Neuroimmune Response Mediated by Cytokines in Natural Scrapie after Chronic Dexamethasone Treatment
Abstract
1. Introduction
2. Material and Methods
2.1. Immunohistochemical Techniques
2.1.1. IL-1, IL-1R, IL-6 and IFNγR Detection
2.1.2. IL-2R, IL-10R and TNFR Detection
2.2. RT-qPCR
2.2.1. RNA Purification
2.2.2. Retrotranscription
2.2.3. RT-qPCR
2.3. Statistical Analysis
3. Results
3.1. Immunohistochemistry
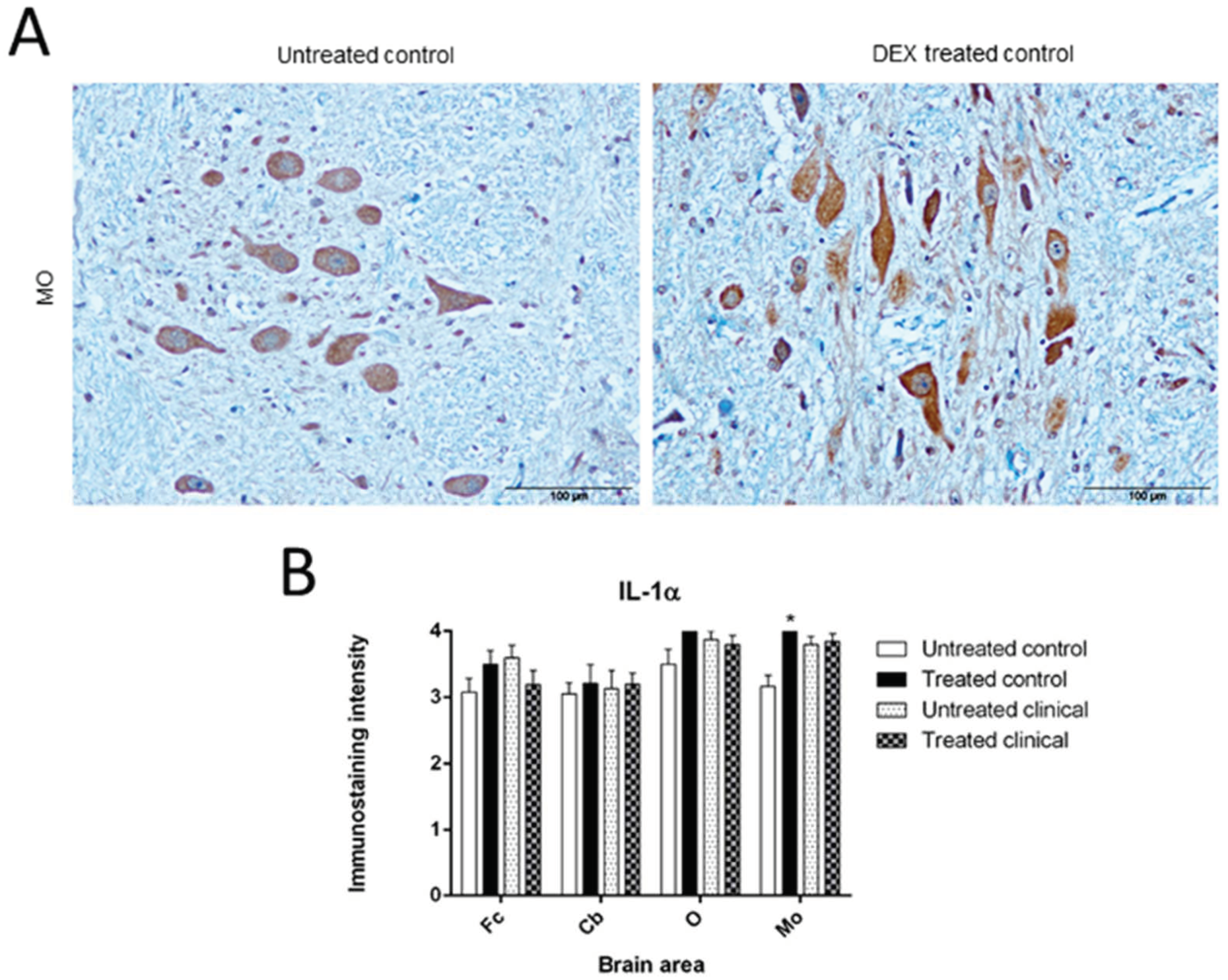
3.1.1. IL-1
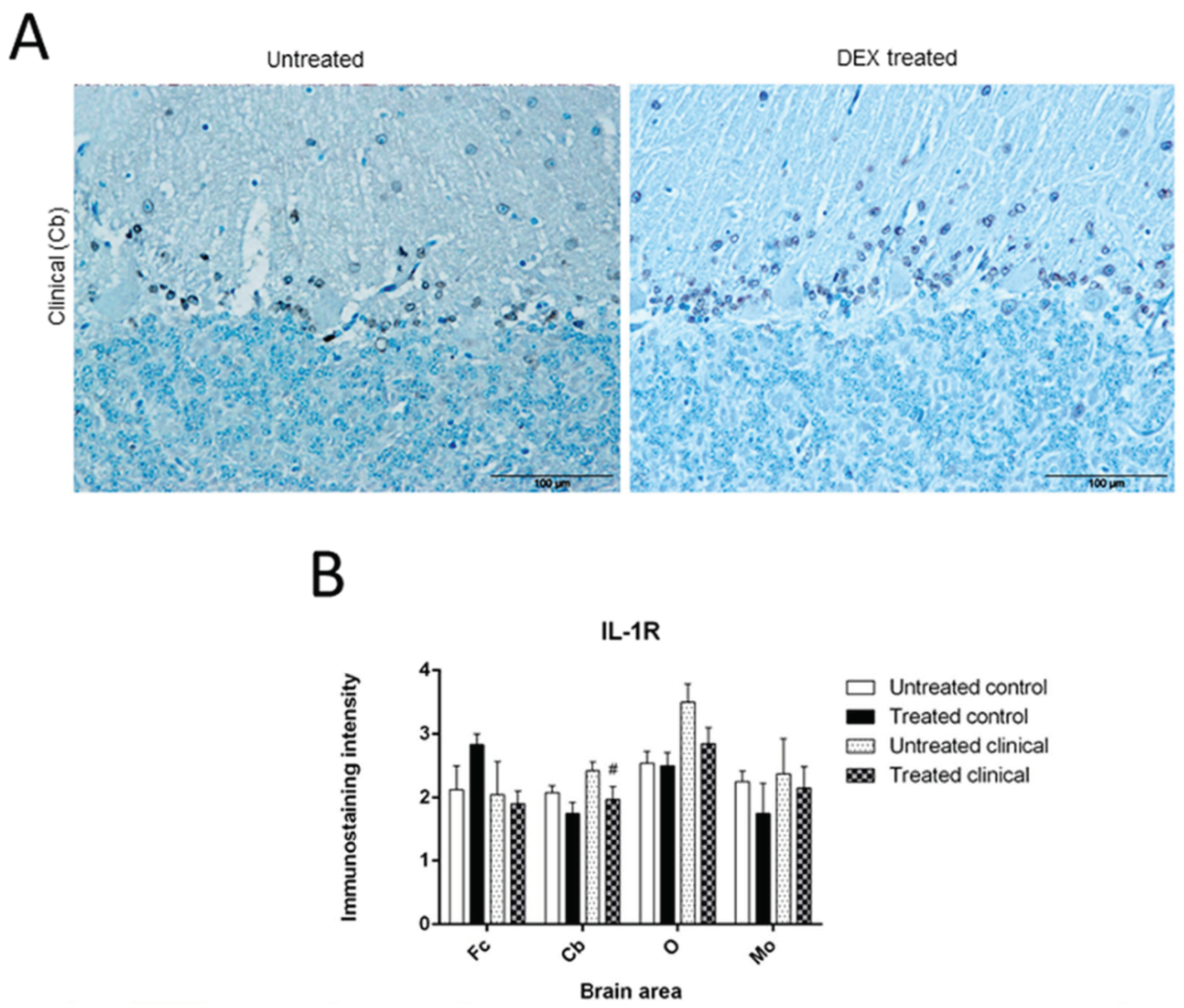
3.1.2. IL-1R
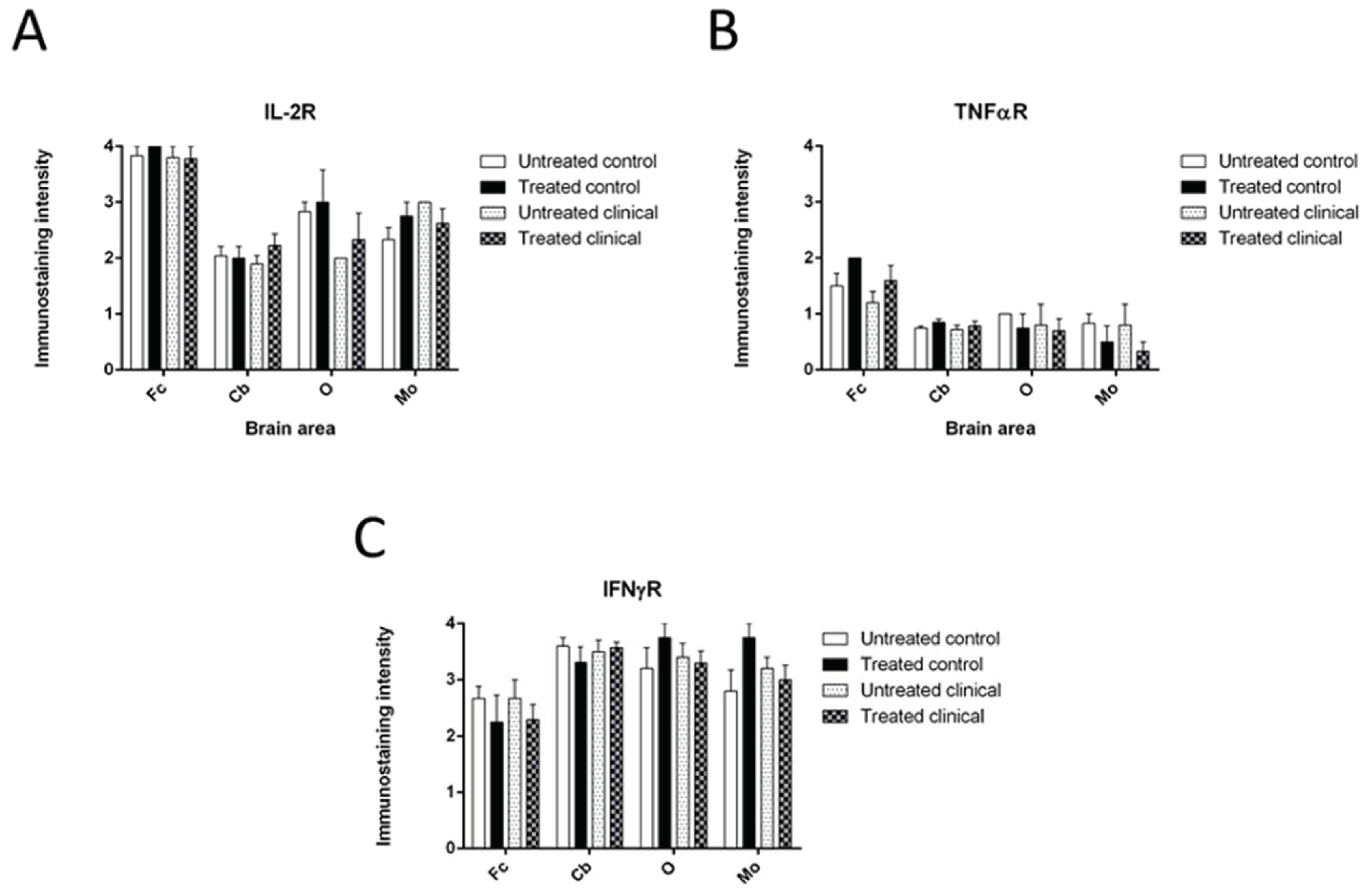
3.1.3. IL-2R
3.1.4. IL-6
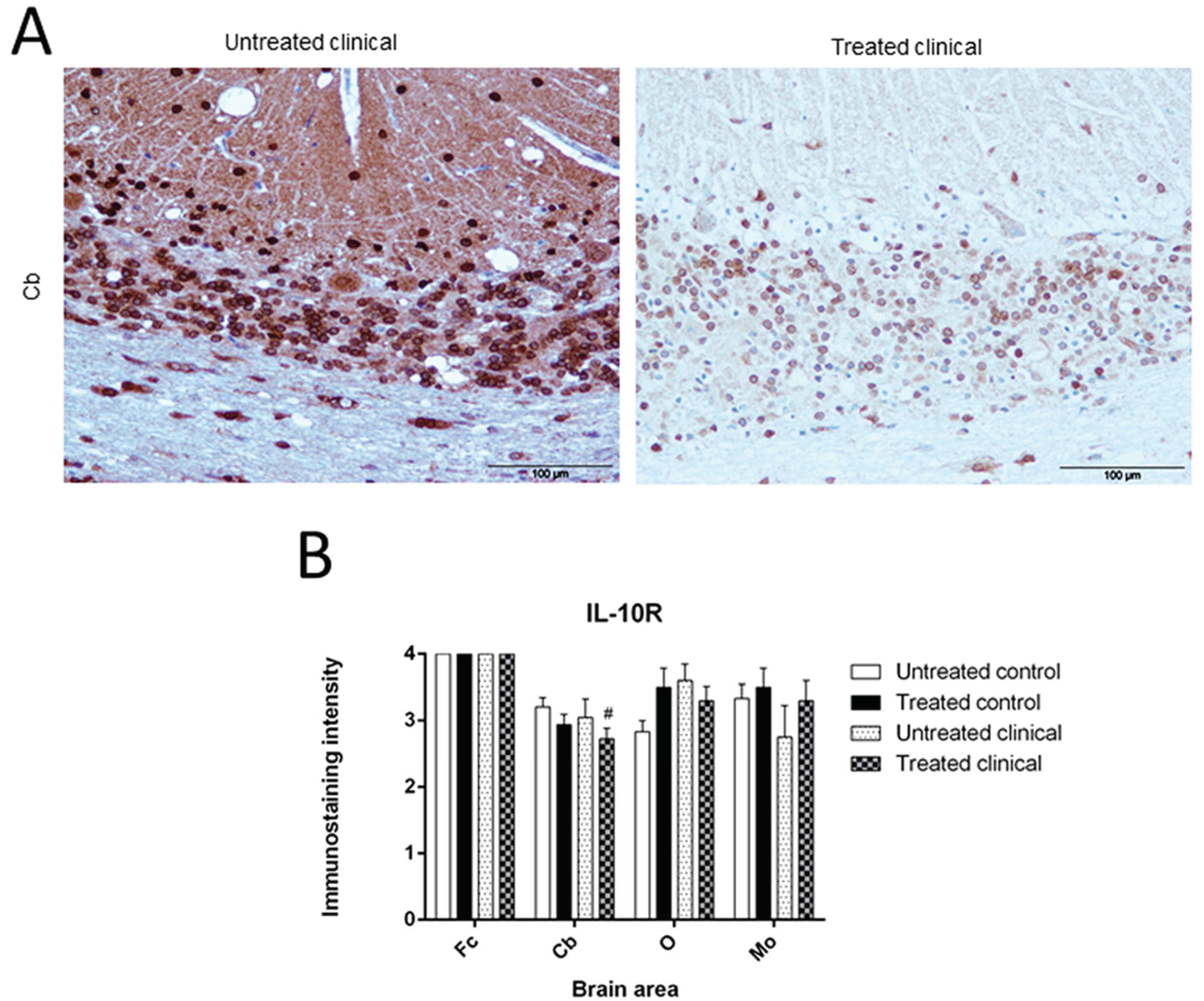
3.1.5. IL-10R
3.1.6. TNFR
3.1.7. IFNγR
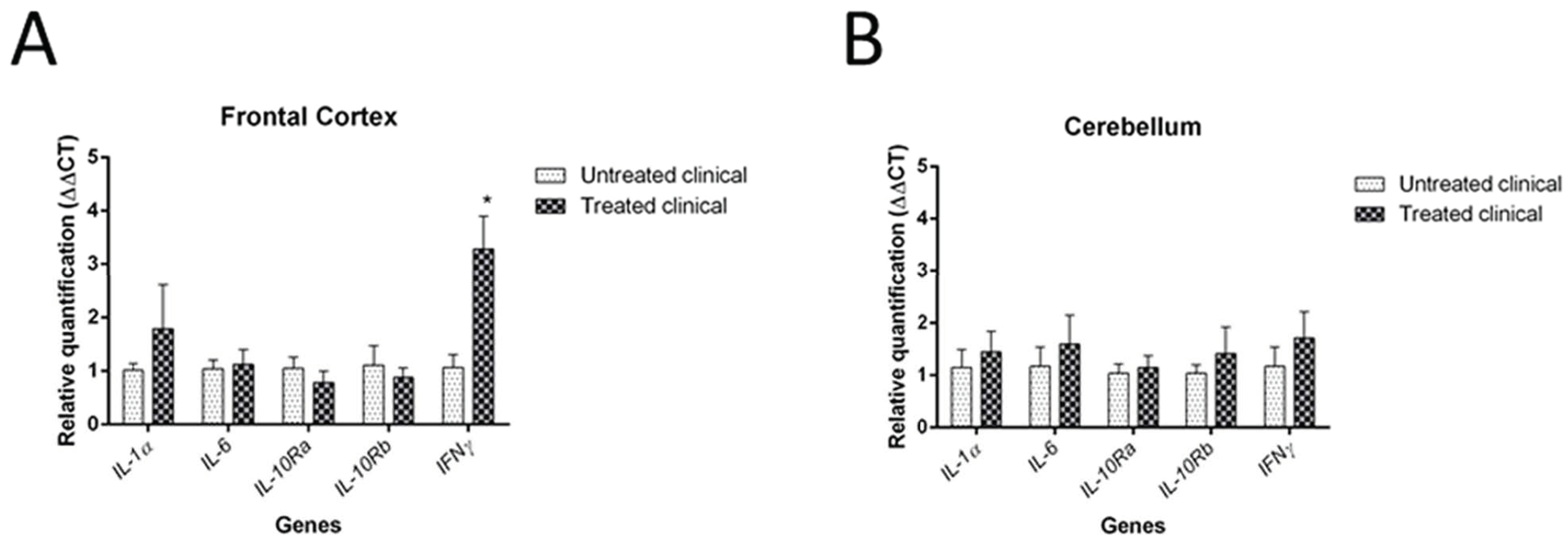
3.2. RT-qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burwinkel, M.; Riemer, C.; Schwarz, A.; Schultz, J.; Neidhold, S.; Bamme, T.; Baier, M. Role of Cytokines and Chemokines in Prion Infections of the Central Nervous System. Int. J. Dev. Neurosci. 2004, 22, 497–505. [Google Scholar] [CrossRef]
- Pasquali, P.; Nonno, R.; Mandara, M.T.; Di Bari, M.A.; Ricci, G.; Petrucci, P.; Capuccini, S.; Cartoni, C.; Macri, A.; Agrimi, U. Intracerebral Administration of Interleukin-12 (IL-12) and IL-18 Modifies the Course of Mouse Scrapie. BMC Vet. Res. 2006, 2, 37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marcos-Carcavilla, A.; Calvo, J.H.; Gonzalez, C.; Moazami-Goudarzi, K.; Laurent, P.; Bertaud, M.; Hayes, H.; Beattie, A.E.; Serrano, C.; Lyahyai, J.; et al. IL-1 Family Members as Candidate Genes Modulating Scrapie Susceptibility in Sheep: Localization, Partial Characterization, and Expression. Mamm. Genome 2007, 18, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Servida, F.; Ravasi, C.; Puricelli, M.; Formentin, E.A.; Dall’Ara, P.; Poli, G. Decrease in Neuroinflammation after Immunisation with Synthetic Prion Peptides in an Animal Model of Scrapie. Vet. Res. Commun. 2007, 31 (Suppl. S1), 265–267. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Deczkowska, A. Neurological Disease as a Failure of Brain-Immune Crosstalk: The Multiple Faces of Neuroinflammation. Trends Immunol. 2016, 37, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Rodriguez, J.J.; Parpura, V. Neurotransmitters and Integration in Neuronal-Astroglial Networks. Neurochem. Res. 2012, 37, 2326–2338. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.E.; Sofroniew, M.V. Reactive Gliosis and the Multicellular Response to CNS Damage and Disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M.; Messing, A.; Steinhauser, C.; Lee, J.M.; Parpura, V.; Hol, E.M.; Sofroniew, M.V.; Verkhratsky, A. Astrocytes: A Central Element in Neurological Diseases. Acta Neuropathol. 2016, 131, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.L.; Eddleston, M.; Kemper, P.; Oldstone, M.B.; Hobbs, M.V. Activation of Cerebral Cytokine Gene Expression and Its Correlation with Onset of Reactive Astrocyte and Acute-Phase Response Gene Expression in Scrapie. J. Virol. 1994, 68, 2383–2387. [Google Scholar] [CrossRef]
- Williams, A.E.; van Dam, A.M.; Man, A.H.W.K.; Berkenbosch, F.; Eikelenboom, P.; Fraser, H. Cytokines, Prostaglandins and Lipocortin-1 Are Present in the Brains of Scrapie-Infected Mice. Brain Res. 1994, 654, 200–206. [Google Scholar] [CrossRef]
- Williams, A.; Van Dam, A.M.; Ritchie, D.; Eikelenboom, P.; Fraser, H. Immunocytochemical Appearance of Cytokines, Prostaglandin E2 and Lipocortin-1 in the CNS during the Incubation Period of Murine Scrapie Correlates with Progressive PrP Accumulations. Brain Res. 1997, 754, 171–180. [Google Scholar] [CrossRef]
- Peyrin, J.M.; Lasmezas, C.I.; Haik, S.; Tagliavini, F.; Salmona, M.; Williams, A.; Richie, D.; Deslys, J.P.; Dormont, D. Microglial Cells Respond to Amyloidogenic PrP Peptide by the Production of Inflammatory Cytokines. NeuroReport 1999, 10, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Veerhuis, R.; Hoozemans, J.J.; Janssen, I.; Boshuizen, R.S.; Langeveld, J.P.; Eikelenboom, P. Adult Human Microglia Secrete Cytokines When Exposed to Neurotoxic Prion Protein Peptide: No Intermediary Role for Prostaglandin E2. Brain Res. 2002, 925, 195–203. [Google Scholar] [CrossRef]
- Brown, A.R.; Webb, J.; Rebus, S.; Walker, R.; Williams, A.; Fazakerley, J.K. Inducible Cytokine Gene Expression in the Brain in the ME7/CV Mouse Model of Scrapie Is Highly Restricted, Is at a Strikingly Low Level Relative to the Degree of Gliosis and Occurs Only Late in Disease. J. Gen. Virol. 2003, 84 Pt 9, 2605–2611. [Google Scholar]
- Kordek, R.; Nerurkar, V.R.; Liberski, P.P.; Isaacson, S.; Yanagihara, R.; Gajdusek, D.C. Heightened Expression of Tumor Necrosis Factor Alpha, Interleukin 1 Alpha, and Glial Fibrillary Acidic Protein in Experimental Creutzfeldt-Jakob Disease in Mice. Proc. Natl. Acad. Sci. USA 1996, 93, 9754–9758. [Google Scholar] [CrossRef]
- Carroll, J.A.; Striebel, J.F.; Race, B.; Phillips, K.; Chesebro, B. Prion Infection of Mouse Brain Reveals Multiple New Upregulated Genes Involved in Neuroinflammation or Signal Transduction. J. Virol. 2015, 89, 2388–2404. [Google Scholar] [CrossRef]
- Liberski, P.P.; Nerurkar, V.R.; Yanagihara, R.; Gajdusek, D.C. Tumor Necrosis Factor Alpha (TNF-Alpha) Is Involved in the Pathogenesis of the Panencephalopathic Type of Creutzfeldt-Jakob Disease. Mol. Chem. Neuropathol. 1995, 24, 223–225. [Google Scholar] [CrossRef]
- Cunningham, C.; Boche, D.; Perry, V.H. Transforming Growth Factor Beta1, the Dominant Cytokine in Murine Prion Disease: Influence on Inflammatory Cytokine Synthesis and Alteration of Vascular Extracellular Matrix. Neuropathol. Appl. Neurobiol. 2002, 28, 107–119. [Google Scholar] [CrossRef]
- Schultz, J.; Schwarz, A.; Neidhold, S.; Burwinkel, M.; Riemer, C.; Simon, D.; Kopf, M.; Otto, M.; Baier, M. Role of Interleukin-1 in Prion Disease-Associated Astrocyte Activation. Am. J. Pathol. 2004, 165, 671–820. [Google Scholar] [CrossRef]
- Thackray, A.M.; McKenzie, A.N.; Klein, M.A.; Lauder, A.; Bujdoso, R. Accelerated Prion Disease in the Absence of Interleukin-10. J. Virol. 2004, 78, 13697–13707. [Google Scholar] [CrossRef]
- Tribouillard-Tanvier, D.; Striebel, J.F.; Peterson, K.E.; Chesebro, B. Analysis of Protein Levels of 24 Cytokines in Scrapie Agent-Infected Brain and Glial Cell Cultures from Mice Differing in Prion Protein Expression Levels. J. Virol. 2009, 83, 11244–11253. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Wilcockson, D.C.; Campion, S.; Lunnon, K.; Perry, V.H. Central and Systemic Endotoxin Challenges Exacerbate the Local Inflammatory Response and Increase Neuronal Death during Chronic Neurodegeneration. J. Neurosci. 2005, 25, 9275–9284. [Google Scholar] [PubMed]
- Hernandez, R.S.; Sarasa, R.; Toledano, A.; Badiola, J.J.; Monzon, M. Morphological Approach to Assess the Involvement of Astrocytes in Prion Propagation. Cell Tissue Res. 2014, 358, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Monzon, M.; Hernandez, R.S.; Garces, M.; Sarasa, R.; Badiola, J.J. Glial Alterations in Human Prion Diseases: A Correlative Study of Astroglia, Reactive Microglia, Protein Deposition, and Neuropathological Lesions. Medicine 2018, 97, e0320. [Google Scholar] [CrossRef]
- Garces, M.; Guijarro, M.I.; Vargas, A.; Badiola, J.J.; Monzon, M. Neuroglial Patterns Are Shared by Cerebella from Prion and Prion-Like Disorder Affected Patients. Mech. Ageing Dev. 2019, 184, 111176. [Google Scholar] [CrossRef]
- Guijarro, I.M.; Garces, M.; Andres-Benito, P.; Marin, B.; Otero, A.; Barrio, T.; Carmona, M.; Ferrer, I.; Badiola, J.J.; Monzon, M. Assessment of Glial Activation Response in the Progress of Natural Scrapie after Chronic Dexamethasone Treatment. Int. J. Mol. Sci. 2020, 21, 3231. [Google Scholar] [CrossRef]
- Morales, I.; Guzman-Martinez, L.; Cerda-Troncoso, C.; Farias, G.A.; Maccioni, R.B. Neuroinflammation in the Pathogenesis of Alzheimer’s Disease. A Rational Framework for the Search of Novel Therapeutic Approaches. Front. Cell. Neurosci. 2014, 8, 112. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil Is in the Details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef]
- Ransohoff, R.M. How Neuroinflammation Contributes to Neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Garwood, C.J.; Pooler, A.M.; Atherton, J.; Hanger, D.P.; Noble, W. Astrocytes Are Important Mediators of Abeta-Induced Neurotoxicity and Tau Phosphorylation in Primary Culture. Cell Death Dis. 2011, 2, e167. [Google Scholar] [CrossRef] [PubMed]
- Romero-Trevejo, J.L.; Gomez-Villamandos, J.C.; Pedrera, M.; Blanco, A.; Bautista, M.J.; Sanchez-Cordon, P.J. Immunohistochemical Study of Macrophage and Cytokine Dynamics in the Gut of Scrapie-Infected Mice. Histol. Histopathol. 2010, 25, 1025–1038. [Google Scholar] [PubMed]
- Lyoo, C.H.; Ikawa, M.; Liow, J.S.; Zoghbi, S.S.; Morse, C.L.; Pike, V.W.; Fujita, M.; Innis, R.B.; Kreisl, W.C. Cerebellum Can Serve as a Pseudo-Reference Region in Alzheimer Disease to Detect Neuroinflammation Measured with PET Radioligand Binding to Translocator Protein. J. Nucl. Med. 2015, 56, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Sarasa, R.; Martinez, A.; Monleon, E.; Bolea, R.; Vargas, A.; Badiola, J.J.; Monzon, M. Involvement of Astrocytes in Transmissible Spongiform Encephalopathies: A Confocal Microscopy Study. Cell Tissue Res. 2012, 350, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, J.E.; Murphy, L.; Grabert, K.; McColl, B.W.; Cancellotti, E.; Freeman, T.C.; Manson, J.C. Defining the Microglia Response during the Time Course of Chronic Neurodegeneration. J. Virol. 2015, 90, 3003–3017. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H. Microglia. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Obst, J.; Simon, E.; Mancuso, R.; Gomez-Nicola, D. The Role of Microglia in Prion Diseases: A Paradigm of Functional Diversity. Front. Aging Neurosci. 2017, 9, 207. [Google Scholar] [CrossRef]
- Walsh, D.T.; Betmouni, S.; Perry, V.H. Absence of Detectable IL-1beta Production in Murine Prion Disease: A Model of Chronic Neurodegeneration. J. Neuropathol. Exp. Neurol. 2001, 60, 173–182. [Google Scholar] [CrossRef]
- Perry, V.H.; Cunningham, C.; Boche, D. Atypical Inflammation in the Central Nervous System in Prion Disease. Curr. Opin. Neurol. 2002, 15, 349–354. [Google Scholar] [CrossRef]
- Boche, D.; Perry, V.H.; Nicoll, J.A. Review: Activation Patterns of Microglia and Their Identification in the Human Brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Rodrigues, J.J.; Pivoriunas, A.; Zorec, R.; Semyanov, A. Astroglial Atrophy in Alzheimer’s Disease. Pflug. Arch. 2019, 471, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Llorens, F.; Lopez-Gonzalez, I.; Thune, K.; Carmona, M.; Zafar, S.; Andreoletti, O.; Zerr, I.; Ferrer, I. Subtype and Regional-Specific Neuroinflammation in Sporadic Creutzfeldt-Jakob Disease. Front. Aging Neurosci. 2014, 6, 198. [Google Scholar] [CrossRef] [PubMed]
- Forster, C.; Waschke, J.; Burek, M.; Leers, J.; Drenckhahn, D. Glucocorticoid Effects on Mouse Microvascular Endothelial Barrier Permeability Are Brain Specific. J. Physiol. 2006, 573 Pt 2, 413–425. [Google Scholar] [CrossRef]
- De Bosscher, K.; Vanden Berghe, W.; Haegeman, G. The Interplay between the Glucocorticoid Receptor and Nuclear Factor-kappaB or Activator Protein-1: Molecular Mechanisms for Gene Repression. Endocr. Rev. 2003, 24, 488–522. [Google Scholar] [CrossRef] [PubMed]
- Munck, A.; Guyre, P.M.; Holbrook, N.J. Physiological Functions of Glucocorticoids in Stress and Their Relation to Pharmacological Actions. Endocr. Rev. 1984, 5, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Guerne, P.A.; Carson, D.A.; Lotz, M. IL-6 production by Human Articular Chondrocytes. Modulation of Its Synthesis by Cytokines, Growth Factors, and Hormones In Vitro. J. Immunol. 1990, 144, 499–505. [Google Scholar] [PubMed]
- Bowers, S.L.; Bilbo, S.D.; Dhabhar, F.S.; Nelson, R.J. Stressor-Specific Alterations in Corticosterone and Immune Responses in Mice. Brain Behav. Immun. 2008, 22, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Caso, J.R.; Munhoz, C.D.; Sapolsky, R.M. The Stressed CNS: When Glucocorticoids Aggravate Inflammation. Neuron 2009, 64, 33–39. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Wu, W.; Yin, Y.; Huang, D.; Wang, Y.; Li, W.; Li, W. Chronic Glucocorticoids Exposure Enhances Neurodegeneration in the Frontal Cortex and Hippocampus via NLRP-1 Inflammasome Activation in Male Mice. Brain Behav. Immun. 2016, 52, 58–70. [Google Scholar] [CrossRef]
- Kirwan, J.R. Glucocorticoid Resistance in Patients with Rheumatoid Arthritis. Scand. J. Rheumatol. 2007, 36, 165–166. [Google Scholar] [CrossRef]
- Mrak, R.E.; Sheng, J.G.; Griffin, W.S. Glial Cytokines in Alzheimer’s Disease: Review and Pathogenic Implications. Hum. Pathol. 1995, 26, 816–823. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s Disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Griffin, W.S.; Mrak, R.E. Interleukin-1 in the Genesis and Progression of and Risk for Development of Neuronal Degeneration in Alzheimer’s Disease. J. Leukoc. Biol. 2002, 72, 233–238. [Google Scholar] [PubMed]
- Sastre, M.; Klockgether, T.; Heneka, M.T. Contribution of Inflammatory Processes to Alzheimer’s Disease: Molecular Mechanisms. Int. J. Dev. Neurosci. 2006, 24, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Serou, M.J.; DeCoster, M.A.; Bazan, N.G. Interleukin-1 Beta Activates Expression of Cyclooxygenase-2 and Inducible Nitric oxide Synthase in Primary Hippocampal Neuronal Culture: Platelet-Activating Factor as a Preferential Mediator of Cyclooxygenase-2 Expression. J. Neurosci. Res. 1999, 58, 593–598. [Google Scholar] [CrossRef]
- Zheng, Y.; Horii, A.; Appleton, I.; Darlington, C.L.; Smith, P.F. Damage to the Vestibular Inner Ear Causes Long-Term Changes in Neuronal Nitric Oxide Synthase Expression in the Rat Hippocampus. Neuroscience 2001, 105, 1–5. [Google Scholar] [CrossRef]
- Dinkel, K.; MacPherson, A.; Sapolsky, R.M. Novel Glucocorticoid Effects on Acute Inflammation in the CNS. J. Neurochem. 2003, 84, 705–716. [Google Scholar] [CrossRef]
- Tamguney, G.; Giles, K.; Glidden, D.V.; Lessard, P.; Wille, H.; Tremblay, P.; Groth, D.F.; Yehiely, F.; Korth, C.; Moore, R.C.; et al. Genes Contributing to Prion Pathogenesis. J. Gen. Virol. 2008, 89 Pt 7, 1777–1788. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Bruttger, J.; Karram, K.; Wortge, S.; Regen, T.; Marini, F.; Hoppmann, N.; Klein, M.; Blank, T.; Yona, S.; Wolf, Y.; et al. Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity 2015, 43, 92–106. [Google Scholar] [CrossRef]
- Ban, E.; Milon, G.; Prudhomme, N.; Fillion, G.; Haour, F. Receptors for Interleukin-1 (Alpha and Beta) in Mouse Brain: Mapping and Neuronal Localization in Hippocampus. Neuroscience 1991, 43, 21–30. [Google Scholar] [PubMed]
- French, R.A.; VanHoy, R.W.; Chizzonite, R.; Zachary, J.F.; Dantzer, R.; Parnet, P.; Bluthe, R.M.; Kelley, K.W. Expression and Localization of p80 and p68 Interleukin-1 Receptor Proteins in the Brain of Adult Mice. J. Neuroimmunol. 1999, 93, 194–202. [Google Scholar] [CrossRef]
- Ravizza, T.; Vezzani, A. Status Epilepticus Induces Time-Dependent Neuronal and Astrocytic Expression of Interleukin-1 Receptor Type I in the Rat Limbic System. Neuroscience 2006, 137, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Spulber, S.; Bartfai, T.; Schultzberg, M. IL-1/IL-1ra Balance in the Brain Revisited—Evidence from Transgenic Mouse Models. Brain Behav. Immun. 2009, 23, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Biologic Basis for Interleukin-1 in Disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef]
- Alves, S.; Churlaud, G.; Audrain, M.; Michaelsen-Preusse, K.; Fol, R.; Souchet, B.; Braudeau, J.; Korte, M.; Klatzmann, D.; Cartier, N. Interleukin-2 Improves Amyloid Pathology, Synaptic Failure and Memory in Alzheimer’s Disease Mice. Brain 2017, 140, 826–842. [Google Scholar] [CrossRef]
- Saadoun, D.; Terrier, B.; Cacoub, P. Interleukin-25: Key Regulator of Inflammatory and Autoimmune Diseases. Curr. Pharm. Des. 2011, 17, 3781–3785. [Google Scholar] [CrossRef]
- Luber-Narod, J.; Rogers, J. Immune System Associated Antigens Expressed by Cells of the Human Central Nervous System. Neurosci. Lett. 1988, 94, 17–22. [Google Scholar] [CrossRef]
- Sarder, M.; Abe, K.; Saito, H.; Nishiyama, N. Comparative Effect of IL-2 and IL-6 on Morphology of Cultured Hippocampal Neurons from Fetal Rat Brain. Brain Res. 1996, 715, 9–16. [Google Scholar] [CrossRef]
- Dansokho, C.; Ahmed, D.A.; Aid, S.; Toly-Ndour, C.; Chaigneau, T.; Calle, V.; Cagnard, N.; Holzenberger, M.; Piaggio, E.; Aucouturier, P.; et al. Regulatory T Cells Delay Disease Progression in Alzheimer-Like Pathology. Brain 2016, 139 Pt 4, 1237–1251. [Google Scholar] [CrossRef]
- Campbell, I.L.; Abraham, C.R.; Masliah, E.; Kemper, P.; Inglis, J.D.; Oldstone, M.B.; Mucke, L. Neurologic Disease Induced in Transgenic Mice by Cerebral Overexpression of Interleukin 6. Proc. Natl. Acad. Sci. USA 1993, 90, 10061–10065. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, F.B.; Brown, D.R. A Model for the Mechanism of Astrogliosis in Prion Disease. Mol. Cell. Neurosci. 2000, 16, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C. Interleukin-6 and Chronic Inflammation. Arthritis Res. Ther. 2006, 8 (Suppl. S2), S3. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Gauldie, J.; Cox, G.; Baumann, H.; Jordana, M.; Lei, X.F.; Achong, M.K. IL-6 Is an Antiinflammatory Cytokine Required for Controlling Local or Systemic Acute Inflammatory Responses. J. Clin. Investig. 1998, 101, 311–320. [Google Scholar] [CrossRef]
- Yasukawa, H.; Ohishi, M.; Mori, H.; Murakami, M.; Chinen, T.; Aki, D.; Hanada, T.; Takeda, K.; Akira, S.; Hoshijima, M.; et al. IL-6 Induces an Anti-Inflammatory Response in the Absence of SOCS3 in Macrophages. Nat. Immunol. 2003, 4, 551–556. [Google Scholar] [CrossRef]
- Tilg, H.; Dinarello, C.A.; Mier, J.W. IL-6 and APPs: Anti-Inflammatory and Immunosuppressive Mediators. Immunol. Today 1997, 18, 428–432. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yoshizaki, K.; Kishimoto, T.; Ito, H. IL-6 Is Required for the Development of Th1 Cell-Mediated Murine Colitis. J. Immunol. 2000, 164, 4878–4882. [Google Scholar] [CrossRef]
- Allan, S.M.; Rothwell, N.J. Cytokines and Acute Neurodegeneration. Nat. Rev. Neurosci. 2001, 2, 734–744. [Google Scholar] [CrossRef]
- Mabbott, N.A.; Williams, A.; Farquhar, C.F.; Pasparakis, M.; Kollias, G.; Bruce, M.E. Tumor Necrosis Factor Alpha-Deficient, But not Interleukin-6-deficient, Mice Resist Peripheral Infection with Scrapie. J. Virol. 2000, 74, 3338–3344. [Google Scholar] [CrossRef]
- Bauer, J.; Strauss, S.; Volk, B.; Berger, M. IL-6-mediated Events in Alzheimer’s Disease Pathology. Immunol. Today 1991, 12, 422. [Google Scholar] [CrossRef]
- Strauss, S.; Bauer, J.; Ganter, U.; Jonas, U.; Berger, M.; Volk, B. Detection of Interleukin-6 and Alpha 2-macroglobulin Immunoreactivity in Cortex and Hippocampus of Alzheimer’s Disease Patients. Lab. Investig. 1992, 66, 223–230. [Google Scholar] [PubMed]
- Wood, J.A.; Wood, P.L.; Ryan, R.; Graff-Radford, N.R.; Pilapil, C.; Robitaille, Y.; Quirion, R. Cytokine Indices in Alzheimer’s Temporal Cortex: No Changes in Mature IL-1 Beta or IL-1RA but Increases in the Associated Acute Phase Proteins IL-6, Alpha 2-macroglobulin and C-reactive Protein. Brain Res. 1993, 629, 245–252. [Google Scholar] [CrossRef]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The Master Regulator of Immunity to Infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, P.; Siegel, M.I.; Egan, R.W.; Billah, M.M. Interleukin (IL)-10 Inhibits Nuclear Factor Kappa B (NF Kappa B) Activation in Human Monocytes. IL-10 and IL-4 Suppress Cytokine Synthesis by Different Mechanisms. J. Biol. Chem. 1995, 270, 9558–9563. [Google Scholar] [CrossRef]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the Immune Response in the Brain: IL-10 and Its Regulation. J. Neuroinflamm. 2016, 13, 297. [Google Scholar] [CrossRef]
- Chakrabarty, P.; Li, A.; Ceballos-Diaz, C.; Eddy, J.A.; Funk, C.C.; Moore, B.; DiNunno, N.; Rosario, A.M.; Cruz, P.E.; Verbeeck, C.; et al. IL-10 Alters Immunoproteostasis in APP Mice, Increasing Plaque Burden and Worsening Cognitive Behavior. Neuron 2015, 85, 519–533. [Google Scholar] [CrossRef]
- Guillot-Sestier, M.V.; Doty, K.R.; Gate, D.; Rodriguez, J., Jr.; Leung, B.P.; Rezai-Zadeh, K.; Town, T. Il10 Deficiency Rebalances Innate Immunity to Mitigate Alzheimer-Like Pathology. Neuron 2015, 85, 534–548. [Google Scholar] [CrossRef]
- Prinz, M.; Montrasio, F.; Klein, M.A.; Schwarz, P.; Priller, J.; Odermatt, B.; Pfeffer, K.; Aguzzi, A. Lymph Nodal Prion Replication and Neuroinvasion in Mice Devoid of Follicular Dendritic Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 919–924. [Google Scholar] [CrossRef]
- Mabbott, N.A.; McGovern, G.; Jeffrey, M.; Bruce, M.E. Temporary Blockade of the Tumor Necrosis Factor Receptor Signaling Pathway Impedes the Spread of Scrapie to the Brain. J. Virol. 2002, 76, 5131–5139. [Google Scholar] [CrossRef]
- Zhao, M.; Cribbs, D.H.; Anderson, A.J.; Cummings, B.J.; Su, J.H.; Wasserman, A.J.; Cotman, C.W. The Induction of the TNFalpha Death Domain Signaling Pathway in Alzheimer’s Disease Brain. Neurochem. Res. 2003, 28, 307–318. [Google Scholar] [CrossRef]
- Cacabelos, R.; Alvarez, X.A.; Franco-Maside, A.; Fernandez-Novoa, L.; Caamano, J. Serum Tumor Necrosis Factor (TNF) in Alzheimer’s Disease and Multi-Infarct Dementia. Methods Find. Exp. Clin. Pharmacol. 1994, 16, 29–35. [Google Scholar] [PubMed]
- Sakudo, A.; Lee, D.C.; Saeki, K.; Matsumoto, Y.; Itohara, S.; Onodera, T. Tumor Necrosis Factor Attenuates Prion Protein-Deficient Neuronal Cell Death by Increases in Anti-Apoptotic Bcl-2 Family Proteins. Biochem. Biophys. Res. Commun. 2003, 310, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Goetz, F.W.; Planas, J.V.; MacKenzie, S. Tumor Necrosis Factors. Dev. Comp. Immunol. 2004, 28, 487–497. [Google Scholar] [CrossRef]
- Scherbel, U.; Raghupathi, R.; Nakamura, M.; Saatman, K.E.; Trojanowski, J.Q.; Neugebauer, E.; Marino, M.W.; McIntosh, T.K. Differential Acute and Chronic Responses of Tumor Necrosis Factor-Deficient Mice to Experimental Brain Injury. Proc. Natl. Acad. Sci. USA 1999, 96, 8721–8726. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.U.; de Vellis, J. Microglia in Health and Disease. J. Neurosci. Res. 2005, 81, 302–313. [Google Scholar] [CrossRef]
- Sato, K. Effects of Microglia on Neurogenesis. Glia 2015, 63, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.G.; Kelly, D.; Rath, E.M.; Baerwald, K.D.; Suzuki, K.; Popko, B. Targeted CNS Expression of Interferon-Gamma in Transgenic Mice Leads to Hypomyelination, Reactive Gliosis, and Abnormal Cerebellar Development. Mol. Cell. Neurosci. 1996, 7, 354–370. [Google Scholar] [CrossRef]
- Hashioka, S.; Klegeris, A.; Schwab, C.; McGeer, P.L. Interferon-Gamma-Dependent Cytotoxic Activation of Human Astrocytes and Astrocytoma Cells. Neurobiol. Aging 2009, 30, 1924–1935. [Google Scholar] [CrossRef]
- Sarna, J.R.; Hawkes, R. Patterned Purkinje Cell Death in the Cerebellum. Prog. Neurobiol. 2003, 70, 473–507. [Google Scholar] [CrossRef]
- Nelson, T.E.; Campbell, I.L.; Gruol, D.L. Altered Physiology of Purkinje Neurons in Cerebellar Slices from Transgenic Mice with Chronic Central Nervous System Expression of Interleukin-6. Neuroscience 1999, 89, 127–136. [Google Scholar]
- Ragagnin, A.; Ezpeleta, J.; Guillemain, A.; Boudet-Devaud, F.; Haeberle, A.M.; Demais, V.; Vidal, C.; Demuth, S.; Beringue, V.; Kellermann, O.; et al. Cerebellar Compartmentation of Prion Pathogenesis. Brain Pathol. 2017, 28, 240–263. [Google Scholar] [CrossRef] [PubMed]
- Munck, A.; Naray-Fejes-Toth, A. The Ups and Downs of Glucocorticoid Physiology. Permissive and Suppressive Effects Revisited. Mol. Cell. Endocrinol. 1992, 90, C1–C4. [Google Scholar] [CrossRef]
- Filaretova, L.; Podvigina, T.; Bagaeva, T.; Morozova, O. Dual Action of Glucocorticoid Hormones on the Gastric Mucosa: How the Gastroprotective Action Can Be Transformed to the Ulcerogenic One. Inflammopharmacology 2009, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]


| Antibody | Antigen | Type | Dilution | Retrieval Method | Source |
|---|---|---|---|---|---|
| IL-1 alpha | IL-1 | Polyclonal | 1:100 | Autoclave 121 °C (citrate buffer 10%) | ThermoFisher |
| Anti-IL-1RN | IL-1R | Polyclonal | 1:100 | Autoclave 121 °C (citrate buffer 10%) | Sigma |
| IL-2R.1 | IL-2R | Monoclonal | 1:1000 | PTLink 96 °C | ThermoFisher |
| 8H12 | IL-6 | Monoclonal | 1:40 | Autoclave 121 °C (citrate buffer 10%) | ThermoFisher |
| OTI1D10 | IL-10R | Monoclonal | 1:250 | PTLink 96 °C | ThermoFisher |
| Ber-H2 | TNFR | Monoclonal | Ready to use | PTLink 96 °C | Dako |
| IFNGR1 | IFNγR | Polyclonal | 1:200 | Autoclave 121 °C (citrate buffer 10%) | ThermoFisher |
| Gene | Full Name | Reference | Source |
|---|---|---|---|
| Gus-β | β-glucuronidase (reference gene) | Oa04828868_m1 | ThermoFisher |
| HPRT-1 | Hypoxanthine Phosphoribosyltransferase 1 (reference gene) | Oa04825272_gH | ThermoFisher |
| IL-1α | Interleukin 1 alpha | Oa04658681_m1 | ThermoFisher |
| IL-6 | Interleukin 6 | Oa04656315_m1 | ThermoFisher |
| IL-10Ra | Interleukin 10 receptor alpha | Oa04822455_m1 | ThermoFisher |
| IL-10Rb | Interleukin 10 receptor beta | Oa04894070_m1 | ThermoFisher |
| IFNγ | Interferon gamma | Oa04657364_m1 | ThermoFisher |
| Group | Marker Assessed (Methodology) | DEX Effect | Brain Area | Statistical Significance |
|---|---|---|---|---|
| Treated control | IL-1α (IHC) | Increase | MO | * p < 0.05 |
| Treated clinical | IL-1R (IHC) | Decrease | Cb | # p = 0.082 |
| Treated control | IL-6 (IHC) | Increase | Fc | * p < 0.05 |
| Decrease | O | # p = 0.071 | ||
| Treated clinical | Decrease | O | * p < 0.05 | |
| Treated clinical | IL-10R (IHC) | Decrease | Cb | # p = 0.074 |
| Treated clinical | IFNγ (RT-qPCR) | Increase | Fc | * p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guijarro, I.M.; Garcés, M.; Andrés-Benito, P.; Marín, B.; Otero, A.; Barrio, T.; Carmona, M.; Ferrer, I.; Badiola, J.J.; Monzón, M. Neuroimmune Response Mediated by Cytokines in Natural Scrapie after Chronic Dexamethasone Treatment. Biomolecules 2021, 11, 204. https://doi.org/10.3390/biom11020204
Guijarro IM, Garcés M, Andrés-Benito P, Marín B, Otero A, Barrio T, Carmona M, Ferrer I, Badiola JJ, Monzón M. Neuroimmune Response Mediated by Cytokines in Natural Scrapie after Chronic Dexamethasone Treatment. Biomolecules. 2021; 11(2):204. https://doi.org/10.3390/biom11020204
Chicago/Turabian StyleGuijarro, Isabel M., Moisés Garcés, Pol Andrés-Benito, Belén Marín, Alicia Otero, Tomás Barrio, Margarita Carmona, Isidro Ferrer, Juan J. Badiola, and Marta Monzón. 2021. "Neuroimmune Response Mediated by Cytokines in Natural Scrapie after Chronic Dexamethasone Treatment" Biomolecules 11, no. 2: 204. https://doi.org/10.3390/biom11020204
APA StyleGuijarro, I. M., Garcés, M., Andrés-Benito, P., Marín, B., Otero, A., Barrio, T., Carmona, M., Ferrer, I., Badiola, J. J., & Monzón, M. (2021). Neuroimmune Response Mediated by Cytokines in Natural Scrapie after Chronic Dexamethasone Treatment. Biomolecules, 11(2), 204. https://doi.org/10.3390/biom11020204







