Low Expression of miR-375 and miR-190b Differentiates Grade 3 Patients with Endometrial Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Approval
2.3. RNA
2.4. NGS
2.5. Bioinformatic Pipeline and Data Analysis
2.6. QPCR
3. Results
3.1. Study Group
3.2. MiRNA Profiling
3.3. Data Cleaning
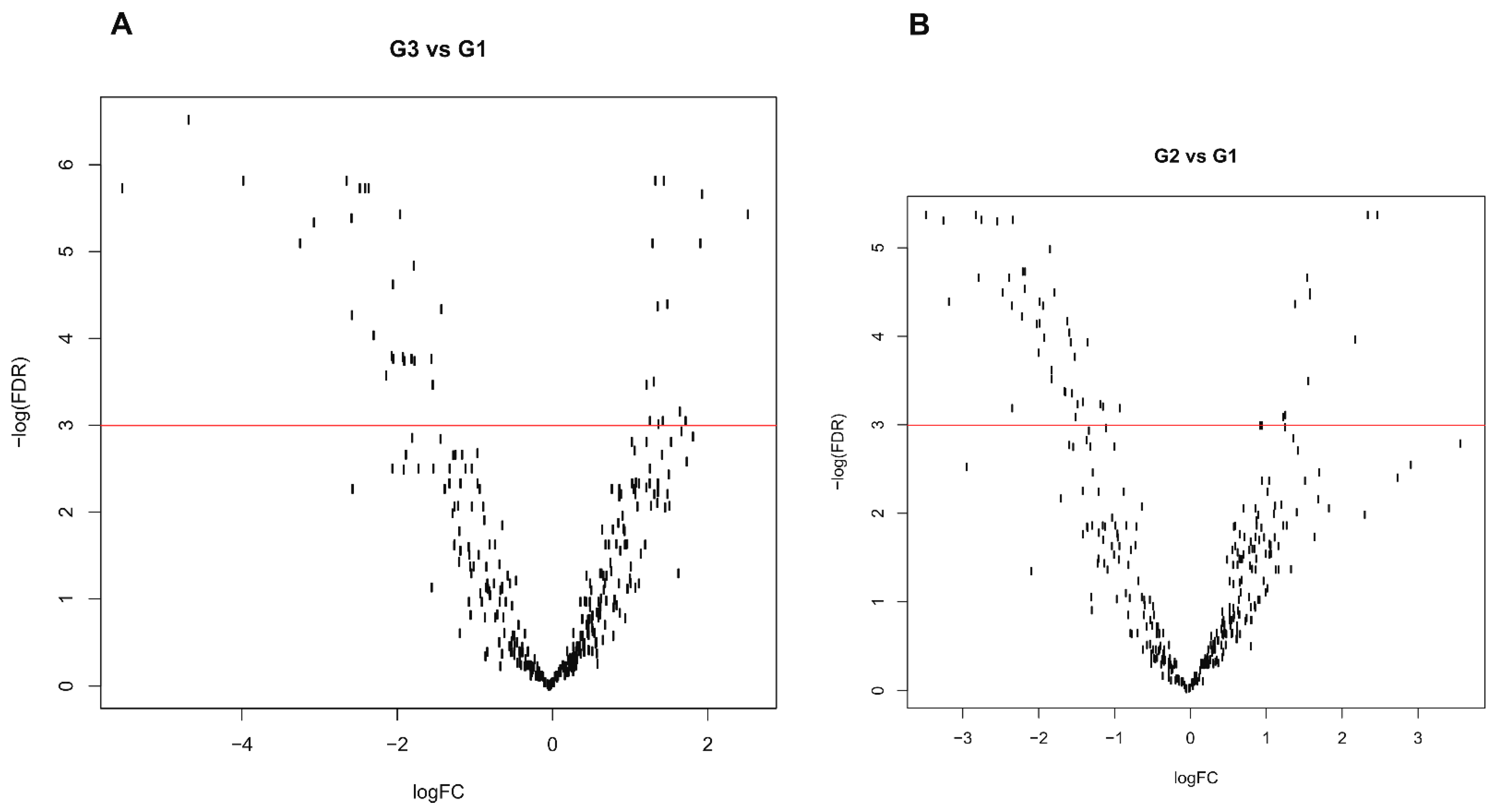
3.4. Differential Expression Profile
3.5. Possible Grade Signature
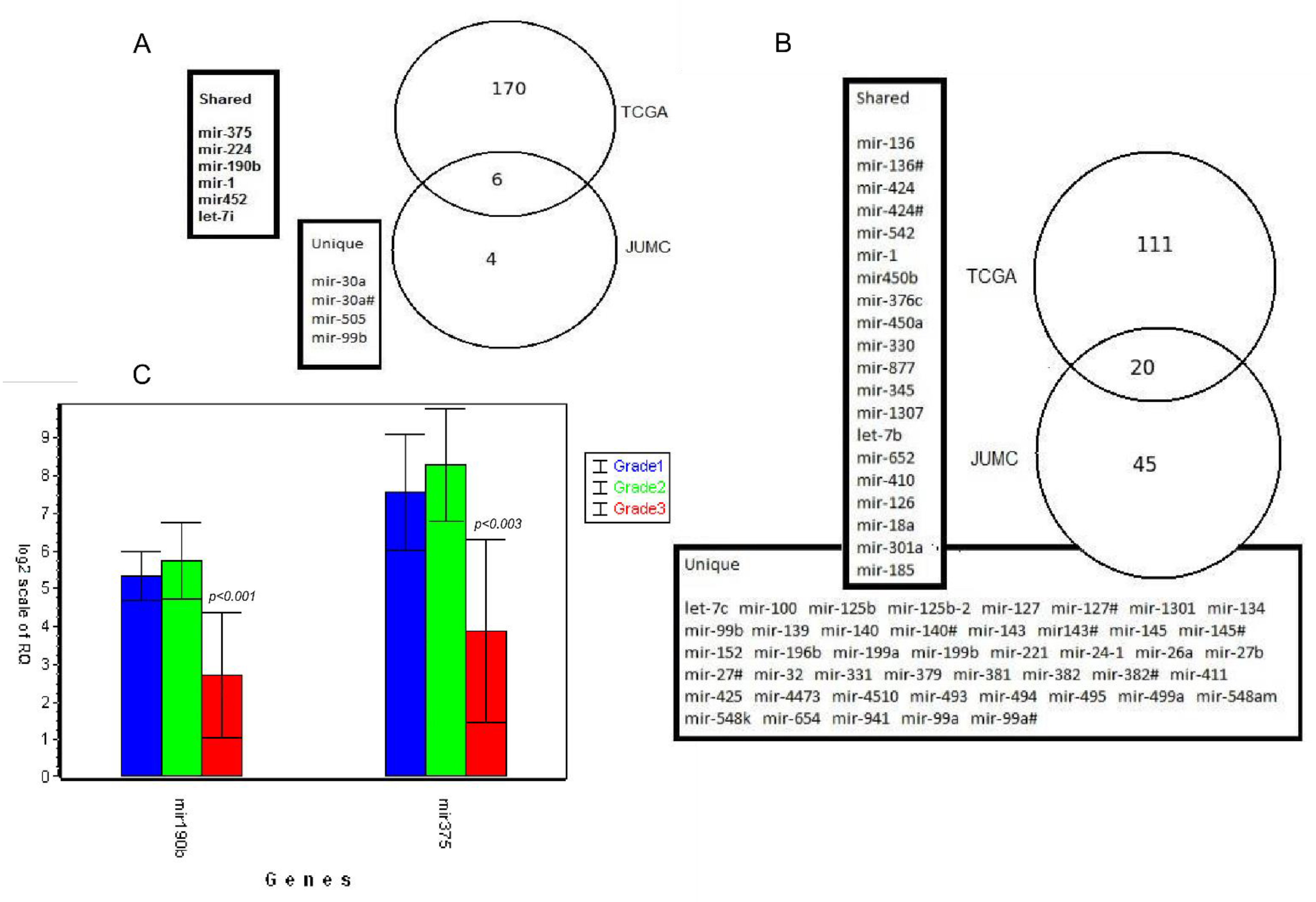
3.6. Validation of Results Using External TCGA Data
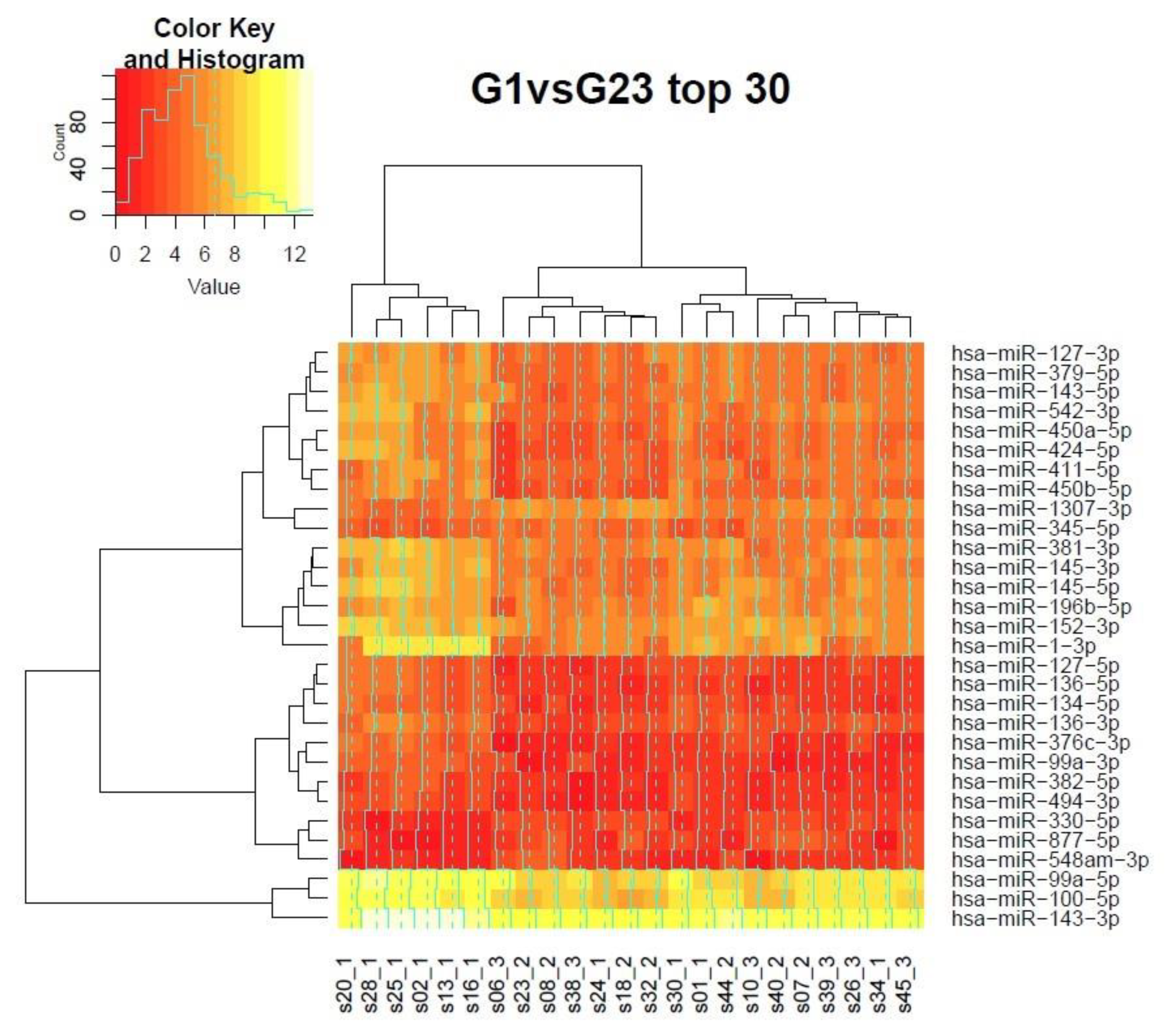
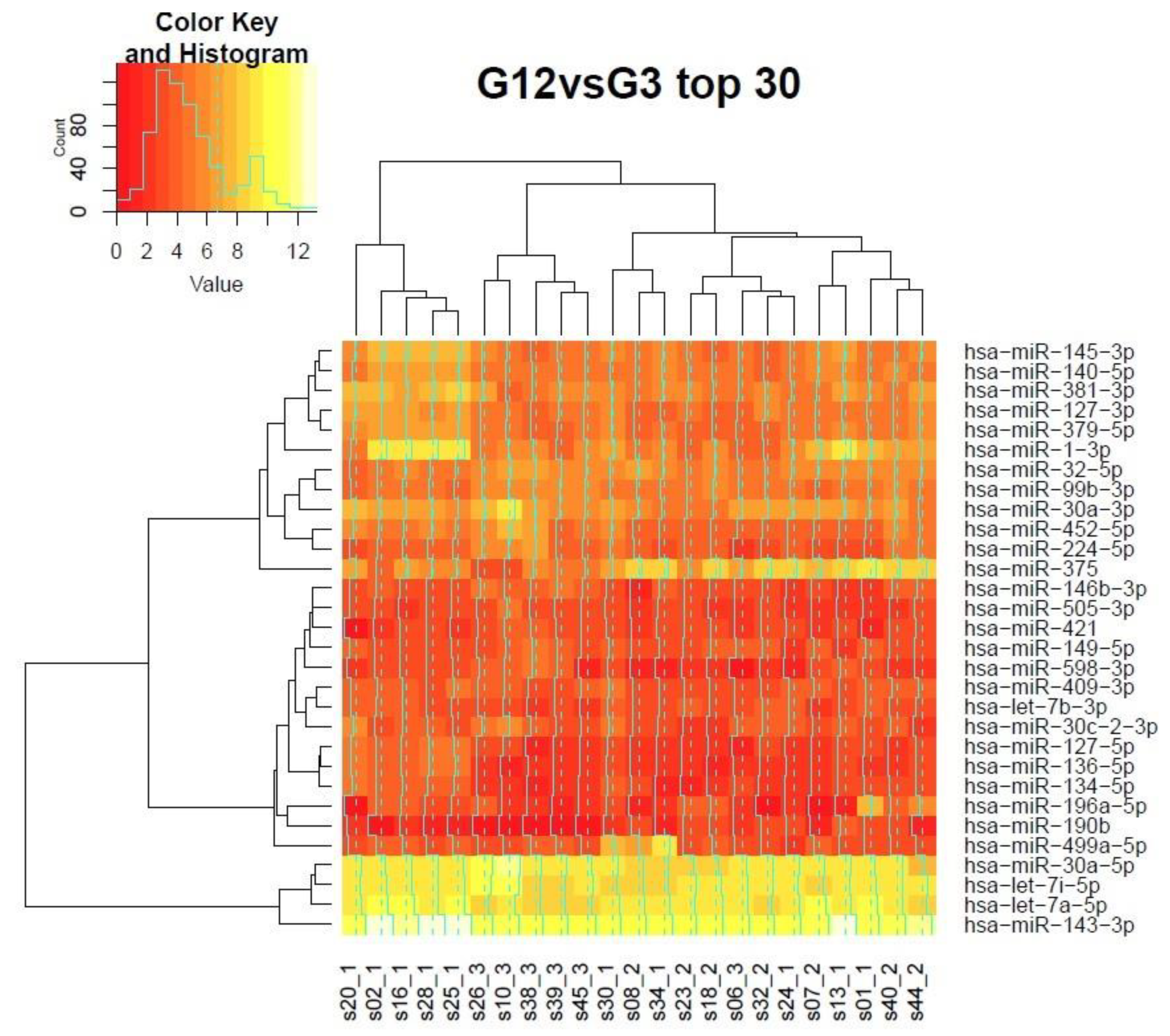
3.7. Clustering of Samples in the Grading Context
3.8. Gene Ontology of Predicted Targets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Scholten, A.N.; Smit, V.T.H.B.M.; Beerman, H.; Van Putten, W.L.J.; Creutzberg, C.L. Prognostic significance and interobserver variability of histologic grading systems for endometrial carcinoma. Cancer 2004, 100, 764–772. [Google Scholar] [CrossRef]
- Soslow, R.A.; Tornos, C.; Park, K.J.; Malpica, A.; Matias-Guiu, X.; Oliva, E.; Parkash, V.; Carlson, J.; McCluggage, W.G.; Gilks, C.B. Endometrial Carcinoma Diagnosis. Int. J. Gynecol. Pathol. 2019, 38, S64–S74. [Google Scholar] [CrossRef] [PubMed]
- Lax, S.F. Pathology of Endometrial Carcinoma. Adv. Exp. Med. Biol. 2017, 943, 75–96. [Google Scholar]
- Thomas, S.; Hussein, Y.; Bandyopadhyay, S.; Cote, M.; Hassan, O.; Abdulfatah, E.; Alosh, B.; Guan, H.; Soslow, R.A.; Ali-Fehmi, R. Interobserver Variability in the Diagnosis of Uterine High-Grade Endometrioid Carcinoma. Arch. Pathol. Lab. Med. 2016, 140, 836–843. [Google Scholar] [CrossRef]
- Hu, S.; Hinson, J.L.; Matnani, R.; Cibull, M.L.; Karabakhtsian, R.G. Are the uterine serous carcinomas underdiagnosed? Histomorphologic and immunohistochemical correlates and clinical follow up in high-grade endometrial carcinomas initially diagnosed as high-grade endometrioid carcinoma. Mod. Pathol. 2018, 31, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Soslow, R.A. Endometrial carcinomas with ambiguous features. Semin. Diagn. Pathol. 2010, 27, 261–273. [Google Scholar] [CrossRef]
- Gurtan, A.M.; Sharp, P.A. The Role of miRNAs in Regulating Gene Expression Networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Ueda, T.; Volinia, S.; Okumura, H.; Shimizu, M.; Taccioli, C.; Rossi, S.; Alder, H.; Liu, C.; Oue, N.; Yasui, W.; et al. Relation between microRNA expression and progression and prognosis of gastric cancer: A microRNA expression analysis. Lancet Oncol. 2010, 11, 136–146. [Google Scholar] [CrossRef]
- Di Meo, A.; Saleeb, R.; Wala, S.J.; Khella, H.W.; Ding, Q.; Zhai, H.; Krishan, K.; Krizova, A.; Gabril, M.; Evans, A.; et al. A miRNA-based classification of renal cell carcinoma subtypes by PCR and in situ hybridization. Oncotarget 2018, 9, 2092. [Google Scholar] [CrossRef] [PubMed]
- Mamatjan, Y.; Agnihotri, S.; Goldenberg, A.; Tonge, P.; Mansouri, S.; Zadeh, G.; Aldape, K. Molecular Signatures for Tumor Classification. J. Mol. Diagn. 2017, 19, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Delangle, R.; De Foucher, T.; Larsen, A.K.; Sabbah, M.; Azaïs, H.; Bendifallah, S.; Daraï, E.; Ballester, M.; Mehats, C.; Uzan, C.; et al. The Use of microRNAs in the Management of Endometrial Cancer: A Meta-Analysis. Cancers 2019, 11, 832. [Google Scholar] [CrossRef]
- Tsukamoto, O.; Miura, K.; Mishima, H.; Abe, S.; Kaneuchi, M.; Higashijima, A.; Miura, S.; Kinoshita, A.; Yoshiura, K.; Masuzaki, H. Identification of endometrioid endometrial carcinoma-associated microRNAs in tissue and plasma. Gynecol. Oncol. 2014, 132, 715–721. [Google Scholar] [CrossRef]
- Xiong, H.; Li, Q.; Liu, S.; Wang, F.; Xiong, Z.; Chen, J.; Chen, H.; Yang, Y.; Tan, X.; Luo, Q.; et al. Integrated microRNA and mRNA Transcriptome Sequencing Reveals the Potential Roles of miRNAs in Stage I Endometrioid Endometrial Carcinoma. PLoS ONE 2014, 9, e110163. [Google Scholar] [CrossRef] [PubMed]
- Piulats, J.M.; Guerra, E.; Gil-Martín, M.; Roman-Canal, B.; Gatius, S.; Sanz-Pamplona, R.; Velasco, A.; Vidal, A.; Matias-Guiu, X. Molecular approaches for classifying endometrial carcinoma. Gynecol. Oncol. 2017, 145, 200–207. [Google Scholar] [CrossRef]
- Gilks, C.B.; Oliva, E.; Soslow, R.A. Poor Interobserver Reproducibility in the Diagnosis of High-grade Endometrial Carcinoma. Am. J. Surg. Pathol. 2013, 37, 874–881. [Google Scholar] [CrossRef]
- Andrews Simon a Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 13 February 2021).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Canlorbe, G.; Wang, Z.; Laas, E.; Bendifallah, S.; Castela, M.; Lefevre, M.; Chabbert-Buffet, N.; Daraï, E.; Aractingi, S.; Méhats, C.; et al. Identification of microRNA expression profile related to lymph node status in women with early-stage grade 1–2 endometrial cancer. Mod. Pathol. 2016, 29, 391–401. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Guo, Z.; Ma, X.; Song, Y.; Guo, Y. Regulation of NEAT1/miR-214-3p on the growth, migration and invasion of endometrial carcinoma cells. Arch. Gynecol. Obstet. 2017, 295, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-S.; Lin, H.; Chen, D.; Yi, Z.; Zeng, B.; Jiang, Y.; Ren, G. A four-miRNA signature as a novel biomarker for predicting survival in endometrial cancer. Gene 2019, 697, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Strobl, C.; Boulesteix, A.-L.; Zeileis, A.; Hothorn, T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinform. 2007, 8, 25. [Google Scholar] [CrossRef]
- Manzoni, C.; Kia, D.A.; Vandrovcova, J.; Hardy, J.; Wood, N.W.; Lewis, P.A.; Ferrari, R. Genome, transcriptome and proteome: The rise of omics data and their integration in biomedical sciences. Brief. Bioinform. 2018, 19, 286–302. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Ali, H.R.; Rueda, O.M.; Chin, S.-F.; Curtis, C.; Dunning, M.J.; Aparicio, S.A.; Caldas, C. Genome-driven integrated classification of breast cancer validated in over 7500 samples. Genome Biol. 2014, 15, 431. [Google Scholar] [CrossRef]
- Teo, W.-Y.; Sekar, K.; Seshachalam, P.; Shen, J.; Chow, W.-Y.; Lau, C.C.; Yang, H.; Park, J.; Kang, S.-G.; Li, X.; et al. Relevance of a TCGA-derived Glioblastoma Subtype Gene-Classifier among Patient Populations. Sci. Rep. 2019, 9, 7442. [Google Scholar] [CrossRef]
- Rosenfeld, N.; Aharonov, R.; Meiri, E.; Rosenwald, S.; Spector, Y.; Zepeniuk, M.; Benjamin, H.; Shabes, N.; Tabak, S.; Levy, A.; et al. MicroRNAs accurately identify cancer tissue origin. Nat. Biotechnol. 2008, 26, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-W.; Lin, J.-S.; He, X.-X. The emerging role of miR-375 in cancer. Int. J. Cancer 2014, 135, 1011–1018. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, L.; Chen, L.; Li, J.; Zhang, F.; Xing, Y.; Zhao, J. miR-190b promotes tumor growth and metastasis via suppressing NLRC3 in bladder carcinoma. FASEB J. 2020, 34, 4072–4084. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, C.; Cui, Q.; Luan, X.; Wang, Q.; Zhou, C. miR-190b promotes colorectal cancer progression through targeting forkhead box protein P2. Exp. Ther. Med. 2019. [Google Scholar] [CrossRef]
- Hong, H.; Yao, S.; Zhang, Y.; Ye, Y.; Li, C.; Hu, L.; Sun, Y.; Huang, H.-Y.; Ji, H. In vivo miRNA knockout screening identifies miR-190b as a novel tumor suppressor. PLoS Genet. 2020, 16, e1009168. [Google Scholar] [CrossRef]
- Yano, M.; Ito, K.; Yabuno, A.; Ogane, N.; Katoh, T.; Miyazawa, M.; Miyazawa, M.; Hasegawa, K.; Narahara, H.; Yasuda, M. Impact of TP53 immunohistochemistry on the histological grading system for endometrial endometrioid carcinoma. Mod. Pathol. 2019, 32, 1023–1031. [Google Scholar] [CrossRef]
- Bosse, T.; Nout, R.A.; McAlpine, J.N.; McConechy, M.K.; Britton, H.; Hussein, Y.R.; Gonzalez, C.; Ganesan, R.; Steele, J.C.; Harrison, B.T.; et al. Molecular Classification of Grade 3 Endometrioid Endometrial Cancers Identifies Distinct Prognostic Subgroups. Am. J. Surg. Pathol. 2018, 1. [Google Scholar] [CrossRef]
- Torres, A.; Torres, K.; Pesci, A.; Ceccaroni, M.; Paszkowski, T.; Cassandrini, P.; Zamboni, G.; Maciejewski, R. Diagnostic and prognostic significance of miRNA signatures in tissues and plasma of endometrioid endometrial carcinoma patients. Int. J. Cancer 2013, 132, 1633–1645. [Google Scholar] [CrossRef]
- Jayaraman, M.; Radhakrishnan, R.; Mathews, C.A.; Yan, M.; Husain, S.; Moxley, K.M.; Song, Y.S.; Dhanasekaran, D.N. Identification of novel diagnostic and prognostic miRNA signatures in endometrial cancer. Genes Cancer 2017. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ma, W.; Luo, L. Establishment of the Prognosis Predicting Signature for Endometrial Cancer Patient. Med. Sci. Monit. 2019, 25, 8248–8259. [Google Scholar] [CrossRef] [PubMed]
- Olesen, T.B.; Svahn, M.F.; Faber, M.T.; Duun-Henriksen, A.K.; Junge, J.; Norrild, B.; Kjaer, S.K. Prevalence of Human Papillomavirus in endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2014, 134, 206–215. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Papazoglou, D.; Koukourakis, M.I.; Maltezos, E. Human Papillomavirus in Endometrial Adenocarcinomas: Infectious Agent or a Mere “Passenger”? Infect. Dis. Obstet. Gynecol. 2007, 2007, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Deligdisch, L.; Marin, T.; Lee, A.T.; Etkind, P.; Holland, J.F.; Melana, S.; Pogo, B.G.T. Human Mammary Tumor Virus (HMTV) in Endometrial Carcinoma. Int. J. Gynecol. Cancer 2013, 23, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Johal, H.; Faedo, M.; Faltas, J.; Lau, A.; Mousina, R.; Cozzi, P.; DeFazio, A.; Rawlinson, W.D. DNA of mouse mammary tumor virus-like virus is present in human tumors influenced by hormones. J. Med. Virol. 2010, 82, 1044–1105. [Google Scholar] [CrossRef]
| Phenotype | Grade 1 | Grade 2 | Grade 3 | Wilcoxon Test (p-Value) |
|---|---|---|---|---|
| Age (years) | 66 (54–78) | 54 (53–65) | 64 (46–78) | 0.1099 |
| Height (cm) | 163 (150–170) | 164 (158–170) | 165 (156–168) | - |
| Weight (kg) | 80 (63–100) | 74 (53–108) | 67 (52–98) | - |
| No. of menstruations | 375 (340–430) | 380 (350–410) | 350 (290–430) | 0.6508 |
| Time since last menstruation (months) | 51.5 (48–57) | 53 (49–53) | 50 (44–57) | 0.5787 |
| Pregnancies | 2 (0–4) | 2 (1–4) | 2 (1–3) | 0.6466 |
| BMI | 29.04 (23.31–40.90) | 28.04 (20.70–40.15) | 24.61 (20.57–38.21) | 0.4982 |
| Grade vs. Grade | 1 | 2 | 3 | Class. Error | miRNAs |
|---|---|---|---|---|---|
| 1 | 9 | 1 | 0 | 0.1 | hsa-miR-652-3p, hsa-miR-4510,hsa-miR-190b, hsa-miR-421,hsa-miR-941,hsa-miR-1307-3p, hsa-miR-375,hsa-miR-345-5p, hsa-miR-99b-3p, hsa-let-7b-3p |
| 2 | 1 | 6 | 0 | 0.1429 | |
| 3 | 0 | 1 | 6 | 0.1429 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrus, M.; Seweryn, M.; Kapusta, P.; Wołkow, P.; Pityński, K.; Wątor, G. Low Expression of miR-375 and miR-190b Differentiates Grade 3 Patients with Endometrial Cancer. Biomolecules 2021, 11, 274. https://doi.org/10.3390/biom11020274
Pietrus M, Seweryn M, Kapusta P, Wołkow P, Pityński K, Wątor G. Low Expression of miR-375 and miR-190b Differentiates Grade 3 Patients with Endometrial Cancer. Biomolecules. 2021; 11(2):274. https://doi.org/10.3390/biom11020274
Chicago/Turabian StylePietrus, Miłosz, Michał Seweryn, Przemysław Kapusta, Paweł Wołkow, Kazimierz Pityński, and Gracjan Wątor. 2021. "Low Expression of miR-375 and miR-190b Differentiates Grade 3 Patients with Endometrial Cancer" Biomolecules 11, no. 2: 274. https://doi.org/10.3390/biom11020274
APA StylePietrus, M., Seweryn, M., Kapusta, P., Wołkow, P., Pityński, K., & Wątor, G. (2021). Low Expression of miR-375 and miR-190b Differentiates Grade 3 Patients with Endometrial Cancer. Biomolecules, 11(2), 274. https://doi.org/10.3390/biom11020274







