Crucial Role of Juvenile Hormone Receptor Components Methoprene-Tolerant and Taiman in Sexual Maturation of Adult Male Desert Locusts
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Rearing
2.2. In Silico Analysis of JH Signaling Components
2.2.1. Amino Acid Sequence Predictions of Scg-Met and Scg-Tai
2.2.2. Multiple Sequence Alignments
2.3. RNA Interference
2.3.1. Production of dsRNA
2.3.2. RNAi Experiment
2.4. Observation of Mating Behavior
2.5. Calculation of the Accessory Gland Somatic and Gonadosomatic Indices
2.6. Corpora Allata Surface Area Measurement
2.7. Volatile Determination
2.8. Transcript Profiling
2.8.1. Sample Collection
2.8.2. RNA Preparation
2.8.3. Quantitative Real-Time (q-)RT-PCR
2.8.4. q-RT-PCR Temporal Profiling of JH Pathway Components
3. Results
3.1. Identification of Scg-Met and Scg-Tai cDNAs in S. gregaria
3.2. Transcript Levels of JH Pathway Components in Untreated Adult Male Locusts
3.3. Expression of MEKRE93 and JH Biosynthesis Genes in dsScg-Met Injected Males
3.4. Absence of Sexual Activity in dsScg-Met Injected Male Locusts
3.5. Scg-Met Knockdown Increases Corpora Allata Size
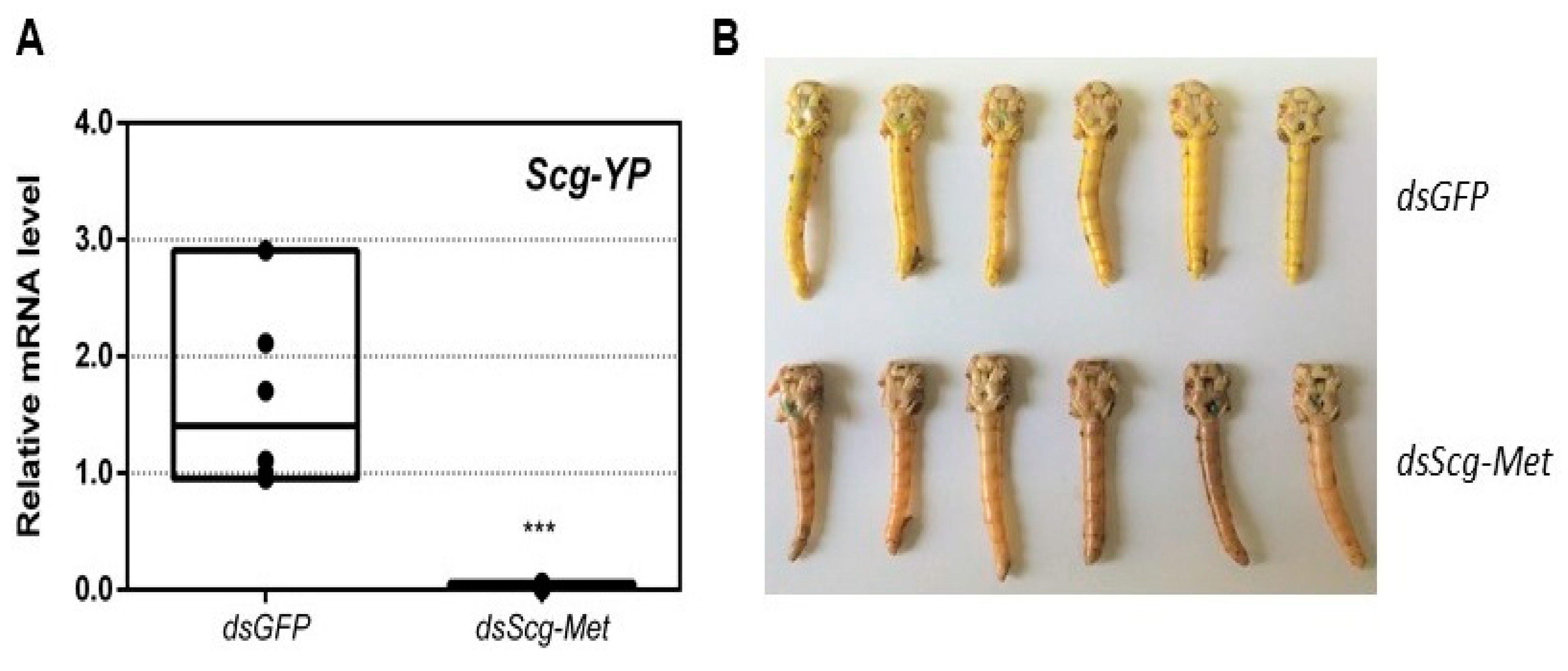
3.6. Scg-Met Knockdown Reduces Yellow Pigmentation in The Cuticle of Adult Male Desert Locusts
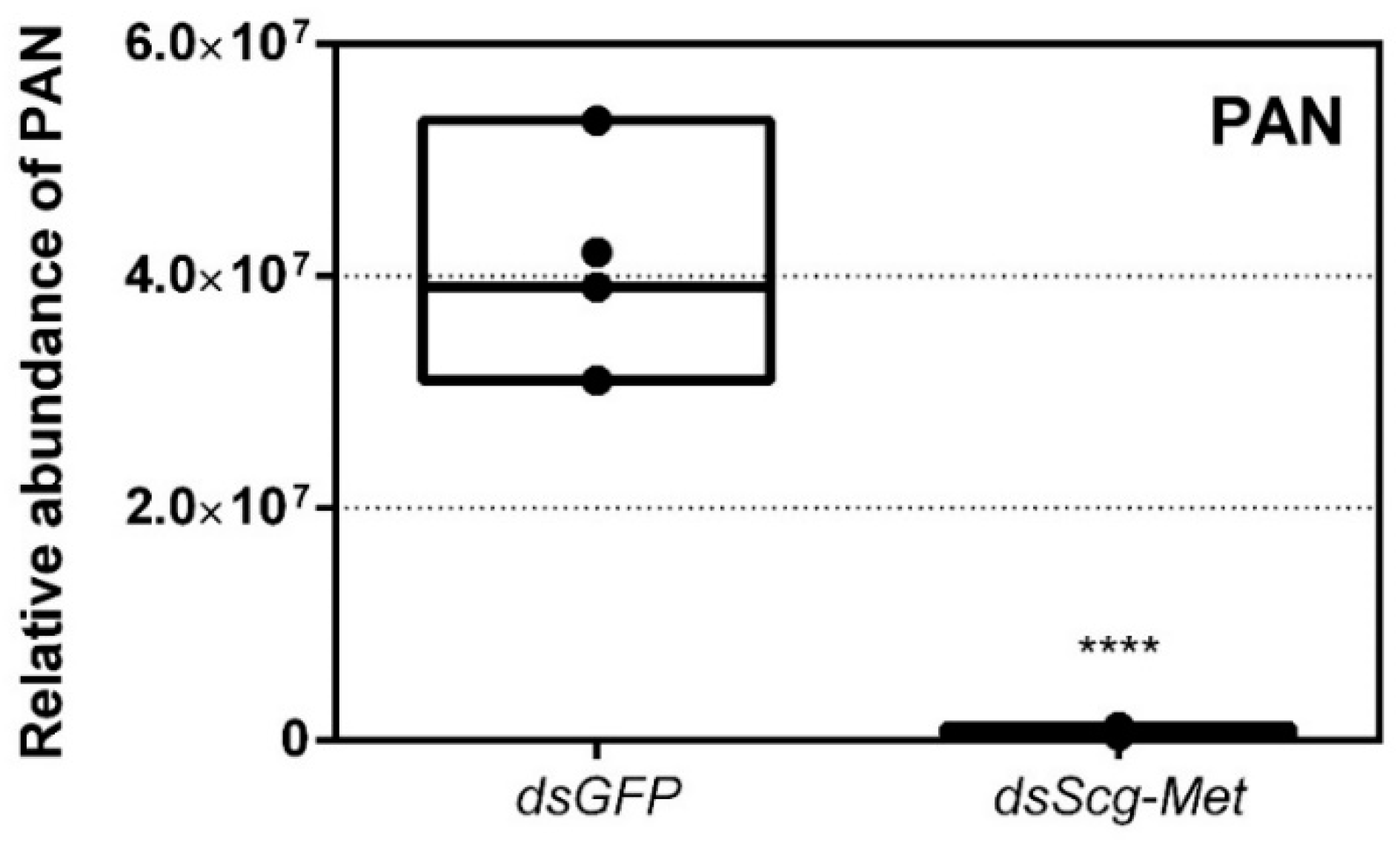
3.7. Effect of Scg-Met Knockdown on Phenylacetonitrile Production
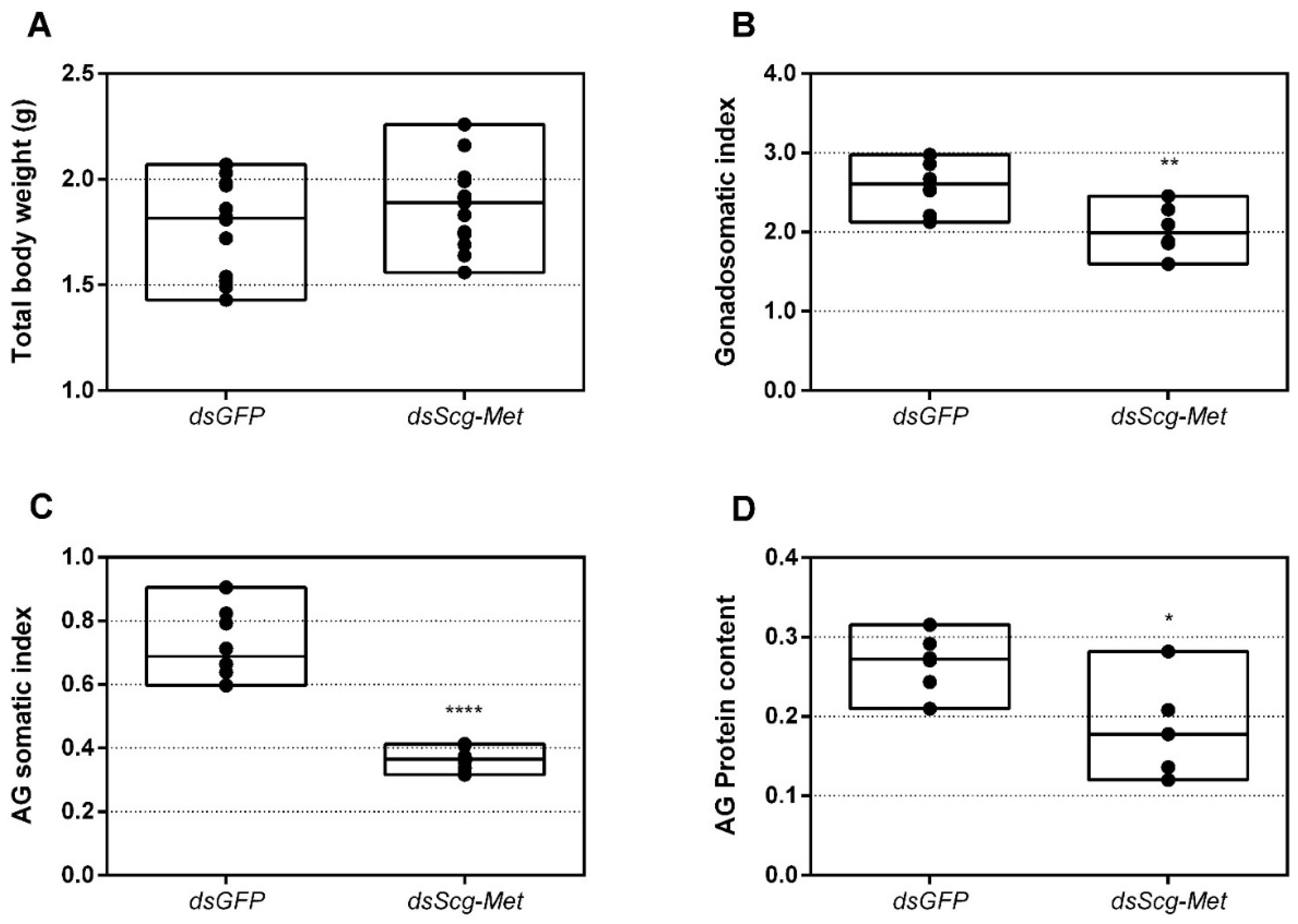
3.8. Effect of Scg-Met Knockdown on Development of Male Reproductive Organs
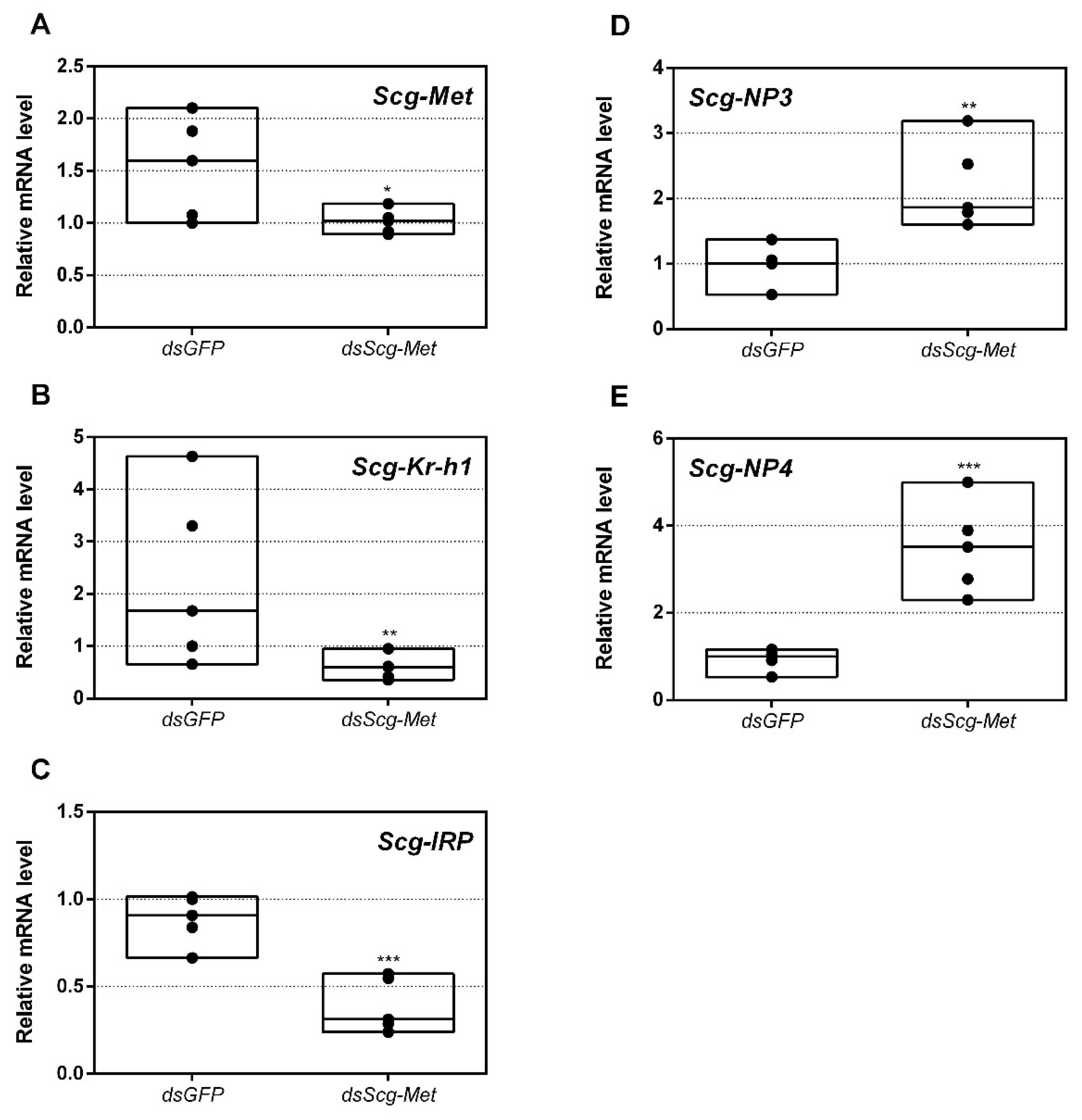
3.9. Effect of Scg-Met Knockdown on Gene Expression Profiles in the Fat Body
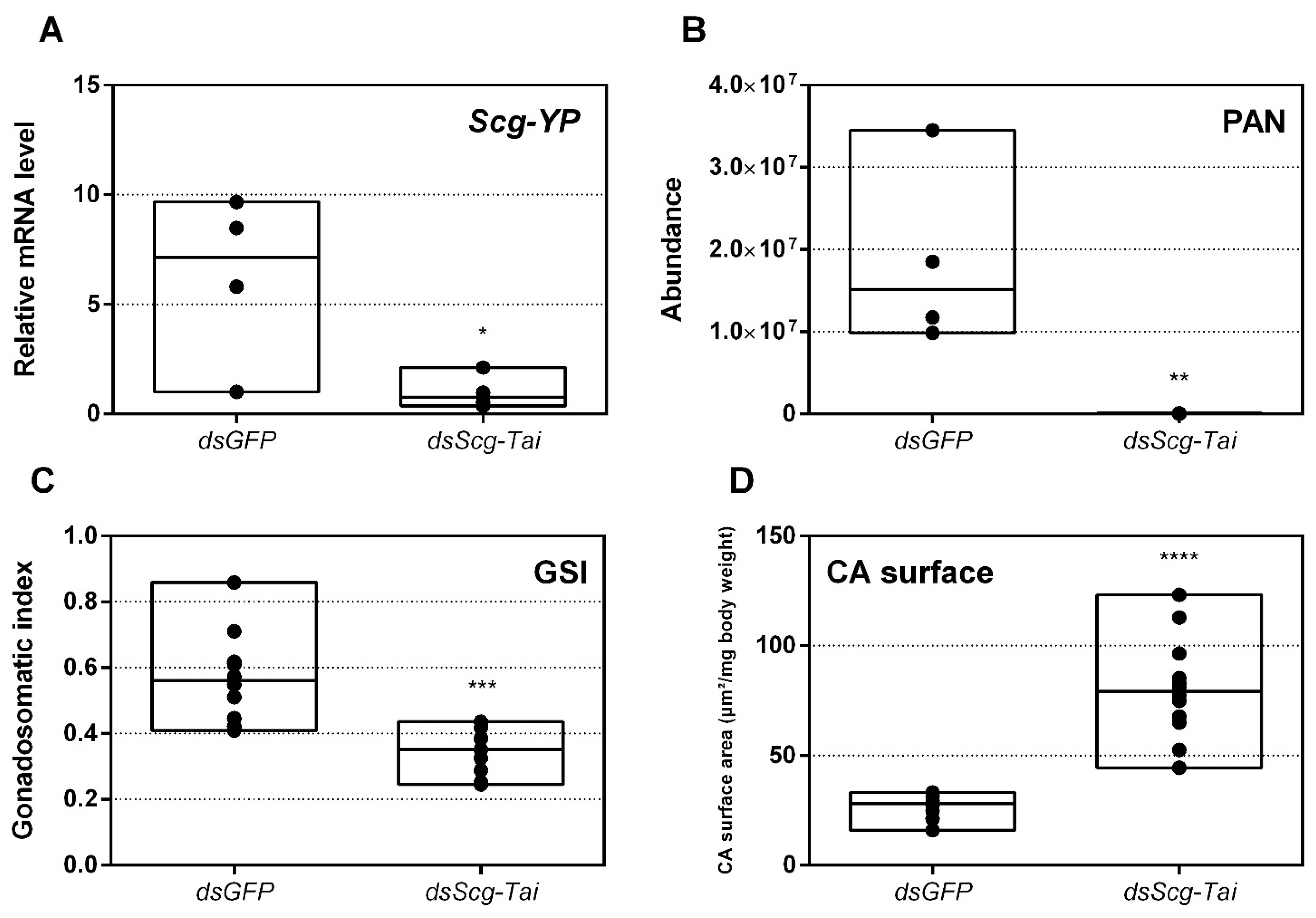
3.10. Knockdown of Scg-Tai also Results in an Identical Phenotype
4. Discussion
4.1. Scg-Met Knockdown Severely Disrupts the Reproductive Development of Adult Male Locusts
- An obvious visual effect of the Scg-Met knockdown was the impaired cuticular pigmentation of the adult males (Figure 3). S. gregaria adults are weakly pigmented after their final molt. Hardening of the cuticle upon molting, results in steady background coloration. In adult gregarious males, a remarkable change to a bright yellow cuticular coloration occurs when they become sexually mature [73]. In our colony, this color change normally manifests itself at days 9–12 after the final molt. Research has highlighted the role of yellow protein (YP) in this remarkable phenotypic transformation. YP has a high binding capacity for carotenoids originating from the diet and will be allocated towards the epidermis and cuticle when gregarious males become sexually mature. High levels of JH during adult male development will induce increasing YP levels, eventually resulting in the deposition of the yellow pigment in the cuticle [74]. The strongly reduced Scg-YP expression in dsScg-Met injected males is fully in line with the observed absence of a bright yellow coloration in the cuticle, in stark contrast with the dsGFP injected (control) males (Figure 3).
- In comparison to dsGFP injected (control) males, the Scg-Met knockdown locusts produced much lower amounts of the pheromone PAN (Figure 4). Gregarious desert locusts live in very dense populations. Besides visual cues, pheromone communication plays a crucial role in mating behavior. One of the best-known pheromones produced by gregarious male desert locusts is phenylacetonitrile (PAN). This pheromone is abundantly released by the legs and wings of gregarious male locusts and plays a crucial role in identifying an appropriate sexual partner by preventing male-male interactions, as well as in the post-copulatory guarding of the inseminated female mate [38]. Knockdown of Scg-Met very strongly affected PAN emission, which illustrates that Scg-Met plays a crucial role in the emission of this male-to-male anti-aphrodisiac pheromone.
- The development of the male reproductive organs, testes and accessory glands, was significantly inhibited by knocking down Scg-Met during the adult stage (Figure 5). Interestingly, a recent study demonstrated that treating male S. gregaria nymphs (final nymphal stage) with a JH mimic resulted in underdeveloped accessory glands and seminal vesicles, and impaired molting to the adult stage [75]. This suggests a different function of JH in the development of reproductive organs in the desert locust during distinct life stages, again highlighting the complex role of JH throughout the life history of insects. The role of JH in protein accumulation in AG has been described in several insect species [76]. A more recent study on D. melanogaster Met27 mutants first demonstrated the role of Met in the development of the male AG. Met27 mutants showed reduced AG sizes. This could be ascribed to the overall reduced protein synthesis resulting from the impaired JH signaling [77]. Similar effects were observed after knocking down Met or Tai in the linden bug, Pyrrhocoris apterus [78,79]. These studies demonstrated the participation of Met and Tai in generating the JH dependent effects on AG size in male P. apterus. Surprisingly, Met knockdown in this species did not reduce locomotor activity or mating behavior, in contrast to what is observed during adult reproductive diapause, which made the authors suggest that an additional photoperiodic control mechanism might be situated in parallel, independent or upstream of JH [79]. In the red flour beetle, T. castaneum, a JHAMT knockdown reduced the AG size and production of seminal proteins, significantly affecting male fitness [80]. Moreover, in locust species, JH has previously been shown to be essential for the growth of the AG and associated protein content in this tissue [44,46,47]. Our results show that Scg-Met knockdown in S. gregaria produced effects opposite to JH itself, clearly illustrating the adverse consequences of a disabled transduction of the JH signal on the development of reproductive organs in adult male locusts.
- Interestingly, both the size of the CA and the expression of Scg-JHAMT, which codes for a rate-limiting JH biosynthetic enzyme, were significantly increased upon sustained knockdown of Scg-Met (Figure 2). These observations suggest the existence of homeostatic regulatory mechanisms for preserving a balance between JH synthesis and JH sensitivity. This is also supported by the observed increase in Scg-JHAMT and Scg-CYP15A1 expression upon Scg-Met knockdown in adult female S. gregaria [14]. A similar stimulation of JH production following Met knockdown was previously also described in the linden bug, P. apterus [78]. This homeostatic regulation may reside within the CA but could also be more complex and include inter-organ communication mechanisms.
- Finally, knockdown of either component of the JH receptor complex induced very similar phenotypic effects in the desert locust (Figure 6). Recent studies revealed that Met forms a complex with Tai to become a functionally active JH-dependent receptor. Once JH is bound to Met, the JH-Met complex will subsequently interact with a Tai transcription factor domain to form an active JH signal transducer [10]. Our findings are in line with this mechanistic model for JH signaling and indicate that reproductive organ growth, yellow coloration and PAN pheromone emission in gregarious males are highly dependent on expression of both Scg-Met and Scg-Tai.
4.2. Profiles of Scg-Met, Scg-Tai, Scg-Krh1, Scg-JHAMT and Scg-CYP15A1 in Untreated Adult Males
4.3. Possible Cross-Talk with Developmental Peptide Hormones?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raikhel, A.S.; Brown, M.R.; Belles, X. Hormonal control of reproductive processes. In Comprehensive Molecular Insect Science; Gilbert, L.I., Iatrou, K., Gill, S.S., Eds.; Elsevier: Oxford, UK, 2005; pp. 433–491. [Google Scholar]
- Brown, M.R.; Sieglaff, D.H.; Rees, H.H. Gonadal Ecdysteroidogenesis in Arthropoda: Occurrence and Regulation. Annu. Rev. Entomol. 2009, 54, 105–125. [Google Scholar] [CrossRef]
- Marchal, E.; Vandersmissen, H.P.; Badisco, L.; Van De Velde, S.; Verlinden, H.; Iga, M.; Van Wielendaele, P.; Huybrechts, R.; Simonet, G.; Smagghe, G.; et al. Control of ecdysteroidogenesis in prothoracic glands of insects: A review. Peptides 2010, 31, 506–519. [Google Scholar] [CrossRef]
- Van de Velde, S.; Badisco, L.; Marchal, E.; Vanden Broeck, J.; Smagghe, G. Diversity in factors regulating ecdysteroidogenesis in insects. In Ecdysone, Structures and Functions; Smagghe, G., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 283–314. [Google Scholar]
- Nation, J.L. Insect Physiology and Biochemistry, Nature; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- Marchal, E.; Zhang, J.R.; Badisco, L.; Verlinden, H.; Hult, E.F.; Van Wielendaele, P.; Yagi, K.J.; Tobe, S.S.; Vanden Broeck, J. Final steps in juvenile hormone biosynthesis in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2011, 41, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory Pathways Controlling Female Insect Reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef]
- Wilson, T.G.; Fabian, J. A Drosophila melanogaster mutant resistant to a chemical analog of juvenile hormone. Dev. Biol. 1986, 118, 190–201. [Google Scholar] [CrossRef]
- Miura, K.; Oda, M.; Makita, S.; Chinzei, Y. Characterization of the Drosophila Methoprene -tolerant gene product. FEBS J. 2005, 272, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.-P.; Iwema, T.; Epa, V.C.; Takaki, K.; Rynes, J.; Jindra, M. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl. Acad. Sci. USA 2011, 108, 21128–21133. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mead, E.A.; Zhu, J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl. Acad. Sci. USA 2010, 108, 638–643. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Sheng, Z.; Sui, Y.; Palli, S.R. Steroid Receptor Co-activator Is Required for Juvenile Hormone Signal Transduction through a bHLH-PAS Transcription Factor, Methoprene Tolerant. J. Biol. Chem. 2011, 286, 8437–8447. [Google Scholar] [CrossRef] [PubMed]
- Minakuchi, C.; Zhou, X.; Riddiford, L.M. Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech. Dev. 2008, 125, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Gijbels, M.; Lenaerts, C.; Vanden Broeck, J.; Marchal, E. Juvenile Hormone receptor Met is essential for ovarian maturation in the Desert Locust, Schistocerca gregaria. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The Juvenile Hormone Signaling Pathway in Insect Development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Konopova, B.; Smykal, V.; Jindra, M. Common and Distinct Roles of Juvenile Hormone Signaling Genes in Metamorphosis of Holometabolous and Hemimetabolous Insects. PLoS ONE 2011, 6, e28728. [Google Scholar] [CrossRef] [PubMed]
- Konopova, B.; Jindra, M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2007, 104, 10488–10493. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Kayukawa, T.; Shinoda, T.; Belles, X. A Role for Taiman in Insect Metamorphosis. PLoS Genet. 2014, 10, e1004769. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Tan, A.; Palli, S.R. bHLH-PAS family transcription factor methoprene-tolerant plays a key role in JH action in preventing the premature development of adult structures during larval–pupal metamorphosis. Mech. Dev. 2008, 125, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M. Rhodnius, Golden Oil, and Met: A History of Juvenile Hormone Research. Front. Cell Dev. Biol. 2020, 8, 679. [Google Scholar] [CrossRef]
- Belles, X.; Santos, C.G. The MEKRE93 (Methoprene tolerant-Krüppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect Biochem. Mol. Biol. 2014, 52, 60–68. [Google Scholar] [CrossRef]
- Gujar, H.; Palli, S.R. Krüppel homolog 1 and E93 mediate Juvenile hormone regulation of metamorphosis in the common bed bug, Cimex lectularius. Sci. Rep. 2016, 6, 26092. [Google Scholar] [CrossRef]
- Ureña, E.; Manjón, C.; Franch-Marro, X.; Martín, D. Transcription factor E93 specifies adult metamorphosis in hemimetabolous and holometabolous insects. Proc. Natl. Acad. Sci. USA 2014, 111, 7024–7029. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.-M.; Yue, Y.; Song, Z.-H.; Wang, J.-J.; Dou, W. The alternative splicing of BdTai and its involvement in the development of Bactrocera dorsalis (Hendel). J. Insect Physiol. 2017, 101, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Marchal, E.; Hult, E.F.; Huang, J.; Pang, Z.; Stay, B.; Tobe, S.S. Methoprene-Tolerant (Met) Knockdown in the Adult Female Cockroach, Diploptera punctata Completely Inhibits Ovarian Development. PLoS ONE 2014, 9, e106737. [Google Scholar] [CrossRef]
- Paim, R.M.; Pereira, M.H.; Di Ponzio, R.; O Rodrigues, J.; Guarneri, A.A.; Gontijo, N.D.F.; Araujo, R.N. Validation of reference genes for expression analysis in the salivary gland and the intestine of Rhodnius prolixus (Hemiptera, Reduviidae) under different experimental conditions by quantitative real-time PCR. BMC Res. Notes 2012, 5, 128. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wu, Z.; Wang, Z.; Deng, S.; Zhou, S. Krüppel-homolog 1 mediates juvenile hormone action to promote vitellogenesis and oocyte maturation in the migratory locust. Insect Biochem. Mol. Biol. 2014, 52, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Sambucaro, M.J.; Riccillo, F.L.; Calderón-Fernández, G.M.; Sterkel, M.; Diambra, L.A.; Ronderos, J.R. Genomic and functional characterization of a methoprene-tolerant gene in the kissing-bug Rhodnius prolixus. Gen. Comp. Endocrinol. 2015, 216, 1–8. [Google Scholar] [CrossRef]
- Wang, J.-L.; Saha, T.T.; Zhang, Y.; Zhang, C.; Raikhel, A.S. Juvenile hormone and its receptor methoprene-tolerant promote ribosomal biogenesis and vitellogenesis in the Aedes aegypti mosquito. J. Biol. Chem. 2017, 292, 10306–10315. [Google Scholar] [CrossRef]
- Yue, Y.; Yang, R.-L.; Wang, W.-P.; Zhou, Q.-H.; Chen, E.-H.; Yuan, G.-R.; Wang, J.-J.; Dou, W. Involvement of Met and Kr-h1 in JH-Mediated Reproduction of Female Bactrocera dorsalis (Hendel). Front. Physiol. 2018, 9, 482. [Google Scholar] [CrossRef]
- Zhu, L.; Yin, T.-Y.; Sun, D.; Liu, W.; Zhu, F.; Lei, C.-L.; Wang, X.-P. Juvenile hormone regulates the differential expression of putative juvenile hormone esterases via methoprene-tolerant in non-diapause-destined and diapause-destined adult female beetle. Gene 2017, 627, 373–378. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Song, J.; Kang, L.; Zhou, S. An isoform of Taiman that contains a PRD-repeat motif is indispensable for transducing the vitellogenic juvenile hormone signal in Locusta migratoria. Insect Biochem. Mol. Biol. 2017, 82, 31–40. [Google Scholar] [CrossRef]
- Goodwin, T.W. The Biochemistry of Locust Pigmentation. Biol. Rev. 1952, 27, 439–460. [Google Scholar] [CrossRef]
- Goodwin, T.W.; Srisukh, S. The Carotenoids of the Locust Integument. Nat. Cell Biol. 1948, 161, 525–526. [Google Scholar] [CrossRef]
- Lange, A.B.; Phillips, D.R.; Loughton, B. The effects of precocene II on early adult development in male Locusta. J. Insect Physiol. 1983, 29, 73–81. [Google Scholar] [CrossRef]
- Norris, M.J.; Pener, M.P. An Inhibitory Effect of Allatectomized Males and Females on the Sexual Maturation of Young Male Adults of Schistocerca gregaria (Forsk.) (Orthoptera: Acrididae). Nat. Cell Biol. 1965, 208, 1122. [Google Scholar] [CrossRef]
- Amerasinghe, F. Effects of J.H.I and J.H.III on yellowing, sexual activity and pheromone production in allatectomized male Schistocerca gregaria. J. Insect Physiol. 1978, 24, 603–611. [Google Scholar] [CrossRef]
- Amwayi, P.W.; Masiga, D.K.; Govender, P.; Teal, P.E.; Torto, B. Mass spectral determination of phenylacetonitrile (PAN) levels in body tissues of adult desert locust, Schistocerca gregaria. J. Insect Physiol. 2012, 58, 1037–1041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loher, W. The chemical acceleration of the maturation process and its hormonal control in the male of the desert locust. Proc. R. Soc. Lond. Ser. B Boil. Sci. 1961, 153, 380–397. [Google Scholar] [CrossRef]
- Mahamat, H.; Hassanali, A.; Odongo, H. The Role of Different Components of the Pheromone Emission of Mature Males of the Desert Locust, Schistocerca gregaria (Forskål) (Orthoptera: Acrididae) in Accelerating Maturation of Immature Adults. Int. J. Trop. Insect Sci. 2000, 20, 1–5. [Google Scholar] [CrossRef]
- Seidelmann, K.; Ferenz, H.-J. Courtship inhibition pheromone in desert locusts, Schistocerca gregaria. J. Insect Physiol. 2002, 48, 991–996. [Google Scholar] [CrossRef]
- Ferenz, H.-J.; Seidelmann, K. Pheromones in relation to aggregation and reproduction in desert locusts. Physiol. Entomol. 2003, 28, 11–18. [Google Scholar] [CrossRef]
- I Tawfik, A.; Treiblmayr, K.; Hassanali, A.; Osir, E. Time-course haemolymph juvenile hormone titres in solitarious and gregarious adults of Schistocerca gregaria, and their relation to pheromone emission, CA volumetric changes and oocyte growth. J. Insect Physiol. 2000, 46, 1143–1150. [Google Scholar] [CrossRef]
- Braun, R.P.; Wyatt, G.R. Growth of the male accessory gland in adult locusts: Roles of juvenile hormone, JH esterase, and JH binding proteins. Arch. Insect Biochem. Physiol. 1995, 30, 383–400. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Avruch, L.; Tobe, S.S. Juvenile hormone biosynthesis by the corpora allata of the male desert locust, Schistocerca gregaria, during sexual maturation. Can. J. Zool. Can. Zool. 1978, 56, 2097–2102. [Google Scholar] [CrossRef]
- Ismail, S.M.; Gillott, C. Identification of a Nuclear Juvenile Hormone-Binding Protein in the Long Hyaline Tubule of Male Melanoplus sanguinipes. Arch. Insect Biochem. Physiol. 1996, 32, 623–631. [Google Scholar] [CrossRef]
- Badisco, L.; Van Wielendaele, P.; Vanden Broeck, J. Eat to reproduce: A key role for the insulin signaling pathway in adult insects. Front. Physiol. 2013, 4, 202. [Google Scholar] [CrossRef]
- Lenaerts, C.; Monjon, E.; Van Lommel, J.; Verbakel, L.; Vanden Broeck, J. Peptides in insect oogenesis. Curr. Opin. Insect Sci. 2019, 31, 58–64. [Google Scholar] [CrossRef]
- Mirth, C.K.; Alves, A.N.; Piper, M.D.W. Turning food into eggs: Insights from nutritional biology and developmental physiology of Drosophila. Curr. Opin. Insect Sci. 2019, 31, 49–57. [Google Scholar] [CrossRef]
- Badisco, L.; Marchal, E.; Van Wielendaele, P.; Verlinden, H.; Vleugels, R.; Vanden Broeck, J. RNA interference of insulin-related peptide and neuroparsins affects vitellogenesis in the desert locust Schistocerca gregaria. Peptides 2011, 32, 573–580. [Google Scholar] [CrossRef]
- Girardie, J.; Boureme, D.; Couillaud, F.; Tamarelle, M. Anti-juvenile effect of neuroparsin A, a neuroprotein isolated from locust corpora cardiaca. Insect Biochem. 1987, 17, 977–983. [Google Scholar] [CrossRef]
- Girardie, J.; Girardie, A.; Huet, J.-C.; Pernollet, J.-C. Amino acid sequence of locust neuroparsins. FEBS Lett. 1989, 245, 4–8. [Google Scholar] [CrossRef]
- Girardie, J.; Huet, J.-C.; Atay-Kadiri, Z.; Ettaouil, S.; Delbecque, J.-P.; Fournier, B.; Pernollet, J.-C.; Girardie, A. Isolation, sequence determination, physical and physiological characterization of the neuroparsins and ovary maturing parsins of Schistocerca gregaria. Insect Biochem. Mol. Biol. 1998, 28, 641–650. [Google Scholar] [CrossRef]
- Badisco, L.; Claeys, I.; Van Loy, T.; Van Hiel, M.; Franssens, V.; Simonet, G.; Vanden Broeck, J. Neuroparsins, a family of conserved arthropod neuropeptides. Gen. Comp. Endocrinol. 2007, 153, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Badisco, L.; Claeys, I.; Van Hiel, M.; Clynen, E.; Huybrechts, J.; Vandersmissen, T.; Van Soest, S.; Vanden Bosch, L.; Simonet, G.; Vanden Broeck, J. Purification and characterization of an insulin-related peptide in the desert locust, Schistocerca gregaria: Immunolocalization, cDNA cloning, transcript profiling and interaction with neuroparsin. J. Mol. Endocrinol. 2008, 40, 137–150. [Google Scholar] [CrossRef]
- Nässel, D.R.; Vanden Broeck, J. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci. 2015, 73, 271–290. [Google Scholar] [CrossRef]
- Lenaerts, C.; Palmans, J.; Marchal, E.; Verdonck, R.; Vanden Broeck, J. Role of the venus kinase receptor in the female reproductive physiology of the desert locust, Schistocerca gregaria. Sci. Rep. 2017, 7, 11730. [Google Scholar] [CrossRef] [PubMed]
- Vogel, K.J.; Brown, M.R.; Strand, M.R. Ovary ecdysteroidogenic hormone requires a receptor tyrosine kinase to activate egg formation in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2015, 112, 5057–5062. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, H.; Sterck, L.; Li, J.; Li, Z.; Yssel, A.; Gansemans, Y.; Verdonck, R.; Holtof, M.; Song, H.; Behmer, S.T.; et al. First draft genome assembly of the desert locust, Schistocerca gregaria. F1000Research 2020, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Park, Y.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.; Potter, S.; Finn, R.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; I Hurwitz, D.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.; von Haeseler, A.; Minh, B. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, 232–235. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, 256–259. [Google Scholar] [CrossRef]
- Rasband, W.S.; ImageJ, U.S. ImageJ; National Institutes of Health: Bethesda, MD, USA, 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 8 January 2021).
- Van Hiel, B.; Van Wielendaele, P.; Temmerman, L.; Van Soest, S.; Vuerinckx, K.; Huybrechts, R.; Vanden Broeck, J.; Simonet, G. Identification and validation of housekeeping genes in brains of the desert locust Schistocerca gregaria under different developmental conditions. BMC Mol. Biol. 2009, 10, 10–56. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef]
- Sas, F.; Begum, M.; Vandersmissen, T.; Geens, M.; Claeys, I.; Van Soest, S.; Huybrechts, J.; Huybrechts, R.; De Loof, A. Development of a real-time PCR assay for measurement of yellow protein mRNA transcription in the desert locust Schistocerca gregaria: A basis for isolation of a peptidergic regulatory factor. Peptides 2007, 28, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Belles, X. Krüppel homolog 1 and E93: The doorkeeper and the key to insect metamorphosis. Arch. Insect Biochem. Physiol. 2020, 103, e21609. [Google Scholar] [CrossRef]
- Gijbels, M.; Schellens, S.; Schellekens, T.; Bruyninckx, E.; Marchal, E.; Vanden Broeck, J. Precocious Downregulation of Krüppel-Homolog 1 in the Migratory Locust, Locusta migratoria, Gives Rise to An Adultoid Phenotype with Accelerated Ovarian Development but Disturbed Mating and Oviposition. Int. J. Mol. Sci. 2020, 21, 6058. [Google Scholar] [CrossRef] [PubMed]
- Gijbels, M.; Marchal, E.; Verdonckt, T.W.; Bruyninckx, E.; Vanden Broeck, J. RNAi-Mediated Knockdown of Transcription Factor E93 in Nymphs of the Desert Locust (Schistocerca gregaria) Inhibits Adult Morphogenesis and Results in Supernumerary Juvenile Stages. Int. J. Mol. Sci. 2020, 21, 7518. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Belles, X. Conserved repressive function of Krüppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci. Rep. 2011, 1, 163. [Google Scholar] [CrossRef] [PubMed]
- Uvarov, B. Grasshoppers and Locusts; Cambridge University Press: London, UK, 1996. [Google Scholar]
- Wybrandt, G.; Andersen, S.O. Purification and sequence determination of a yellow protein from sexually mature males of the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2001, 31, 1183–1189. [Google Scholar] [CrossRef]
- Hiroyoshi, S.; Kokwaro, E.; Mettupalli, S.; Mitsunaga, T.; Yagi, S.; Reddy, G.V.P. Effects of the juvenile hormone mimic NC-184 on the development of the reproductive organs and mating behaviour of nymphs of the desert locust, Schistocerca gregaria (Orthoptera: Acrididae). Eur. J. Entomol. 2019, 116, 477–485. [Google Scholar] [CrossRef]
- Chen, P.S. The Functional Morphology and Biochemistry of Insect Male Accessory Glands and their Secretions. Annu. Rev. Entomol. 1984, 29, 233–255. [Google Scholar] [CrossRef]
- Wilson, T.G.; Demoor, S.; Lei, J. Juvenile hormone involvement in Drosophila melanogaster male reproduction as suggested by the Methoprene-tolerant(27) mutant phenotype. Insect Biochem. Mol. Biol. 2003, 33, 1167–1175. [Google Scholar] [CrossRef]
- Hejnikova, M.; Paroulek, M.; Hodkova, M. Decrease in Methoprene tolerant and Taiman expression reduces juvenile hormone effects and enhances the levels of juvenile hormone circulating in males of the linden bug Pyrrhocoris apterus. J. Insect Physiol. 2016, 94, 72–80. [Google Scholar] [CrossRef]
- Urbanová, V.; Bazalová, O.; Vaněčková, H.; Dolezel, D. Photoperiod regulates growth of male accessory glands through juvenile hormone signaling in the linden bug, Pyrrhocoris apterus. Insect Biochem. Mol. Biol. 2016, 70, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Tan, A.; Sun, Z.; Chen, Z.; Rainkin, M.; Palli, S.R. Juvenile hormone regulation of male accessory gland activity in the red flour beetle, Tribolium castaneum. Mech. Dev. 2009, 126, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Claeys, I.; Breugelmans, B.; Simonet, G.; Van Soest, S.; Sas, F.; De Loof, A.; Vanden Broeck, J. Neuroparsin transcripts as molecular markers in the process of desert locust (Schistocerca gregaria) phase transition. Biochem. Biophys. Res. Commun. 2006, 341, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Van Wielendaele, P.; Badisco, L.; Vanden Broeck, J. Neuropeptidergic regulation of reproduction in insects. Gen. Comp. Endocrinol. 2013, 188, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Tatar, M. A Mutant Drosophila Insulin Receptor Homolog That Extends Life-Span and Impairs Neuroendocrine Function. Science 2001, 292, 107–110. [Google Scholar] [CrossRef]
- Sim, C.; Denlinger, D.L. A shut-down in expression of an insulin-like peptide, ILP-1, halts ovarian maturation during the overwintering diapause of the mosquito, Culex pipiens. Insect Mol. Biol. 2009, 18, 325–332. [Google Scholar] [CrossRef]
- Cheng, D.; Peng, J.; Meng, M.; Wei, L.; Kang, L.; Qian, W.; Xia, Q. Microarray Analysis of the Juvenile Hormone Response in Larval Integument of the Silkworm, Bombyx mori. Int. J. Genom. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Xu, J.; Sheng, Z.; Palli, S.R. Juvenile Hormone and Insulin Regulate Trehalose Homeostasis in the Red Flour Beetle, Tribolium castaneum. PLoS Genet. 2013, 9, e1003535. [Google Scholar] [CrossRef]
- Sheng, Z.; Xu, J.; Bai, H.; Zhu, F.; Palli, S.R. Juvenile Hormone Regulates Vitellogenin Gene Expression through Insulin-like Peptide Signaling Pathway in the Red Flour Beetle, Tribolium castaneum. J. Biol. Chem. 2011, 286, 41924–41936. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Raikhel, A.S.; Zhu, J. Characterization of a juvenile hormone-regulated chymotrypsin-like serine protease gene in Aedes aegypti mosquito. Insect Biochem. Mol. Biol. 2008, 38, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.-P.; Wang, J.-X.; Zhao, X.-F. The impacts of classical insect hormones on the expression profiles of a new digestive trypsin-like protease (TLP) from the cotton bollworm, Helicoverpa armigera. Insect Mol. Biol. 2009, 18, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, D.; Zheng, S.; Liu, L.; Tao, S.; Yang, L.; Hu, S.; Feng, Q. A chymotrypsin-like serine protease cDNA involved in food protein digestion in the common cutworm, Spodoptera litura: Cloning, characterization, developmental and induced expression patterns, and localization. J. Insect Physiol. 2010, 56, 788–799. [Google Scholar] [CrossRef]
- Cornette, R.; Hayashi, Y.; Koshikawa, S.; Miura, T. Differential gene expression in response to juvenile hormone analog treatment in the damp-wood termite Hodotermopsis sjostedti (Isoptera, Archotermopsidae). J. Insect Physiol. 2013, 59, 509–518. [Google Scholar] [CrossRef]
- Spit, J.; Holtof, M.; Badisco, L.; Vergauwen, L.; Vogel, E.; Knapen, I.; Vanden Broeck, J. Transcriptional Analysis of The Adaptive Digestive System of The Migratory Locust in Response to Plant Defensive Protease Inhibitors. Sci. Rep. 2016, 6, 32460. [Google Scholar] [CrossRef]
- Meunier, N.; Belgacem, Y.H.; Martin, J.-R. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. J. Exp. Biol. 2007, 210, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Janssen, T.; Claeys, I.; Simonet, G.; De Loof, A.; Girardie, J.; Vanden Broeck, J. cDNA cloning and transcript distribution of two different neuroparsin precursors in the desert locust, Schistocerca gregaria. Insect Mol. Biol. 2001, 10, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Claeys, I.; Simonet, G.; Van Loy, T.; De Loof, A.; Vanden Broeck, J. cDNA cloning and transcript distribution of two novel members of the neuroparsin family in the desert locust, Schistocerca gregaria. Insect Mol. Biol. 2003, 12, 473–481. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holtof, M.; Van Lommel, J.; Gijbels, M.; Dekempeneer, E.; Nicolai, B.; Vanden Broeck, J.; Marchal, E. Crucial Role of Juvenile Hormone Receptor Components Methoprene-Tolerant and Taiman in Sexual Maturation of Adult Male Desert Locusts. Biomolecules 2021, 11, 244. https://doi.org/10.3390/biom11020244
Holtof M, Van Lommel J, Gijbels M, Dekempeneer E, Nicolai B, Vanden Broeck J, Marchal E. Crucial Role of Juvenile Hormone Receptor Components Methoprene-Tolerant and Taiman in Sexual Maturation of Adult Male Desert Locusts. Biomolecules. 2021; 11(2):244. https://doi.org/10.3390/biom11020244
Chicago/Turabian StyleHoltof, Michiel, Joachim Van Lommel, Marijke Gijbels, Elfie Dekempeneer, Bart Nicolai, Jozef Vanden Broeck, and Elisabeth Marchal. 2021. "Crucial Role of Juvenile Hormone Receptor Components Methoprene-Tolerant and Taiman in Sexual Maturation of Adult Male Desert Locusts" Biomolecules 11, no. 2: 244. https://doi.org/10.3390/biom11020244
APA StyleHoltof, M., Van Lommel, J., Gijbels, M., Dekempeneer, E., Nicolai, B., Vanden Broeck, J., & Marchal, E. (2021). Crucial Role of Juvenile Hormone Receptor Components Methoprene-Tolerant and Taiman in Sexual Maturation of Adult Male Desert Locusts. Biomolecules, 11(2), 244. https://doi.org/10.3390/biom11020244





