Rosmarinic Acid, a Bioactive Phenolic Compound, Inhibits Glutamate Release from Rat Cerebrocortical Synaptosomes through GABAA Receptor Activation
Abstract
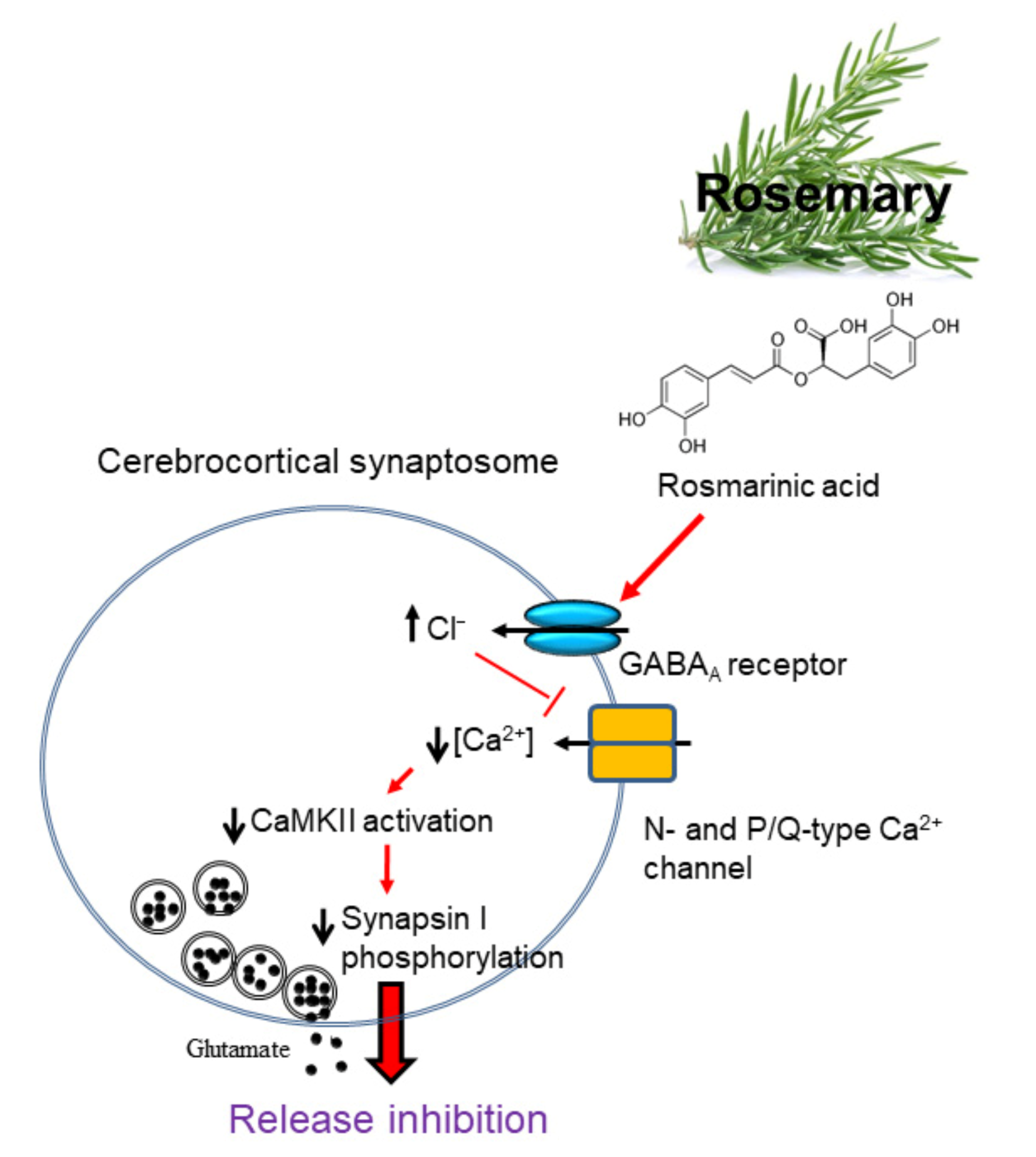
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation of Synaptosomes from the Rat
2.3. Measurement of Glutamate Release
2.4. Measurement of Intrasynaptosomal Ca2+ Concentration ([Ca2+]i)
2.5. Measurement of Membrane Potential
2.6. Western Blot
2.7. Immunocytochemistry
2.8. Molecular Docking Study
2.9. Statistical Analysis
2.10. Chemicals
3. Results
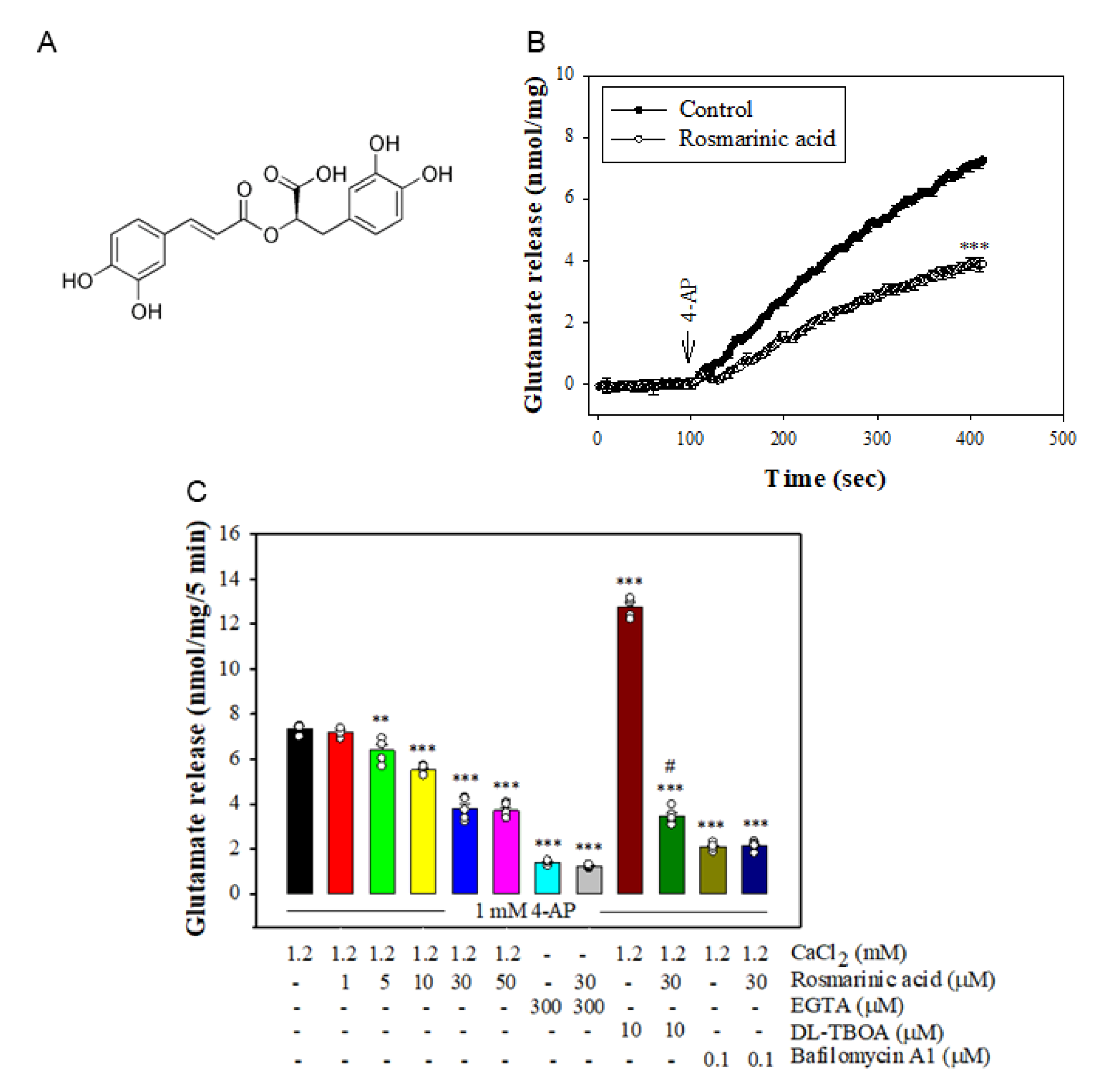
3.1. Effect of Rosmarinic Acid on the 4-AP-Evoked Glutamate Release in Rat Cerebrocortical Synaptosomes
3.2. Effect of Rosmarinic Acid on [Ca2+]i and Membrane Potential
3.3. Effect of Rosmarinic Acid on Glutamate Release in the Presence of VGCC Blockers or Intracellular Ca2+ Release Inhibitors
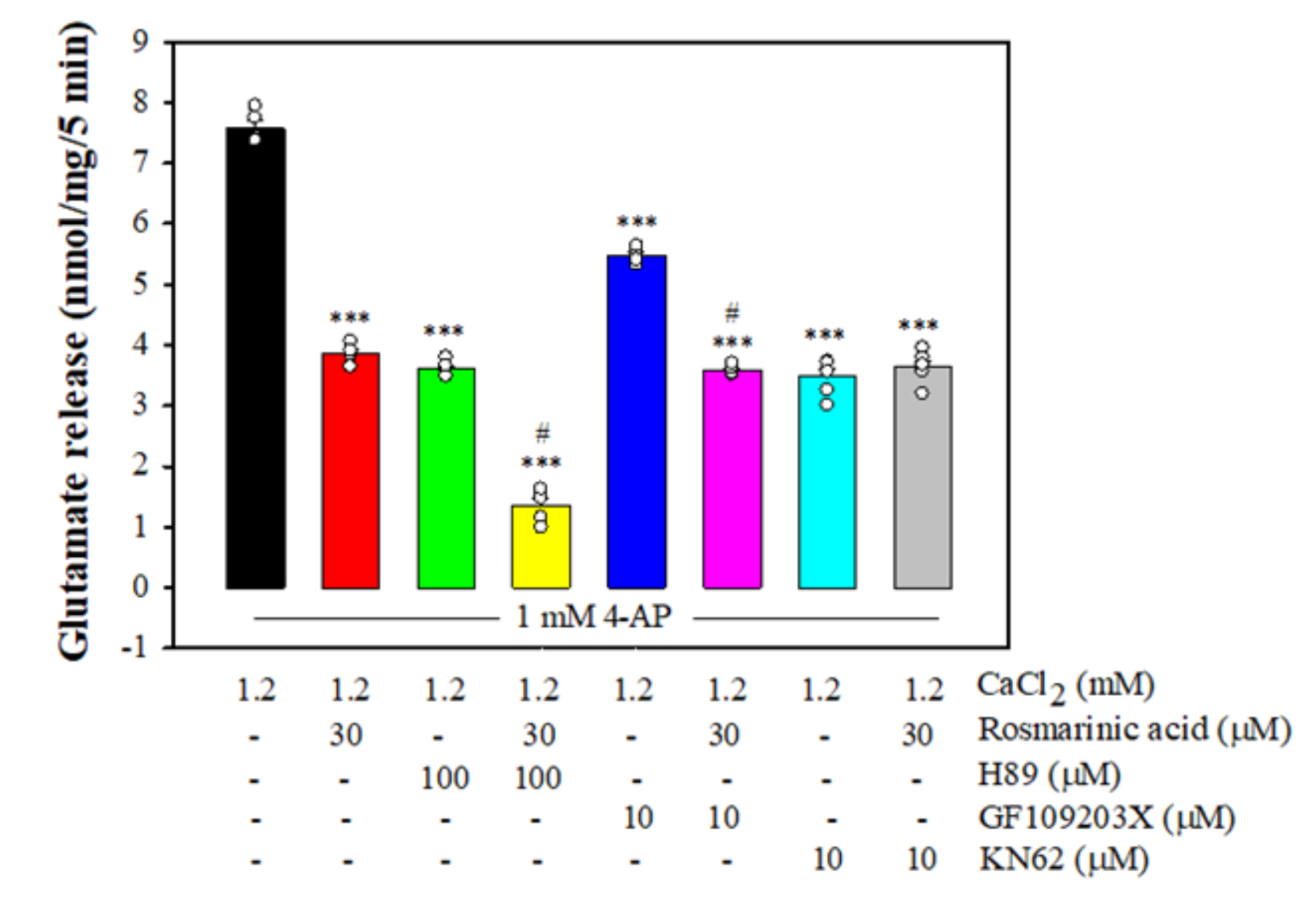
3.4. Effect of Rosmarinic Acid on Glutamate Release in the Presence of Protein Kinase Inhibitors
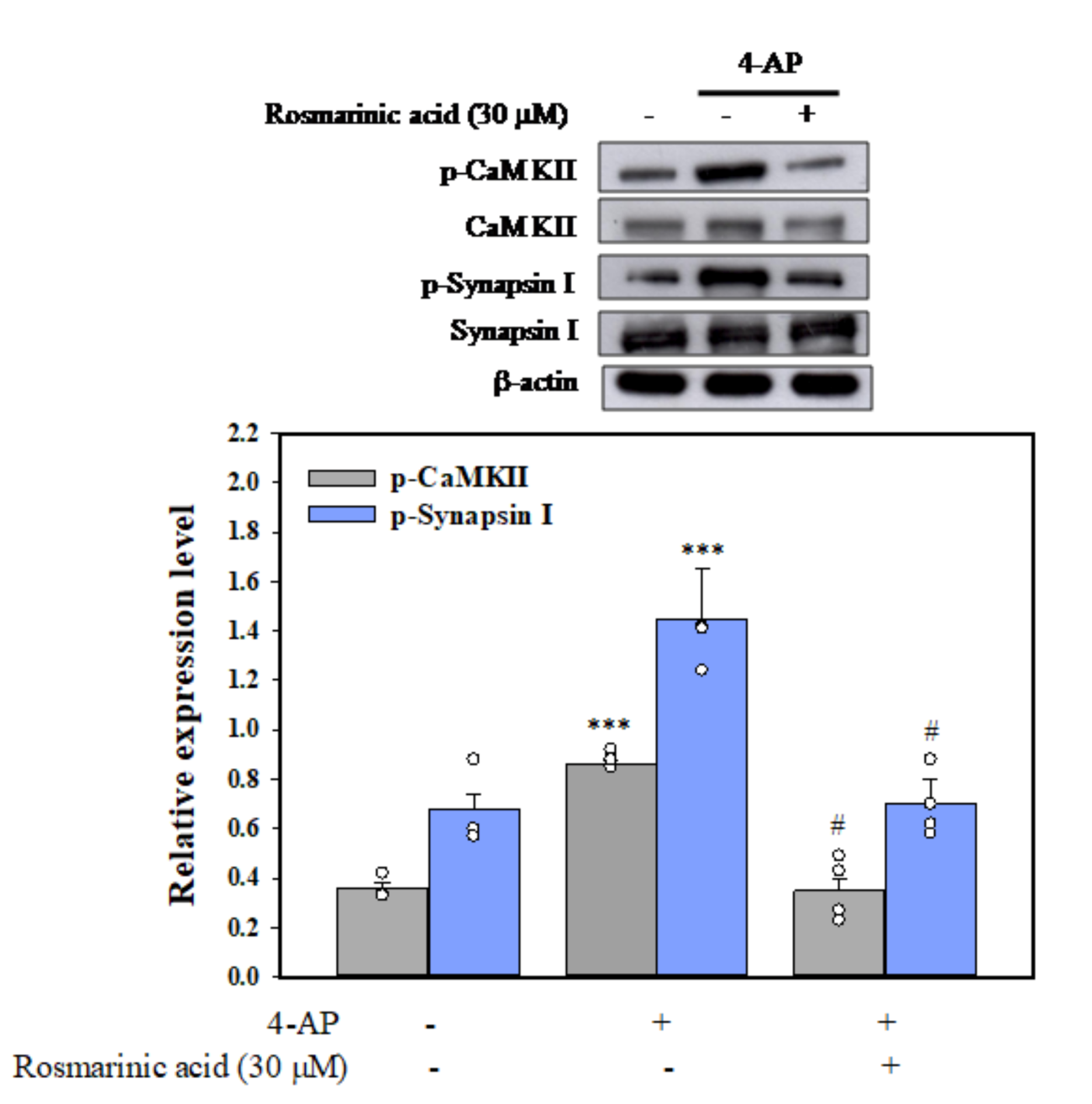
3.5. Effect of Rosmarinic Acid on the Phosphorylation of CaMKII and Synapsin I (Serine 603, a Substrate Site of CaMKII) in Synaptosomes
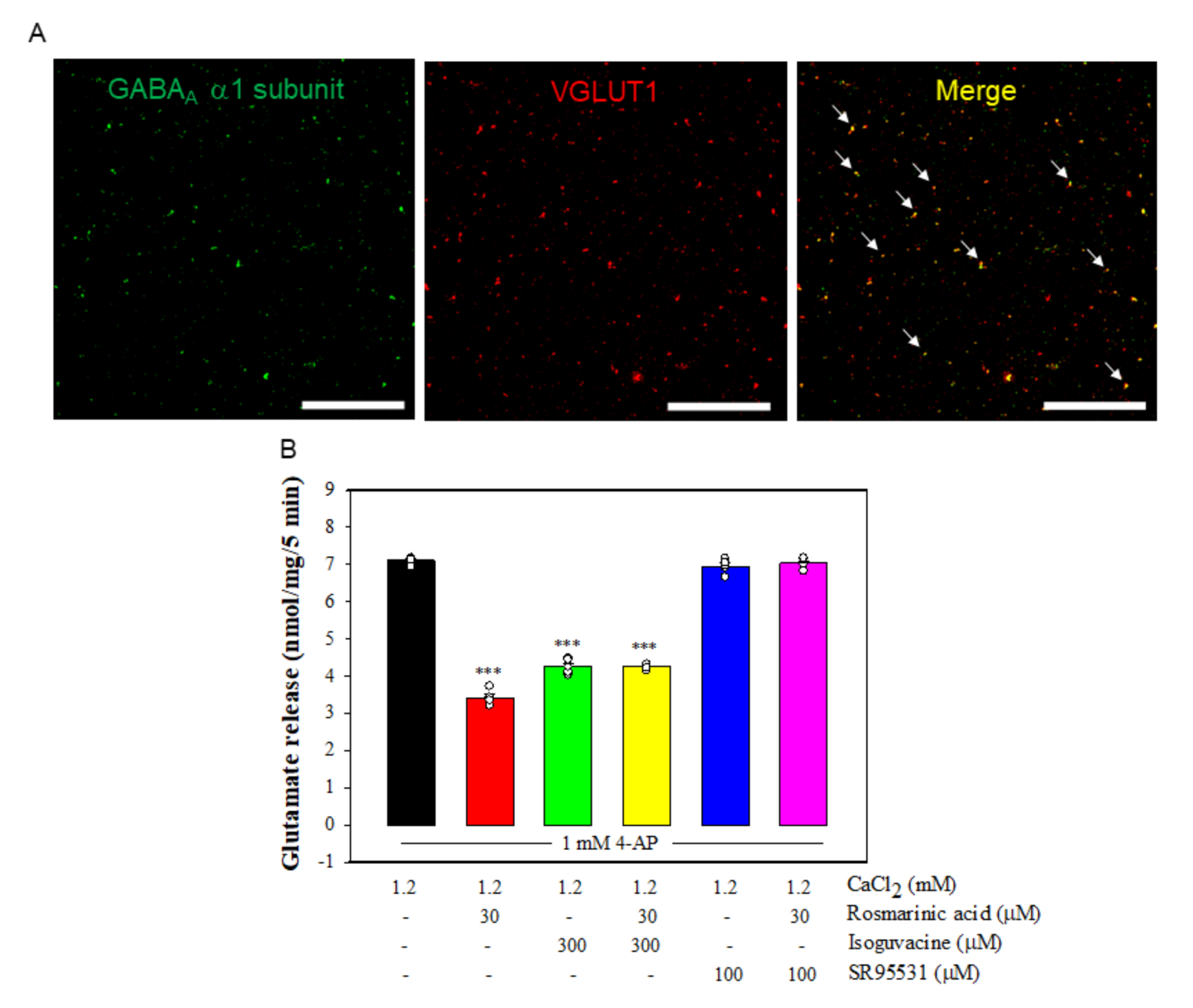
3.6. Effect of Rosmarinic Acid on Glutamate Release in the Presence of GABAAReceptor Agonist and Antagonist
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, S.; Zhang, Y.; Zhang, J.; Wang, H.; Ren, B. Glutamate receptors and signal transduction in learning and memory. Mol. Biol. Rep. 2011, 38, 453–460. [Google Scholar] [CrossRef]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1998, 1, 623–634. [Google Scholar] [CrossRef]
- Bano, D.; Ankarcrona, M. Beyond the critical point: An overview of excitotoxicity, calcium overload and the downstream consequences. Neurosci. Lett. 2018, 663, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch. Eur. J. Physiol. 2010, 460, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Maher, P. Chronic glutamate toxicity in neurodegenerative diseases-what is the evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef]
- Mdzinarishvili, A.; Sumbria, R.; Lang, D.; Klein, J. Ginkgo extract EGb761 confers neuroprotection by reduction of glutamate release in ischemic brain. J. Pharm. Pharm. Sci. 2012, 15, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.B.; Cheng, S.J.; Hung, W.C.; Lee, W.T.; Min, M.Y. Rosiglitazone suppresses in vitro seizures in hippocampal slice by inhibiting presynaptic glutamate release in a model of temporal lobe epilepsy. PLoS ONE 2015, 10, e0144806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarevic, V.; Yang, Y.; Ivanova, D.; Fejtova, A.; Svenningsson, P. Riluzole attenuates the efficacy of glutamatergic transmission by interfering with the size of the readily releasable neurotransmitter pool. Neuropharmacology 2018, 143, 38–48. [Google Scholar] [CrossRef]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective strategies for neurological disorders by natural products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Tóth, F.; Cseh, E.K.; Vécsei, L. Natural molecules and neuroprotection: Kynurenic acid, pantethine and α-lipoic acid. Int. J. Mol. Sci. 2021, 22, 403. [Google Scholar] [CrossRef]
- Al-Sereiti, M.R.; Abu-Amer, K.M.; Sen, P. Pharmacology of rosemary (Rosmarinus officinalis Linn) and its therapeutic potentials. Indian J. Exp. Biol. 1999, 37, 124–130. [Google Scholar] [PubMed]
- Rahbardar, M.G.; Hosseinzadeh, H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran J. Basic Med. Sci. 2020, 23, 1100–1112. [Google Scholar]
- Aruoma, O.; Spencer, J.; Rossi, R.; Aeschbach, R.; Khan, A.; Mahmood, N.; Halliwell, B. An evaluation of the antioxidant and antiviral action of extracts of rosemary and provencal herbs. Food Chem. Toxicol. 1996, 34, 449–456. [Google Scholar] [CrossRef]
- Bakirel, T.; Bakirel, U.; Keles, O.U.; Ulgen, S.G.; Yardibi, H. In Vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J. Ethnopharmacol. 2008, 116, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Rahbardar, M.G.; Amin, B.; Mehri, S.; Mirnajafi-Zadeh, S.J.; Hosseinzadeh, H. Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain. Biomed. Pharmacother. 2017, 86, 441–449. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary Extract polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- González-Trujano, M.; Peña, E.; Martínez, A.; Moreno, J.; Guevara-Fefer, P.; Deciga-Campos, M.; López-Muñoz, F. Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents. J. Ethnopharmacol. 2007, 111, 476–482. [Google Scholar] [CrossRef]
- Machado, D.G.; Bettio, L.E.; Cunha, M.P.; Capra, J.C.; Dalmarco, J.B.; Pizzolatti, M.G.; Rodrigues, A.L.S. Antidepressant-like effect of the extract of Rosmarinus officinalis in mice: Involvement of the monoaminergic system. Prog. Neuro-Psychopharmacol. 2009, 33, 642–650. [Google Scholar] [CrossRef]
- Naderali, E.; Nikbakht, F.; Ofogh, S.N.; Rasoolijazi, H. The role of rosemary extract in degeneration of hippocampal neurons induced by kainic acid in the rat: A behavioral and histochemical approach. J. Integr. Neurosci. 2018, 17, 19–25. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Karimi, G.; Noubakht, M. Effects of Rosmarinus officinalis L. aerial parts essential oil on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. J. Med. Plants 2004, 3, 51–57. [Google Scholar]
- Fonteles, A.A.; de Souza, C.M.; de Sousa Neves, J.C.; Menezes, A.P.F.; Santos do Carmo, M.R.; Fernandes, F.D.P.; de Araújo, P.R.; de Andrade, G.M. Rosmarinic acid prevents against memory deficits in ischemic mice. Behav. Brain Res. 2016, 297, 91–103. [Google Scholar] [CrossRef]
- Shimojo, Y.; Kosaka, K.; Noda, Y.; Shimizu, T.; Shirasawa, T. Effect of rosmarinic acid in motor dysfunction and life span in a mouse model of familial amyotrophic lateral sclerosis. J. Neurosci. Res. 2010, 88, 896–904. [Google Scholar] [CrossRef]
- Rong, H.; Liang, Y.; Niu, Y. Rosmarinic acid attenuates β-amyloid-induced oxidative stress via Akt/GSK-3β/Fyn-mediated Nrf2 activation in PC12 cells. Free Radic. Biol. Med. 2018, 120, 114–123. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, H.S.; Park, E.; Kim, S.; Lee, S.Y.; Kim, C.S.; Kim, D.K.; Kim, S.J.; Chun, H.S. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology 2008, 250, 109–115. [Google Scholar] [CrossRef]
- Taram, F.; Ignowski, E.; Duval, N.; Linseman, D.A. Neuroprotection comparison of rosmarinic acid and carnosic acid in primary cultures of cerebellar granule neurons. Molecules 2018, 23, 2956. [Google Scholar] [CrossRef] [Green Version]
- Su, I.C.; Hung, C.F.; Lin, C.N.; Huang, S.K.; Wang, S.J. Cycloheterophyllin inhibits the release of glutamate from nerve terminals of the rat hippocampus. Chem. Res. Toxicol. 2019, 32, 1591–1598. [Google Scholar] [CrossRef]
- Chiu, K.M.; Lin, T.Y.; Lee, M.Y.; Lu, C.W.; Wang, M.J.; Wang, S.J. Typhaneoside suppresses glutamate release through inhibition of voltage-dependent calcium entry in rat cerebrocortical nerve terminal. Chem. Res. Toxicol. 2021, 34, 1286–1295. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Akerman, K.E.; Scott, I.G.; Heikkilä, J.E.; Heinonen, E. Ionic dependence of membrane potential and glutamate receptor-linked responses in synaptoneurosomes as measured with a cyanine dye, DiS-C2-(5). J. Neurochem. 1987, 48, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Lin, T.Y.; Chiu, K.M.; Lee, M.Y.; Huang, J.H.; Wang, S.J. Silymarin inhibits glutamate release and prevents against kainic acid-induced excitotoxic injury in rats. Biomedicines 2020, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Tibbs, G.R.; Barrie, A.P.; Van Mieghem, F.J.; McMahon, H.T.; Nicholls, D.G. Repetitive action potentials in isolated nerve terminals in the presence of 4-aminopyridine: Effects on cytosolic free Ca2+ and glutamate release. J. Neurochem. 1989, 53, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, E.; Sánchez-Prieto, J. Presynaptic modulation of glutamate release targets different calcium channels in rat cerebrocortical nerve terminals. Eur. J. Neurosci. 1997, 9, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Neuronal calcium signaling. Neuron 1998, 21, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Barrie, A.P.; Nicholls, D.G.; Sanchez-Prieto, J.; Sihra, T.S. An ion channel locus for the protein kinase C potentiation of transmitter glutamate release from guinea pig cerebrocortical synaptosomes. J. Neurochem. 1991, 57, 1398–1404. [Google Scholar] [CrossRef]
- Kwon, Y.O.; Hong, J.T.; Oh, K.W. Rosmarinic acid potentiates pentobarbital-induced sleep behaviors and non-rapid eye movement (NREM) sleep through the activation of GABAA-ergic systems. Biomol. Ther. (Seoul) 2017, 25, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liu, C.; Wu, H.; Wang, J.; Sun, Y.; Liu, R.; Li, T.; Yu, X.; Geng, D.; Sun, Y.K. Global analysis the potential medicinal substances of Shuangxia Decoction and the process in vivo via mass spectrometry technology. Front. Pharmacol 2021, 12, 654807. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G.; Sihra, T.S.; Sanchez-Prieto, J. Calcium-dependent and -independent release of glutamate from synaptosomes monitored by continuous fluorometry. J. Neurochem. 1987, 49, 50–57. [Google Scholar] [CrossRef]
- Nicholls, D.G. Presynaptic modulation of glutamate release. Prog. Brain Res. 1998, 116, 15–22. [Google Scholar]
- Millán, C.; Sánchez-Prieto, J. Differential coupling of N- and P/Q-type calcium channels to glutamate exocytosis in the rat cerebral cortex. Neurosci. Lett. 2002, 330, 29–32. [Google Scholar] [CrossRef]
- Hudmon, A.; Schulman, H. Neuronal Ca2+/calmodulin-dependent protein kinase II: The role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 2002, 71, 473–510. [Google Scholar] [CrossRef]
- Nichols, R.A.; Sihra, T.S.; Czernik, A.J.; Nairn, A.C.; Greengard, P. Calcium/calmodulin-dependent protein kinase II increases glutamate and noradrenaline release from synaptosomes. Nature 1990, 343, 647–651. [Google Scholar] [CrossRef]
- Hinds, H.L.; Goussakov, I.; Nakazawa, K.; Tonegawa, S.; Bolshakov, V.Y. Essential function of alpha-calcium/calmodulin-dependent protein kinase II in neurotransmitter release at a glutamatergic central synapse. Proc. Natl. Acad. Sci. 2003, 100, 4275–4280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León, D.; Sánchez-Nogueiro, J.; Marín-García, P.; Miras-Portugal, M.A. Glutamate release and synapsin-I phosphorylation induced by P2X7 receptors activation in cerebellar granule neurons. Neurochem. Int. 2008, 52, 1148–1159. [Google Scholar] [CrossRef]
- Llinás, R.; Gruner, J.A.; Sugimori, M.; McGuinness, T.L.; Greengard, P. Regulation by synapsin I and Ca2+-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J. Physiol. 1991, 436, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Chi, P.; Greengard, P.; Ryan, T.A. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron 2003, 38, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Leenders, A.G.; Sheng, Z.H. Modulation of neurotransmitter release by the second messenger-activated protein kinases: Implications for presynaptic plasticity. Pharmacol. Ther. 2005, 105, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Ohyama, A.; Hosaka, K.; Komiya, Y.; Akagawa, K.; Yamauchi, E.; Taniguchi, H.; Sasagawa, N.; Kumakura, K.; Mochida, S.; Yamauchi, T.; et al. Regulation of exocytosis through Ca2+/ATP-dependent binding of autophosphorylated Ca2+/calmodulin-activated protein kinase II to syntaxin 1A. J. Neurosci. 2002, 22, 3342–3351. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.W. Regulation of synaptic transmission by presynaptic CaMKII and BK channels. Mol. Neurobiol. 2008, 38, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Sieghart, W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv. Pharmacol. 2006, 54, 231–263. [Google Scholar]
- Olsen, R.W.; Sieghart, W. GABAA receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacol. 2009, 56, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Sieghart, W.; Sperk, G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr. Top. Med. Chem. 2002, 2, 795–816. [Google Scholar] [CrossRef]
- Chang, C.Y.; Lin, T.Y.; Lu, C.W.; Wang, C.C.; Wang, Y.C.; Chou, S.S.; Wang, S.J. Apigenin, a natural flavonoid, inhibits glutamate release in the rat hippocampus. Eur. J. Pharmacol. 2015, 762, 72–81. [Google Scholar] [CrossRef]
- Chang, Y.; Lin, T.Y.; Lu, C.W.; Huang, S.K.; Wange, Y.C.; Wang, S.J. Xanthohumol-induced presynaptic reduction of glutamate release in the rat hippocampus. Food Funct. 2016, 7, 212–226. [Google Scholar] [CrossRef]
- David, A.; Wagner, D.A.; Czajkowski, C. Structure and dynamics of the GABA binding pocket: Anarrowing cleft that constricts during activation. J. Neurosci. 2001, 21, 67–74. [Google Scholar]
- Padgett, C.L.; Lummis, S.C.R. The F-loop of the GABAA receptor gamma2 subunit contributes to benzodiazepine modulation. J. Biol. Chem. 2008, 283, 2702–2708. [Google Scholar] [CrossRef] [Green Version]
- Long, P.; Mercer, A.; Begum, R.; Stephens, G.J.; Sihra, T.S.; Jovanovic, J.N. Nerve terminal GABAA receptors activate Ca2+/calmodulin-dependent signaling to inhibit voltage-gated Ca2+ influx and glutamate release. J. Biol. Chem. 2009, 284, 8726–8737. [Google Scholar] [CrossRef] [Green Version]
- Garlet, Q.I.; Rodrigues, P.; Barbosa, L.B.; Londero, A.L.; Mello, C.F.; Heinzmann, B.M. Nectandra grandiflora essential oil and its isolated sesquiterpenoids minimize anxiety-related behaviors in mice through GABAergic mechanisms. Toxicol. Appl. Pharmacol. 2019, 375, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Sonar, V.P.; Fois, B.; Distinto, S.; Maccioni, E.; Meleddu, R.; Cottiglia, F.; Acquas, E.; Kasture, S.; Floris, C.; Colombo, D.; et al. Ferulic acid esters and Withanolides: In search of Withania somnifera GABAA receptor modulators. J. Nat. Prod. 2019, 82, 1250–1257. [Google Scholar] [CrossRef] [PubMed]


| Cytosolic [Ca2+] (nM) | DiSC3(5) Fluorescence | |||||
|---|---|---|---|---|---|---|
| Basal | 4-AP (1 mM) | n | Basal | 4-AP (1 mM) | n | |
| Control | 148.6±1.1 | 196.7± 3.1 | 5 | 0.6 ± 0.3 | 24.7 ± 1.6 | 5 |
| Rosmarinic acid | 147.9±2.1 | 170.7 ± 4.0 *** | 5 | 0.5 ± 0.8 | 24.3 ± 1.8 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Hsieh, P.-W.; Kuo, J.-R.; Wang, S.-J. Rosmarinic Acid, a Bioactive Phenolic Compound, Inhibits Glutamate Release from Rat Cerebrocortical Synaptosomes through GABAA Receptor Activation. Biomolecules 2021, 11, 1029. https://doi.org/10.3390/biom11071029
Wang C-C, Hsieh P-W, Kuo J-R, Wang S-J. Rosmarinic Acid, a Bioactive Phenolic Compound, Inhibits Glutamate Release from Rat Cerebrocortical Synaptosomes through GABAA Receptor Activation. Biomolecules. 2021; 11(7):1029. https://doi.org/10.3390/biom11071029
Chicago/Turabian StyleWang, Che-Chuan, Pei-Wen Hsieh, Jinn-Rung Kuo, and Su-Jane Wang. 2021. "Rosmarinic Acid, a Bioactive Phenolic Compound, Inhibits Glutamate Release from Rat Cerebrocortical Synaptosomes through GABAA Receptor Activation" Biomolecules 11, no. 7: 1029. https://doi.org/10.3390/biom11071029
APA StyleWang, C.-C., Hsieh, P.-W., Kuo, J.-R., & Wang, S.-J. (2021). Rosmarinic Acid, a Bioactive Phenolic Compound, Inhibits Glutamate Release from Rat Cerebrocortical Synaptosomes through GABAA Receptor Activation. Biomolecules, 11(7), 1029. https://doi.org/10.3390/biom11071029







