1. Introduction
Endothelial cells (ECs) constitute the inner cell layer within the blood vessels and are primarily involved in vascular homeostasis, regulating blood flow, hemostasis, blood cells’ luminal adherence and vascular permeability [
1]. The endothelial dysfunction is considered the triggering event in the initiation of atherosclerotic disease [
2], and it is associated with the loss of the EC barrier, allowing the recruitment of monocytes and the infiltration of low-density lipoproteins (LDLs) within the intima. Oxidation of LDLs is followed by foam cell generation, a powerful stimulus initiating the inflammatory cascade. Smooth muscle cells (SMCs) are induced to migrate and proliferate into the intima, forming the major component of the atherosclerotic plaque. Arterial calcification is commonly associated with vascular disease, and currently the coronary calcification score is used to define the burden of atherosclerotic disease.
However, it is not clear whether SMCs are the only cells involved in the mineralization of the intima. Several studies have pointed out the presence of circulating osteogenic progenitors able to migrate into areas of vascular injury [
2], while others reported the presence of resident mesengenic progenitors in the adult arterial wall [
3,
4]. One additional emerging mechanism is the occurrence of endothelial to mesenchymal transition (End–MT) [
5]. In fact, ECs are highly plastic cells, able to de-differentiate into a multipotent mesenchymal progenitor, and End–MT is an essential step in cardiogenesis and vasculogenesis during the embryonic development [
1,
6]. In this process, ECs undergo molecular rearrangements, by losing their cobblestone morphology and tight junctions, and acquiring mesenchymal features, including elongated shape, migration, matrix remodeling abilities, as well as cytoskeletal alterations [
5]. These structural and functional changes are accompanied by the downregulation of typical endothelial markers, i.e., CD-31, vascular endothelial cadherin (VE-cadherin) and the acquisition of mesenchymal markers, like fibroblast-specific protein (FSP) and α-smooth muscle actin (α-SMA). End–MT can be triggered by different factors, like the members of the transforming growth factor (TGF)-β family, which includes growth factors, bone morphogenetic proteins (BMP) and activins [
7]. The binding between TGF-β members and relative receptors activates the receptor-regulated SMADs, which translocate to the nucleus and regulate downstream gene expression [
8]. The occurrence of End–MT has already been described in systemic and organ-specific fibrotic diseases [
9], pulmonary hypertension [
10] and atherosclerosis [
11], supporting the association between endothelial plasticity and vascular injury due to inflammation or surgical procedures. In atherosclerosis, End–MT can be one of the sources of mesenchymal progenitors, which critically drive the pathological remodeling of the vascular wall. In this regard, we previously demonstrated that MSCs derived from normal and atherosclerotic aortas have increased osteogenic potential when exposed to inflammatory conditions [
12], hypothesizing a further mechanism contributing to plaque calcification. End–MT is modulated by different signaling pathways, transcription factors and microenvironment cues. Micro-RNAs (miRNAs) are small non-coding RNAs (18–24 nucleotides) that act as endogenous regulators of gene expression by binding to the 3′untranslated region (UTR) of the mRNA target. miRNAs can control different biological processes, like cell proliferation, differentiation and migration. It has been recently shown that miRNAs influence the endothelial cell plasticity by targeting transcripts involved in End–MT [
13]. We recently discovered a differential deregulated miR-30a-5p and miR-30d signature in carotid artery plaques that was associated with distinct histological calcification patterns [
14]. Interestingly, by using miRNA prediction target tools, we found End–MT mediators (VIMENTIN, Snail family transcriptional repressor-1 (SNAI1)) and regulators of the osteogenic and chondrogenic commitment (Runt-related transcription factor, RUNX-2; SRY box transcription factor, SOX-9) among miR-30a-5p and miR-30d predicted targets [
15]. Further, miR-30a-5p was reported to downregulate epithelial–mesenchymal transition (EMT) [
16], a process occurring during morphogenesis and oncogenesis in epithelial cell lines; this process, correlating with cancer progression and metastasis, is analogous to End–MT. Due to their regulatory role on different processes with relevance to the cardiovascular system, miRNAs have been proposed as potential biomarkers for staging cardiovascular diseases, for patient stratification, or as therapeutic targets. In this regard, literature studies performed in animal models showed that miR-21 inhibition was associated with reduced in-stent restenosis [
17], whereas miR-29b inhibition reduced the growth of abdominal aortic aneurysm [
18]. However, the introduction of miRNAs in the clinical practice is challenging for many technical issues, including the need for standardized protocols for miRNA analysis. Moreover, miRNA-based therapeutic approaches require an accurate evaluation of miRNA signaling mechanisms; indeed, miRNAs are broadly expressed among tissues and their manipulation can lead to side effects [
19]. For this reason, unveiling the biology of miRNAs and their role in disease models would be crucial to their utilization for diagnostic and therapeutic purposes in the field of cardiovascular diseases.
In order to investigate the link between End–MT and plaque calcification, we firstly established an in vitro model of End–MT driven by inflammatory soluble factors: in addition to the well-known TGF-β1, we included TGF-β3, belonging to the same family, and Tumor Necrosis Factor-α (TNF-α), as an inflammatory cytokine mimicking the atherosclerotic milieu. Then, we explored the miRNA biology during End–MT and osteogenic differentiation, by inducing the ectopic expression of miR-30a-5p and miR-30d in this cell model.
2. Materials and Methods
2.1. Cells and Culture Conditions
Human Umbilical Vein Endothelial Cells (HUVEC; Lonza, Basel, Switzerland), were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich, St Louis, MO, USA) enriched with 10% Fetal Bovine Serum (FBS, Sigma-Aldrich, St Louis, MO, USA) and 1% penicillin/streptomycin (Sigma-Aldrich, St Louis, MO, USA), in a humidified incubator (5% CO2, 37 °C). To induce End–MT, HUVEC were treated with 10 ng/mL TGF-β1 (Peprotech, Rocky Hill, NJ, USA), TGF-β3 (Peprotech, Rocky Hill, NJ, USA) and TNF-α (Sigma-Aldrich, St Louis, MO, USA) separately, in DMEM without serum, for 24 h and 6 days.
2.2. Cell Viability Assay
The effect of miRNA transfection on cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Vybrant MTT Cell Proliferation Assay Kit, Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. To this aim, cells were seeded in 96-well plates at 104 cells/well in 100 μL growth medium. The MTT assay was performed on HUVECs after 24 h and 6 days exposure to TGF-β1, TGF-β3 and TNF-α, and after 24 and 48 h from miRNA over-expression. Briefly, cell medium was replaced with fresh growth medium after treatment, and 10 μL of 12 mM MTT component A (3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide) were added to each well and left in an incubator for 4 h. Then, 100 μL of MTT component B sodium dodecyl sulfate (SDS)-hydrochloride acid (HCl) were added for a further 18 h at 37 °C. Absorbance was read at optical density (O.D.) 570 nm by a micro- plate reader.
2.3. Flow Cytometry
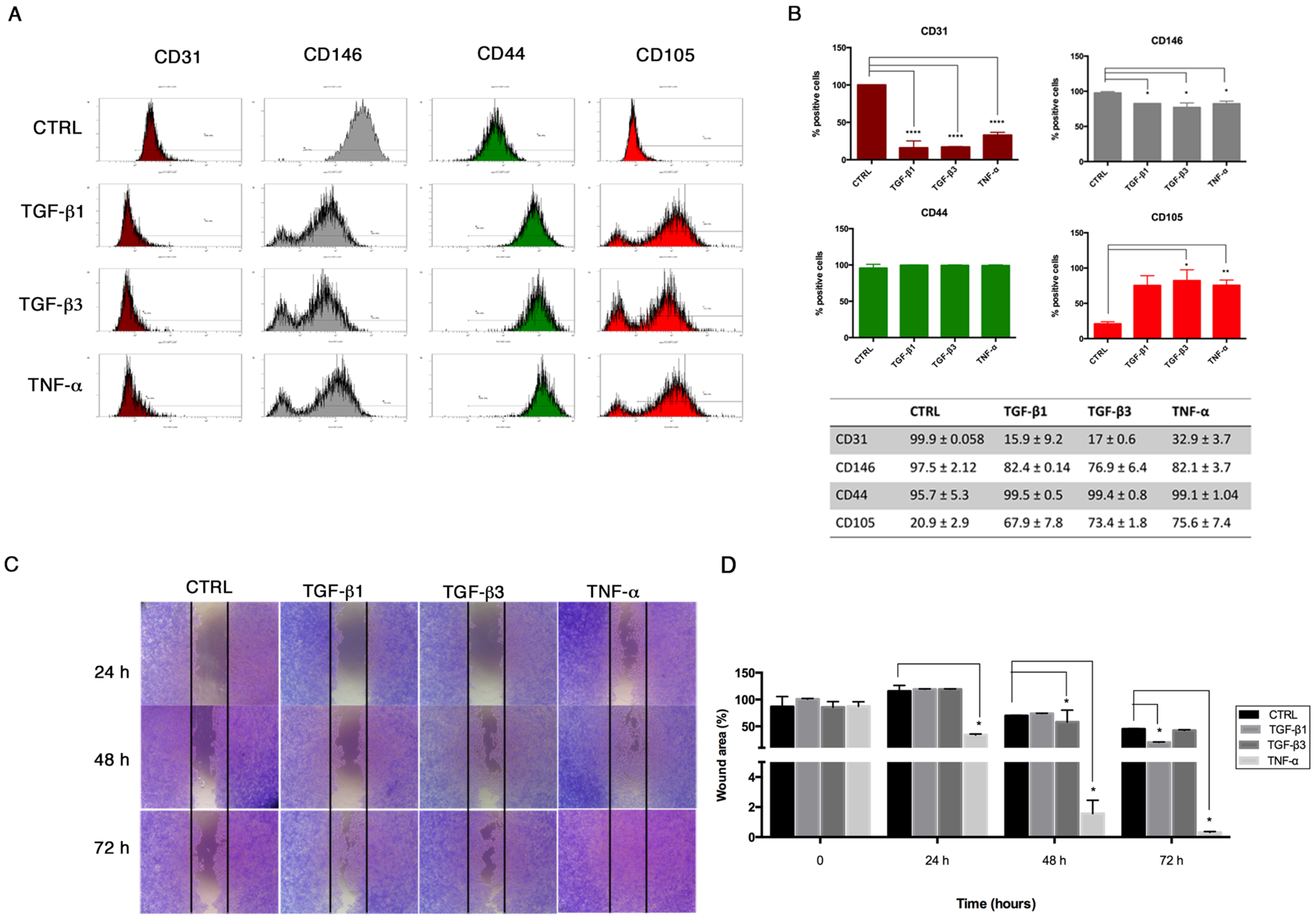
Flow cytometry was performed on HUVECs to investigate the expression of endothelial (CD31, CD146) and mesenchymal (CD44, CD105) surface markers after End-MT for 6 days. Briefly, cells were fixed by using a Fixation Kit (Beckman-Coulter, Brea, CA, USA) and stored at 4 °C. Fixed cells were incubated for 20 min using the following conjugated panel of antibodies: anti-CD31-phycoerythrin (PE) (Clone L 133.1, Beckton Dikinson BD, Franklin Lakes, NJ, USA), anti-CD146-PE (Clone s-Endo1, Biocytex), anti-CD105-phycoerythrin (PE) (Clone TEA3/17.1.1, Beckman-Coulter, Brea, CA, USA) and anti-CD44-fluorescein isothiocyanate (FITC) (Clone J.173, Beckman-Coulter, Brea, CA, USA). Negative controls were performed using appropriate conjugated irrelevant antibodies (IgG1 (Mouse)-FITC; IgG1 (Mouse)-PE, Beckman-Coulter, Brea, CA, USA). Samples were analyzed using a Navios flow cytometer equipped with 3 lasers (10-Color) for data acquisition.
2.4. Cell Transfection
HUVECs were seeded at a density of 1.5 × 105 and 1 × 105 in 12- and 24-well plates respectively, and left to adhere overnight. Transfection was performed with mirVana mimics (Thermo Fisher Scientific, Waltham, MA, USA) and Lipofectamine RNAiMAX (Thermo Fisher Scientific, Waltham, MA, USA) (1:1 ratio), according to the manufacturer’s instructions. Mimics used were: miR-30a-5p-mimic (hsa-miR-30a-5p, assay ID: MC11062, mature miRNA sequence: UGUAAACAUCCUCGACUGGAAG), miR-30d-mimic (hsa-miR-30d, assay ID: MC10756, mature miRNA sequence: UGUAAACAUCCCCGACUGGAAG) and mimic negative control (miR-NC) (10 pmol/μL). After transfection, HUVECs were processed for functional assays and RNA/protein extraction.
2.5. In Vitro Migration Assay
In order to evaluate the migration property of HUVECs following End–MT induction and miRNA transfection, we performed a scratch assay. Briefly, 1 × 10
5 cells were seeded in a 24-well plate. For the End–MT study, the cell monolayer was wounded with a sterile p200 pipette tip, washed with PBS, treated with TGF-β1, TGF-β3 and TNF-α (10 ng/mL) in serum-free DMEM and incubated for additional 24–48–72 h. Cells were fixed with formalin at room temperature (rt), washed with Phosphate Buffer Saline (PBS), stained with 0.1% Crystal Violet in 25% methanol for 25 min, and air-dried. The migration property was analyzed under the phase-contrast light microscope and images were taken with a digital camera (Nikon, Tokyo, Japan). The quantification of the wounded area was performed on three fields of each treatment group and experimental time point. The wounded area was measured using the image analysis software ImageJ [
20], and expressed as percentage relative to the control group at time 0.
2.6. Osteogenic Differentiation Assay
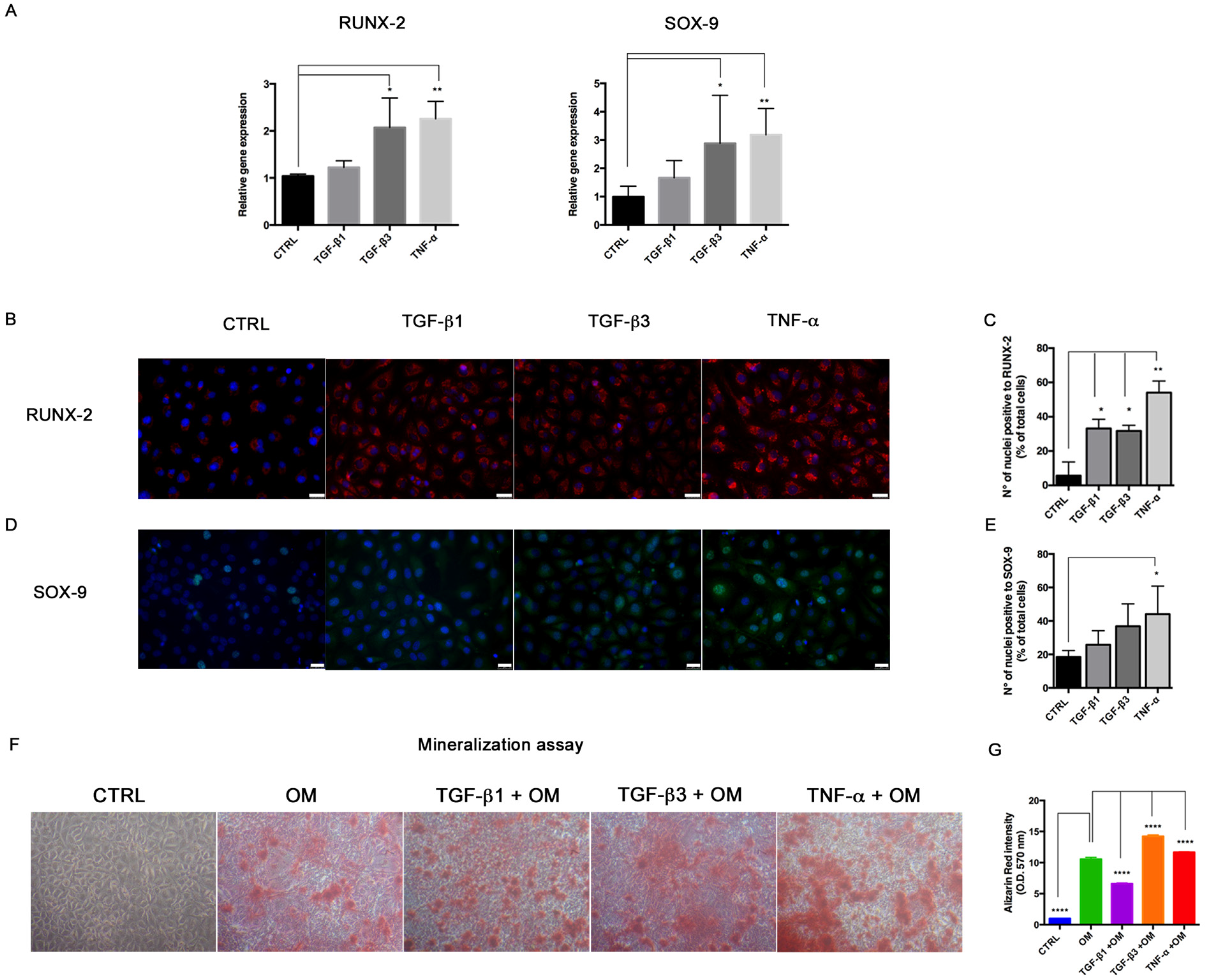
In order to test the effects of miRNA overexpression on the osteogenic differentiation potential, HUVECs were seeded at a density of 4 × 104 cells/well on 24-well plates and left to adhere. After miRNA transfection, Optimem medium was changed with the Stem Pro Osteogenic Differentiation Kit (Thermo Fisher Scientific, Waltham, MA, USA). The miRNA transfection was repeated every three days to keep miRNA levels in cell culture. HUVECs cultured in DMEM with 10% FBS were used as controls. After 14 days, HUVECs were processed for Alizarin Red staining to detect the mineralization process, immunofluorescence, RNA and protein extraction to investigate osteogenic marker expression. For mineralization activity, cells were fixed with formalin, washed twice with PBS and stained with Alizarin Red for 30 min at rt. Images were taken under a phase-contrast light microscope and pictures were taken with a digital camera (Nikon, Tokyo, Japan). Then, Alizarin Red was dissolved by adding 10% cetylpiridinium chloride (in Na2HPO4 10 mM, pH 7) to wells for dissolving Alizarin Red, for 15 min in the dark at rt. Calcium-bound Alizarin Red was measured by reading absorbance O.D. 570 nm by spectrophotometer.
2.7. RNA Extraction and Quantitative Real-Time Analysis
Total RNA was extracted from cell cultures through TRIreagent (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. Reverse Transcription was performed from one µg of total RNA in 20 µL reaction volume using the High-Capacity Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA). Real-Time PCR was carried out in a CFX Connect Real-Time PCR Detection System (BioRad Laboratories, Hercules, CA, USA) using the SYBR green mix (BioRad Laboratories) and specific couples of primers were designed using the Basic Local Alignment Tool (BLAST) from National Center for Biotechnology Information (NCBI) (purchased from Sigma-Aldrich, St Louis, MO, USA;
Table 1). Each assay was performed in triplicate and target gene expression was normalized to glyceraldehyde 3-phospate dehydrogenase (GAPDH). Final results were determined by the comparative 2
^−ΔΔCt method and expressed as fold changes relative to untreated controls, and to control without lipofectamine in transfection experiments.
2.8. MicroRNA Expression Analysis
For the analysis of miRNA expression levels during End-MT, osteogenic differentiation and for the validation of miR over-expression, cDNA reverse-transcription was performed with miRNA-specific primers in the same reaction using TaqMan microRNA Assays and TaqMan microRNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer’s instructions. miRNA expression was performed through Real Time PCR, using TaqMan MicroRNA Assay-specific probes for each target and TaqMan Universal Master Mix II No AmpErase UNG. The reaction was carried out in CFX Connect Real-Time PCR (BioRad Laboratories, CA, USA). Each assay was executed in triplicate and target miRNA expression was normalized to non-coding small nuclear (snRNA) U6 gene. Final results were determined by the comparative 2^−ΔΔCt method and expressed as fold changes relative to controls.
2.9. Luciferase Activity Assay
The human 3′untranslated (UTR) portion of the SLUG gene was amplified in pGEM-T Easy Vector (Promega Corporation, Madison, WI, USA), with the following primer sequences: 3′UTR FWD: GAATCTCGAGGCTGTGTAGC; 3′UTR REV: ATCTAGATGGTCAGCACAGGAG.
For the luciferase assay, HUVECs were plated at a density of 1 × 105 in 96-well plates and, after 24 h, co-transfected with pmiR-GLO SLUG 3′-UTR and 10 pmol of miR-30a-5p mimics, or miR-30d mimic, or miR-negative control. Transfection was assessed with Lipofectamine RNAiMAX (Thermo Fisher Scientific, Waltham, MA, USA). After 24 h, the Firefly and Renilla luciferase activity were measured though the Dual-Glo Luciferase Assay System (Promega Corporation, Madison, WI, USA), according to the manufacturer’s instructions.
2.10. Immunofluorescence
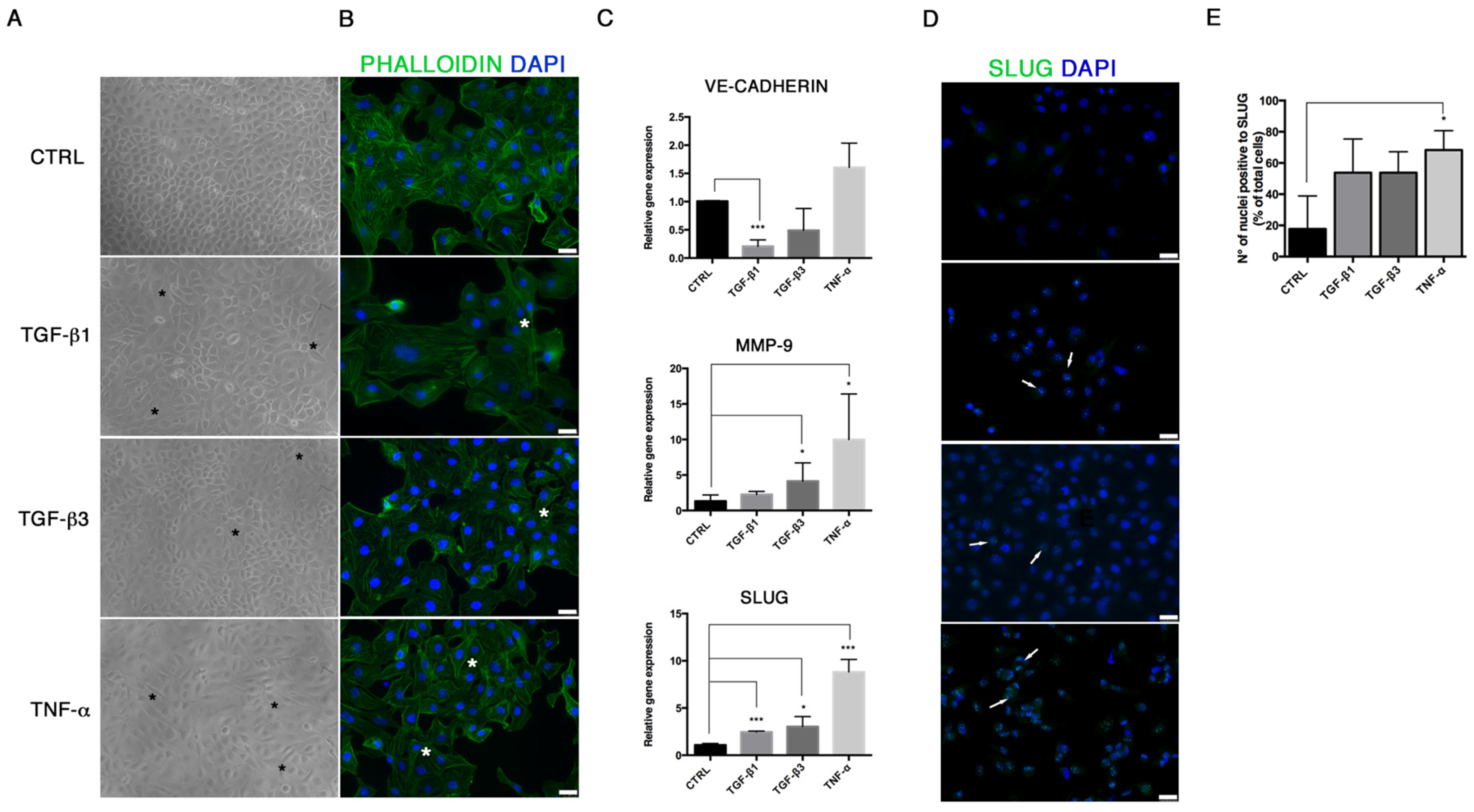
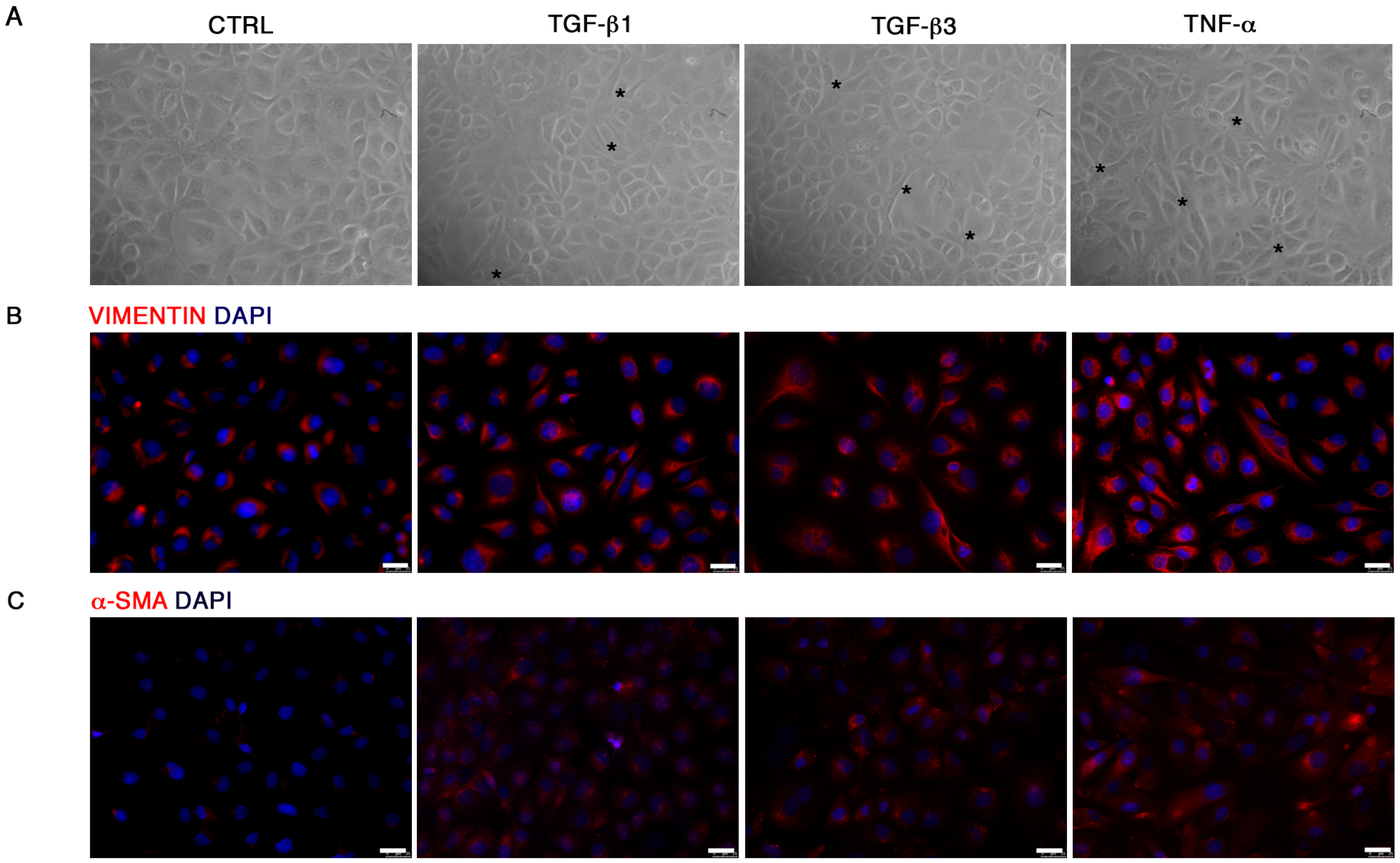
For immunofluorescence, cells were fixed with cold absolute methanol for 10 min at rt, or 2% paraformaldehyde for 4 min at rt for the analysis of filamentous (F)-actin. Then, cells were permeabilized with Triton X-100 1% in PBS for an additional 10 min at rt. The blocking of non-specific binding sites was performed with bovine serum albumin (BSA) 1% in PBS for 30 min at rt. After blocking, cells were incubated with primary antibodies. For staining of F-actin, Alexa fluor 488 Phalloidin was added (1:500, Thermo Fisher Scientific, Waltham, MA, USA) for 20 min at rt, washed with PBS and counterstained with Pro Long anti-fade reagent with 4′-6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific, Waltham, MA, USA). For VIMENTIN (1:500; Cell Signaling), SLUG (1:100, A7, Santa Cruz Biotechnology, Dallas, TX, USA), RUNX-2 (1:100; NBP1-77461SS, Novus Biologicals, Littleton, CO, USA), SOX-9 (1:500, Abcam, Cambridge, UK) and α-SMA (1:100, Sigma-Aldrich, St Louis, MO, USA) detection, the primary antibody was incubated for 1 h at 37 °C. Samples were then washed with PBS and incubated with anti-mouse Alexa Fluor 488 and anti-rabbit Alexa Fluor 546 (Thermo Fisher Scientific, Waltham, MA, USA) secondary antibodies in 1% BSA/PBS for 1 h at 37 °C in the dark. After washing with PBS, nuclei were counterstained with DAPI. Images were acquired by a Leica DMI4000 B inverted fluorescence microscope (Leica Microsystems, Wetzlar, Germany). Quantification of SLUG, RUNX-2 and SOX-9 positive cells was performed on digitalized images randomly acquired at 40× magnification, and a minimum of 5 fields were examined for each sample. Results were expressed as percentage of nuclei positive to the target protein and expressed as percentage of positive cells/total cells.
2.11. Western Blot
Total cellular proteins were extracted by HUVEC using lysis buffer (0.1 M KH2PO4, pH 7.5, 1% NP-40, 0.1 mM β-glycerolphosphate, supplemented with protease inhibitor cocktail; Sigma-Aldrich, St Louis, MO, USA) and quantified spectrophometrically by the Bio-Rad Protein Assay (BioRad Laboratories, CA, USA). Thirty micrograms of proteins were separated on 12% TGX FastCast Acrylamide Solutions (BioRad Laboratories, CA, USA). Gels were imaged and the ratio of SLUG and VIMENTIN to the total protein concentration in miRNA transfection assays was measured by using Image Lab Software (BioRad Laboratories, CA, USA). Then, proteins were transferred to nitrocellulose membrane (GE Healthcare Life Sciences, Chicago, IL, USA), blocked with 5% non-fat dry milk in TBS-Tween for 1 h at rt and incubated with the following primary antibodies: VIMENTIN (1:1000; D21H3, Cell Signaling Technology, Danvers, MA, USA), SLUG (1:1000; A7, Santa Cruz Biotechnology, Dallas, TX, USA) and β-actin (1:4000; AC-74, Sigma-Aldrich, St. Louis, MO, USA) at 4 °C over night (o/n). Incubation with secondary antibody human anti-rabbit/mouse horseradish peroxidase-conjugated (GE Healthcare, Chicago, IL, USA) was performed for 1 h at rt. The protein signal was detected using Westar ηC chemiluminescent substrate (Cyanagen, Bologna, Italy).
2.12. Immunohistochemistry
SLUG detection by immunohistochemistry was performed on pre-existing tissue blocks obtained from archival tissues of healthy multiorgan donors and patients affected by atherosclerosis aneurysm (APP-13-01, Di.Ce. 3868-2015; St. Orsola-Malpighi Ethic Committee). All samples were anonymous and treated according to the ethical guidelines of the 1975 Declaration of Helsinki and following revisions. Immunohistochemistry was assessed using a non-biotin-amplified method (Novolink, Leica Biosystems, Wetzlar, Germany). Briefly, sections (4 μm thick) of formalin-fixed and paraffin-embedded tissues were deparaffinized and rehydrated through a series of graded ethanol and rinsed in distilled water. Endogenous peroxidase activity was blocked in 3% H2O2 in absolute methanol for 10 min at rt, antigen retrieval was performed using citrate buffer (pH 6) in autoclave (120 °C) for 20 min and, after cooling, slides were washed with Tris Buffered Saline (TBS). Sections were subsequently incubated with SLUG primary antibody (1:500; Santa Cruz Biotechnology, Dallas, TX, USA) in a moist chamber at 4 °C o/n, incubated with NovoLink Polymer for 30 min at rt and then exposed to the substrate/chromogen 3,3′-diaminobenzidine (DAB) prepared from Novocastra DAB Chromogen and NovoLink DAB buffer. Nuclei were counterstained with Mayer’s hematoxylin. Samples were dehydrated, cover slipped and observed under a light microscope using the Image Pro Plus program. Quantification of SLUG-positive cells was performed on digitalized images randomly acquired at 25× magnification, and a minimum of 5 histological sections was examined for each sample, by using the Image Pro Plus measurement tool. Results were expressed as mean of SLUG-positive areas.
2.13. Statistical Analysis
Each experiment was executed at least in triplicate, and all data were expressed as mean ± standard deviation (SD). The graphs and the statistical analyses were performed by GraphPad Prism 6 statistical software. The significance of differences between two experimental conditions was evaluated using the unpaired Student’s t test, whereas ordinary one-way analysis of variance (ANOVAs) followed by Bonferroni and Dunnett’s test was applied for multiple comparisons. Results were considered statistically significant at the 95% confidence level (p < 0.05).
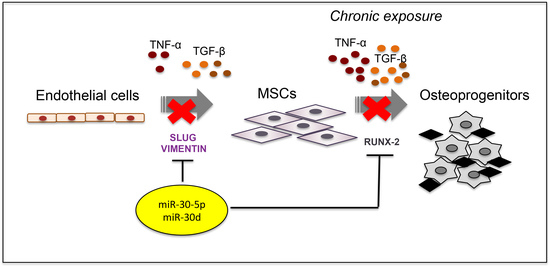
4. Discussion
In the present study, we investigated the involvement of miR-30a-5p and miR-30d during End–MT and vascular calcification. To this aim, we firstly established a cell model for studying End–MT and its potential association with the in vitro osteogenic process. We therefore explored the endothelial cell plasticity and phenotype transition in the presence of growth factors and cytokines mimicking the inflammatory microenvironment expected to be present during the development of the atheromatous plaque. During End–MT, endothelial cells lose their typical cobblestone shape and markers, switching to a MSC phenotype, including markers, cytoskeleton rearrangements and properties, i.e., migration, multilineage differentiation. End–MT occurs under vascular injury, representing an intermediate step in atherosclerosis and calcification [
21]. We demonstrated that TGF-β1, TGF-β3 and TNF-α reproduce the inflammatory setting in vitro and induce End–MT in HUVECs, which acquire morphology (evidenced by F-actin staining), markers (MMP-9, SLUG, VIMENTIN, CD105) and motility, typical of MSCs. In parallel, we detected the loss of endothelial markers in all experimental conditions (CD31, CD146). VE-CADHERIN expression resulted affected only in TGF-β1 and TGF-β3, whereas it was increased in cells treated with TNF-α, indicating a possible compensatory response to keep the endothelial barrier functionality.
Interestingly, we also detected the upregulation of the transcriptional factors RUNX-2 and SOX-9, commonly associated with the osteo/chondrogenic lineage. We further demonstrated that the combination of TGF-β3 and TNF-α with osteogenic induction medium significantly increased the mineralization activity in HUVECs. These results suggest the ability of transitional endothelial cells to become a significant contributor of specialized cells able to release pro-calcific matrix. Since vascular calcification is currently considered a stem cell-driven process, endothelial cells seem to acquire a novel and dynamic role during vascular remodeling and disease progression. Thus, End–MT represents a novel and promising target for preventing the pathological bone formation. Comparing all data, we noticed that TNF-α and TGF-β3 were more effective than TGF-β1 at triggering endothelial cell plasticity versus a mesenchymal/osteogenic phenotype. These data confirm the pivotal role of inflammation to the vascular remodeling and calcification process, and also suggest the contribution of the TGF-β3 signaling to the atherosclerosis complications.
Once established this in vitro model of End–MT, we investigated the role of miR-30a-5p and miR-30d as possible modulators of endothelial cell behavior. Indeed, miRNAs recently emerged as novel and intriguing regulators of many biological processes, including End–MT, through their ability to target genes that are critical to endothelial cell trans-differentiation [
22]. As a first step, we analyzed the expression levels of miR-30a-5p and miR-30d, members of the miR-30 family that controls the development and biology of bone, adipose tissue and blood vessels [
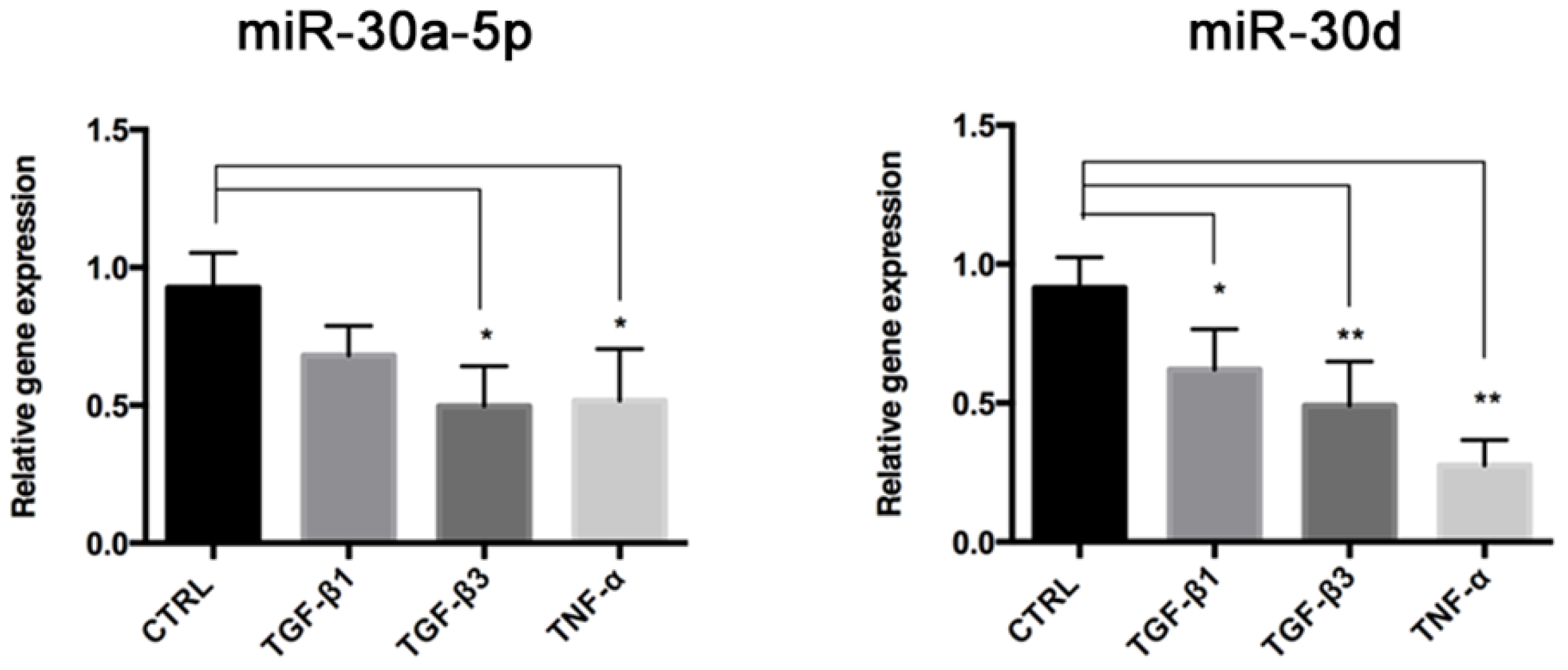
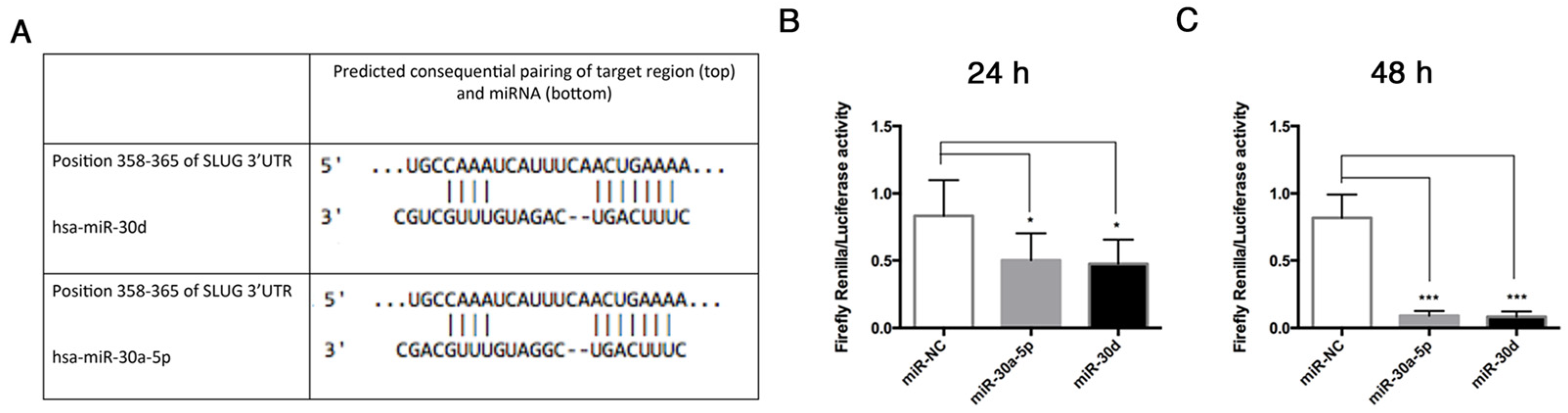
23]. We found that these miRNAs were both downregulated in End–MT, especially in TGF-β3 and TNF-α conditions. This result strengthens the hypothesis on a direct link between the osteo/chondrogenic commitment, inflammation and End–MT. The analysis performed through TargetScan identified that VIMENTIN and SNAI1 are predicted targets of miR-30a-5p and miR-30d [
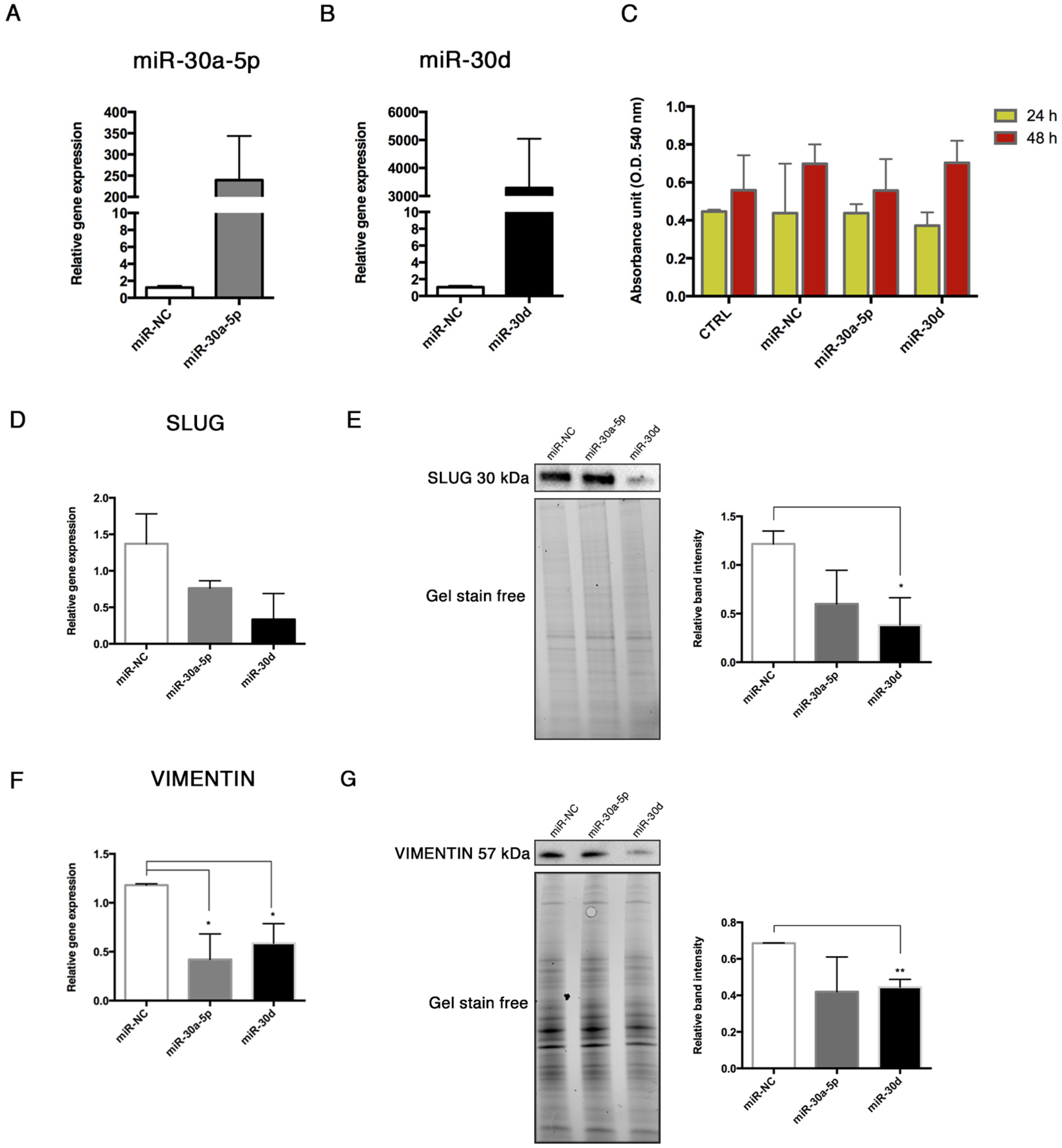
15], thus we explored the interactions between these miRNAs and some End–MT mediators by performing gain-of-function experiments in HUVECs. The over-expression of miR-30a-5p and miR-30d in HUVECs was associated with the downregulation of SLUG, notably with miR-30d. Interestingly, we observed a significant reduction of the relative luciferase activity in HUVECs co-transfected with the pmiR-Glo 3′UTR SLUG and miR-30a-5p, and miR-30, after 24 and 48 h. These data demonstrated that a direct interaction exists between SLUG and both miRNAs, therefore SLUG is a direct target of miR-30a-5p and miR-30d. We also observed a significant decrease of VIMENTIN following miRNA over-expression, and this result was in accordance with literature studies that have already investigated that VIMENTIN is a direct target of miR-30a-5p [
24,
25]. These data imply that miR-30a-5p and miR-30d may target the pathological differentiation of endothelial cells. The role of the miR-30 family in End–MT has been poorly investigated. More data are available with regard to EMT. Chung et al. demonstrated the inhibitory role of miR-30a-5p on EMT by increasing the tight-junction claudin-5 in human upper tract urothelial carcinoma cells [
16]. Further, miR-30a-5p targets the ROR1, the orphan like receptor tyrosine kinase (RTK), reducing EMT and metastasis formation in triple-negative breast cancer [
26]. However, data on endothelial cells are few. Jiang et al. observed that miR-30a stimulates arteriolar branching [
27], whereas another work on miR-30b showed its inhibitory role on capillary morphogenesis acting on TGF-β2 signaling [
28]. Thus, members of the same miRNA family may exploit distinct functions.
Considering that End–MT is strongly connected with the osteogenic differentiation process and atherosclerotic calcification, we further explored this issue. The members of the miR-30 group can regulate the osteogenic process, indeed their over-expression regulated the BMP-2-induced osteogenic process by targeting RUNX-2 and Smad1 in mouse osteoblasts and bone marrow mesenchymal stem cells [
29]. Moreover, it was shown that Bone Morphogenetic Protein 2 (BMP-2) was able to downregulate miR-30b and miR-30c, consequently increasing RUNX-2 expression in a model of human coronary artery SMCs [
30]. A recent work also demonstrated that miR-30b is able to reduce vascular calcification in vivo [
31]. Another study demonstrated that miR-30a-5p levels were decreased in serum of atherosclerotic patients, and the same research explored the role of miR-30a-5p in a monocyte–endothelial cell co-culture system, showing that miRNA overexpression in the human monocyte cell line THP-1 cells protects endothelial cells from apoptosis [
32]. Our recently published study reported that miR-30a-5p and miR-30d are differentially expressed in carotid plaques exhibiting different calcification patterns. In addition, the expression levels of miR-30a-5p and miR-30d were reduced in endothelial cells after osteogenic differentiation [
14]. These preliminary observations and previous studies, which elucidated the involvement of the miR-30 family in MSC osteogenic differentiation, prompted us to study miR-30a-5p and miR-30d in endothelial cells under osteogenic conditions. To this purpose, we transfected HUVECs with miR-30a-5p-mimic and miR-30d-mimic during osteogenic induction, for 14 days. At the end of treatment, we noticed a significant lowering of the mineralization ability in transfected HUVECs, in accordance with the reduced expression of RUNX-2 and BMP-2 genes.
In this study, we also highlight the relevance of SLUG to atheroma calcification, whose involvement during arterial calcification has not been elucidated yet. Sànchez-Duffhues et al. demonstrated that SLUG is involved in endothelial cell-mediated calcification [
33]. Here, we report that SLUG is highly expressed in human atherosclerosis, both at the aortic and carotid level, as well as in vitro when HUVECs differentiated into osteoprogenitors. These data implicate the involvement of SLUG during the pathological remodeling occurring in atherosclerosis and calcification processes, providing novel cues for investigating the atherosclerotic calcification and the search for new therapeutic approaches.
To the best of our knowledge, this is the first study addressing the role of miR-30a-5p and miR-30d in endothelial cells with respect to the End–MT and calcification process. We showed that End–MT transformation is tightly associated with the vascular bone formation under the inflammatory milieu and represents a potential clinically relevant target. Our results, in accordance with the literature data, support a negative correlation between the investigated miRNA and the atherosclerotic calcification. miRNAs are attractive candidates for promising therapeutic approaches aimed at controlling the disease progression, by regulating the main upstream mechanisms, as well as possible stage-specific markers. The present study can therefore be preliminary to future investigations for elucidating the role of miR-30a-5p and miR-30d, novel signaling pathways connected with atherosclerosis and calcification and the possible delivery approach in the light of their utilization in the clinical practice.