Protective Role of Sphingomyelin in Eye Lens Cell Membrane Model against Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Source and Treatment Procedure
2.3. Liposome Solution Preparation and Characterization
2.4. Preparation of Flat Gold Surfaces
2.5. Fabrication of Solid Supported Lipid Bilayers
2.6. Electrochemical Measurements
2.7. Lipid Bilayer Extraction
2.8. Liquid Chromatography-Tandem Mass Spectrometry Analysis
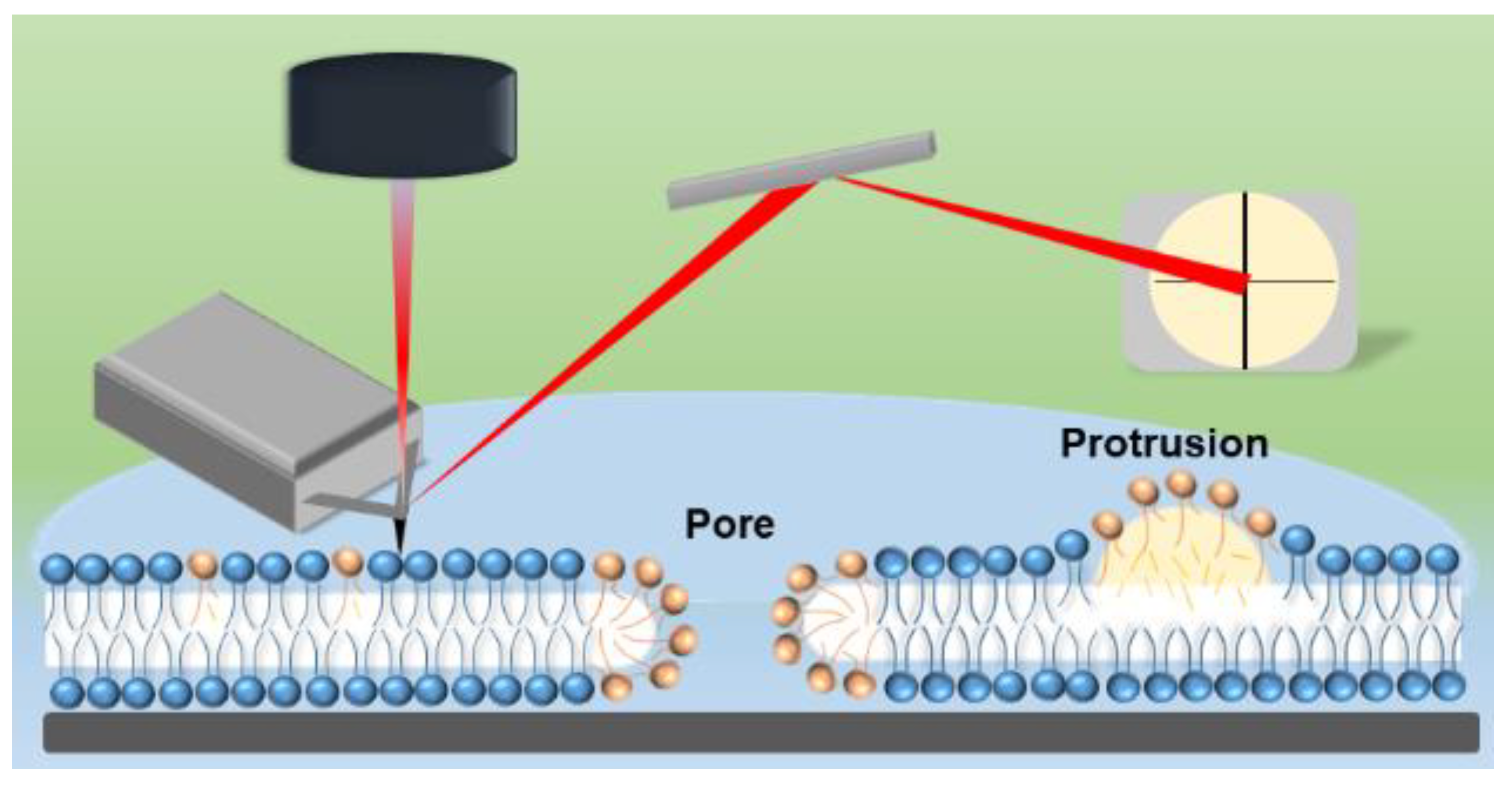
2.9. Atomic Force Microscopy (AFM) Imaging and Analysis
3. Results and Discussion
3.1. Role of SM in Size and Stability of Liposomes
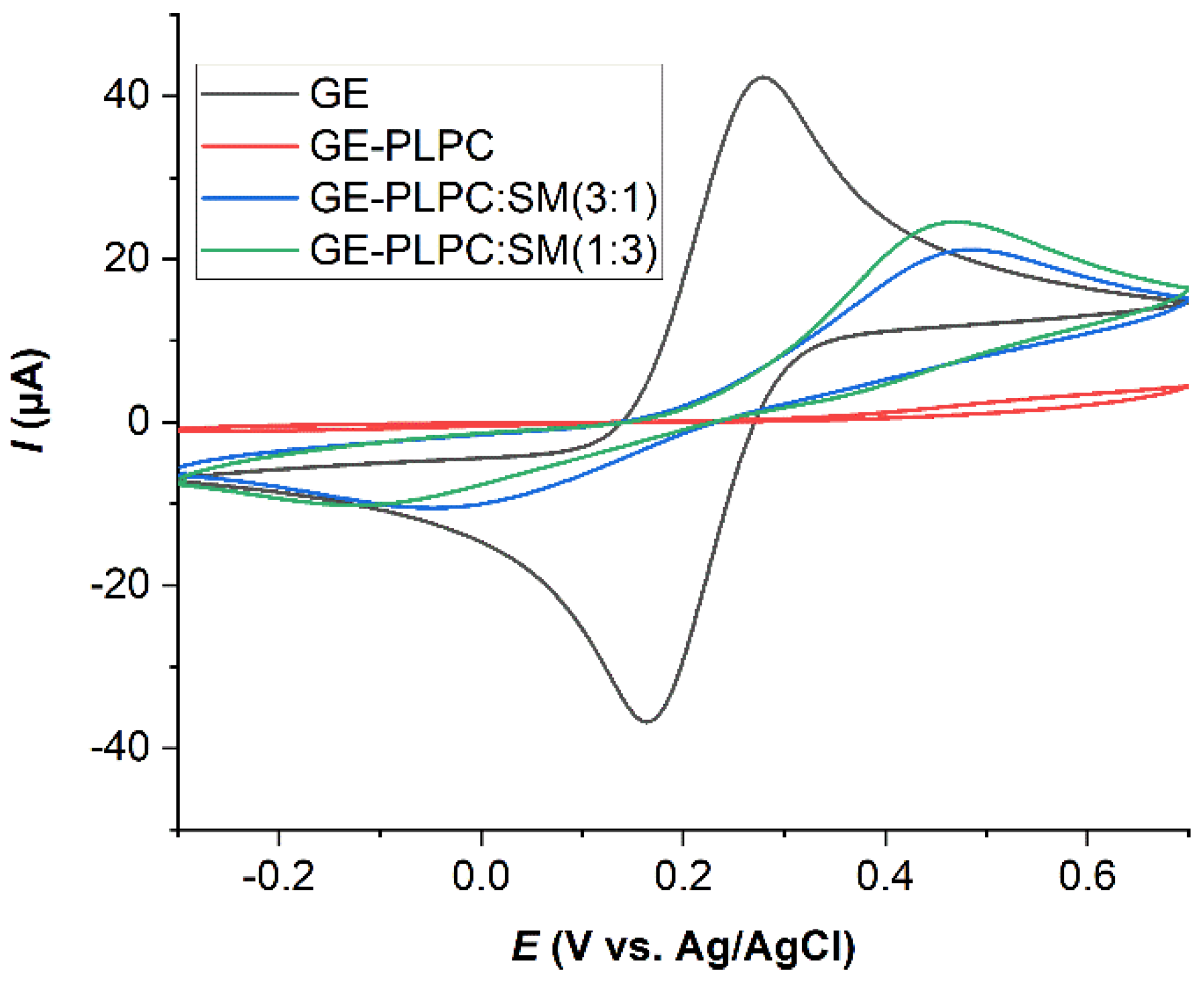
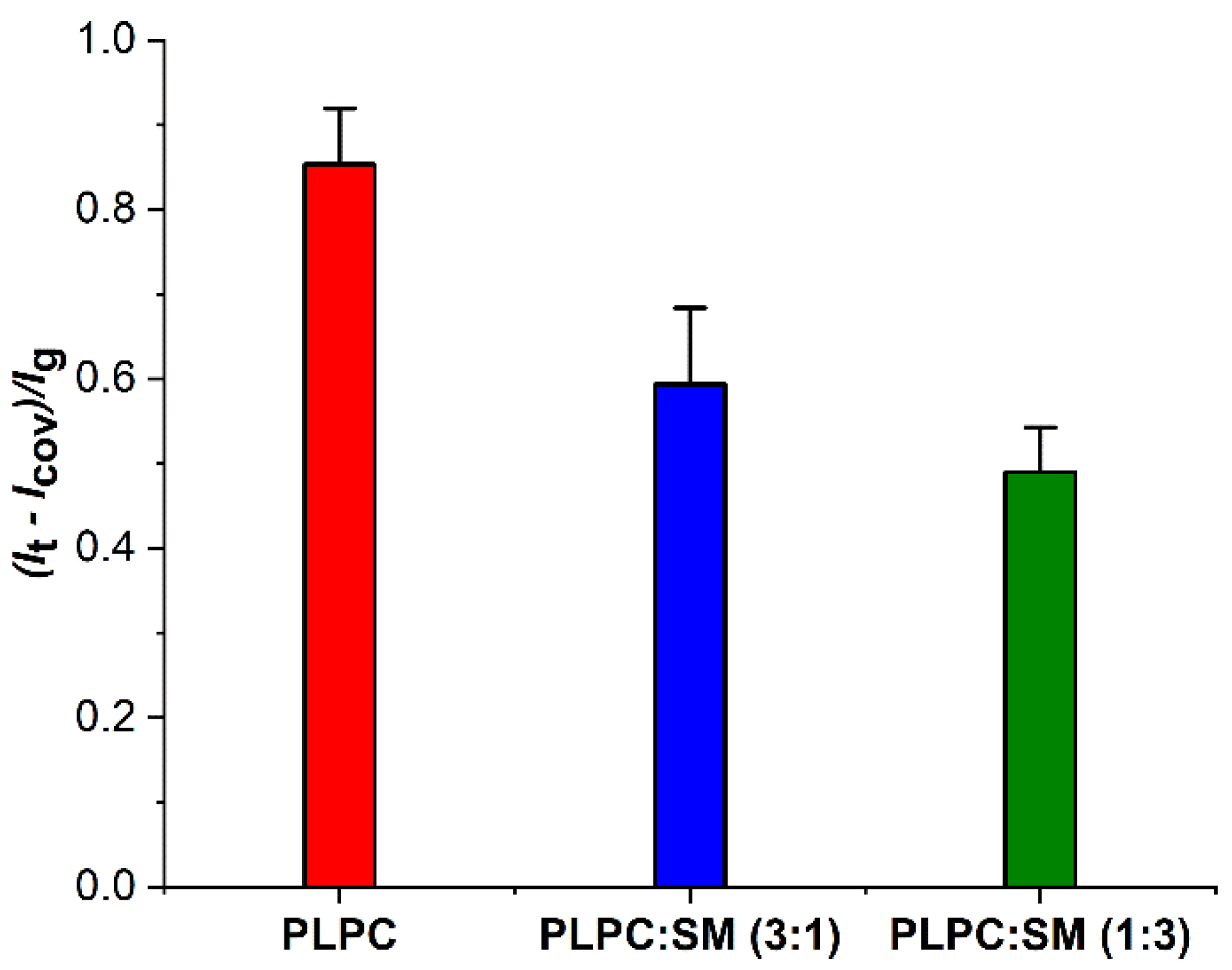
3.2. Effect of ROS on Model Eye Lens Cell Membrane in Presence and Absence of SM: Electrochemistry
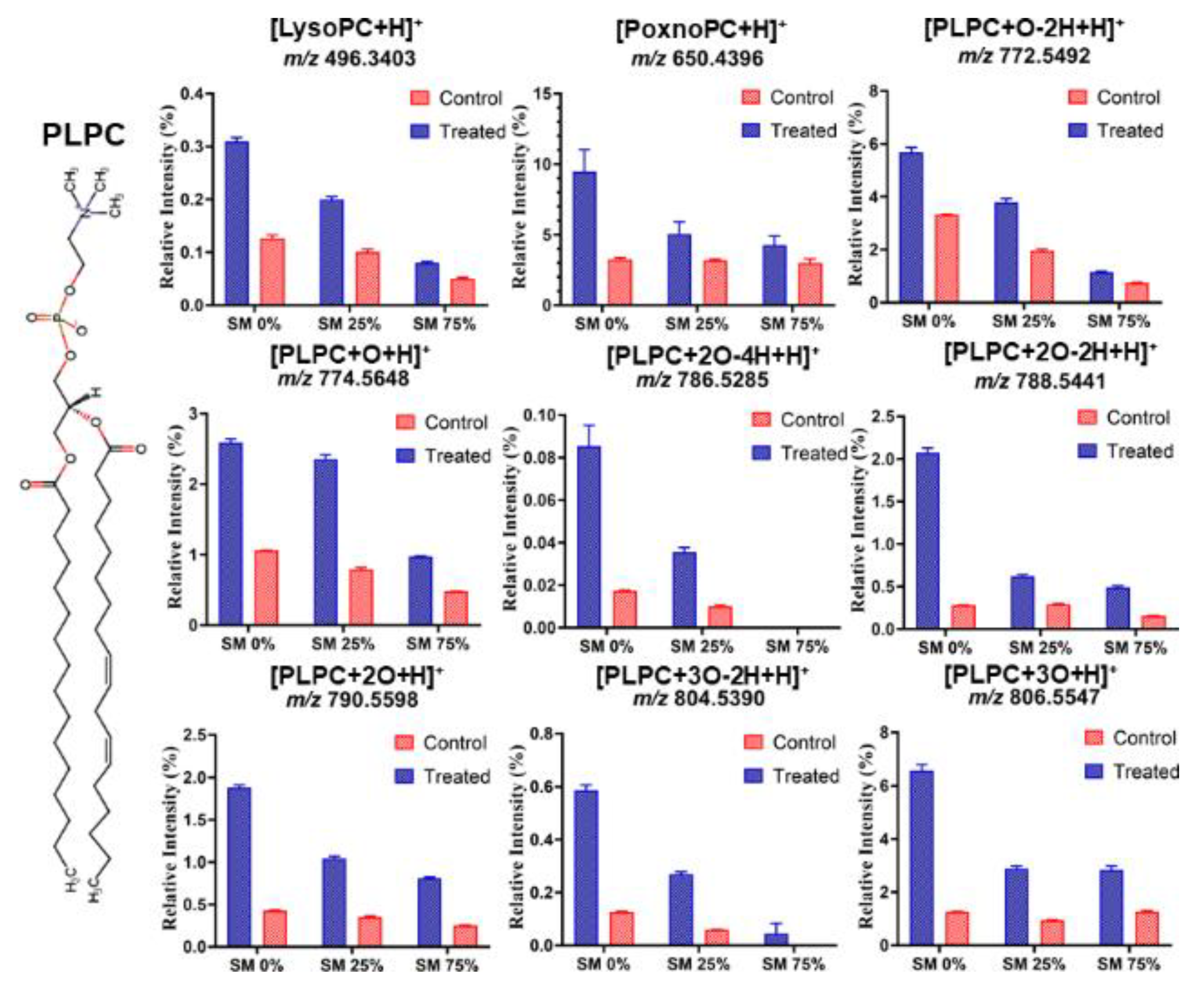
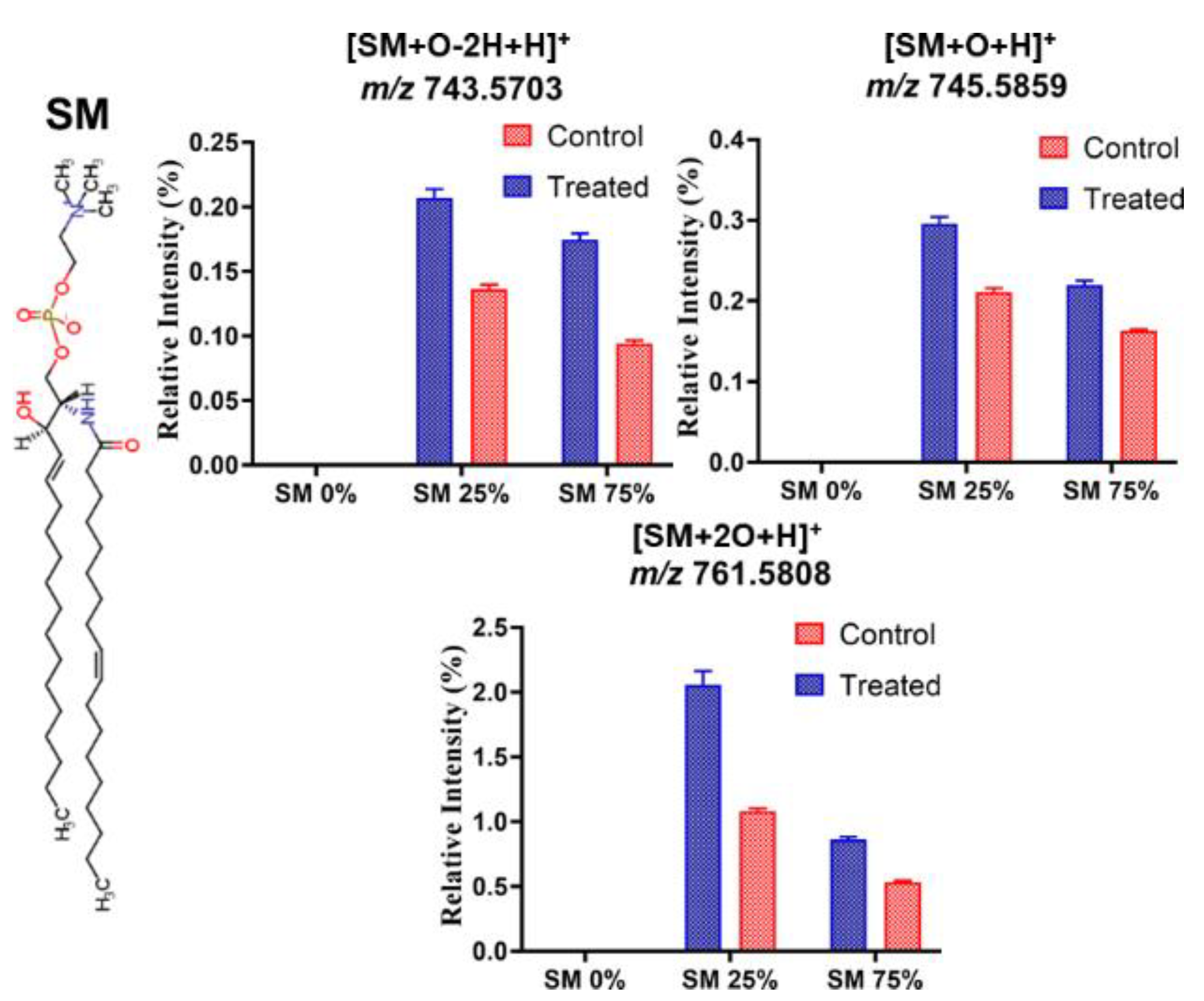
3.3. Effect of ROS on Model Eye Lens Cell Membrane in Presence and Absence of SM: HR-Mass Spectrometry
3.4. Effect of ROS on Model Eye Lens Cell Membrane in Presence and Absence of SM: Atomic Force Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babizhayev, M.A.; Yegorov, Y.E. Reactive oxygen species and the aging eye: Specific role of metabolically active mitochondria in maintaining lens function and in the initiation of the oxidation-induced maturity onset cataract—A novel platform of mitochondria-targeted antioxidants with broad therapeutic potential for redox regulation and detoxification of oxidants in eye diseases. Am. J. Ther. 2016, 23, e98–e117. [Google Scholar] [CrossRef]
- Flatt, T. A new definition of aging? Front. Genet. 2012, 3, 148. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef]
- Truscott, R.J. Age-related nuclear cataract-oxidation is the key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef]
- D’Avanzo, N. Lipid regulation of sodium channels. Curr. Top. Membr. 2016, 78, 353–407. [Google Scholar] [CrossRef]
- Liu, K.; Han, D.N.; Huang, S.H.; Fang, D.J. Enzyme-modified microelectrodes for electrochemical detection of sphingomyelin in the plasma membranes of single cells. Electrochem. Commun. 2020, 112. [Google Scholar] [CrossRef]
- Talbott, C.M.; Vorobyov, I.; Borchman, D.; Taylor, K.G.; DuPre, D.B.; Yappert, M.C. Conformational studies of sphingolipids by NMR spectroscopy. II. Sphingomyelin. Biochim. Biophys. Acta BBA Biomembranes. 2000, 1467, 326–337. [Google Scholar] [CrossRef]
- Borchman, D.; Byrdwell, W.C.; Yappert, M.C. Regional and age-dependent differences in the phospholipid composition of human lens membranes. Invest. Ophthalmol. Vis. Sci. 1994, 35, 3938–3942. [Google Scholar] [PubMed]
- Perry, R.H.; Borchman, D.; Byrdwell, W.C. Changes in the phospholipid composition of the human lens with age and cataract formation. Invest. Ophthalmol. Vis. Sci. 2003, 44, U534. [Google Scholar]
- Borchman, D.; Yappert, M.C. Age-related lipid oxidation in human lenses. Invest. Ophthalmol. Vis. Sci. 1998, 39, 1053–1058. [Google Scholar]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Milliner, S.E. Changes in human meibum lipid composition with age using nuclear magnetic resonance spectroscopy. Invest. Ophthalmol. Vis. Sci. 2012, 53, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Cavallotti, C.; Cerulli, L. Age-Related Changes of the Human Eye; Humana Press: Totowa, NJ, USA, 2008; p. 410. [Google Scholar]
- Perluigi, M.; Di Domenico, F.; Giorgi, A.; Schinina, M.E.; Coccia, R.; Cini, C.; Bellia, F.; Cambria, M.T.; Cornelius, C.; Butterfield, D.A.; et al. Redox proteomics in aging rat brain: Involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. J. Neurosci. Res. 2010, 88, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Yappert, M.C.; Afzal, M. Lens lipids and maximum lifespan. Exp. Eye Res. 2004, 79, 761–768. [Google Scholar] [CrossRef]
- Shichi, H. Cataract formation and prevention. Expert Opin. Investig. Drugs 2004, 13, 691–701. [Google Scholar] [CrossRef]
- Berthoud, V.M.; Beyer, E.C. Oxidative stress, lens gap junctions, and cataracts. Antioxid. Redox Signal. 2009, 11, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.X.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic membranes: A review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Kornberg, R.D.; McConnell, H.M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry 1971, 10, 1111–1120. [Google Scholar] [CrossRef]
- Hamai, C.; Yang, T.; Kataoka, S.; Cremer, P.S.; Musser, S.M. Effect of average phospholipid curvature on supported bilayer formation on glass by vesicle fusion. Biophys. J. 2006, 90, 1241–1248. [Google Scholar] [CrossRef]
- Pautot, S.; Frisken, B.J.; Weitz, D.A. Engineering asymmetric vesicles. Proc. Natl. Acad. Sci. USA 2003, 100, 10718–10721. [Google Scholar] [CrossRef]
- Dimova, R.; Marques, C. The Giant Vesicle Book; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019. [Google Scholar]
- Lipkowski, J. Building biomimetic membrane at a gold electrode surface. Phys. Chem. Chem. Phys. 2010, 12, 13874–13887. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E. Supported membranes: Scientific and practical applications. Science 1996, 271, 43–48. [Google Scholar] [CrossRef]
- Groves, J.T.; Ulman, N.; Boxer, S.G. Micropatterning fluid lipid bilayers on solid supports. Science 1997, 275, 651–653. [Google Scholar] [CrossRef]
- Mueller, P.; Rudin, D.O.; Tien, H.T.; Wescott, W.C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef]
- Jeong, D.W.; Jang, H.; Choi, S.Q.; Choi, M.C. Enhanced stability of freestanding lipid bilayer and its stability criteria. Sci. Rep. 2016, 6, 38158. [Google Scholar] [CrossRef]
- Simons, K.; Vaz, W.L. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef]
- Bloom, M.; Evans, E.; Mouritsen, O.G. Physical-properties of the fluid lipid-bilayer component of cell-membranes—A perspective. Q. Rev. Biophys. 1991, 24, 293–397. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, J.; De Souza, J.F.V.; Dang, A.T.; Liu, G.Y.; Kuhl, T.L. Preparation and characterization of solid-supported lipid bilayers formed by langmuir-blodgett deposition: A tutorial. Langmuir 2018, 34, 15622–15639. [Google Scholar] [CrossRef]
- Mingeot-Leclercq, M.P.; Deleu, M.; Brasseur, R.; Dufrene, Y.F. Atomic force microscopy of supported lipid bilayers. Nat. Protoc. 2008, 3, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.J.; Frank, C.W.; Kasemo, B.; Hook, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010, 5, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Hirst, D.J.; Kulkarni, K.; Del Borgo, M.P.; Aguilar, M.I. Exploring molecular-biomembrane interactions with surface plasmon resonance and dual polarization interferometry technology: Expanding the spotlight onto biomembrane structure. Chem. Rev. 2018, 118, 5392–5487. [Google Scholar] [CrossRef] [PubMed]
- Ferhan, A.R.; Yoon, B.K.; Park, S.; Sut, T.N.; Chin, H.; Park, J.H.; Jackman, J.A.; Cho, N.J. Solvent-assisted preparation of supported lipid bilayers. Nat. Protoc. 2019, 14, 2091–2118. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.P.; Berat, R.; Brisson, A.R. Formation of solid-supported lipid bilayers: An integrated view. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef]
- Chan, Y.H.M.; Boxer, S.G. Model membrane systems and their applications. Curr. Opin. Chem. Biol. 2007, 11, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Hanna, P.M.; Mason, R.P. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic. Bio. Med. 1997, 22, 885–888. [Google Scholar] [CrossRef]
- Miller, C.J.; Rose, A.L.; Waite, T.D. Importance of iron complexation for fenton-mediated hydroxyl radical production at circumneutral pH. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Attri, P.; Kim, Y.H.; Park, D.H.; Park, J.H.; Hong, Y.J.; Uhm, H.S.; Kim, K.N.; Fridman, A.; Choi, E.H. Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Sci. Rep. 2015, 5, 9332. [Google Scholar] [CrossRef]
- Nakamura, K.; Shirato, M.; Tenkumo, T.; Kanno, T.; Westerlund, A.; Ortengren, U.; Sasaki, K.; Niwano, Y. Hydroxyl radicals generated by hydrogen peroxide photolysis recondition biofilm-contaminated titanium surfaces for subsequent osteoblastic cell proliferation. Sci. Rep. 2019, 9, 4688. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M.; Fridman, A. Plasma medicine. Plasma Process. Polym. 2008, 5, 501. [Google Scholar] [CrossRef]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine-current state of research and medical application. Plasma Phys. Control Fusion. 2017, 59, 014031. [Google Scholar] [CrossRef]
- Nasri, Z.; Bruno, G.; Bekeschus, S.; Weltmann, K.-D.; von Woedtke, T.; Wende, K. Development of an electrochemical sensor for in-situ monitoring of reactive species produced by cold physical plasma. Sens. Actuators B Chem. 2021, 326. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schafer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma medicine: Applications of cold atmospheric pressure plasma in dermatology. Oxid. Med. Cell Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef]
- Assadian, O.; Ousey, K.J.; Daeschlein, G.; Kramer, A.; Parker, C.; Tanner, J.; Leaper, D.J. Effects and safety of atmospheric low-temperature plasma on bacterial reduction in chronic wounds and wound size reduction: A systematic review and meta-analysis. Int. Wound J. 2019, 16, 103–111. [Google Scholar] [CrossRef]
- Dai, X.; Bazaka, K.; Richard, D.J.; Thompson, E.R.W.; Ostrikov, K.K. The emerging role of gas plasma in oncotherapy. Trends Biotechnol. 2018, 36, 1183–1198. [Google Scholar] [CrossRef]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.-P.; Laurencin-Dalicieux, S.; Cousty, S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918786475. [Google Scholar] [CrossRef]
- Keidar, M. A prospectus on innovations in the plasma treatment of cancer. Phys. Plasmas 2018, 25, 083504. [Google Scholar] [CrossRef]
- Semmler, M.L.; Bekeschus, S.; Schafer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular mechanisms of the efficacy of cold atmospheric pressure plasma (CAP) in cancer treatment. Cancers 2020, 12. [Google Scholar] [CrossRef]
- Kim, G.C.; Lee, H.W.; Byun, J.H.; Chung, J.; Jeon, Y.C.; Lee, J.K. Dental applications of low-temperature nonthermal plasmas. Plasma Process. Polym. 2013, 10, 199–206. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from physical plasmas: Redox chemistry for biomedical therapy. Oxid. Med. Cell Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef]
- Busco, G.; Fasani, F.; Dozias, S.; Ridou, L.; Douat, C.; Pouvesle, J.; Robert, E.; Grillon, C. Changes in oxygen level upon cold plasma treatments: Consequences for RONS production. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 147–152. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kINPen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51. [Google Scholar] [CrossRef]
- Breden, D.; Raja, L.L. Computational study of the interaction of cold atmospheric helium plasma jets with surfaces. Plasma Sources Sci. Technol. 2014, 23. [Google Scholar] [CrossRef]
- Groning, A.; Ahrens, H.; Ortmann, T.; Lawrenz, F.; Brezesinski, G.; Scholz, F.; Helm, C.A. Molecular mechanisms of phosphatidylcholine monolayer solidification due to hydroxyl radicals. Soft Matter 2011, 7, 6467–6476. [Google Scholar] [CrossRef]
- Tero, R.; Yamashita, R.; Hashizume, H.; Suda, Y.; Takikawa, H.; Hori, M.; Ito, M. Nanopore formation process in artificial cell membrane induced by plasma-generated reactive oxygen species. Arch. Biochem. Biophys. 2016, 605, 26–33. [Google Scholar] [CrossRef]
- Tero, R.; Suda, Y.; Kato, R.; Tanoue, H.; Takikawa, H. Plasma irradiation of artificial cell membrane system at solid-liquid interface. Appl. Phys. Express 2014, 7, 077001. [Google Scholar] [CrossRef]
- Scholz, F.; de Lopez Lara Gonzalez, G.; de Machado Carvalho, L.; Hilgemann, M.; Brainina, K.Z.; Kahlert, H.; Jack, R.S.; Minh, D.T. Indirect electrochemical sensing of radicals and radical scavengers in biological matrices. Angew. Chem. Int. Ed. Engl. 2007, 46, 8079–8081. [Google Scholar] [CrossRef] [PubMed]
- Ravandeh, M.; Thal, D.; Kahlert, H.; Wende, K.; Lalk, M. Self-assembled mono- and bilayers on gold electrodes to assess antioxidants—a comparative study. J. Solid State Electrochem. 2020. [Google Scholar] [CrossRef]
- Barroso, M.F.; Luna, M.A.; Moyano, F.; Delerue-Matos, C.; Correa, N.M.; Molina, P.G. Study of lipid peroxidation and ascorbic acid protective role in large unilamellar vesicles from a new electrochemical performance. Bioelectrochemistry 2018, 120, 120–126. [Google Scholar] [CrossRef]
- Coliva, G.; Lange, M.; Colombo, S.; Chervet, J.-P.; Domingues, M.R.; Fedorova, M. Sphingomyelins prevent propagation of lipid peroxidation—LC-MS/MS evaluation of inhibition mechanisms. Molecules 2020, 25, 1925. [Google Scholar] [CrossRef]
- Yusupov, M.; Wende, K.; Kupsch, S.; Neyts, E.C.; Reuter, S.; Bogaerts, A. Effect of head group and lipid tail oxidation in the cell membrane revealed through integrated simulations and experiments. Sci. Rep. 2017, 7, 5761. [Google Scholar] [CrossRef]
- Reis, A.; Domingues, P.; Ferrer-Correia, A.J.; Domingues, M.R. Tandem mass spectrometry of intact oxidation products of diacylphosphatidylcholines: Evidence for the occurrence of the oxidation of the phosphocholine head and differentiation of isomers. J. Mass Spectrom. 2004, 39, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Hermes, M.; Scholz, F.; Hardtner, C.; Walther, R.; Schild, L.; Wolke, C.; Lendeckel, U. Electrochemical signals of mitochondria: A new probe of their membrane properties. Angew. Chem. Int. Ed. Engl. 2011, 50, 6872–6875. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraj, K.; Roman Silva, J.I.; Kahlert, H.; Lendeckel, U.; Scholz, F. The acid-base and redox properties of menaquinone MK-4, MK-7, and MK-9 (vitamin K2) in DMPC monolayers on mercury. Eur. Biophys. J. 2020, 49, 279–288. [Google Scholar] [CrossRef]
- Ravandeh, M.; Kahlert, H.; Jablonowski, H.; Lackmann, J.W.; Striesow, J.; Agmo Hernandez, V.; Wende, K. A combination of electrochemistry and mass spectrometry to monitor the interaction of reactive species with supported lipid bilayers. Sci. Rep. 2020, 10, 18683. [Google Scholar] [CrossRef] [PubMed]
- Widomska, J.; Subczynski, W.K.; Mainali, L.; Raguz, M. Cholesterol bilayer domains in the eye lens health: A review. Cell Biochem. Biophys. 2017, 75, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Grami, V.; Marrero, Y.; Tang, D.; Yappert, M.C.; Rasi, V.; Borchman, D. Human lens phospholipid changes with age and cataract. Invest. Ophthalmol. Vis. Sci. 2005, 46, 1682–1689. [Google Scholar] [CrossRef]
- Yappert, M.C.; Rujoi, M.; Borchman, D.; Vorobyov, I.; Estrada, R. Glycero-versus sphingo-phospholipids: Correlations with human and non-human mammalian lens growth. Exp. Eye Res. 2003, 76, 725–734. [Google Scholar] [CrossRef]
- Deeley, J.M.; Hankin, J.A.; Friedrich, M.G.; Murphy, R.C.; Truscott, R.J.; Mitchell, T.W.; Blanksby, S.J. Sphingolipid distribution changes with age in the human lens. J. Lipid Res. 2010, 51, 2753–2760. [Google Scholar] [CrossRef]
- Borchman, D.; Yappert, M.C. Lipids and the ocular lens. J. Lipid Res. 2010, 51, 2473–2488. [Google Scholar] [CrossRef]
- Byrdwell, W.C.; Borchman, D. Liquid chromatography/mass-spectrometric characterization of sphingomyelin and dihydrosphingomyelin of human lens membranes. Ophthalmic Res. 1997, 29, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Deeley, J.M.; Mitchell, T.W.; Wei, X.; Korth, J.; Nealon, J.R.; Blanksby, S.J.; Truscott, R.J. Human lens lipids differ markedly from those of commonly used experimental animals. Biochim. Biophys. Acta 2008, 1781, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E. Lipid Peroxidation in Biomembranes; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Martensson, C.; Agmo Hernandez, V. Ubiquinone-10 in gold-immobilized lipid membrane structures acts as a sensor for acetylcholine and other tetraalkylammonium cations. Bioelectrochemistry 2012, 88, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.; Kahlert, H.; Chinnaya, J.; Ahrens, P.; Hasse, U. Impact of gold-1-decanethiol-SAM formation and removal cycles on the surface properties of polycrystalline gold and SAM quality. J. Solid State Electrochem. 2018, 22, 1149–1154. [Google Scholar] [CrossRef]
- Sill, A.; Nestler, P.; Azinfar, A.; Helm, C.A. Tailorable polyanion diffusion coefficient in LbL films: The role of polycation molecular weight and polymer conformation. Macromolecules 2019, 52, 9045–9052. [Google Scholar] [CrossRef]
- Kern, W. The evolution of silicon-wafer cleaning technology. J. Electrochem. Soc. 1990, 137, 1887–1892. [Google Scholar] [CrossRef]
- Chiu, S.W.; Vasudevan, S.; Jakobsson, E.; Mashl, R.J.; Scott, H.L. Structure of sphingomyelin bilayers: A simulation study. Biophys. J. 2003, 85, 3624–3635. [Google Scholar] [CrossRef]
- Burgess, I.; Li, M.; Horswell, S.L.; Szymanski, G.; Lipkowski, J.; Majewski, J.; Satija, S. Electric field-driven transformations of a supported model biological membrane—An electrochemical and neutron reflectivity study. Biophys. J. 2004, 86, 1763–1776. [Google Scholar] [CrossRef]
- Bard, A.J.; Inzelt, G.R.; Scholz, F. Electrochemical Dictionary, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2012; p. 991. [Google Scholar]
- Oborina, E.M.; Yappert, M.C. Effect of sphingomyelin versus dipalmitoylphosphatidylcholine on the extent of lipid oxidation. Chem. Phys. Lipids 2003, 123, 223–232. [Google Scholar] [CrossRef]
- Hammer, M.U.; Forbrig, E.; Kupsch, S.; Weltmann, K.-D.; Reuter, S. Influence of plasma treatment on the structure and function of lipids. Plasma Med. 2013, 3, 97–114. [Google Scholar] [CrossRef]
- Van der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef]

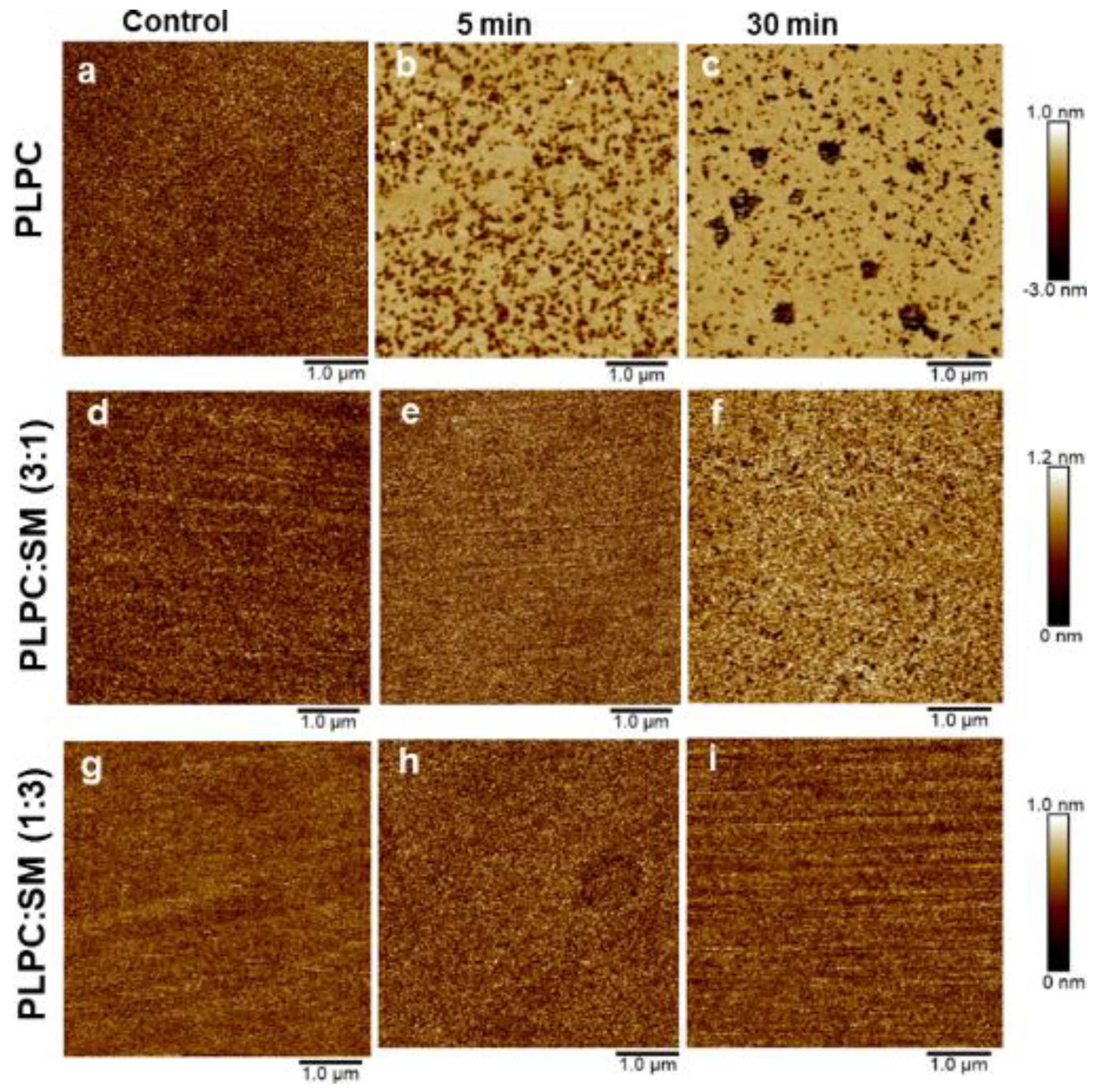
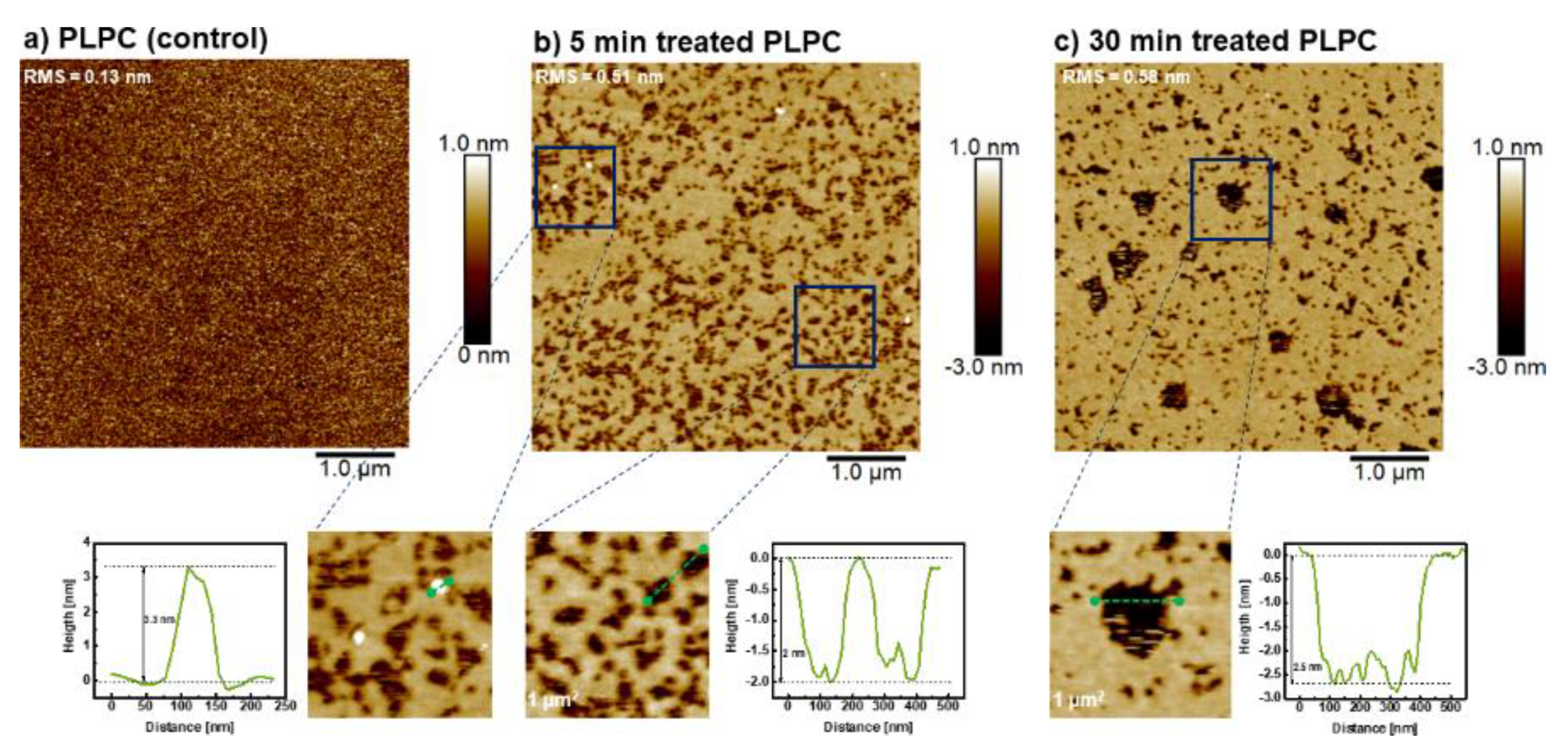
| Liposomes | Mean Radius (nm) | Standard Deviation (nm) |
|---|---|---|
| 100 mol% PLPC | 76.3 | 1.2 |
| 75% mol% PLPC + 25% mol% SM | 75.8 | 0.7 |
| 25% mol% PLPC + 75% mol% SM | 96.1 | 1.2 |
| PLPC Peroxidation Product | m/z (Positive Mode) |
|---|---|
| [LysoPC + H]+ | 496.3403 |
| [PoxnoPC + H]+ | 650.4396 |
| [PLPC + O − 2H + H]+ | 772.5492 |
| [PLPC + O + H]+ | 774.5648 |
| [PLPC + 2O − 4H + H]+ | 786.5285 |
| [PLPC + 2O − 2H + H]+ | 788.5441 |
| [PLPC + 2O − 2H + H]+ | 790.5598 |
| [PLPC + 2O − 2H + H]+ | 804.5390 |
| [PLPC + 3O + H]+ | 806.5547 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravandeh, M.; Coliva, G.; Kahlert, H.; Azinfar, A.; Helm, C.A.; Fedorova, M.; Wende, K. Protective Role of Sphingomyelin in Eye Lens Cell Membrane Model against Oxidative Stress. Biomolecules 2021, 11, 276. https://doi.org/10.3390/biom11020276
Ravandeh M, Coliva G, Kahlert H, Azinfar A, Helm CA, Fedorova M, Wende K. Protective Role of Sphingomyelin in Eye Lens Cell Membrane Model against Oxidative Stress. Biomolecules. 2021; 11(2):276. https://doi.org/10.3390/biom11020276
Chicago/Turabian StyleRavandeh, Mehdi, Giulia Coliva, Heike Kahlert, Amir Azinfar, Christiane A. Helm, Maria Fedorova, and Kristian Wende. 2021. "Protective Role of Sphingomyelin in Eye Lens Cell Membrane Model against Oxidative Stress" Biomolecules 11, no. 2: 276. https://doi.org/10.3390/biom11020276
APA StyleRavandeh, M., Coliva, G., Kahlert, H., Azinfar, A., Helm, C. A., Fedorova, M., & Wende, K. (2021). Protective Role of Sphingomyelin in Eye Lens Cell Membrane Model against Oxidative Stress. Biomolecules, 11(2), 276. https://doi.org/10.3390/biom11020276









