Thermosonication for the Production of Sulforaphane Rich Broccoli Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Thermosonication and Thermal Treatments
2.4. Broccoli Puree Processing
2.5. Glucoraphanin Extraction and Analysis
2.5.1. Aqueous Extraction
2.5.2. Methanolic Extraction
2.5.3. Analysis and Quantification of Glucoraphanin
2.6. Sulforaphane Extraction and Analysis
2.6.1. Extraction
2.6.2. Analysis and Quantification
2.7. Determination of Myrosinase Activity
2.7.1. Myrosinase Extraction
2.7.2. Myrosinase Activity Assay
2.8. Statistical Analysis
3. Results and Discussion
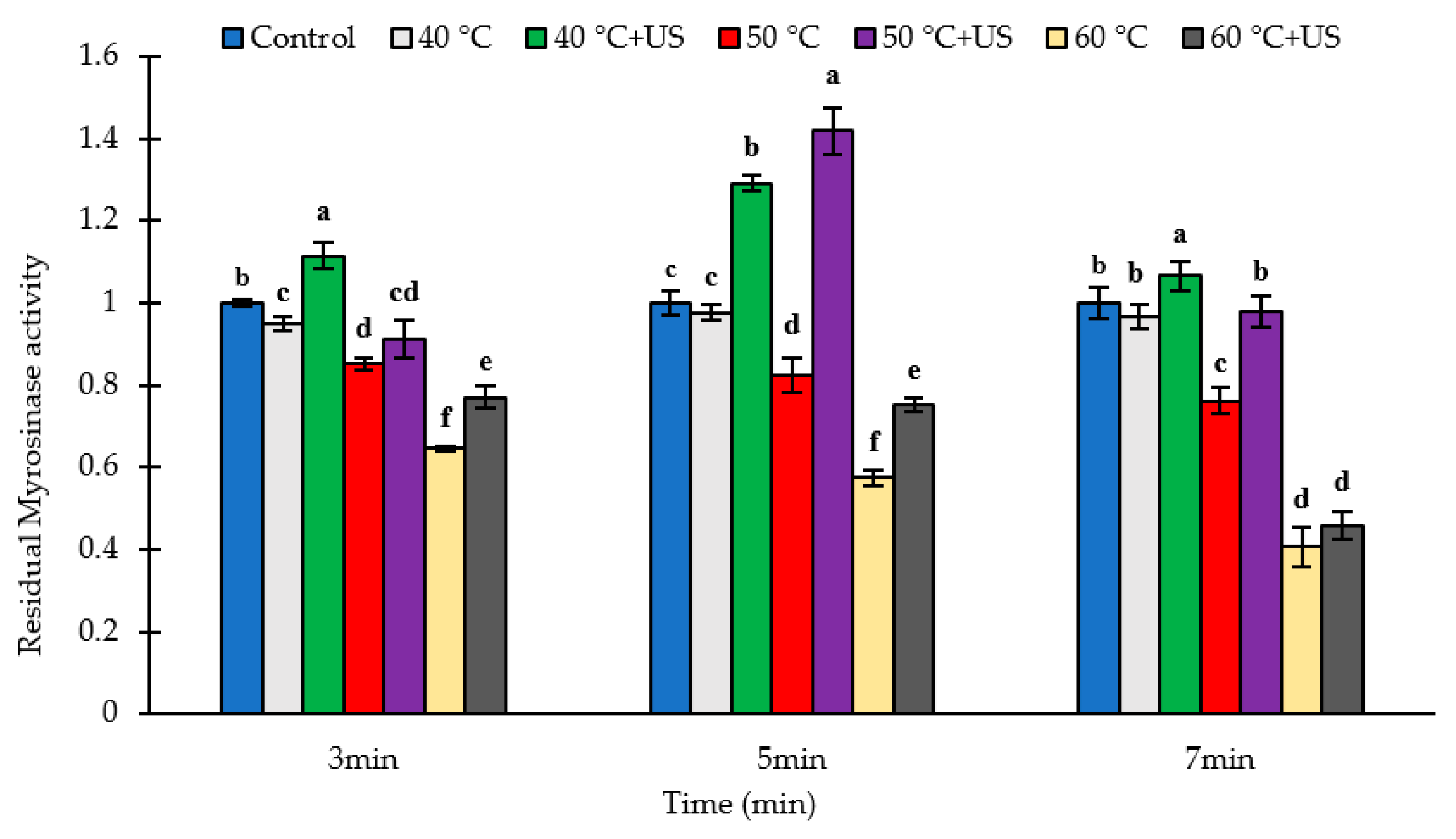
3.1. The Stability of Broccoli Myrosinase Subject to Thermosonication and Thermal Processing
3.2. Effect of Thermosonication and Heat on Sulforaphane Yield
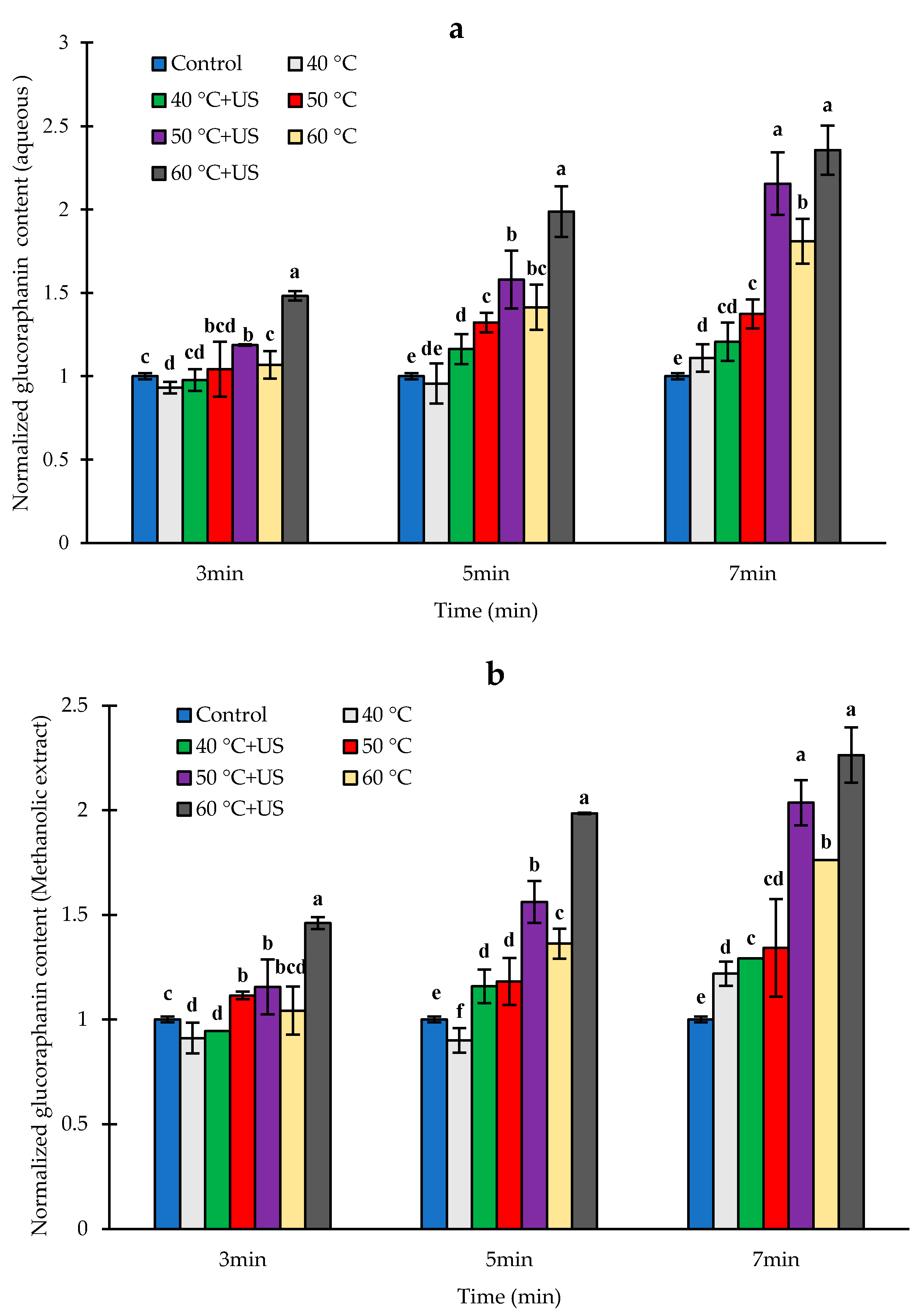
3.3. Effect of Heat and Thermosonication Treatments on Glucoraphanin Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogers, G.; Ekman, J.; Titley, M. Identifying New Products, Uses and Markets for Australian Vegetables: A Desktop Study; Horticulture Australia Ltd.: Sydney, Australia, 2013; pp. 30–32. Available online: https://ausveg.com.au/app/data/technical-insights/docs/130052_VG12046.pdf (accessed on 20 February 2021).
- Shi, M.; Hlaing, M.M.; Ying, D.; Ye, J.; Sanguansri, L.; Augustin, M.A. New food ingredients from broccoli by-products: Physical, chemical and technological properties. Int. J. Food Sci. Technol. 2019, 54, 1423–1432. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Valverde, J.; Kehoe, K.; Reilly, K.; Rai, D.K.; Barry-Ryan, C. Development of a Novel Functional Soup Rich in Bioactive Sulforaphane Using Broccoli (Brassica oleracea L. ssp. italica) Florets and Byproducts. Food Bioprocess. Technol. 2013, 7, 1310–1321. [Google Scholar] [CrossRef] [Green Version]
- Tahata, S.; Singh, S.V.; Lin, Y.; Hahm, E.-R.; Beumer, J.H.; Christner, S.M.; Rao, U.N.; Sander, C.; Tarhini, A.A.; Tawbi, H.; et al. Evaluation of Biodistribution of Sulforaphane after Administration of Oral Broccoli Sprout Extract in Melanoma Patients with Multiple Atypical Nevi. Cancer Prev. Res. 2018, 11, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Armah, C.N.; Derdemezis, C.; Traka, M.H.; Dainty, J.R.; Doleman, J.F.; Saha, S.; Leung, W.; Potter, J.F.; Lovegrove, J.A.; Mithen, R.F. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: Evidence from randomised controlled trials. Mol. Nutr. Food Res. 2015, 59, 918–926. [Google Scholar] [CrossRef]
- Sivapalan, T.; Melchini, A.; Saha, S.; Needs, P.W.; Traka, M.H.; Tapp, H.; Dainty, J.R.; Mithen, R.F. Bioavailability of Glucoraphanin and Sulforaphane from High-Glucoraphanin Broccoli. Mol. Nutr. Food Res. 2017, 62, e1700911. [Google Scholar] [CrossRef] [Green Version]
- Ghawi, S.K.; Methven, L.; Niranjan, K. The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba). Food Chem. 2013, 138, 1734–1741. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Baluch, N.; Homayouni, T.S.; Morgatskaya, E.; Kumar, S.; Kazemi, P.; Yeger, H. The role of Sulforaphane in cancer chemoprevention and health benefits: A mini-review. J. Cell Commun. Signal. 2017, 12, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 2017, 9, eaah4477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, T.; Colaço, B.; Venâncio, C.; Pires, M.J.; Oliveira, P.A.; Rosa, E.; Antunes, L.M. Potential effects of sulforaphane to fight obesity. J. Sci. Food Agric. 2018, 98, 2837–2844. [Google Scholar] [CrossRef]
- Román, J.; Castillo, A.; Cottet, L.; Mahn, A. Kinetic and structural study of broccoli myrosinase and its interaction with different glucosinolates. Food Chem. 2018, 254, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kissen, R.; Rossiter, J.T.; Bones, A.M. The ‘mustard oil bomb’: Not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem. Rev. 2008, 8, 69–86. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol. Plant. 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Bello, C.; Maldini, M.; Baima, S.; Scaccini, C.; Natella, F. Glucoraphanin and sulforaphane evolution during juice preparation from broccoli sprouts. Food Chem. 2018, 268, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Juvik, J.A.; Jeffery, E.H. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochem. 2004, 65, 1273–1281. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Swarup, R.; Juvik, J.A.; Mithen, R.; Bennett, A.M.; Jeffery, E.H. Epithiospecifier Protein from Broccoli (Brassica oleracea L. ssp. italica) Inhibits Formation of the Anticancer Agent Sulforaphane. J. Agric. Food Chem. 2006, 54, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Barrientos, H.; Román, J.; Mahn, A. Optimization of a blanching step to maximize sulforaphane synthesis in broccoli florets. Food Chem. 2014, 145, 264–271. [Google Scholar] [CrossRef]
- Pongmalai, P.; Fu, N.; Soponronnarit, S.; Chiewchan, N.; Devahastin, S.; Chen, X.D. Microwave pretreatment enhances the formation of cabbage sulforaphane and its bioaccessibility as shown by a novel dynamic soft rat stomach model. J. Funct. Foods 2018, 43, 186–195. [Google Scholar] [CrossRef]
- Pongmalai, P.; Devahastin, S.; Chiewchan, N.; Soponronnarit, S. Enhancing the recovery of cabbage glucoraphanin through the monitoring of sulforaphane content and myrosinase activity during extraction by different methods. Sep. Purif. Technol. 2017, 174, 338–344. [Google Scholar] [CrossRef]
- Wang, J.; Barba, F.J.; Sørensen, J.C.; Frandsen, H.B.; Sørensen, S.; Olsen, K.; Orlien, V. High pressure effects on myrosinase activity and glucosinolate preservation in seedlings of Brussels sprouts. Food Chem. 2018, 245, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Sarvan, I.; Verkerk, R.; Van Boekel, M.; Dekker, M. Comparison of the degradation and leaching kinetics of glucosinolates during processing of four Brassicaceae (broccoli, red cabbage, white cabbage, Brussels sprouts). Innov. Food Sci. Emerg. Technol. 2014, 25, 58–66. [Google Scholar] [CrossRef]
- Van Eylen, D.; Oey, I.; Hendrickx, M.; Van Loey, A. Effects of pressure/temperature treatments on stability and activity of endogenous broccoli (Brassica oleracea L. cv. Italica) myrosinase and on cell permeability. J. Food Eng. 2008, 89, 178–186. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Velázquez-Estrada, R.M.; Roig, A.X.; García-Galindo, H.S.; Sayago-Ayerdi, S.G.; Montalvo-González, E. Thermosonication: An alternative processing for fruit and vegetable juices. Trends Food Sci. Technol. 2017, 61, 26–37. [Google Scholar] [CrossRef]
- Sinisterra, J. Application of ultrasound to biotechnology: An overview. Ultrasonics 1992, 30, 180–185. [Google Scholar] [CrossRef]
- Terefe, N.S.; Buckow, R.; Versteeg, C. Quality-Related Enzymes in Plant-Based Products: Effects of Novel Food-Processing Technologies Part 3: Ultrasonic Processing. Crit. Rev. Food Sci. Nutr. 2014, 55, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, Z.; Sun, D.-W. Kinetic modeling of ultrasound-assisted extraction of phenolic compounds from grape marc: Influence of acoustic energy density and temperature. Ultrason. Sonochem. 2014, 21, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Miao, H.; Qian, H.; Yao, L.; Wang, B.; Wang, Q. Effects of industrial pre-freezing processing and freezing handling on glucosinolates and antioxidant attributes in broccoli florets. Food Chem. 2016, 210, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.X.; Wang, J.H.; McAuley, C.; Augustin, M.A.; Terefe, N.S. Fermentation for enhancing the bioconversion of glucoraphanin into sulforaphane and improve the functional attributes of broccoli puree. J. Funct. Foods 2019, 61, 103461. [Google Scholar] [CrossRef]
- Ludikhuyze, L.; Ooms, V.; Weemaes, C.; Hendrickx, M. Kinetic study of the irreversible thermal and pressure inactivation of myrosinase from broccoli (Brassica oleracea L. Cv. italica). J. Agric. Food Chem. 1999, 47, 1794–1800. [Google Scholar] [CrossRef]
- Terefe, N.S.; Gamage, M.; Vilkhu, K.; Simons, L.; Mawson, R.; Versteeg, C. The kinetics of inactivation of pectin methylesterase and polygalacturonase in tomato juice by thermosonication. Food Chem. 2009, 117, 20–27. [Google Scholar] [CrossRef]
- Sarvan, I.; Van Der Klauw, M.; Oliviero, T.; Dekker, M.; Verkerk, R. The effect of chewing on oral glucoraphanin hydrolysis in raw and steamed broccoli. J. Funct. Foods 2018, 45, 306–312. [Google Scholar] [CrossRef]
- Cai, Y.X.; Augustin, M.A.; Jegasothy, H.; Wang, J.H.; Terefe, N.S. Mild heat combined with lactic acid fermentation: A novel approach for enhancing sulforaphane yield in broccoli puree. Food Funct. 2020, 11, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Latté, K.P.; Appel, K.-E.; Lampen, A. Health benefits and possible risks of broccoli—An overview. Food Chem. Toxicol. 2011, 49, 3287–3309. [Google Scholar] [CrossRef]
- Jones, R.; Frisina, C.; Winkler, S.; Imsic, M.; Tomkins, R. Cooking method significantly effects glucosinolate content and sulforaphane production in broccoli florets. Food Chem. 2010, 123, 237–242. [Google Scholar] [CrossRef]
- Sarvan, I.; Kramer, E.; Bouwmeester, H.; Dekker, M.; Verkerk, R. Sulforaphane formation and bioaccessibility are more affected by steaming time than meal composition during in vitro digestion of broccoli. Food Chem. 2017, 214, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Bones, A.M.; Rossiter, J.T. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Aguilar-Camacho, M.; Welti-Chanes, J.; Jacobo-Velázquez, D.A. Combined effect of ultrasound treatment and exogenous phytohormones on the accumulation of bioactive compounds in broccoli florets. Ultrason. Sonochem. 2019, 50, 289–301. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokri, S.; Jegasothy, H.; Augustin, M.A.; Terefe, N.S. Thermosonication for the Production of Sulforaphane Rich Broccoli Ingredients. Biomolecules 2021, 11, 321. https://doi.org/10.3390/biom11020321
Shokri S, Jegasothy H, Augustin MA, Terefe NS. Thermosonication for the Production of Sulforaphane Rich Broccoli Ingredients. Biomolecules. 2021; 11(2):321. https://doi.org/10.3390/biom11020321
Chicago/Turabian StyleShokri, Sajad, Hema Jegasothy, Mary Ann Augustin, and Netsanet Shiferaw Terefe. 2021. "Thermosonication for the Production of Sulforaphane Rich Broccoli Ingredients" Biomolecules 11, no. 2: 321. https://doi.org/10.3390/biom11020321
APA StyleShokri, S., Jegasothy, H., Augustin, M. A., & Terefe, N. S. (2021). Thermosonication for the Production of Sulforaphane Rich Broccoli Ingredients. Biomolecules, 11(2), 321. https://doi.org/10.3390/biom11020321





