRef]
- Bahk, S.; Jones, W.D. Insect odorant receptor trafficking requires calmodulin. BMC Biol. 2016, 14, 83. [Google Scholar] [CrossRef]
- Pallen, C.J.; Steele, J.E. A putative role for calmodulin in corpus cardiacum stimulated trehalose synthesis in fat body of the american cockroach (Periplaneta americana). Insect Biochem. 1988, 18, 577–584. [Google Scholar] [CrossRef]
- Doğan, C.; Hänniger, S.; Heckel, D.G.; Coutu, C.; Hegedus, D.D.; Crubaugh, L.; Groves, R.L.; Mutlu, D.A.; Suludere, Z.; Bayram, Ş.; et al. Characterization of calcium signaling proteins from the fat body of the Colorado Potato Beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae): Implications for diapause and lipid metabolism. Insect Biochem. Mol. Biol. 2021, 133, 103549. [Google Scholar] [CrossRef]
- Doğan, C.; Hänniger, S.; Heckel, D.G.; Coutu, C.; Hegedus, D.D.; Crubaugh, L.; Groves, R.L.; Bayram, Ş.; Toprak, U. Two calcium-binding chaperones from the fat body of the Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) involved in diapause. Arch. Insect Biochem. Physiol. 2021, 106, e21755. [Google Scholar] [CrossRef]
- Bootman, M.D.; Lipp, P.; Berridge, M.J. The organization and functions of local Ca2+ signales. J. Cell Sci. 2001, 114, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.M.; Taylor, C.W. IP3 receptors-lessons from analyses ex cellula. J. Cell Sci. 2018, 132, jcs222463. [Google Scholar] [CrossRef]
- Pizzo, P.; Lissandron, V.; Capitanio, P.; Pozzan, T. Ca2+ signalling in the Golgi apparatus. Cell Calcium 2011, 50, 184–192. [Google Scholar] [CrossRef]
- Glitsch, M.D.; Bakowski, D.; Parekh, A.B. Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake. EMBO J. 2002, 21, 6744–6754. [Google Scholar] [CrossRef]
- Haller, T.; Dietl, P.; Deetjen, P.; Völkl, H. The lysosomal compartment as intracellular calcium store in MDCK cells: A possible involvement in InsP3-mediated Ca2+ release. Cell Calcium 1996, 19, 157–165. [Google Scholar] [CrossRef]
- Fill, M.; Copello, J.A. Ryanodine receptor calcium release channels. Physiol. Rev. 2002, 82, 893–922. [Google Scholar] [CrossRef]
- Kobrinsky, E.; Ondrias, K.; Marks, A.R. Expressed ryanodine receptor can substitute for the inositol 1,4,5-trisphosphate receptor in Xenopus laevis oocytes during progesterone-induced maturation. Dev. Biol. 1995, 172, 531–540. [Google Scholar] [CrossRef][Green Version]
- Hamilton, S.L.; Serysheva, I.I. Ryanodine receptor structure: Progress and challenges. J. Biol. Chem. 2009, 284, 4047–4051. [Google Scholar] [CrossRef] [PubMed]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef] [PubMed]
- Paknejad, N.; Hite, R.K. Structural basis for the regulation of inositol trisphosphate receptors by Ca2+ and IP3. Nat. Struct. Mol Biol. 2018, 25, 660–668. [Google Scholar] [CrossRef]
- Prole, D.L.; Taylor, C.W. Structure and Function of IP3 Receptors. Cold Spring Harb. Perspect. Biol. 2019, 11, a035063. [Google Scholar] [CrossRef]
- Beutner, G.; Sharma, V.K.; Giovannucci, D.R.; Yule, D.I.; Sheu, S.S. Identification of a ryanodine receptor in rat heart mitochondria. J. Biol. Chem. 2001, 276, 21482–21488. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Beutner, G.; Dirksen, R.T.; Kinnally, K.W.; Sheu, S.S. Mitochondrial ryanodine receptors and other mitochondrial Ca2+ permeable channels. FEBS Lett. 2010, 584, 1948–1955. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, D.; He, X.; Huang, Y.; Shao, H. Transport of calcium ions into mitochondria. Curr. Genom. 2016, 17, 215–219. [Google Scholar] [CrossRef]
- Roest, G.; La Rovere, R.M.; Bultynck, G.; Parys, J.B. IP3 receptor properties and function at membrane contact sites. Adv. Exp. Med. Biol. 2017, 981, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Cremer, T.; Neefjes, J.; Berlin, I. The journey of Ca2+ through the cell—Pulsing through the network of ER membrane contact sites. J. Cell Sci. 2020, 133, jcs249136. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.D.; Velamakanni, S.; Ishiyama, N.; Stathopulos, P.B.; Rossi, A.M.; Khan, S.A.; Dale, P.; Li, C.; Ames, J.B.; Ikura, M.; et al. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature 2012, 483, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, H.; Nishimura, S.; Matsumoto, T.; Ishida, H.; Kangawa, K.; Minamino, N.; Matsuo, H.; Ueda, M.; Hanaoka, M.; Hirose, T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 1989, 339, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Otsu, K.; Willard, H.F.; Khanna, V.K.; Zorzato, F.; Green, N.M.; MacLennan, D.H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 13472–13483. [Google Scholar] [CrossRef]
- Hakamata, Y.; Nakai, J.; Takeshima, H.; Imoto, K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett. 1992, 312, 229–235. [Google Scholar] [CrossRef]
- Hasan, G.; Rosbash, M. Drosophila homologs of two mammalian intracellular Ca(2+)-release channels: Identification and expression patterns of the inositol 1,4,5-triphosphate and the ryanodine receptor genes. Development 1992, 116, 967–975. [Google Scholar] [CrossRef]
- Takeshima, H.; Nishi, M.; Iwabe, N.; Miyata, T.; Hosoya, T.; Masai, I.; Hotta, Y. Isolation and characterization of a gene for a ryanodine receptor/calcium release channel in Drosophila melanogaster. FEBS Lett. 1994, 337, 81–87. [Google Scholar] [CrossRef]
- Puente, E.; Suner, M.; Evans, A.D.; McCaffery, A.R.; Windass, J.D. Identification of a polymorphic ryanodine receptor gene from Heliothis virescens (Lepidoptera: Noctuidae). Insect Biochem. Mol. Biol. 2000, 30, 335–347. [Google Scholar] [CrossRef]
- Scott-Ward, T.S.; Dunbar, S.J.; Windass, J.D.; Williams, A.J. Characterization of the ryanodine receptor-Ca2+ release channel from the thoracic tissues of the lepidopteran insect Heliothis virescens. J. Membr. Biol. 2001, 179, 127–141. [Google Scholar] [CrossRef]
- Kato, K.; Kiyonaka, S.; Sawaguchi, Y.; Tohnishi, M.; Masaki, T.; Yasokawa, N.; Mizuno, Y.; Mori, E.; Inoue, K.; Hamachi, I.; et al. Molecular characterization of flubendiamide sensitivity in the lepidopterous ryanodine receptor Ca(2+) release channel. Biochemistry 2009, 48, 10342–10352. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Han, Z.; Zhu, Y.; Xie, Z.; Wang, J.; Liu, Y.; Li, X. Molecular characterization of a ryanodine receptor gene in the rice leaffolder, Cnaphalocrocis medinalis (Guenée). PLoS ONE 2012, 7, e36623. [Google Scholar] [CrossRef]
- Sun, L.; Cui, L.; Rui, C.; Yan, X.; Yang, D.; Yuan, H. Modulation of the expression of ryanodine receptor mRNA from Plutella xylostella as a result of diamide insecticide application. Gene 2012, 511, 265–273. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Yang, Y.; Wu, Y. Molecular cloning, characterization and mRNA expression of a ryanodine receptor gene from diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2012, 102, 204–212. [Google Scholar] [CrossRef]
- Cui, L.; Yang, D.; Yan, X.; Rui, C.; Wang, Z.; Yuan, H. Molecular cloning, characterization and expression profiling of a ryanodine receptor gene in Asian corn borer, Ostrinia furnacalis (Guenée). PLoS ONE 2013, 8, e75825. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Gao, J.; Xie, Z.; Huang, L.; Wang, W.; Wang, J. Molecular cloning and mRNA expression of a ryanodine receptor gene in the cotton bollworm, Helicoverpa armigera. Pestic. Biochem. Physiol. 2013, 107, 327–333. [Google Scholar] [CrossRef]
- Wu, S.; Wang, F.; Huang, J.; Fang, Q.; Shen, Z.; Ye, G. Molecular and cellular analyses of a ryanodine receptor from hemocytes of Pieris rapae. Dev. Comp. Immunol. 2013, 41, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Shahzad, M.F.; Zhang, L.; Li, F.; Lin, K. Amplifying long transcripts of ryanodine receptors of five agricultural pests by transcriptome analysis and gap filling. Genome 2013, 56, 651–658. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, L.; Chen, Q.; Qin, W.; Huang, S.; Jiang, Y.; Qin, H. Chlorantraniliprole resistance and its biochemical and new molecular target mechanisms in laboratory and field strains of Chilo suppressalis (Walker). Pest Manag. Sci. 2018, 74, 1416–1423. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, G.; Cui, L.; Ma, C.; Yuan, H. Molecular characterization of a ryanodine receptor gene from Spodoptera exigua and its upregulation by chlorantraniliprole. Pestic. Biochem. Physiol. 2015, 123, 56–63. [Google Scholar] [CrossRef]
- Sun, L.N.; Zhang, H.J.; Quan, L.F.; Yan, W.T.; Yue, Q.; Li, Y.Y.; Qiu, G.S. Characterization of the ryanodine receptor gene with a unique 3’-UTR and alternative splice site from the oriental fruit moth. J. Insect Sci. 2016, 16, 16. [Google Scholar] [CrossRef]
- Roditakis, E.; Steinbach, D.; Moritz, G.; Vasakis, E.; Stavrakaki, M.; Ilias, A.; Garcia-Vidal, L. Ryanodine receptor point mutations confer diamide insecticide resistance in tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Insect Biochem. Mol. Biol. 2017, 80, 11–20. [Google Scholar] [CrossRef]
- Boaventura, D.; Bolzan, A.; Padovez, F.E.; Okuma, D.M.; Omoto, C.; Nauen, R. Detection of a ryanodine receptor target-site mutation in diamide insecticide resistant fall armyworm, Spodoptera frugiperda. Pest Manag. Sci. 2020, 76, 47–54. [Google Scholar] [CrossRef]
- Yuan, G.R.; Shi, W.Z.; Yang, W.J.; Jiang, X.Z.; Dou, W.; Wang, J.J. Molecular characteristics, mRNA expression, and alternative splicing of a ryanodine receptor gene in the oriental fruit fly, Bactrocera dorsalis (Hendel). PLoS ONE 2014, 9, e95199. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Gao, J.; Wang, W.; Huang, L.; Guo, X.; Li, B.; Wang, J. Comparative characterization of two intracellular Ca2+-release channels from the red flour beetle, Tribolium castaneum. Sci. Rep. 2014, 4, 6702. [Google Scholar] [CrossRef]
- Wan, P.J.; Guo, W.Y.; Yang, Y.; Lü, F.G.; Lu, W.P.; Li, G.Q. RNAi suppression of the ryanodine receptor gene results in decreased susceptibility to chlorantraniliprole in Colorado potato beetle Leptinotarsa decemlineata. J. Insect Physiol. 2014, 63, 48–55. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Z.; Gao, J.; Liu, Y.; Wang, W.; Huang, L.; Wang, J. Molecular cloning and characterization of a ryanodine receptor gene in brown planthopper (BPH), Nilaparvata lugens (Stål). Pest Manag. Sci. 2014, 70, 790–797. [Google Scholar] [CrossRef]
- Yang, Y.; Wan, P.J.; Hu, X.X.; Li, G.Q. RNAi mediated knockdown of the ryanodine receptor gene decreases chlorantraniliprole susceptibility in Sogatella furcifera. Pestic. Biochem. Physiol. 2014, 108, 58–65. [Google Scholar] [CrossRef]
- Troczka, B.J.; Williams, A.J.; Bass, C.; Williamson, M.S.; Field, L.M.; Davies, T.G. Molecular cloning, characterisation and mRNA expression of the ryanodine receptor from the peach-potato aphid, Myzus persicae. Gene 2015, 556, 106–112. [Google Scholar] [CrossRef]
- Wang, K.Y.; Jiang, X.Z.; Yuan, G.R.; Shang, F.; Wang, J.J. Molecular Characterization, mRNA expression and alternative splicing of ryanodine receptor gene in the brown citrus aphid, Toxoptera citricida (Kirkaldy). Int. J. Mol. Sci. 2015, 16, 15220–15234. [Google Scholar] [CrossRef]
- Yuan, G.R.; Wang, K.Y.; Mou, X.; Luo, R.Y.; Dou, W.; Wang, J.J. Molecular cloning, mRNA expression and alternative splicing of a ryanodine receptor gene from the citrus whitefly, Dialeurodes citri (Ashmead). Pestic. Biochem. Physiol. 2017, 142, 59–66. [Google Scholar] [CrossRef]
- Supattapone, S.; Worley, P.F.; Baraban, J.M.; Snyder, S.H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J. Biol. Chem. 1988, 263, 1530–1534. [Google Scholar] [CrossRef]
- Furuichi, T.; Yoshikawa, S.; Miyawaki, A.; Wada, K.; Maeda, N.; Mikoshiba, K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature 1989, 342, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C.; Newton, C.L.; Archer, B.T., 3rd; Ushkaryov, Y.A.; Mignery, G.A. Structure of a novel InsP3 receptor. EMBO J. 1991, 10, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Blondel, O.; Takeda, J.; Janssen, H.; Seino, S.; Bell, G.I. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J. Biol. Chem. 1993, 268, 11356–11363. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Tanimura, T.; Miyawaki, A.; Nakamura, M.; Yuzaki, M.; Furuichi, T.; Mikoshiba, K. Molecular cloning and characterization of the inositol 1,4,5-trisphosphate receptor in Drosophila melanogaster. J. Biol. Chem. 1992, 267, 16613–16619. [Google Scholar] [CrossRef]
- Guo, L.; Liang, P.; Fang, K.; Chu, D. Silence of inositol 1,4,5-trisphosphate receptor expression decreases cyantraniliprole susceptibility in Bemisia tabaci. Pestic. Biochem. Physiol. 2017, 142, 162–169. [Google Scholar] [CrossRef]
- Troczka, B.J.; Richardson, E.; Homem, R.A.; Davies, T.G.E. An analysis of variability in genome organisation of intracellular calcium release channels across insect orders. Gene 2018, 670, 70–86. [Google Scholar] [CrossRef]
- Peng, Y.C.; Sheng, C.W.; Casida, J.E.; Zhao, C.Q.; Han, Z.J. Ryanodine receptor genes of the rice stem borer, Chilo suppressalis: Molecular cloning, alternative splicing and expression profiling. Pestic. Biochem. Physiol. 2017, 135, 69–77. [Google Scholar] [CrossRef]
- Xu, X.; Bhat, M.B.; Nishi, M.; Takeshima, H.; Ma, J. Molecular cloning of cDNA encoding a drosophila ryanodine receptor and functional studies of the carboxyl-terminal calcium release channel. Biophys. J. 2000, 78, 1270–1281. [Google Scholar] [CrossRef]
- Hamilton, S.L. Ryanodine receptors. Cell Calcium 2005, 38, 253–260. [Google Scholar] [CrossRef]
- Mikoshiba, K. The IP3 receptor/Ca2+ channel and its cellular function. Biochem. Soc. Symp. 2007, 74, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.C.; Lobo, P.A.; Kimlicka, L.; Van Petegem, F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature 2010, 468, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Yuchi, Z.; Lau, K.; Van Petegem, F. Disease mutations in the ryanodine receptor central region: Crystal structures of a phosphorylation hot spot domain. Structure 2012, 20, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Van Petegem, F. Crystal structures of wild type and disease mutant forms of the ryanodine receptor SPRY2 domain. Nat. Commun. 2014, 5, 5397. [Google Scholar] [CrossRef] [PubMed]
- Yuchi, Z.; Yuen, S.M.; Lau, K.; Underhill, A.Q.; Cornea, R.L.; Fessenden, J.D.; Van Petegem, F. Crystal structures of ryanodine receptor SPRY1 and tandem-repeat domains reveal a critical FKBP12 binding determinant. Nat. Commun. 2015, 6, 7947. [Google Scholar] [CrossRef]
- Yan, Z.; Bai, X.; Yan, C.; Wu, J.; Li, Z.; Xie, T.; Peng, W.; Yin, C.; Li, X.; Scheres, S. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 2015, 517, 50–55. [Google Scholar] [CrossRef]
- Zalk, R.; Clarke, O.B.; des Georges, A.; Grassucci, R.A.; Reiken, S.; Mancia, F.; Hendrickson, W.A.; Frank, J.; Marks, A.R. Structure of a mammalian ryanodine receptor. Nature 2015, 517, 44–49. [Google Scholar] [CrossRef]
- Bosanac, I.; Yamazaki, H.; Matsu-Ura, T.; Michikawa, T.; Mikoshiba, K.; Ikura, M. Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Mol. Cell. 2005, 17, 193–203. [Google Scholar] [CrossRef]
- Chan, J.; Whitten, A.E.; Jeffries, C.M.; Bosanac, I.; Mal, T.K.; Ito, J.; Porumb, H.; Michikawa, T.; Mikoshiba, K.; Trewhella, J.; et al. Ligand-induced conformational changes via flexible linkers in the amino-terminal region of the inositol 1,4,5-trisphosphate receptor. J. Mol. Biol. 2007, 373, 1269–1280. [Google Scholar] [CrossRef]
- Lin, C.C.; Baek, K.; Lu, Z. Apo and InsP3-bound crystal structures of the ligand-binding domain of an InsP3 receptor. Nat. Struct. Mol. Biol. 2011, 18, 1172–1174. [Google Scholar] [CrossRef]
- Ludtke, S.J.; Tran, T.P.; Ngo, Q.T.; Moiseenkova-Bell, V.Y.; Chiu, W.; Serysheva, I.I. Flexible architecture of IP3R1 by Cryo-EM. Structure 2011, 19, 1192–1199. [Google Scholar] [CrossRef]
- Li, C.; Enomoto, M.; Rossi, A.M.; Seo, M.; Rahman, T.; Stathopulos, P.B.; Taylor, C.W.; Ikura, M.; Ames, J.B. CaBP1, a neuronal Ca2+ sensor protein, inhibits inositol trisphosphate receptors by clamping intersubunit interactions. Proc. Natl. Acad. Sci. USA 2013, 110, 8507–8512. [Google Scholar] [CrossRef]
- Des Georges, A.; Clarke, O.B.; Zalk, R.; Yuan, Q.; Condon, K.J.; Grassucci, R.A.; Hendrickson, W.A.; Marks, A.R.; Frank, J. Structural Basis for gating and activation of RyR1. Cell 2016, 167, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Baker, M.R.; Wang, Z.; Seryshev, A.B.; Ludtke, S.J.; Baker, M.L.; Serysheva, I.I. Cryo-EM reveals ligand induced allostery underlying InsP3R channel gating. Cell Res. 2018, 28, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Bosanac, I.; Alattia, J.R.; Mal, T.K.; Chan, J.; Talarico, S.; Tong, F.K.; Tong, K.I.; Yoshikawa, F.; Furuichi, T.; Iwai, M.; et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature 2002, 420, 696–700. [Google Scholar] [CrossRef]
- Uchida, K.; Miyauchi, H.; Furuichi, T.; Michikawa, T.; Mikoshiba, K. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2003, 278, 16551–16560. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Wang, Z.; Tu, H.; Nair, S.; Mathew, M.K.; Hasan, G.; Bezprozvanny, I. Functional properties of the Drosophila melanogaster inositol 1,4,5-trisphosphate receptor mutants. Biophys. J. 2004, 86, 3634–3646. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Yamazaki, H.; Ishiyama, N.; Seo, M.D.; Mal, T.K.; Michikawa, T.; Mikoshiba, K.; Ikura, M. Structural studies of inositol 1,4,5-trisphosphate receptor: Coupling ligand binding to channel gating. J. Biol. Chem. 2010, 285, 36092–36099. [Google Scholar] [CrossRef]
- Yuchi, Z.; Van Petegem, F. Common allosteric mechanisms between ryanodine and inositol-1,4,5-trisphosphate receptors. Channels 2011, 5, 120–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rossi, A.M.; Riley, A.M.; Tovey, S.C.; Rahman, T.; Dellis, O.; Taylor, E.J.; Veresov, V.G.; Potter, B.V.; Taylor, C.W. Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat. Chem. Biol. 2009, 5, 631–639. [Google Scholar] [CrossRef]
- Seo, M.D.; Enomoto, M.; Ishiyama, N.; Stathopulos, P.B.; Ikura, M. Structural insights into endoplasmic reticulum stored calcium regulation by inositol 1,4,5-trisphosphate and ryanodine receptors. Biochim. Biophys. Acta 2015, 1853, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.B.; Zhao, J.; Takeshima, H.; Ma, J. Functional calcium release channel formed by the carboxyl-terminal portion of ryanodine receptor. Biophys. J. 1997, 73, 1329–1336. [Google Scholar] [CrossRef]
- Rossi, D.; Sorrentino, V. Molecular genetics of ryanodine receptors Ca2+-release channels. Cell Calcium 2002, 32, 307–319. [Google Scholar] [CrossRef]
- Ponting, C.; Schultz, J.; Bork, P. SPRY domains in ryanodine receptors (Ca(2+)-release channels). Trends Biochem. Sci. 1997, 22, 193–194. [Google Scholar] [CrossRef]
- Sorrentino, V.; Barone, V.; Rossi, D. Intracellular Ca(2+) release channels in evolution. Curr. Opin. Genet. Dev. 2000, 10, 662–667. [Google Scholar] [CrossRef]
- Santulli, G.; Nakashima, R.; Yuan, Q.; Marks, A.R. Intracellular calcium release channels: An update. J. Physiol. 2017, 595, 3041–3051. [Google Scholar] [CrossRef] [PubMed]
- Ikenoue, T.; Inoki, K.; Yang, Q.; Zhou, X.; Guan, K.L. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008, 27, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P. Novel repeats in ryanodine and IP3 receptors and protein O-mannosyltransferases. Trends Biochem. Sci. 2000, 25, 48–50. [Google Scholar] [CrossRef]
- Woo, J.S.; Suh, H.Y.; Park, S.Y.; Oh, B.H. Structural basis for protein recognition by B30.2/SPRY domains. Mol. Cell. 2006, 24, 967–976. [Google Scholar] [CrossRef]
- Cui, Y.; Tae, H.S.; Norris, N.C.; Karunasekara, Y.; Pouliquin, P.; Board, P.G.; Dulhunty, A.F.; Casarotto, M.G. A dihydropyridine receptor α1s loop region critical for skeletal muscle contraction is intrinsically unstructured and binds to a SPRY domain of the type 1 ryanodine receptor. Int. J. Biochem. Cell Biol. 2009, 41, 677–686. [Google Scholar] [CrossRef]
- Callaway, C.; Seryshev, A.; Wang, J.P.; Slavik, K.J.; Needleman, D.H.; Cantu, C.; Wu, Y., 3rd; Jayaraman, T.; Marks, A.R.; Hamilton, S.L. Localization of the high and low affinity [3H]ryanodine binding sites on the skeletal muscle Ca2+ release channel. J. Biol. Chem. 1994, 269, 15876–15884. [Google Scholar] [CrossRef]
- Smith, J.S.; Rousseau, E.; Meissner, G. Calmodulin modulation of single sarcoplasmic reticulum Ca-release channels from cardiac and skeletal muscle. Circ. Res. 1989, 64, 352–359. [Google Scholar] [CrossRef]
- Tripathy, A.; Xu, L.; Mann, G.; Meissner, G. Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor). Biophys. J. 1995, 69, 106–119. [Google Scholar] [CrossRef]
- Wagenknecht, T.; Radermacher, M.; Grassucci, R.; Berkowitz, J.; Xin, H.B.; Fleischer, S. Locations of calmodulin and FK506-binding protein on the three-dimensional architecture of the skeletal muscle ryanodine receptor. J. Biol. Chem. 1997, 272, 32463–32471. [Google Scholar] [CrossRef] [PubMed]
- Balshaw, D.M.; Xu, L.; Yamaguchi, N.; Pasek, D.A.; Meissner, G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J. Biol. Chem. 2001, 276, 20144–20153. [Google Scholar] [CrossRef]
- Sipma, H.; De Smet, P.; Sienaert, I.; Vanlingen, S.; Missiaen, L.; Parys, J.B.; De Smedt, H. Modulation of inositol 1,4,5-trisphosphate binding to the recombinant ligand-binding site of the type-1 inositol 1,4,5-trisphosphate receptor by Ca2+ and calmodulin. J. Biol. Chem. 1999, 274, 12157–12162. [Google Scholar] [CrossRef]
- Lin, L.; Liu, C.; Qin, J.; Wang, J.; Dong, S.; Chen, W.; He, W.; Gao, Q.; You, M.; Yuchi, Z. Crystal structure of ryanodine receptor N-terminal domain from Plutella xylostella reveals two potential species-specific insecticide-targeting sites. Insect Biochem. Mol. Biol. 2018, 92, 73–83. [Google Scholar] [CrossRef]
- Xu, T.; Yuchi, Z. Crystal structure of diamondback moth ryanodine receptor Repeat34 domain reveals insect-specific phosphorylation sites. BMC Biol. 2019, 17, 77. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, D.; Lin, L.; You, M.; Yuchi, Z.; You, S. Crystal Structure of the ryanodine receptor SPRY2 domain from the diamondback moth provides insights into the development of novel insecticides. J. Agric. Food Chem. 2020, 68, 1731–1740. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, W.; Salauddin, N.M.; Lin, L.; You, M.; You, S.; Yuchi, Z. Crystal structure of the N-terminal domain of ryanodine receptor from the honeybee, Apis mellifera. Insect Biochem. Mol. Biol. 2020, 125, 103454. [Google Scholar] [CrossRef]
- Lin, L.; Hao, Z.; Cao, P.; Yuchi, Z. Homology modeling and docking study of diamondback moth ryanodine receptor reveals the mechanisms for channel activation, insecticide binding and resistance. Pest Manag. Sci. 2020, 76, 1291–1303. [Google Scholar] [CrossRef]
- Zhao, M.; Li, P.; Li, X.; Zhang, L.; Winkfein, R.J.; Chen, S.R. Molecular identification of the ryanodine receptor pore-forming segment. J. Biol. Chem. 1999, 274, 25971–25974. [Google Scholar] [CrossRef]
- Troczka, B.J.; Williams, A.J.; Williamson, M.S.; Field, L.M.; Luemmen, P.; Davies, T.G. Stable expression and functional characterisation of the diamondback moth ryanodine receptor G4946E variant conferring resistance to diamide insecticides. Sci. Rep. 2015, 5, 14680. [Google Scholar] [CrossRef]
- Du, G.G.; Guo, X.; Khanna, V.K.; MacLennan, D.H. Functional characterization of mutants in the predicted pore region of the rabbit cardiac muscle Ca(2+) release channel (ryanodine receptor isoform 2). J. Biol. Chem. 2001, 276, 31760–31771. [Google Scholar] [CrossRef] [PubMed]
- Schug, Z.T.; da Fonseca, P.C.; Bhanumathy, C.D.; Wagner, L., 2nd; Zhang, X.; Bailey, B.; Morris, E.P.; Yule, D.I.; Joseph, S.K. Molecular characterization of the inositol 1,4,5-trisphosphate receptor pore-forming segment. J. Biol. Chem. 2008, 283, 2939–2948. [Google Scholar] [CrossRef]
- Gao, L.; Balshaw, D.; Xu, L.; Tripathy, A.; Xin, C.; Meissner, G. Evidence for a role of the lumenal M3-M4 loop in skeletal muscle Ca(2+) release channel (ryanodine receptor) activity and conductance. Biophys. J. 2000, 79, 828–840. [Google Scholar] [CrossRef]
- Du, G.G.; MacLennan, D.H. Functional consequences of mutations of conserved, polar amino acids in transmembrane sequences of the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1998, 273, 31867–31872. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Ebisawa, K.; Li, X.; Zhang, L. Molecular identification of the ryanodine receptor Ca2+ sensor. J. Biol. Chem. 1998, 273, 14675–14678. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Casida, J.E. Species differences in chlorantraniliprole and flubendiamide insecticide binding sites in the ryanodine receptor. Pestic. Biochem. Physiol. 2013, 107, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Lümmen, P.; Nauen, R.; Casida, J.E. Diamide insecticide target site specificity in the Heliothis and Musca ryanodine receptors relative to toxicity. J. Agric. Food Chem. 2014, 62, 4077–4082. [Google Scholar] [CrossRef]
- Wang, R.; Bolstad, J.; Kong, H.; Zhang, L.; Brown, C.; Chen, S.R. The predicted TM10 transmembrane sequence of the cardiac Ca2+ release channel (ryanodine receptor) is crucial for channel activation and gating. J. Biol. Chem. 2004, 279, 3635–3642. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, V.; Volpe, P. Ryanodine receptors: How many, where and why? Trends Pharmacol. Sci. 1993, 14, 98–103. [Google Scholar] [CrossRef]
- Xiong, H.; Feng, X.; Gao, L.; Xu, L.; Pasek, D.A.; Seok, J.H.; Meissner, G. Identification of a two EF-hand Ca2+ binding domain in lobster skeletal muscle ryanodine receptor/Ca2+ release channel. Biochemistry 1998, 37, 4804–4814. [Google Scholar] [CrossRef]
- Guo, W.; Sun, B.; Xiao, Z.; Liu, Y.; Wang, Y.; Zhang, L.; Wang, R.; Chen, S.R. The EF-hand Ca2+ binding domain is not required for cytosolic Ca2+ activation of the cardiac ryanodine receptor. J. Biol. Chem. 2016, 291, 2150–2160. [Google Scholar] [CrossRef]
- Xu, L.; Gomez, A.C.; Pasek, D.A.; Meissner, G.; Yamaguchi, N. Two EF-hand motifs in ryanodine receptor calcium release channels contribute to isoform-specific regulation by calmodulin. Cell Calcium 2017, 66, 62–70. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Xin, C.; Meissner, G. Identification of apocalmodulin and Ca2+-calmodulin regulatory domain in skeletal muscle Ca2+ release channel, ryanodine receptor. J. Biol. Chem. 2001, 276, 22579–22585. [Google Scholar] [CrossRef]
- Ladenburger, E.M.; Plattner, H. Calcium-release channels in paramecium. Genomic expansion, differential positioning and partial transcriptional elimination. PLoS ONE 2011, 6, e27111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiurillo, M.A.; Lander, N.; Vercesi, A.E.; Docampo, R. IP3 receptor-mediated Ca2+ release from acidocalcisomes regulates mitochondrial bioenergetics and prevents autophagy in Trypanosoma cruzi. Cell Calcium 2020, 92, 102284. [Google Scholar] [CrossRef] [PubMed]
- Futatsugi, A.; Kuwajima, G.; Mikoshiba, K. Tissue-specific and developmentally regulated alternative splicing in mouse skeletal muscle ryanodine receptor mRNA. Biochem. J. 1995, 305, 373–378. [Google Scholar] [CrossRef]
- George, C.H.; Rogers, S.A.; Bertrand, B.M.A.; Tunwell, R.E.A.; Thomas, N.L.; Steele, D.S.; Cox, E.V.; Pepper, C.; Hazeel, C.J.; Claycomb, W.C.; et al. Alternative splicing of ryanodine receptors modulates cardiomyocyte Ca2+ signaling and susceptibility to apoptosis. Circ. Res. 2007, 100, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Lueck, J.D.; Harvey, P.J.; Pace, S.M.; Ikemoto, N.; Casarotto, M.G.; Dirksen, R.T.; Dulhunty, A.F. Alternative splicing of RyR1 alters the efficacy of skeletal EC coupling. Cell Calcium 2009, 45, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Takasawa, S.; Kuroki, M.; Nata, K.; Noguchi, N.; Ikeda, T.; Yamauchi, A.; Ota, H.; Itaya-Hironaka, A.; Sakuramoto-Tsuchida, S.; Takahashi, I.; et al. A novel ryanodine receptor expressed in pancreatic islets by alternative splicing from type 2 ryanodine receptor gene. Biochem. Biophys. Res. Commun. 2010, 397, 140–145. [Google Scholar] [CrossRef]
- Foskett, J.K.; White, C.; Cheung, K.H.; Mak, D.O. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007, 87, 593–658. [Google Scholar] [CrossRef]
- Wang, X.; Khakame, S.K.; Ye, C.; Yang, Y.; Wu, Y. Characterization of field evolved resistance to chlorantraniliprole in the diamondback moth, Plutella xylostella, from China. Pest Manag. Sci. 2013, 69, 661–665. [Google Scholar] [CrossRef]
- D’Cruz, A.A.; Kershaw, N.J.; Chiang, J.J.; Wang, M.K.; Nicola, N.A.; Babon, J.J.; Gack, M.U.; Nicholson, S.E. Crystal structure of the TRIM25 B30.2 (PRYSPRY) domain: A key component of antiviral signalling. Biochem. J. 2013, 456, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, F.C., Jr.; Li, S.H.; Danoff, S.; Ullrich, A.; Ross, C.A. Molecular cloning of a cDNA for the human inositol 1,4,5-trisphosphate receptor type 1, and the identification of a third alternatively spliced variant. Brain Res. Mol. Brain Res. 1995, 32, 291–296. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. Mega X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Le, S.Q.; Gascuel, O. An improved general amino acid replacement matrix. Mol. Biol. Evol. 2008, 25, 1307–1320. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783. [Google Scholar] [CrossRef]
- Meur, G.; Parker, A.K.; Gergely, F.V.; Taylor, C.W. Targeting and retention of type 1 ryanodine receptors to the endoplasmic reticulum. J. Biol. Chem. 2007, 282, 23096–23103. [Google Scholar] [CrossRef]
- Cárdenas, C.; Miller, R.A.; Smith, I.; Bui, T.; Molgó, J.; Müller, M.; Vais, H.; Cheung, K.H.; Yang, J.; Parker, I.; et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 2010, 142, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Csordás, G.; Weaver, D.; Hajnóczky, G. Endoplasmic reticulum-mitochondrial contactology: Structure and signaling functions. Trends Cell Biol. 2018, 28, 523–540. [Google Scholar] [CrossRef] [PubMed]
- López-Sanjurjo, C.I.; Tovey, S.C.; Prole, D.L.; Taylor, C.W. Lysosomes shape Ins(1,4,5)P3-evoked Ca2+ signals by selectively sequestering Ca2+ released from the endoplasmic reticulum. J. Cell Sci. 2013, 126 Pt 1, 289–300. [Google Scholar] [CrossRef]
- Garrity, A.G.; Wang, W.; Collier, C.M.; Levey, S.A.; Gao, Q.; Xu, H. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. Elife 2016, 5, e15887. [Google Scholar] [CrossRef] [PubMed]
- Atakpa, P.; Thillaiappan, N.B.; Mataragka, S.; Prole, D.L.; Taylor, C.W. IP3 receptors preferentially associate with ER-lysosome contact sites and selectively deliver Ca2+ to lysosomes. Cell Rep. 2018, 25, 3180–3193.e7. [Google Scholar] [CrossRef]
- Meissner, G. The structural basis of ryanodine receptor ion channel function. J. Gen. Physiol. 2017, 149, 1065–1089. [Google Scholar] [CrossRef] [PubMed]
- Arruda, A.P.; Pers, B.M.; Parlakgul, G.; Güney, E.; Goh, T.; Cagampan, E.; Lee, G.Y.; Goncalves, R.L.; Hotamisligil, G.S. Defective STIM-mediated store operated Ca2+ entry in hepatocytes leads to metabolic dysfunction in obesity. Elife 2017, 6, e29968. [Google Scholar] [CrossRef]
- Parekh, A.B.; Putney, J.W., Jr. Store-operated calcium channels. Physiol. Rev. 2005, 85, 757–810. [Google Scholar] [CrossRef]
- Woodard, G.E.; López, J.J.; Jardín, I.; Salido, G.M.; Rosado, J.A. TRPC3 regulates agonist-stimulated Ca2+ mobilization by mediating the interaction between type I inositol 1,4,5-trisphosphate receptor, RACK1, and Orai1. J. Biol. Chem. 2010, 285, 8045–8053. [Google Scholar] [CrossRef]
- Béliveau, É.; Lessard, V.; Guillemette, G. STIM1 positively regulates the Ca2+ release activity of the inositol 1,4,5-trisphosphate receptor in bovine aortic endothelial cells. PLoS ONE 2014, 9, e114718. [Google Scholar] [CrossRef] [PubMed]
- Thillaiappan, N.B.; Chavda, A.P.; Tovey, S.C.; Prole, D.L.; Taylor, C.W. Ca2+ signals initiate at immobile IP3 receptors adjacent to ER-plasma membrane junctions. Nat. Commun. 2017, 8, 1505. [Google Scholar] [CrossRef] [PubMed]
- Sampieri, A.; Santoyo, K.; Asanov, A.; Vaca, L. Association of the IP3R to STIM1 provides a reduced intraluminal calcium microenvironment, resulting in enhanced store-operated calcium entry. Sci. Rep. 2018, 8, 13252. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Store-operated calcium channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, G.; Yang, Y.; Fu, S.; Liu, X.; Kang, H.; Yang, X.; Su, X.C.; Shen, Y. Calmodulin dissociates the STIM1-Orai1 complex and STIM1 oligomers. Nat. Commun. 2017, 8, 1042. [Google Scholar] [CrossRef]
- Qazi, S.; Trimmer, B.A. The role of inositol 1,4,5-trisphosphate 5-phosphatase in inositol signaling in the CNS of larval Manduca sexta. Insect Biochem. Mol. Biol. 1999, 29, 161–175. [Google Scholar] [CrossRef]
- Sartain, C.V.; Wolfner, M.F. Calcium and egg activation in Drosophila. Cell Calcium 2013, 53, 10–15. [Google Scholar] [CrossRef]
- Berridge, M.J. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef]
- Megha Hasan, G. Control of protein translation by IP3R-mediated Ca2+ release in Drosophila neuroendocrine cells. Fly 2017, 11. [Google Scholar] [CrossRef]
- Alzayady, K.J.; Wang, L.; Chandrasekhar, R.; Wagner, L.E.; Van Petegem, F.; Yule, D.I. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci. Signal 2016, 9, ra35. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, R.; Alzayady, K.J.; Wagner, L.E.; Yule, D.I. Unique regulatory properties of heterotetrameric inositol 1,4,5-trisphosphate receptors revealed by studying concatenated receptor constructs. J. Biol. Chem. 2016, 291, 4846–4860. [Google Scholar] [CrossRef]
- Marchant, J.S.; Taylor, C.W. Cooperative activation of IP3 receptors by sequential binding of IP3 and Ca2+ safeguards against spontaneous activity. Curr. Biol. 1997, 7, 510–518. [Google Scholar] [CrossRef]
- Adkins, C.E.; Taylor, C.W. Lateral inhibition of inositol 1,4,5-trisphosphate receptors by cytosolic Ca(2+). Curr. Biol. 1999, 9, 1115–1118. [Google Scholar] [CrossRef]
- Furutama, D.; Shimoda, K.; Yoshikawa, S.; Miyawaki, A.; Furuichi, T.; Mikoshiba, K. Functional expression of the type 1 inositol 1,4,5-trisphosphate receptor promoter-lacZ fusion genes in transgenic mice. J. Neurochem. 1996, 66, 1793–1801. [Google Scholar] [CrossRef]
- Parker, I.; Choi, J.; Yao, Y. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: Hot spots, puffs and blips. Cell Calcium 1996, 20, 105–121. [Google Scholar] [CrossRef]
- Bootman, M.D.; Berridge, M.J.; Lipp, P. Cooking with calcium: The recipes for composing global signals from elementary events. Cell 1997, 91, 367–373. [Google Scholar] [CrossRef]
- Marchant, J.S.; Parker, I. Role of elementary Ca(2+) puffs in generating repetitive Ca(2+) oscillations. EMBO J. 2001, 20, 65–76. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol trisphosphate and calcium oscillations. Biochem. Soc. Symp. 2007, 74, 1–7. [Google Scholar] [CrossRef]
- Rapp, P.E.; Berridge, M.J. The control of transepithelial potential oscillations in the salivary gland of Calliphora erythrocephala. J. Exp. Biol. 1981, 93, 119–132. [Google Scholar] [CrossRef]
- Rosay, P.; Armstrong, J.D.; Wang, Z.; Kaiser, K. Synchronized neural activity in the Drosophila memory centers and its modulation by amnesiac. Neuron 2001, 30, 759–770. [Google Scholar] [CrossRef]
- Goldammer, J.; Mantziaris, C.; Büschges, A.; Schmidt, J. Calcium imaging of CPG-evoked activity in efferent neurons of the stick insect. PLoS ONE 2018, 13, e0202822. [Google Scholar] [CrossRef]
- Vanderheyden, V.; Devogelaere, B.; Missiaen, L.; De Smedt, H.; Bultynck, G.; Parys, J.B. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim. Biophys. Acta 2009, 1793, 959–970. [Google Scholar] [CrossRef]
- DeSouza, N.; Reiken, S.; Ondrias, K.; Yang, Y.M.; Matkovich, S.; Marks, A.R. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J. Biol. Chem. 2002, 277, 39397–39400. [Google Scholar] [CrossRef]
- Khan, M.T.; Wagner, L., 2nd; Yule, D.I.; Bhanumathy, C.; Joseph, S.K. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 2006, 281, 3731–3737. [Google Scholar] [CrossRef]
- Arguin, G.; Regimbald-Dumas, Y.; Fregeau, M.O.; Caron, A.Z.; Guillemette, G. Protein kinase C phosphorylates the inositol 1,4,5-trisphosphate receptor type 2 and decreases the mobilization of Ca2+ in pancreatoma AR4-2J cells. J. Endocrinol. 2007, 192, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.M.; Maroja, L.S.; Cottrill, S.; Bomkamp, B.E.; Westervelt, K.A.; Deitcher, D.L. The wavy mutation maps to the inositol 1,4,5-trisphosphate 3-kinase 2 (IP3K2) gene of Drosophila and interacts with IP3R to affect wing development. G3 2016, 6, 299–310. [Google Scholar] [CrossRef]
- Adkins, C.E.; Morris, S.A.; De Smedt, H.; Sienaert, I.; Török, K.; Taylor, C.W. Ca2+-calmodulin inhibits Ca2+ release mediated by type-1, -2 and -3 inositol trisphosphate receptors. Biochem. J. 2000, 345, 357–363. [Google Scholar] [CrossRef]
- Kasri, N.N.; Török, K.; Galione, A.; Garnham, C.; Callewaert, G.; Missiaen, L.; Parys, J.B.; De Smedt, H. Endogenously bound calmodulin is essential for the function of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2006, 281, 8332–8338. [Google Scholar] [CrossRef]
- Tang, J.; Lin, Y.; Zhang, Z.; Tikunova, S.; Birnbaumer, L.; Zhu, M.X. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J. Biol. Chem. 2001, 276, 21303–21310. [Google Scholar] [CrossRef] [PubMed]
- Meissner, G.; Rios, E.; Tripathy, A.; Pasek, D.A. Regulation of skeletal muscle Ca2+ release channel (ryanodine receptor) by Ca2+ and monovalent cations and anions. J. Biol. Chem. 1997, 272, 1628–1638. [Google Scholar] [CrossRef]
- Gutteridge, S.; Caspar, T.; Cordova, D.; Tao, Y.; Wu, L.; Smith, R.M. Nucleic Acids Encoding Ryanodine Receptors. U.S. Patent 7,205,147, 2003. [Google Scholar]
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M.; Flexner, L.; Gutteridge, S.; et al. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Physiol. 2006, 84, 196–214. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M.; Flexner, L.; Gutteridge, S.; et al. The novel mode of action of anthranilic diamide insecticides: Ryanodine receptor activation. ACS Symp. Ser. 2007, 948, 223–234. [Google Scholar]
- Meissner, G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J. Biol. Chem. 1986, 261, 6300–6306. [Google Scholar] [CrossRef]
- Schmitt, M.; Turberg, A.; Londershausen, M.; Dorn, A. Binding sites for Ca2+ channel effectors and ryanodine in Periplaneta americana-possible targets for new insecticides. Pestic. Sci. 1996, 48, 375–385. [Google Scholar] [CrossRef]
- Ebbinghaus-Kintscher, U.; Luemmen, P.; Lobitz, N.; Schulte, T.; Funke, C.; Fischer, R.; Masaki, T.; Yasokawa, N.; Tohnishi, M. Phthalic acid diamides activate ryanodine-sensitive Ca2+ release channels in insects. Cell Calcium 2006, 39, 21–33. [Google Scholar] [CrossRef]
- Zimanyi, I.; Pessah, I.N. Pharmacological characterization of the specific binding of [3H]ryanodine to rat brain microsomal membranes. Brain Res. 1991, 561, 181–191. [Google Scholar] [CrossRef]
- Sharma, P.; Ishiyama, N.; Nair, U.; Li, W.; Dong, A.; Miyake, T.; Wilson, A.; Ryan, T.; MacLennan, D.H.; Kislinger, T.; et al. Structural determination of the phosphorylation domain of the ryanodine receptor. FEBS J. 2012, 279, 3952–3964. [Google Scholar] [CrossRef]
- Andersson, D.C.; Betzenhauser, M.J.; Reiken, S.; Umanskaya, A.; Shiomi, T.; Marks, A.R. Stress-induced increase in skeletal muscle force requires protein kinase A phosphorylation of the ryanodine receptor. J. Physiol. 2012, 590, 6381–6387. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Li, X.; Ebisawa, K.; Zhang, L. Functional characterization of the recombinant type 3 Ca2+ release channel (ryanodine receptor) expressed in HEK293 cells. J. Biol. Chem. 1997, 272, 24234–24246. [Google Scholar] [CrossRef]
- Fruen, B.R.; Bardy, J.M.; Byrem, T.M.; Strasburg, G.M.; Louis, C.F. Differential Ca(2+) sensitivity of skeletal and cardiac muscle ryanodine receptors in the presence of calmodulin. Am. J. Physiol. Cell Physiol. 2000, 279, C724–C733. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sullivan, K.M.; Beckingham, K. Drosophila calmodulin mutants with specific defects in the musculature or in the nervous system. Genetics 2003, 165, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Arnon, A.; Cook, B.; Montell, C.; Selinger, Z.; Minke, B. Calmodulin regulation of calcium stores in phototransduction of Drosophila. Science 1997, 275, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Arnon, A.; Cook, B.; Gillo, B.; Montell, C.; Selinger, Z.; Minke, B. Calmodulin regulation of light adaptation and store-operated dark current in Drosophila photoreceptors. Proc. Natl. Acad. Sci. USA 1997, 94, 5894–5899. [Google Scholar] [CrossRef]
- Scott, K.; Sun, Y.; Beckingham, K.; Zuker, C.S. Calmodulin regulation of Drosophila light-activated channels and receptor function mediates termination of the light response in vivo. Cell 1997, 91, 375–383. [Google Scholar] [CrossRef]
- Karagas, N.E.; Venkatachalam, K. Roles for the endoplasmic reticulum in regulation of neuronal calcium homeostasis. Cells 2019, 8, 1232. [Google Scholar] [CrossRef] [PubMed]
- Inui, M.; Saito, A.; Fleischer, S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J. Biol. Chem. 1987, 262, 15637–15642. [Google Scholar] [CrossRef]
- Zorzato, F.; Fujii, J.; Otsu, K.; Phillips, M.; Green, N.M.; Lai, F.A.; Meissner, G.; MacLennan, D.H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 2244–2256. [Google Scholar] [CrossRef]
- Giannini, G.; Conti, A.; Mammarella, S.; Scrobogna, M.; Sorrentino, V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J. Cell Biol. 1995, 128, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Martínez, O.; Cañedo-Merino, R.; Díaz-Muñoz, M.; Riesgo-Escovar, J.R. Biochemical characterization, distribution and phylogenetic analysis of Drosophila melanogaster ryanodine and IP3 receptors, and thapsigargin-sensitive Ca2+ ATPase. J. Cell Sci. 2003, 116, 2483–2494. [Google Scholar] [CrossRef]
- Chintapalli, V.R.; Wang, J.; Dow, J.A. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007, 39, 715–720. [Google Scholar] [CrossRef] [PubMed]
- McQuilton, P.; St Pierre, S.E.; Thurmond, J. FlyBase Consortium. FlyBase 101--the basics of navigating FlyBase. Nucleic Acids Res. 2012, 40, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.F. The Insects: Structure and Function, 4th ed.; Cambridge University Press: New York, NY, USA, 1998. [Google Scholar]
- Miyatake, R.; Furukawa, A.; Matsushita, M.; Iwahashi, K.; Nakamura, K.; Ichikawa, Y.; Suwaki, H. Tissue-specific alternative splicing of mouse brain type ryanodine receptor/calcium release channel mRNA. FEBS Lett. 1996, 395, 123–126. [Google Scholar] [CrossRef]
- Jiang, D.; Xiao, B.; Li, X.; Chen, S.R. Smooth muscle tissues express a major dominant negative splice variant of the type 3 Ca2+ release channel (ryanodine receptor). J. Biol. Chem. 2003, 278, 4763–4769. [Google Scholar] [CrossRef]
- Verma, A.; Hirsch, D.J.; Snyder, S.H. Calcium pools mobilized by calcium or inositol 1,4,5-trisphosphate are differentially localized in rat heart and brain. Mol. Biol. Cell 1992, 3, 621–631. [Google Scholar] [CrossRef][Green Version]
- Furuichi, T.; Simon-Chazottes, D.; Fujino, I.; Yamada, N.; Hasegawa, M.; Miyawaki, A.; Yoshikawa, S.; Guénet, J.L.; Mikoshiba, K. Widespread expression of inositol 1,4,5-trisphosphate receptor type 1 gene (Insp3r1) in the mouse central nervous system. Recept. Channels 1993, 1, 11–24. [Google Scholar]
- Gorza, L.; Schiaffino, S.; Volpe, P. Inositol 1,4,5-trisphosphate receptor in heart: Evidence for its concentration in Purkinje myocytes of the conduction system. J. Cell Biol. 1993, 121, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Ferreri-Jacobia, M.; Mak, D.O.; Foskett, J.K. Translational mobility of the type 3 inositol 1,4,5-trisphosphate receptor Ca2+ release channel in endoplasmic reticulum membrane. J. Biol. Chem. 2005, 280, 3824–3831. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.; Siddhartha, G.; Joshi, R.; Patel, S.; Hasan, G. Interactions between the inositol 1,4,5-trisphosphate and cyclic AMP signaling pathways regulate larval molting in Drosophila. Genetics 2001, 158, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Raghu, P.; Hasan, G. The inositol 1,4,5-triphosphate receptor expression in Drosophila suggests a role for IP3 signalling in muscle development and adult chemosensory functions. Dev. Biol. 1995, 171, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.O.; Beck, R.R. Role of calcium ion in hormone-stimulated lipolysis. Biochem. Pharmacol. 1986, 35, 767–772. [Google Scholar] [CrossRef]
- Shi, H.; Dirienzo, D.; Zemel, M.B. Effects of dietary calcium on adipocyte lipid metabolism and body weight regulation in energy-restricted aP2-agouti transgenic mice. FASEB J. 2001, 15, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B. Regulation of adiposity and obesity risk by dietary calcium: Mechanisms and implications. J. Am. Coll Nutr. 2002, 21, 146S–151S. [Google Scholar] [CrossRef]
- Jacqmain, M.; Doucet, E.; Després, J.P.; Bouchard, C.; Tremblay, A. Calcium intake, body composition, and lipoprotein-lipid concentrations in adults. Am. J. Clin. Nutr. 2003, 77, 1448–1452. [Google Scholar] [CrossRef]
- Arruda, A.P.; Hotamisligil, G.S. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab. 2015, 22, 381–397. [Google Scholar] [CrossRef]
- Maus, M.; Cuk, M.; Patel, B.; Lian, J.; Ouimet, M.; Kaufmann, U.; Yang, J.; Horvath, R.; Hornig-Do, H.T.; Chrzanowska-Lightowlers, Z.M.; et al. Store-operated Ca2+ entry controls induction of lipolysis and the transcriptional reprogramming to lipid metabolism. Cell Metab. 2017, 25, 698–712. [Google Scholar] [CrossRef]
- Alomaim, H.; Griffin, P.; Swist, E.; Plouffe, L.J.; Vandeloo, M.; Demonty, I.; Kumar, A.; Bertinato, J. Dietary calcium affects body composition and lipid metabolism in rats. PLoS ONE 2019, 14, e0210760. [Google Scholar] [CrossRef] [PubMed]
- Toprak, U.; Hegedus, D.; Doğan, C.; Güney, G. A journey into the world of insect lipid metabolism. Arch. Insect Biochem. Physiol. 2020, 104, e21682. [Google Scholar] [CrossRef] [PubMed]
- Toprak, U.; Güz, N.; Gürkan, M.O.; Hegedus, D.D. Identification and coordinated expression of perilipin genes in the biological cycle of sunn pest, Eurygaster maura (Hemiptera: Scutelleridae): Implications for lipolysis and lipogenesis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 171, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Güney, G.; Toprak, U.; Hegedus, D.D.; Bayram, Ş.; Coutu, C.; Bekkaoui, D.; Baldwin, D.; Heckel, D.G.; Hänniger, S.; Cedden, D.; et al. A look into Colorado potato beetle lipid metabolism through the lens of lipid storage droplet proteins. Insect Biochem. Mol. Biol. 2021, 133, 103473. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Jayakumar, S.; Richhariya, S.; Hasan, G. Loss of IP3 receptor function in neuropeptide secreting neurons leads to obesity in adult Drosophila. BMC Neurosci. 2013, 14, 157. [Google Scholar] [CrossRef]
- Subramanian, M.; Metya, S.K.; Sadaf, S.; Kumar, S.; Schwudke, D.; Hasan, G. Altered lipid homeostasis in Drosophila InsP3 receptor mutants leads to obesity and hyperphagia. Dis. Model Mech. 2013, 6, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Baumbach, J.; Hummel, P.; Bickmeyer, I.; Kowalczyk, K.M.; Frank, M.; Knorr, K.; Hildebrandt, A.; Riedel, D.; Jäckle, H.; Kühnlein, R.P. A Drosophila in vivo screen identifies store-operated calcium entry as a key regulator of adiposity. Cell Metab. 2014, 19, 331–343. [Google Scholar] [CrossRef]
- Baumbach, J.; Xu, Y.; Hehlert, P.; Kühnlein, R.P. Gαq, Gγ1 and Plc21C control Drosophila body fat storage. J. Genet. Genom. 2014, 41, 283–292. [Google Scholar] [CrossRef]
- Bi, J.; Xiang, Y.; Chen, H.; Liu, Z.; Grönke, S.; Kühnlein, R.P.; Huang, X. Opposite and redundant roles of the two Drosophila perilipins in lipid mobilization. J. Cell Sci. 2012, 125, 3568–3577. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Wang, W.; Liu, Z.; Huang, X.; Jiang, Q.; Liu, G.; Wang, Y.; Huang, X. Seipin promotes adipose tissue fat storage through the ER Ca2+-ATPase SERCA. Cell Metab. 2014, 19, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Borcherding, A.F.; Heier, C.; Tian, G.; Roeder, T.; Kühnlein, R.P. Chronic dysfunction of stromal interaction molecule by pulsed RNAi induction in fat tissue impairs organismal energy homeostasis in Drosophila. Sci. Rep. 2019, 9, 6989. [Google Scholar] [CrossRef]
- Toprak, U. The role of peptide hormones in insect lipid metabolism. Front. Physiol. 2020, 11, 434. [Google Scholar] [CrossRef]
- Arrese, E.L.; Flowers, M.T.; Gazard, J.L.; Wells, M.A. Calcium and cAMP are second messengers in the adipokinetic hormone-induced lipolysis of triacylglycerols in Manduca sexta fat body. J. Lipid Res. 1999, 40, 556–564. [Google Scholar] [CrossRef]
- Venkiteswaran, G.; Hasan, G. Intracellular Ca2+ signaling and store-operated Ca2+ entry are required in Drosophila neurons for flight. Proc. Natl. Acad. Sci. USA 2009, 106, 10326–10331. [Google Scholar] [CrossRef]
- Agrawal, N.; Venkiteswaran, G.; Sadaf, S.; Padmanabhan, N.; Banerjee, S.; Hasan, G. Inositol 1,4,5-trisphosphate receptor and dSTIM function in Drosophila insulin-producing neurons regulates systemic intracellular calcium homeostasis and flight. J. Neurosci. 2010, 30, 1301–1313. [Google Scholar] [CrossRef]
- Bednářová, A.; Kodrík, D.; Krishnan, N. Adipokinetic hormone exerts its anti-oxidative effects using a conserved signal-transduction mechanism involving both PKC and cAMP by mobilizing extra- and intracellular Ca2+ stores. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 158, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Gáliková, M.; Diesner, M.; Klepsatel, P.; Hehlert, P.; Xu, Y.; Bickmeyer, I.; Predel, R.; Kühnlein, R.P. Energy homeostasis control in Drosophila adipokinetic hormone mutants. Genetics 2015, 201, 665–683. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Bi, J.; Shui, G.; Liu, Z.; Xiang, Y.; Liu, Y.; Wenk, M.R.; Yang, H.; Huang, X. Tissue-autonomous function of Drosophila seipin in preventing ectopic lipid droplet formation. PLoS Genet. 2011, 7, e1001364. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Yang, L.; Li, P.; Hofmann, O.; Dicker, L.; Hide, W.; Lin, X.; Watkins, S.M.; Ivanov, A.R.; Hotamisligil, G.S. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011, 473, 528–531. [Google Scholar] [CrossRef]
- Starling, R.C.; Hammer, D.F.; Altschuld, R.A. Human myocardial ATP content and in vivo contractile function. Mol. Cell Biochem. 1998, 180, 171–177. [Google Scholar] [CrossRef]
- Cao, T.; Jin, J.P. Evolution of flight muscle contractility and energetic efficiency. Front. Physiol. 2020, 11, 1038. [Google Scholar] [CrossRef]
- Palade, P.; Györke, S. Excitation-contraction coupling in crustacea: Do studies on these primitive creatures offer insights about EC coupling more generally? J. Muscle Res. Cell Motil. 1993, 14, 283–287. [Google Scholar] [CrossRef]
- Maryon, E.B.; Coronado, R.; Anderson, P. unc-68 encodes a ryanodine receptor involved in regulating C. elegans body-wall muscle contraction. J. Cell Biol. 1996, 134, 885–893. [Google Scholar] [CrossRef]
- Devlin, C.L.; Amole, W.; Anderson, S.; Shea, K. Muscarinic acetylcholine receptor compounds alter net Ca2+ flux and contractility in an invertebrate smooth muscle. Invert. Neurosci. 2003, 5, 9–17. [Google Scholar] [CrossRef]
- Tamashiro, H.; Yoshino, M. Involvement of plasma membrane Ca2+ channels, IP3 receptors, and ryanodine receptors in the generation of spontaneous rhythmic contractions of the cricket lateral oviduct. J. Insect Physiol. 2014, 71, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ellington, C.P. Power and efficiency of insect flight muscle. J. Exp. Biol. 1985, 115, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H. Structure, function and evolution of insect flight muscle. Biophysics 2011, 7, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Yamazawa, T.; Takeshima, H.; Shimuta, M.; Iino, M. A region of the ryanodine receptor critical for excitation-contraction coupling in skeletal muscle. J. Biol. Chem. 1997, 272, 8161–8164. [Google Scholar] [CrossRef]
- Calderón, J.C.; Bolaños, P.; Caputo, C. The excitation-contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.M.; Scott, K.; Zuker, C.S.; Rubin, G.M. The ryanodine receptor is essential for larval development in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 5942–5947. [Google Scholar] [CrossRef] [PubMed]
- Wegener, C.; Nässel, D.R. Peptide-induced Ca(2+) movements in a tonic insect muscle: Effects of proctolin and periviscerokinin-2. J. Neurophysiol. 2000, 84, 3056–3066. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Adebiyi, A.; Jaggar, J.H. Inositol trisphosphate receptors in smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, 2190–2210. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Collins, T.J.; Mackenzie, L.; Roderick, H.L.; Berridge, M.J.; Peppiatt, C.M. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002, 16, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, L.; Prevarskaya, N.; Mazurier, J.; Shuba, Y.; Skryma, R. 2-APB inhibits volume-regulated anion channels independently from intracellular calcium signaling modulation. FEBS Lett. 2004, 556, 121–126. [Google Scholar] [CrossRef]
- Lange, A.B. Inositol phospholipid hydrolysis may mediate the action of proctolin on insect visceral muscle. Arch. Insect Biochem. Physiol. 1988, 9, 201–209. [Google Scholar] [CrossRef]
- Lange, A.B.; Nykamp, D.A. Signal transduction pathways regulating the contraction of an insect visceral muscle. Arch. Insect Biochem. Physiol. 1996, 33, 183–196. [Google Scholar] [CrossRef]
- Nykamp, D.A.; Lange, A.B. The effects of octopamine are mediated via a G protein in the oviducts of Locusta migratoria. Biog. Amines 1998, 14, 177. [Google Scholar]
- Nykamp, D.A.; Lange, A.B. Interaction between octopamine and proctolin on the oviducts of Locusta migratoria. J. Insect Physiol. 2000, 46, 809–816. [Google Scholar] [CrossRef]
- Lange, A.B. A review of the involvement of proctolin as a cotransmitter and local neurohormone in the oviduct of the locust, Locusta migratoria. Peptides 2002, 23, 2063–2070. [Google Scholar] [CrossRef]
- Hinton, J.M.; Nejad, M.; Issberner, J.P.; Hancock, J.T.; Osborne, R.H. Muscarinic acetylcholine and proctolin receptors in the foregut of the locust Schistocerca gregaria: Role of inositol phosphates, protein kinase C and calcium in second messenger effects. Insect Biochem. Mol. Biol. 1998, 28, 331–343. [Google Scholar] [CrossRef]
- Peron, S.; Zordan, M.A.; Magnabosco, A.; Reggiani, C.; Megighian, A. From action potential to contraction: Neural control and excitation-contraction coupling in larval muscles of Drosophila. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 154, 173–183. [Google Scholar] [CrossRef]
- Banerjee, S.; Lee, J.; Venkatesh, K.; Wu, C.F.; Hasan, G. Loss of flight and associated neuronal rhythmicity in inositol 1,4,5-trisphosphate receptor mutants of Drosophila. J. Neurosci. 2004, 24, 7869–7878. [Google Scholar] [CrossRef]
- Agrawal, T.; Sadaf, S.; Hasan, G.A. Genetic RNAi screen for IP3/Ca2+ coupled GPCRs in Drosophila identifies the PdfR as a regulator of insect flight. PLoS Genet. 2013, 9, e1003849. [Google Scholar] [CrossRef]
- Chakraborty, S.; Hasan, G. Spontaneous Ca2+ influx in Drosophila pupal neurons is modulated by IP3-receptor function and influences maturation of the flight circuit. Front. Mol. Neurosci. 2017, 10, 111. [Google Scholar] [CrossRef]
- Banerjee, S.; Hasan, G. The InsP3 receptor: Its role in neuronal physiology and neurodegeneration. Bioessays 2005, 27, 1035–1047. [Google Scholar] [CrossRef]
- Brembs, B.; Christiansen, F.; Pflüger, H.J.; Duch, C. Flight initiation and maintenance deficits in flies with genetically altered biogenic amine levels. J. Neurosci. 2007, 27, 11122–11131. [Google Scholar] [CrossRef]
- Sharma, A.; Hasan, G. Modulation of flight and feeding behaviours requires presynaptic IP3Rs in dopaminergic neurons. Elife 2020, 9, e62297. [Google Scholar] [CrossRef]
- Hoyle, G. Evidence that insect dorsal unpaired medican (DUM) neurons are octopaminergic. J. Exp. Zool. 1975, 193, 425–431. [Google Scholar] [CrossRef]
- Evans, P.D.; O’Shea, M. An octopaminergic neurone modulates neuromuscular transmission in the locust. Nature 1977, 270, 257–259. [Google Scholar] [CrossRef]
- Hoyle, G. Distributions of nerve and muscle fibre types in locust jumping muscle. J. Exp. Biol. 1978, 73, 205–233. [Google Scholar] [CrossRef]
- Evans, P.D.; Siegler, M.V. Octopamine mediated relaxation of maintained and catch tension in locust skeletal muscle. J. Physiol. 1982, 324, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Ryglewski, S.; Pflueger, H.J.; Duch, C. Expanding the neuron’s calcium signaling repertoire: Intracellular calcium release via voltage-induced PLC and IP3R activation. PLoS Biol. 2007, 5, e66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baines, R.A.; Walther, C.; Hinton, J.M.; Osborne, R.H.; Konopińska, D. Selective activity of a proctolin analogue reveals the existence of two receptor subtypes. J. Neurophysiol. 1996, 75, 2647–2650. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Vinberg, F. Transduction and adaptation mechanisms in the cilium or microvilli of photoreceptors and olfactory receptors from ınsects to humans. Front. Cell Neurosci. 2021, 15, 662453. [Google Scholar] [CrossRef]
- Honkanen, A.; Immonen, E.V.; Salmela, I.; Heimonen, K.; Weckström, M. Insect photoreceptor adaptations to night vision. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160077. [Google Scholar] [CrossRef]
- Sokolinskaya, E.L.; Kolesov, D.V.; Lukyanov, K.A.; Bogdanov, A.M. Molecular principles of insect chemoreception. Acta Nat. 2020, 12, 81–91. [Google Scholar] [CrossRef]
- Paulsen, R.; Schwemer, J. Studies on the insect visual pigment sensitive to ultraviolet light: Retinal as the chromophoric group. Biochim. Biophys. Acta 1972, 283, 520–529. [Google Scholar] [CrossRef]
- Charlton-Perkins, M.; Cook, T.A. Building a fly eye: Terminal differentiation events of the retina, corneal lens, and pigmented epithelia. Curr. Top. Dev. Biol. 2010, 93, 129–173. [Google Scholar] [CrossRef]
- Scott, K.; Zuker, C. TRP, TRPL and trouble in photoreceptor cells. Curr. Opin. Neurobiol. 1998, 8, 383–388. [Google Scholar] [CrossRef]
- Henderson, S.R.; Reuss, H.; Hardie, R.C. Single photon responses in Drosophila photoreceptors and their regulation by Ca2+. J. Physiol. 2000, 524, 179–194. [Google Scholar] [CrossRef]
- Fain, G.L.; Hardie, R.; Laughlin, S.B. Phototransduction and the evolution of photoreceptors. Curr. Biol. 2010, 20, 114–124. [Google Scholar] [CrossRef]
- Huang, J.; Liu, C.H.; Hughes, S.A.; Postma, M.; Schwiening, C.J.; Hardie, R.C. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr. Biol. 2010, 20, 189–197. [Google Scholar] [CrossRef]
- Hardie, R.C.; Franze, K. Photomechanical responses in Drosophila photoreceptors. Science 2012, 338, 260–263. [Google Scholar] [CrossRef]
- Acharya, J.K.; Jalink, K.; Hardy, R.W.; Hartenstein, V.; Zuker, C.S. InsP3 receptor is essential for growth and differentiation but not for vision in Drosophila. Neuron 1997, 18, 881–887. [Google Scholar] [CrossRef]
- Raghu, P.; Colley, N.J.; Webel, R.; James, T.; Hasan, G.; Danin, M.; Selinger, Z.; Hardie, R.C. Normal phototransduction in Drosophila photoreceptors lacking an InsP(3) receptor gene. Mol. Cell Neurosci. 2000, 15, 429–445. [Google Scholar] [CrossRef]
- Hardie, R.C.; Raghu, P. Visual transduction in Drosophila. Nature 2001, 413, 186–193. [Google Scholar] [CrossRef]
- Kohn, E.; Katz, B.; Yasin, B.; Peters, M.; Rhodes, E.; Zaguri, R.; Weiss, S.; Minke, B. Functional cooperation between the IP3 receptor and phospholipase C secures the high sensitivity to light of Drosophila photoreceptors in vivo. J. Neurosci. 2015, 35, 2530–2546. [Google Scholar] [CrossRef]
- Bollepalli, M.K.; Kuipers, M.E.; Liu, C.H.; Asteriti, S.; Hardie, R.C. Phototransduction in Drosophila is compromised by Gal4 expression but not by InsP3 receptor knockdown or mutation. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Baumann, O. Distribution of ryanodine receptor Ca(2+) channels in insect photoreceptor cells. J. Comp. Neurol. 2000, 421, 347–361. [Google Scholar] [CrossRef]
- Menini, A. Calcium signalling and regulation in olfactory neurons. Curr. Opin. Neurobiol. 1999, 9, 419–426. [Google Scholar] [CrossRef]
- Matthews, H.R.; Reisert, J. Calcium, the two-faced messenger of olfactory transduction and adaptation. Curr. Opin. Neurobiol. 2003, 13, 469–475. [Google Scholar] [CrossRef]
- Pézier, A.; Acquistapace, A.; Renou, M.; Rospars, J.P.; Lucas, P. Ca2+ stabilizes the membrane potential of moth olfactory receptor neurons at rest and is essential for their fast repolarization. Chem. Senses 2007, 32, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Murmu, M.S.; Stinnakre, J.; Martin, J.R. Presynaptic Ca2+ stores contribute to odor-induced responses in Drosophila olfactory receptor neurons. J. Exp. Biol. 2010, 21, 4163–4173. [Google Scholar] [CrossRef]
- Clyne, P.J.; Certel, S.J.; de Bruyne, M.; Zaslavsky, L.; Johnson, W.A.; Carlson, J.R. The odor specificities of a subset of olfactory receptor neurons are governed by Acj6, a POU-domain transcription factor. Neuron 1999, 22, 339–347. [Google Scholar] [CrossRef]
- Gao, Q.; Chess, A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics 1999, 60, 31–39. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Amrein, H.; Morozov, P.S.; Rzhetsky, A.; Axel, R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 1999, 96, 725–736. [Google Scholar] [CrossRef]
- Neuhaus, E.M.; Gisselmann, G.; Zhang, W.; Dooley, R.; Störtkuhl, K.; Hatt, H. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat. Neurosci. 2005, 8, 15–17. [Google Scholar] [CrossRef]
- Sato, K.; Pellegrino, M.; Nakagawa, T.; Nakagawa, T.; Vosshall, L.B.; Touhara, K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 2008, 452, 1002–1006. [Google Scholar] [CrossRef]
- Wicher, D.; Schäfer, R.; Bauernfeind, R.; Stensmyr, M.C.; Heller, R.; Heinemann, S.H.; Hansson, B.S. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 2008, 452, 1007–1011. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Hansson, B.S. A unified nomenclature system for the insect olfactory coreceptor. Chem. Senses 2011, 36, 497–498. [Google Scholar] [CrossRef]
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 2009, 136, 149–162. [Google Scholar] [CrossRef]
- Gomez-Diaz, C.; Martin, F.; Garcia-Fernandez, J.M.; Alcorta, E. The two main olfactory receptor families in Drosophila, ORs and IRs: A comparative approach. Front. Cell Neurosci. 2018, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Boekhoff, I.; Seifert, E.; Göggerle, S.; Lindemann, M.; Krüger, B.W.; Breer, H. Pheromone-induced second-messenger signaling in insect antennae. Insect Biochem. Mol. Biol. 1993, 23, 757–762. [Google Scholar] [CrossRef]
- Kain, P.; Chakraborty, T.S.; Sundaram, S.; Siddiqi, O.; Rodrigues, V.; Hasan, G. Reduced odor responses from antennal neurons of G(q)alpha, phospholipase Cbeta, and rdgA mutants in Drosophila support a role for a phospholipid intermediate in insect olfactory transduction. J. Neurosci. 2008, 28, 4745–4755. [Google Scholar] [CrossRef]
- Smart, R.; Kiely, A.; Beale, M.; Vargas, E.; Carraher, C.; Kralicek, A.V.; Christie, D.L.; Chen, C.; Newcomb, R.D.; Warr, C.G. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem. Mol. Biol. 2008, 38, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Vosshall, L.B. Controversy and consensus: Noncanonical signaling mechanisms in the insect olfactory system. Curr. Opin. Neurobiol. 2009, 19, 284–292. [Google Scholar] [CrossRef]
- Chatterjee, A.; Roman, G.; Hardin, P.E. Go contributes to olfactory reception in Drosophila melanogaster. BMC Physiol. 2009, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, W.; Farhat, K.; Oberland, S.; Gisselmann, G.; Neuhaus, E.M. The stimulatory Gα(s) protein is involved in olfactory signal transduction in Drosophila. PLoS ONE 2011, 6, e18605. [Google Scholar] [CrossRef]
- Sargsyan, V.; Getahun, M.N.; Llanos, S.L.; Olsson, S.B.; Hansson, B.S.; Wicher, D. Phosphorylation via PKC regulates the function of the Drosophila odorant co-receptor. Front. Cell Neurosci. 2011, 5, 5. [Google Scholar] [CrossRef]
- Miazzi, F.; Hansson, B.S.; Wicher, D. Odor-induced cAMP production in Drosophila melanogaster olfactory sensory neurons. J. Exp. Biol. 2016, 219, 1798–1803. [Google Scholar] [CrossRef]
- Murmu, M.S.; Martin, J.R. Interaction between cAMP and intracellular Ca(2+)-signaling pathways during odor-perception and adaptation in Drosophila. Biochim. Biophys. Acta. 2016, 1863, 2156–2174. [Google Scholar] [CrossRef]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef]
- Murmu, M.S.; Stinnakre, J.; Réal, E.; Martin, J.R. Calcium-stores mediate adaptation in axon terminals of olfactory receptor neurons in Drosophila. BMC Neurosci. 2011, 12, 105. [Google Scholar] [CrossRef]
- Fadool, D.A.; Ache, B.W. Plasma membrane inositol 1,4,5-trisphosphate-activated channels mediate signal transduction in lobster olfactory receptor neurons. Neuron 1992, 9, 907–918. [Google Scholar] [CrossRef][Green Version]
- Cunningham, A.M.; Ryugo, D.K.; Sharp, A.H.; Reed, R.R.; Snyder, S.H.; Ronnett, G.V. Neuronal inositol 1,4,5-trisphosphate receptor localized to the plasma membrane of olfactory cilia. Neuroscience 1993, 57, 339–352. [Google Scholar] [CrossRef]
- Schild, D.; Restrepo, D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol. Rev. 1998, 78, 429–466. [Google Scholar] [CrossRef]
- Deshpande, M.; Venkatesh, K.; Rodrigues, V.; Hasan, G. The inositol 1,4,5-trisphosphate receptor is required for maintenance of olfactory adaptation in Drosophila antennae. J. Neurobiol. 2000, 43, 282–288. [Google Scholar] [CrossRef]
- Kurahashi, T.; Menini, A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature 1997, 385, 725–729. [Google Scholar] [CrossRef]
- Devaud, J.M.; Acebes, A.; Ferrús, A. Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J. Neurosci. 2001, 21, 6274–6282. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Diaz, C.; Martin, F.; Alcorta, E. The cAMP transduction cascade mediates olfactory reception in Drosophila melanogaster. Behav. Genet. 2004, 34, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Stengl, M. Pheromone transduction in moths. Front. Cell Neurosci. 2010, 4, 133. [Google Scholar] [CrossRef]
- Sklar, P.B.; Anholt, R.R.; Snyder, S.H. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J. Biol. Chem. 1986, 261, 15538–15543. [Google Scholar] [CrossRef]
- Venkatesh, K.; Hasan, G. Disruption of the IP3 receptor gene of Drosophila affects larval metamorphosis and ecdysone release. Curr. Biol. 1997, 7, 500–509. [Google Scholar] [CrossRef]
- Lane, M.E.; Kalderon, D. Genetic investigation of cAMP-dependent protein kinase function in Drosophila development. Genes. Dev. 1993, 7, 1229–1243. [Google Scholar] [CrossRef]
- Liu, P.C.; Wang, J.X.; Song, Q.S.; Zhao, X.F. The participation of calponin in the cross talk between 20-hydroxyecdysone and juvenile hormone signaling pathways by phosphorylation variation. PLoS ONE 2011, 6, e19776. [Google Scholar] [CrossRef]
- Jing, Y.P.; Liu, W.; Wang, J.X.; Zhao, X.F. The steroid hormone 20-hydroxyecdysone via nongenomic pathway activates Ca2+/calmodulin-dependent protein kinase II to regulate gene expression. J. Biol. Chem. 2015, 290, 8469–8481. [Google Scholar] [CrossRef]
- Wang, D.; Pei, X.Y.; Zhao, W.L.; Zhao, X.F. Steroid hormone 20-hydroxyecdysone promotes higher calcium mobilization to induce apoptosis. Cell Calcium 2016, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Pei, X.Y.; Wang, D.; Chen, C.H.; Cai, M.J.; Wang, J.X.; Zhao, X.F. The steroid hormone 20-hydroxyecdysone upregulates calcium release-activated calcium channel modulator 1 expression to induce apoptosis in the midgut of Helicoverpa armigera. Cell Calcium 2017, 68, 24–33. [Google Scholar] [CrossRef]
- Jayakumar, S.; Richhariya, S.; Reddy, O.V.; Texada, M.J.; Hasan, G. Drosophila larval to pupal switch under nutrient stress requires IP3R/Ca(2+) signalling in glutamatergic interneurons. Elife 2016, 5, e17495. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Venkatesh, K.; Srinivas, R.; Nair, S.; Hasan, G. Genetic dissection of itpr gene function reveals a vital requirement in aminergic cells of Drosophila larvae. Genetics 2004, 166, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Vermassen, E.; Parys, J.B.; Mauger, J.P. Subcellular distribution of the inositol 1,4,5-trisphosphate receptors: Functional relevance and molecular determinants. Biol. Cell 2004, 96, 3–17. [Google Scholar] [CrossRef]
- Restrepo, S.; Basler, K. Drosophila wing imaginal discs respond to mechanical injury via slow InsP3R-mediated intercellular calcium waves. Nat. Commun. 2016, 7, 12450. [Google Scholar] [CrossRef]
- Sass, M. Autophagy research on insects. Autophagy 2008, 4, 265–267. [Google Scholar] [CrossRef][Green Version]
- Tettamanti, G.; Saló, E.; González-Estévez, C.; Felix, D.A.; Grimaldi, A.; de Eguileor, M. Autophagy in invertebrates: Insights into development, regeneration and body remodeling. Curr. Pharm. Des. 2008, 14, 116–125. [Google Scholar] [CrossRef]
- Li, Y.B.; Li, X.R.; Yang, T.; Wang, J.X.; Zhao, X.F. The steroid hormone 20-hydroxyecdysone promotes switching from autophagy to apoptosis by increasing intracellular calcium levels. Insect Biochem. Mol. Biol. 2016, 79, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.M.; Ren, D.; Feng, G.; Eberl, D.F.; Dubald, M.; Yang, M.; Hannan, F.; Kousky, C.T.; Zheng, W. Calcium channel as a new potential target for insecticides. In Molecular Action of Insecticides on Ion Channels; Clark, J.M., Ed.; ACS Symposium Series; ACS Publications: Washington, DC, USA, 1995; pp. 162–172. [Google Scholar]
- Bloomquist, J.R. Ion channels as targets for insecticides. Annu. Rev. Entomol. 1996, 41, 163–190. [Google Scholar] [CrossRef] [PubMed]
- Lümmen, P. Calcium channels as molecular target sites of novel ınsecticides. Adv. Insect Physiol. 2013, 44, 287–347. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Williamson, M.S.; Davies, T.G.; Bass, C. Ion channels as insecticide targets. J. Neurogenet. 2016, 30, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R. Insecticide mode of action: Return of the ryanodine receptor. Pest Manag. Sci. 2006, 62, 690–692. [Google Scholar] [CrossRef]
- Jenden, D.J.; Fairhurst, A.S. The pharmacology of ryanodine. Pharmacol. Rev. 1969, 21, 1–25. [Google Scholar]
- Folkers, K.; Rogers, E.; Heal, R.E. Ryania Insecticides. U.S. Patent 2,400,295, 1946. [Google Scholar]
- Rogers, E.F.; Konıuszy, F.R. Plant insecticides; ryanodine, a new alkaloid from Ryania speciosa Vahl. J. Am. Chem. Soc. 1948, 70, 3086–3088. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lahm, G.P.; Selby, T.P.; Freudenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.; Smith, B.K.; Flexner, L.; Clark, C.E.; Cordova, D. Anthranilic diamides: A new class of potent ryanodine receptor activators. Bioorg. Med. Chem. Lett. 2005, 15, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Sattelle, D.B.; Cordova, D.; Cheek, T.R. Insect ryanodine receptors: Molecular targets for novel pest control chemicals. Invert. Neurosci. 2008, 8, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Jeanguenat, A. The story of a new insecticidal chemistry class: The diamides. Pest Manag. Sci. 2013, 69, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Tohnishi, M.; Nakao, H.; Furuya, T.; Seo, A.; Kodama, H.; Tsubata, K.; Fujioka, S.; Kodama, H.; Hirooka, T.; Nishimatsu, T. Flubendiamide, a novel insecticide highly active against lepidopterous insect pests. J. Pestic. Sci. 2005, 30, 354–360. [Google Scholar] [CrossRef]
- Masaki, T.; Yasokawa, N.; Tohnishi, M.; Nishimatsu, T.; Tsubata, K.; Inoue, K.; Motoba, K.; Hirooka, T. Flubendiamide, a novel Ca2+ channel modulator, reveals evidence for functional cooperation between Ca2+ pumps and Ca2+ release. Mol. Pharmacol. 2006, 69, 1733–1739. [Google Scholar] [CrossRef]
- Hirooka, T.; Nishimatsu, T.; Kodama, H.; Reckmann, U.; Nauen, R. The biological profile of flubendiamide, a new benzenedicarboxamide insecticide. Pflanzenschutz Nachr. Bayer 2007, 60, 183–202. [Google Scholar]
- Lahm, G.P.; Stevenson, T.M.; Selby, T.P.; Freudenberger, J.H.; Dubas, C.M.; Smith, B.K.; Cordova, D.; Flexner, L.; Clark, C.E.; Bellin, C.A.; et al. RynaxypyrTM: A new anthranilic diamide insecticide acting at the ryanodine receptor. In Pesticide Chemistry: Crop Protection, Public Health, Environmental Safety; Ohkawa, H., Miyagawa, H., Lee, P.W., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp. 111–120. [Google Scholar] [CrossRef]
- Lahm, G.P.; Cordova, D.; Barry, J.D. New and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem. 2009, 17, 4127–4133. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Clark, D.A.; Bolgunas, S.P.; Lahm, G.P.; Gutteridge, S.; Rhoades, D.F.; Wu, L.; Sopa, J.S.; Rauh, J.J.; et al. Pyrrole-2 carboxamides—A novel class of insect ryanodine receptor activators. Pestic. Biochem. Physiol. 2021, 174, 104798. [Google Scholar] [CrossRef]
- Cameron, R.A.; Williams, C.J.; Portillo, H.E.; Marçon, P.C.; Teixeira, L.A. Systemic application of chlorantraniliprole to cabbage transplants for control of foliar-feeding lepidopteran pests. Crop Prot. 2015, 67, 13–19. [Google Scholar] [CrossRef]
- Foster, S.P.; Denholm, I.; Rison, J.L.; Portillo, H.E.; Margaritopoulis, J.; Slater, R. Susceptibility of standard clones and European field populations of the green peach aphid, Myzus persicae, and the cotton aphid, Aphis gossypii (Hemiptera: Aphididae), to the novel anthranilic diamide insecticide cyantraniliprole. Pest Manag. Sci. 2012, 68, 629–633. [Google Scholar] [CrossRef]
- Selby, T.P.; Lahm, G.P.; Stevenson, T.M.; Hughes, K.A.; Cordova, D.; Annan, I.B.; Barry, J.D.; Benner, E.A.; Currie, M.J.; Pahutski, T.F. Discovery of cyantraniliprole, a potent and selective anthranilic diamide ryanodine receptor activator with cross-spectrum insecticidal activity. Bioorg. Med. Chem. Lett. 2013, 23, 6341–6345. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.D.; Portillo, H.E.; Annan, I.B.; Cameron, R.A.; Clagg, D.G.; Dietrich, R.F.; Watson, L.J.; Leighty, R.M.; Ryan, D.L.; McMillan, J.A.; et al. Movement of cyantraniliprole in plants after foliar applications and its impact on the control of sucking and chewing insects. Pest Manag. Sci. 2015, 71, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Grávalos, C.; Fernández, E.; Belando, A.; Moreno, I.; Ros, C.; Bielza, P. Cross-resistance and baseline susceptibility of Mediterranean strains of Bemisia tabaci to cyantraniliprole. Pest Manag. Sci. 2015, 71, 1030–1036. [Google Scholar] [CrossRef]
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D. Insecticides, biologics and nematicides: Updates to IRAC’s mode of action classification—A tool for resistance management. Pestic. Biochem. Physiol. 2020, 167, 104587. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Qi, S.; Zhang, H.; Liu, K.; Liu, R.; Liang, P.; Casida, J.E.; Liu, S. Fluorescent probes for insect ryanodine receptors: Candidate anthranilic diamides. Molecules 2014, 19, 4105–4114. [Google Scholar] [CrossRef] [PubMed]
- Samurkas, A.; Fan, X.; Ma, D.; Sundarraj, R.; Lin, L.; Yao, L.; Ma, R.; Jiang, H.; Cao, P.; Gao, Q.; et al. Discovery of potential species-specific green insecticides targeting the lepidopteran ryanodine receptor. J. Agric. Food Chem. 2020, 68, 4528–4537. [Google Scholar] [CrossRef] [PubMed]
- Kadala, A.; Charretona, M.; Colleta, C. Flubendiamide, the first phthalic acid diamide insecticide, impairs neuronal calcium signalling in the honey bee’s antennae. J. Insect Physiol. 2020, 125, 104086. [Google Scholar] [CrossRef]
- Masaki, T.; Yasokawa, N.; Ebbinghaus-Kintscher, U.; Luemmen, P. Flubendiamide stimulates Ca2+ pump activity coupled to RyR-mediated calcium release in lepidopterous insects. In Pesticide Chemistry; Ohkawa, H., Miyagawa, H., Lee, P.W., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar] [CrossRef]
- Tao, Y.; Gutteridge, S.; Benner, E.A.; Wu, L.; Rhoades, D.F.; Sacher, M.D.; Rivera, M.A.; Desaeger, J.; Cordova, D. Identification of a critical region in the Drosophila ryanodine receptor that confers sensitivity to diamide insecticides. Insect Biochem. Mol. Biol. 2013, 43, 820–828. [Google Scholar] [CrossRef]
- Chen, S.R.; Li, P.; Zhao, M.; Li, X.; Zhang, L. Role of the proposed pore-forming segment of the Ca2+ release channel (ryanodine receptor) in ryanodine interaction. Biophys. J. 2002, 82, 2436–2447. [Google Scholar] [CrossRef]
- Steinbach, D.; Gutbrod, O.; Lümmen, P.; Matthiesen, S.; Schorn, C.; Nauen, R. Geographic spread, genetics and functional characteristics of ryanodine receptor based target-site resistance to diamide insecticides in diamondback moth, Plutella xylostella. Insect Biochem. Mol. Biol. 2015, 63, 14–22. [Google Scholar] [CrossRef]
- Casida, J.E. Radioligand recognition of insecticide targets. J. Agric. Food. Chem. 2018, 66, 3277–3290. [Google Scholar] [CrossRef]
- Isaacs, A.K.; Qi, S.; Sarpong, R.; Casida, J.E. Insect ryanodine receptor: Distinct but coupled insecticide binding sites for [N-C(3)H(3)]chlorantraniliprole, flubendiamide, and [(3)H]ryanodine. Chem. Res. Toxicol. 2012, 25, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Haji-ghassemi, O.; Ma, D.; Jiang, H.; Lin, L.; Yao, L.; Samurkas, A.; Li, Y.; Wang, Y.; Cao, P.; et al. Structural basis for diamide modulation of ryanodine receptor. Nat. Chem. Biol. 2020, 16, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Y.; Zhou, X.; Li, Z.; Liu, S.; Pei, L.; Gao, X. Functional analysis of a point mutation in the ryanodine receptor of Plutella xylostella (L.) associated with resistance to chlorantraniliprole. Pest Manag. Sci 2014, 70, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Steinbach, D. Resistance to Diamide Insecticides in Lepidopteran Pests. In Advances in Insect Control and Resistance Management; Horowitz, A.R., Ishaaya, I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; p. 219. [Google Scholar] [CrossRef]
- Richardson, E.B.; Troczka, B.J.; Gutbrod, O.; Emyr Davies, T.G.; Nauen, R. Diamide resistance: 10 years of lessons from lepidopteran pests. J. Pest Sci. 2020, 93, 911–928. [Google Scholar] [CrossRef]
- Uchiyama, T.; Ozawa, A. Rapid development of resistance to diamide insecticides in the smaller tea tortrix, Adoxophyes honmai (Lepidoptera: Tortricidae), in the tea fields of Shizuoka Prefecture, Japan. Appl. Entomol. Zool. 2014, 49, 529–534. [Google Scholar] [CrossRef]
- Sial, A.A.; Brunner, J.F.; Doerr, M.D. Susceptibility of Choristoneura rosaceana (Lepidoptera: Tortricidae) to two new reduced-risk insecticides. J. Econ. Entomol. 2010, 103, 140–146. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Shen, A.; Wu, Y. Baseline susceptibility of the diamondback moth (Lepidoptera: Plutellidae) to chlorantraniliprole in China. J. Econ. Entomol. 2010, 103, 843–848. [Google Scholar] [CrossRef]
- Su, J.; Lai, T.; Li, J. Susceptibility of field populations of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in China to chlorantraniliprole and the activities of detoxification enzymes. Crop Prot. 2012, 42, 217–222. [Google Scholar] [CrossRef]
- Lai, T.C.; Li, J.; Su, J.Y. Monitoring of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) resistance to chlorantraniliprole in China. Pestic. Biochem. Physiol. 2011, 101, 198–205. [Google Scholar] [CrossRef]
- Che, W.; Shi, T.; Wu, Y.; Yang, Y. Insecticide resistance status of field populations of Spodoptera exigua (Lepidoptera: Noctuidae) from China. J. Econ. Entomol. 2013, 106, 1855–1862. [Google Scholar] [CrossRef]
- Li, X.; Degain, B.A.; Harpold, V.S.; Marçon, P.G.; Nichols, R.L.; Fournier, A.J.; Naranjo, S.E.; Palumbo, J.C.; Ellsworth, P.C. Baseline susceptibilities of B- and Q-biotype Bemisia tabaci to anthranilic diamides in Arizona. Pest Manag. Sci. 2012, 68, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sukonthabhirom, S.; Dumrongsak, D.; Jumroon, S.; Saroch, T.; Chaweng, A.; Tanaka, T. Update on DBM diamide resistance from Thailand: Causal factors and learnings. In Proceedings of the Sixth International Workshop on Management of the Diamondback Moth and Other Crucifer Insect Pests, Nakhon Pathom, Thailand, 21–25 March 2011; Srinivasan, R., Shelton, A.M., Collins, H.L., Eds.; AVRDC-The World Vegetable Center: Tailem, Taiwan, 2011; pp. 202–212. [Google Scholar]
- Guo, L.; Liang, P.; Zhou, X.; Gao, X. Novel mutations and mutation combinations of ryanodine receptor in a chlorantraniliprole resistant population of Plutella xylostella (L.). Sci. Rep. 2014, 4, 6924. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y. High levels of resistance to chlorantraniliprole evolved in field populations of Plutella xylostella. J. Econ. Entomol. 2012, 105, 1019–1023. [Google Scholar] [CrossRef]
- Su, J.; Zhang, Z.; Wu, M.; Gao, C. Geographic susceptibility of Chilo suppressalis Walker (Lepidoptera: Crambidae), to chlorantraniliprole in China. Pest Manag. Sci. 2014, 70, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Troczka, B.; Zimmer, C.T.; Elias, J.; Schorn, C.; Bass, C.; Davies, T.G.E.; Field, L.M.; Williamson, M.S.; Slater, R.; Nauen, R. Resistance to diamide insecticides in diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) is associated with a mutation in the membrane-spanning domain of the ryanodine receptor. Insect Biochem. Mol. Biol. 2012, 42, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yao, R.; Zhang, Z.; Wu, M.; Zhang, S.; Su, J. Susceptibility baseline and chlorantraniliprole resistance monitoring in Chilo suppressalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2013, 106, 2190–2194. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Yan, H.H.; Gao, L.; Guo, Y.Y.; Xue, C.B. Chlorantraniliprole resistance in the diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 2014, 107, 806–814. [Google Scholar] [CrossRef]
- He, Y.; Zhang, J.; Chen, J. Effect of synergists on susceptibility to chlorantraniliprole in field populations of Chilo suppressalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2014, 107, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.M.; Wanderley-Teixeira, V.; Ferreira, H.N.; Teixeira, A.A.; Siqueira, H.A. Fitness costs associated with field-evolved resistance to chlorantraniliprole in Plutella xylostella (Lepidoptera: Plutellidae). Bull. Entomol. Res. 2014, 104, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.H.; Xue, C.B.; Li, G.Y.; Zhao, X.L.; Che, X.Z.; Wang, L.L. Flubendiamide resistance and Bi-PASA detection of ryanodine receptor G4946E mutation in the diamondback moth (Plutella xylostella L.). Pestic. Biochem. Physiol. 2014, 115, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, S.; Yao, R.; Wu, S.; Su, J.; Gao, C. Susceptibility of the rice stem borer, Chilo suppressalis (Lepidoptera: Crambidae), to flubendiamide in China. J. Econ. Entomol. 2014, 107, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Roditakis, E.; Vasakis, E.; Grispou, M.; Stavrakaki, M.; Nauen, R.; Gravouil, M.; Bassi, A. First report of Tuta absoluta resistance to diamide insecticides. J. Pest Sci. 2015, 88, 9–16. [Google Scholar] [CrossRef]
- Sang, S.; Shu, B.; Yi, X.; Liu, J.; Hu, M.; Zhong, G. Cross-resistance and baseline susceptibility of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) to cyantraniliprole in the south of China. Pest Manag. Sci. 2016, 72, 922–928. [Google Scholar] [CrossRef]
- Silva, J.E.; Assis, C.P.; Ribeiro, L.M.; Siqueira, H.A. Field-Evolved Resistance and Cross-resistance of Brazilian Tuta absoluta (Lepidoptera: Gelechiidae) populations to diamide insecticides. J. Econ. Entomol. 2016, 109, 2190–2195. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, G.; Zhong, L.; Zhang, F.; Bai, Q.; Zheng, X.; Lu, Z. Resistance monitoring of Chilo suppressalis (Walker) (Lepidoptera: Crambidae) to chlorantraniliprole in eight field populations from East and Central China. Crop Prot. 2017, 100, 196–202. [Google Scholar] [CrossRef]
- Ribeiro, L.M.S.; Siqueira, H.A.A.; Wanderley-Teixeira, V.; Ferreira, H.N.; Silva, W.M.; Silva, J.E.; Teixeira, A.A.C. Field resistance of Brazilian Plutella xylostella to diamides is not metabolism-mediated. Crop Prot. 2017, 93, 82–88. [Google Scholar] [CrossRef]
- Roditakis, E.; Mavridis, K.; Riga, M.; Vasakis, E.; Morou, E.; Rison, J.L.; Vontas, J. Identification and detection of indoxacarb resistance mutations in the para sodium channel of the tomato leafminer, Tuta absoluta. Pest Manag. Sci. 2017, 73, 1679–1688. [Google Scholar] [CrossRef]
- Yao, R.; Zhao, D.D.; Zhang, S.; Zhou, L.Q.; Wang, X.; Gao, C.F.; Wu, S.F. Monitoring and mechanisms of insecticide resistance in Chilo suppressalis (Lepidoptera: Crambidae), with special reference to diamides. Pest Manag. Sci. 2017, 73, 1169–1178. [Google Scholar] [CrossRef]
- Cho, S.R.; Kyung, Y.; Shin, S.; Kang, W.J.; Jung, D.H.; Lee, S.J.; Park, G.H.; Kim, S.I.I.; Cho, S.W.; Kim, H.K.; et al. Susceptibility of field populations of Plutella xylostella and Spodoptera exigua to four diamide insecticides. J. Appl. Entomol. 2018, 57, 43–50. [Google Scholar] [CrossRef]
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, J.; Che, W.; Sun, Y.; Li, W.; Luo, C. Characterization of field-evolved resistance to cyantraniliprole in Bemisia tabaci MED from China. J. Integr. Agric. 2019, 18, 2571–2578. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Ma, H.H.; Lu, W.J.; Wang, X.L.; Wu, S.W.; Nauen, R.; Wu, Y.D.; Yang, Y.H. Identification of the ryanodine receptor mutation I4743M and its contribution to diamide insecticide resistance in Spodoptera exigua (Lepidoptera: Noctuidae). Insect Sci. 2020, 4, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.M.; Zhao, Y.X.; Sun, H.; Ni, H.; Liu, C.; Wang, X.; Gao, C.F.; Wu, S.F. Monitoring and mechanisms of insecticide resistance in Spodoptera exigua (Lepidoptera: Noctuidae), with special reference to diamides. Pestic. Biochem. Physiol. 2021, 174, 104831. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nam, H.Y.; Kwon, M.; Choi, J.H.; Cho, S.R.; Kim, G.H. Development of a diamide resistance diagnostic method using LAMP based on a resistance-specific indel in ryanodine receptors for Spodoptera exigua (Lepidoptera: Noctuidae). bioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, P.; Yang, F.; Wang, Y.; Zhou, L.L.; Luo, H.B.; Zhang, S.; Si, S.Y. Monitoring the resistance of the beet armyworm (Lepidoptera: Noctuidae) to four insecticides in Southern China from 2014 to 2018. J. Econ. Entomol. 2021, 114, 332–338. [Google Scholar] [CrossRef]
- Zhang, S.K.; Ren, X.B.; Wang, Y.C.; Su, J. Resistance in Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) to new chemistry insecticides. J. Econ. Entomol. 2014, 107, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.N.; Catchot, A.L.; Musser, F.R.; Gore, J.; Cook, D.C.; Jackson, R. Susceptibility of Chrysodeixis includens (Lepidoptera: Noctuidae) to reduced-risk insecticides. Fla. Entomol. 2013, 96, 554–559. [Google Scholar] [CrossRef]
- Zhang, R.; He, S.; Chen, J. Monitoring of Bactrocera dorsalis (Diptera: Tephritidae) resistance to cyantraniliprole in the south of China. J. Econ. Entomol. 2014, 107, 1233–1238. [Google Scholar] [CrossRef]
- Liu, X.; Ning, Y.; Wang, H.; Wang, K. Cross-resistance, mode of inheritance, synergism, and fitness effects of cyantraniliprole resistance in Plutella xylostella. Entomol. Exp. Appl. 2015, 157, 271–278. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Shen, J.; Mao, K.; You, H.; Li, J. Susceptibility of field populations of the diamondback moth, Plutella xylostella, to a selection of insecticides in Central China. Pestic. Biochem. Physiol. 2016, 132, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.Y.; Wang, H.; Xu, Y.; Huang, J.; Wu, S.; Wu, Y.; Yang, Y. CRISPR/Cas9 mediated G4946E substitution in the ryanodine receptor of Spodoptera exigua confers high levels of resistance to diamide insecticides. Insect Biochem. Mol. Biol. 2017, 89, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Bolzan, A.; Padovez, F.E.; Nascimento, A.R.; Kaiser, I.S.; Lira, E.C.; Amaral, F.S.; Kanno, R.H.; Malaquias, J.B.; Omoto, C. Selection and characterization of the inheritance of resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to chlorantraniliprole and cross-resistance to other diamide insecticides. Pest Manag. Sci. 2019, 75, 2682–2689. [Google Scholar] [CrossRef]
- Teixeira, L.A.; Andaloro, J.T. Diamide insecticides: Global efforts to address insect resistance stewardship challenges. Pestic. Biochem. Physiol. 2013, 106, 76–78. [Google Scholar] [CrossRef]
- Douris, V.; Papapostolou, K.M.; Ilias, A.; Roditakis, E.; Kounadi, S.; Riga, M.; Nauen, R.; Vontas, J. Investigation of the contribution of RyR target-site mutations in diamide resistance by CRISPR/Cas9 genome modification in Drosophila. Insect Biochem. Mol. Biol. 2017, 87, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Douris, V.; Denecke, S.; Van Leeuwen, T.; Bass, C.; Nauen, R.; Vontas, J. Using CRISPR/Cas9 genome modification to understand the genetic basis of insecticide resistance: Drosophila and beyond. Pestic. Biochem. Physiol. 2020, 167, 104595. [Google Scholar] [CrossRef]
- Jiang, W.H.; Lu, W.P.; Guo, W.C.; Xia, Z.H.; Fu, W.J.; Li, G.Q. Chlorantraniliprole susceptibility in Leptinotarsa decemlineata in the north Xinjiang Uygur autonomous region in China. J. Econ. Entomol. 2012, 105, 549–554. [Google Scholar] [CrossRef][Green Version]
- Sial, A.A.; Brunner, J.F.; Garczynski, S.F. Biochemical characterization of chlorantraniliprole and spinetoram resistance in laboratory-selected obliquebanded leafroller, Choristoneura rosaceana (Harris) (Lepidoptera: Tortricidae). Pestic. Biochem. Physiol. 2011, 99, 274–279. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.Y.; Ning, Y.B.; Qiao, K.; Wang, K.Y. Resistance selection and characterization of chlorantraniliprole resistance in Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2015, 108, 1978–1985. [Google Scholar] [CrossRef]
- Lin, Q.; Jin, F.; Hu, Z.; Chen, H.; Yin, F.; Li, Z.; Dong, X.; Zhang, D.; Ren, S.; Feng, X. Transcriptome analysis of chlorantraniliprole resistance development in the diamondback moth Plutella xylostella. PLoS ONE 2013, 8, e72314. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lin, Q.; Chen, H.; Li, Z.; Yin, F.; Feng, X. Identification of a novel cytochrome P450 gene, CYP321E1 from the diamondback moth, Plutella xylostella (L.) and RNA interference to evaluate its role in chlorantraniliprole resistance. Bull. Entomol. Res. 2014, 104, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, R.; Zhu, B.; Gao, X.; Liang, P. Overexpression of cytochrome P450 CYP6BG1 may contribute to chlorantraniliprole resistance in Plutella xylostella (L.). Pest Manag. Sci. 2018, 74, 1386–1393. [Google Scholar] [CrossRef]
- Wang, J.D.; Chen, L.F.; Wang, Y.R.; Fu, H.Y.; Ali, A.; Xiao, D.; Wang, R.; Gao, S.J. Silence of ryanodine receptor gene decreases susceptibility to chlorantraniliprole in the oriental armyworm, Mythimna separata Walker. Pestic. Biochem. Physiol. 2018, 148, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.Y.; Huang, J.L.; Wang, J.; Feng, Y.; Han, T.T.; Wu, Y.D.; Yang, Y.H. Knockout of a P-glycoprotein gene increases susceptibility to abamectin and emamectin benzoate in Spodoptera exigua. Insect Mol. Biol. 2018, 27, 36–45. [Google Scholar] [CrossRef]
- Meng, X.; Yang, X.; Wu, Z.; Shen, Q.; Miao, L.; Zheng, Y.; Qian, K.; Wang, J. Identification and transcriptional response of ATP-binding cassette transporters to chlorantraniliprole in the rice striped stem borer, Chilo suppressalis. Pest Manag. Sci. 2020, 76, 3626–3635. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Zhou, X.; Gao, X.; Liang, P. miRNAs regulated overexpression of ryanodine receptor is involved in chlorantraniliprole resistance in Plutella xylostella (L.). Sci. Rep. 2015, 5, 14095. [Google Scholar] [CrossRef]
- Ma, S.; Liu, J.; Lu, X.; Zhang, X.; Ma, Z. Effect of wilforine on the calcium signaling pathway in Mythimna separata Walker myocytes using the calcium imaging technique. J. Agric. Food Chem. 2019, 67, 13751–13757. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Y.; Wu, L.; Zhang, X. Isolation and insecticidal activity of sesquiterpenes alkaloids from Tripterygium wilfordii Hook f. Ind. Crops Prod. 2014, 52, 642–648. [Google Scholar] [CrossRef]
- Li, Y.; Lian, X.; Wan, Y.; Wang, D.; Chen, W.; Di, F.; Wu, W.; Li, Z. Modulation of the Ca(2+) signaling pathway by celangulin I in the central neurons of Spodoptera exigua. Pestic. Biochem. Physiol. 2016, 127, 76–81. [Google Scholar] [CrossRef]
- Lapied, B.; Pennetier, C.; Apaire-Marchais, V.; Licznar, P.; Corbel, V. Innovative applications for insect viruses: Towards insecticide sensitization. Trends Biotechnol. 2009, 27, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Licznar, P.; List, O.; Goven, D.; Nna, R.N.; Lapied, B.; Apaire-Marchais, V. A novel method using Autographa californica multiple nucleopolyhedrovirus for increasing the sensitivity of insecticide through calcium influx in insect cell line. J. Virol. Methods. 2014, 195, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Abd-Ella, A.; Stankiewicz, M.; Mikulska, K.; Nowak, W.; Pennetier, C.; Goulu, M.; Fruchart-Gaillard, C.; Licznar, P.; Apaire-Marchais, V.; List, O.; et al. The Repellent DEET potentiates carbamate effects via insect muscarinic receptor ınteractions: An alternative strategy to control insect vector-borne diseases. PLoS ONE 2015, 10, e0126406. [Google Scholar] [CrossRef] [PubMed]
- Apaire-Marchais, V.; Ogliastro, M.; Chandre, F.; Pennetier, C.; Raymond, V.; Lapied, B. Virus and calcium: An unexpected tandem to optimize insecticide efficacy. Environ. Microbiol. Rep. 2016, 8, 168–178. [Google Scholar] [CrossRef]
- Deshayes, C.; Moreau, E.; Pitti-Caballero, J.; Froger, J.A.; Apaire-Marchais, V.; Lapied, B. Synergistic agent and intracellular calcium, a successful partnership in the optimization of insecticide efficacy. Curr. Opin. Insect Sci. 2018, 30, 52–58. [Google Scholar] [CrossRef]
- Monette, R.; Potvin, L.; Baines, D.; Laprade, R.; Schwartz, J.L. Interaction between calcium ions and Bacillus thuringiensis toxin activity against Sf9 cells (Spodoptera frugiperda, Lepidoptera). Appl. Environ. Microbiol. 1997, 63, 440–447. [Google Scholar] [CrossRef]
- Toprak, U.; Bayram, S.; Gürkan, O.M. Comparative biological activities of a plaque-purified variant and a Turkish native isolate of SpliNPV-B against Spodoptera littoralis (Lepidoptera: Noctuidae). Pest Manag. Sci. 2006, 62, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.; Shabbir, M.Z.; Wang, Z.; He, K. Analysis of Cry1Ah toxin-binding reliability to midgut membrane proteins of the Asian corn borer. Toxins 2020, 12, 418. [Google Scholar] [CrossRef]
- Erlandson, M.; Toprak, U.; Hegedus, D.D. Role of the peritrophic matrix in insect-pathogen interactions. J. Insect Physiol. 2019, 117, 103894. [Google Scholar] [CrossRef]
- Güney, G.; Cedden, D.; Hänniger, S.; Heckel, D.G.; Coutu, C.; Hegedus, D.D.; Amutkan Mutlu, D.; Suludere, Z.; Sezen, K.; Güney, E.; et al. Silencing of an ABC transporter, but not a cadherin, decreases the susceptibility of Colorado potato beetle larvae to Bacillus thuringiensis ssp. tenebrionis Cry3Aa toxin. Arch. Insect Biochem. Physiol. 2021, in press. [Google Scholar] [CrossRef]
- Toprak, U.; Baldwin, D.; Erlandson, M.; Gillott, C.; Harris, S.; Hegedus, D.D. In vitro and in vivo application of RNA interference for targeting genes involved in peritrophic matrix synthesis in a lepidopteran system. Insect Sci. 2013, 20, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wan, P.; Lai, F.; Zhu, T.; Fu, Q. Double-stranded RNA targeting calmodulin reveals a potential target for pest management of Nilaparvata lugens. Pest Manag. Sci. 2018, 74, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A perspective on RNAi-based biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).