Lysosomal Function and Axon Guidance: Is There a Meaningful Liaison?
Abstract
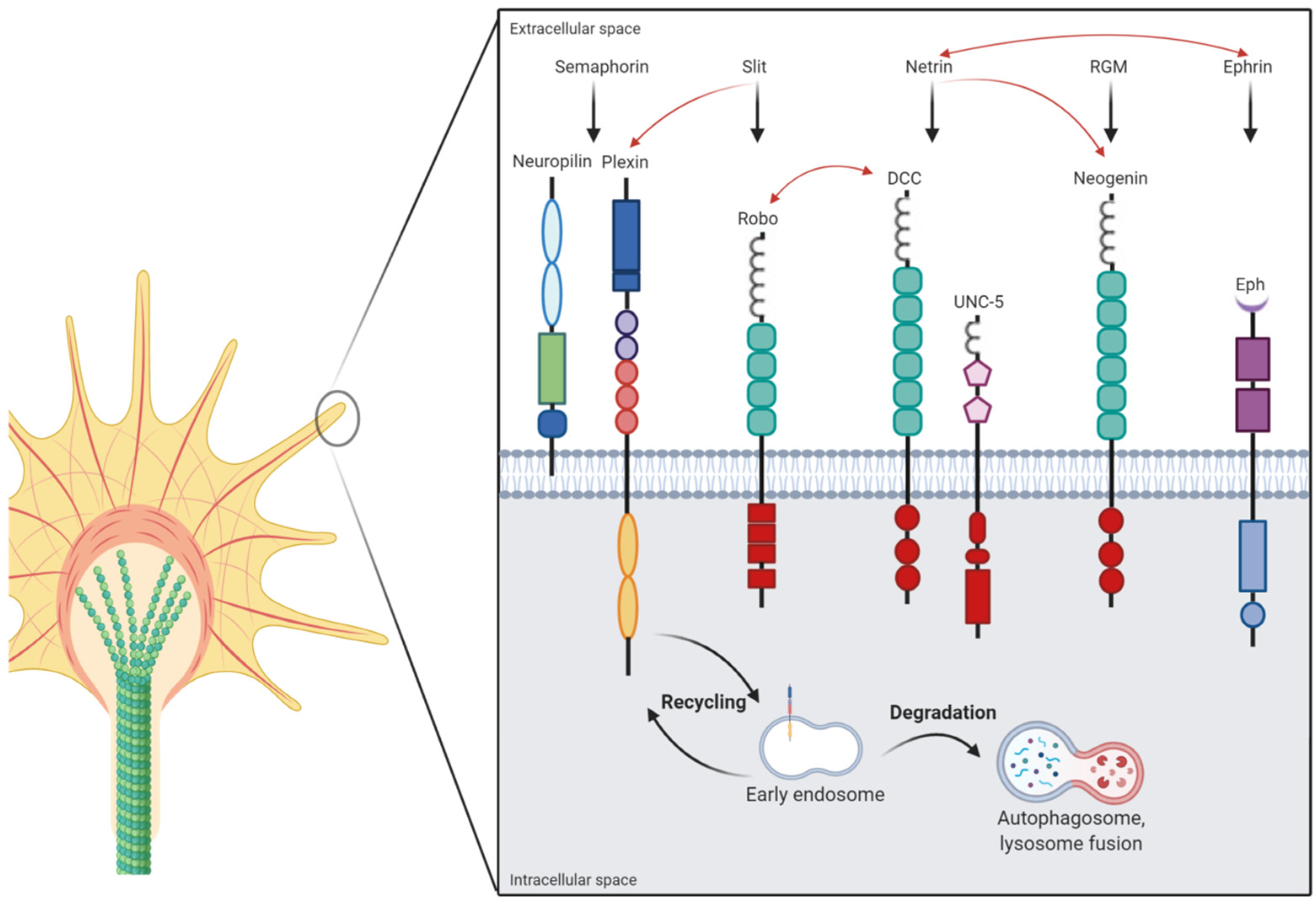
1. Introduction
2. Axonal Guidance Cues
2.1. Semaphorins
2.2. Ephrins
2.3. Repulsive Guidance Molecule (RGM)
2.4. Netrins
2.5. Slits
3. Axonal Guidance Cue Integration and Crosstalk
4. Lysosomal Function in Axonal Development
5. Brain Disorders with Axonal Guidance Defects
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Purves, D.; Lichtman, J.W. Elimination of synapses in the developing nervous system. Science 1980, 210, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Yaron, A.; Schuldiner, O. Common and Divergent Mechanisms in Developmental Neuronal Remodeling and Dying Back Neurodegeneration. Curr. Biol. 2016, 26, R628–R639. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.A.; Ma, L. Developmental regulation of axon branching in the vertebrate nervous system. Development 2011, 138, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Friedman, L.; Komatsu, M.; Tanaka, K. The cellular pathways of neuronal autophagy and their implication in neurodegenerative diseases. Biochim. Biophys. Acta 2009, 1793, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Watzlawik, J.O.; Fiesel, F.C.; Springer, W. Autophagy in Parkinson’s Disease. J. Mol. Biol. 2020, 432, 2651–2672. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Misgeld, T.; Kang, H.; Knecht, S.; Lu, J.; Cao, Y.; Cotman, S.L.; Bishop, D.L.; Lichtman, J.W. Lysosomal activity associated with developmental axon pruning. J. Neurosci. 2008, 28, 8993–9001. [Google Scholar] [CrossRef]
- Farfel-Becker, T.; Roney, J.C.; Cheng, X.T.; Li, S.; Cuddy, S.R.; Sheng, Z.H. Neuronal Soma-Derived Degradative Lysosomes Are Continuously Delivered to Distal Axons to Maintain Local Degradation Capacity. Cell Rep. 2019, 28, 51–64.e54. [Google Scholar] [CrossRef]
- O’Donnell, M.; Chance, R.K.; Bashaw, G.J. Axon growth and guidance: Receptor regulation and signal transduction. Annu. Rev. Neurosci. 2009, 32, 383–412. [Google Scholar] [CrossRef]
- Kolodkin, A.L.; Matthes, D.J.; O’Connor, T.P.; Patel, N.H.; Admon, A.; Bentley, D.; Goodman, C.S. Fasciclin IV: Sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron 1992, 9, 831–845. [Google Scholar] [CrossRef]
- Polleux, F.; Morrow, T.; Ghosh, A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature 2000, 404, 567–573. [Google Scholar] [CrossRef]
- Tamagnone, L.; Artigiani, S.; Chen, H.; He, Z.; Ming, G.I.; Song, H.; Chedotal, A.; Winberg, M.L.; Goodman, C.S.; Poo, M.; et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 1999, 99, 71–80. [Google Scholar] [CrossRef]
- Fujisawa, H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J. Neurobiol. 2004, 59, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Verhaagen, J.; Harvey, A.R. Receptor complexes for each of the Class 3 Semaphorins. Front. Cell Neurosci. 2012, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, R.J.; Peschon, J.J.; Spriggs, M.K.; Kolodkin, A.L. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 2003, 424, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Chak, K.; Andreone, B.J.; Wooley, J.R.; Kolodkin, A.L. The extracellular matrix proteoglycan perlecan facilitates transmembrane semaphorin-mediated repulsive guidance. Genes Dev. 2012, 26, 2222–2235. [Google Scholar] [CrossRef]
- Zhang, H.; Vreeken, D.; Junaid, A.; Wang, G.; Sol, W.; de Bruin, R.G.; van Zonneveld, A.J.; van Gils, J.M. Endothelial Semaphorin 3F Maintains Endothelial Barrier Function and Inhibits Monocyte Migration. Int. J. Mol. Sci. 2020, 21, 1471. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, C.; Lama, G.; Sica, G. Multifaceted Functional Role of Semaphorins in Glioblastoma. Int. J. Mol. Sci. 2019, 20, 2144. [Google Scholar] [CrossRef]
- Cheng, H.J.; Nakamoto, M.; Bergemann, A.D.; Flanagan, J.G. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell 1995, 82, 371–381. [Google Scholar] [CrossRef]
- Egea, J.; Klein, R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007, 17, 230–238. [Google Scholar] [CrossRef]
- Klein, R. Eph/ephrin signalling during development. Development 2012, 139, 4105–4109. [Google Scholar] [CrossRef]
- Chenaux, G.; Henkemeyer, M. Forward signaling by EphB1/EphB2 interacting with ephrin-B ligands at the optic chiasm is required to form the ipsilateral projection. Eur. J. Neurosci. 2011, 34, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph receptors and ephrins in cancer: Bidirectional signalling and beyond. Nat. Rev. Cancer. 2010, 10, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; McLaughlin, T.; Sung, T.C.; Santiago, A.; Lee, K.F.; O’Leary, D.D. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron 2008, 59, 746–758. [Google Scholar] [CrossRef]
- Marler, K.J.; Becker-Barroso, E.; Martínez, A.; Llovera, M.; Wentzel, C.; Poopalasundaram, S.; Hindges, R.; Soriano, E.; Comella, J.; Drescher, U. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J. Neurosci. 2008, 28, 12700–12712. [Google Scholar] [CrossRef]
- Bonanomi, D.; Chivatakarn, O.; Bai, G.; Abdesselem, H.; Lettieri, K.; Marquardt, T.; Pierchala, B.A.; Pfaff, S.L. Ret is a multifunctional coreceptor that integrates diffusible- and contact-axon guidance signals. Cell 2012, 148, 568–582. [Google Scholar] [CrossRef]
- Monnier, P.P.; Sierra, A.; Macchi, P.; Deitinghoff, L.; Andersen, J.S.; Mann, M.; Flad, M.; Hornberger, M.R.; Stahl, B.; Bonhoeffer, F.; et al. RGM is a repulsive guidance molecule for retinal axons. Nature 2002, 419, 392–395. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Deitinghoff, L.; Davis, D.; Conrad, S.; Skutella, T.; Chedotal, A.; Mueller, B.K.; Strittmatter, S.M. Neogenin mediates the action of repulsive guidance molecule. Nat. Cell Biol. 2004, 6, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.; Wilson, N.; De Vries, M.; Bradford, D.; Key, B.; Cooper, H.M. Neogenin and RGMa control neural tube closure and neuroepithelial morphology by regulating cell polarity. J. Neurosci. 2008, 28, 12643–12653. [Google Scholar] [CrossRef]
- Lah, G.J.; Key, B. Novel roles of the chemorepellent axon guidance molecule RGMa in cell migration and adhesion. Mol. Cell Biol. 2012, 32, 968–980. [Google Scholar] [CrossRef]
- Muramatsu, R.; Kubo, T.; Mori, M.; Nakamura, Y.; Fujita, Y.; Akutsu, T.; Okuno, T.; Taniguchi, J.; Kumanogoh, A.; Yoshida, M.; et al. RGMa modulates T cell responses and is involved in autoimmune encephalomyelitis. Nat. Med. 2011, 17, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Malinauskas, T.; Peer, T.V.; Bishop, B.; Mueller, T.D.; Siebold, C. Repulsive guidance molecules lock growth differentiation factor 5 in an inhibitory complex. Proc. Natl. Acad. Sci. USA 2020, 117, 15620–15631. [Google Scholar] [CrossRef]
- Lai Wing Sun, K.; Correia, J.P.; Kennedy, T.E. Netrins: Versatile extracellular cues with diverse functions. Development 2011, 138, 2153–2169. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Ho, W.H.; Gurney, A.; Rosenthal, A. The netrin-G1 ligand NGL-1 promotes the outgrowth of thalamocortical axons. Nat. Neurosci. 2003, 6, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Nishimura-Akiyoshi, S.; Niimi, K.; Nakashiba, T.; Itohara, S. Axonal netrin-Gs transneuronally determine lamina-specific subdendritic segments. Proc. Natl. Acad. Sci. USA 2007, 104, 14801–14806. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Nakanishi, T.; Ueno, M.; Itohara, S.; Yamashita, T. Netrin-G1 Regulates Microglial Accumulation along Axons and Supports the Survival of Layer V Neurons in the Postnatal Mouse Brain. Cell Rep. 2020, 31, 107580. [Google Scholar] [CrossRef] [PubMed]
- Hedgecock, E.M.; Culotti, J.G.; Hall, D.H. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 1990, 4, 61–85. [Google Scholar] [CrossRef]
- Finci, L.I.; Kruger, N.; Sun, X.; Zhang, J.; Chegkazi, M.; Wu, Y.; Schenk, G.; Mertens, H.D.T.; Svergun, D.I.; Zhang, Y.; et al. The crystal structure of netrin-1 in complex with DCC reveals the bifunctionality of netrin-1 as a guidance cue. Neuron 2014, 83, 839–849. [Google Scholar] [CrossRef]
- Keino-Masu, K.; Masu, M.; Hinck, L.; Leonardo, E.D.; Chan, S.S.; Culotti, J.G.; Tessier-Lavigne, M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 1996, 87, 175–185. [Google Scholar] [CrossRef]
- Gad, J.M.; Keeling, S.L.; Shu, T.; Richards, L.J.; Cooper, H.M. The spatial and temporal expression patterns of netrin receptors, DCC and neogenin, in the developing mouse retina. Exp. Eye Res. 2000, 70, 711–722. [Google Scholar] [CrossRef]
- Shu, T.; Valentino, K.M.; Seaman, C.; Cooper, H.M.; Richards, L.J. Expression of the netrin-1 receptor, deleted in colorectal cancer (DCC), is largely confined to projecting neurons in the developing forebrain. J. Comp. Neurol. 2000, 416, 201–212. [Google Scholar] [CrossRef]
- Hamelin, M.; Zhou, Y.; Su, M.W.; Scott, I.M.; Culotti, J.G. Expression of the UNC-5 guidance receptor in the touch neurons of C. elegans steers their axons dorsally. Nature 1993, 364, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, P.A.; Timpe, L.C.; Mitchell, K.J.; Fried, S.R.; Goodman, C.S.; Jan, L.Y.; Jan, Y.N. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 1996, 87, 197–204. [Google Scholar] [CrossRef]
- Fazeli, A.; Dickinson, S.L.; Hermiston, M.L.; Tighe, R.V.; Steen, R.G.; Small, C.G.; Stoeckli, E.T.; Keino-Masu, K.; Masu, M.; Rayburn, H.; et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 1997, 386, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Colavita, A.; Krishna, S.; Zheng, H.; Padgett, R.W.; Culotti, J.G. Pioneer axon guidance by UNC-129, a C. elegans TGF-beta. Science 1998, 281, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, J.M.; Hartley, D.A.; Walther, Z.; Artavanis-Tsakonas, S. Slit: An EGF-homologous locus of D. melanogaster involved in the development of the embryonic central nervous system. Cell 1988, 55, 1047–1059. [Google Scholar] [CrossRef]
- Dickson, B.J.; Gilestro, G.F. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu. Rev. Cell Dev. Biol. 2006, 22, 651–675. [Google Scholar] [CrossRef]
- Nguyen Ba-Charvet, K.T.; Brose, K.; Ma, L.; Wang, K.H.; Marillat, V.; Sotelo, C.; Tessier-Lavigne, M.; Chedotal, A. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J. Neurosci. 2001, 21, 4281–4289. [Google Scholar] [CrossRef]
- Delloye-Bourgeois, C.; Jacquier, A.; Charoy, C.; Reynaud, F.; Nawabi, H.; Thoinet, K.; Kindbeiter, K.; Yoshida, Y.; Zagar, Y.; Kong, Y.; et al. PlexinA1 is a new Slit receptor and mediates axon guidance function of Slit C-terminal fragments. Nat. Neurosci. 2015, 18, 36–45. [Google Scholar] [CrossRef]
- Kidd, T.; Brose, K.; Mitchell, K.J.; Fetter, R.D.; Tessier-Lavigne, M.; Goodman, C.S.; Tear, G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 1998, 92, 205–215. [Google Scholar] [CrossRef]
- Zelina, P.; Blockus, H.; Zagar, Y.; Peres, A.; Friocourt, F.; Wu, Z.; Rama, N.; Fouquet, C.; Hohenester, E.; Tessier-Lavigne, M.; et al. Signaling switch of the axon guidance receptor Robo3 during vertebrate evolution. Neuron 2014, 84, 1258–1272. [Google Scholar] [CrossRef]
- Pak, J.S.; DeLoughery, Z.J.; Wang, J.; Acharya, N.; Park, Y.; Jaworski, A.; Ozkan, E. NELL2-Robo3 complex structure reveals mechanisms of receptor activation for axon guidance. Nat. Commun. 2020, 11, 1489. [Google Scholar] [CrossRef] [PubMed]
- Morlot, C.; Thielens, N.M.; Ravelli, R.B.; Hemrika, W.; Romijn, R.A.; Gros, P.; Cusack, S.; McCarthy, A.A. Structural insights into the Slit-Robo complex. Proc. Natl. Acad. Sci. USA 2007, 104, 14923–14928. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.W.; Mathivet, T.; Larrivee, B.; Tong, R.K.; Kowalski, J.; Pibouin-Fragner, L.; Bouvree, K.; Stawicki, S.; Nicholes, K.; Rathore, N.; et al. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev. Cell 2011, 20, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, N.; Howitt, J.A.; Hussain, S.A.; Hohenester, E. Structural and functional analysis of slit and heparin binding to immunoglobulin-like domains 1 and 2 of Drosophila Robo. J. Biol. Chem. 2008, 283, 16226–16234. [Google Scholar] [CrossRef] [PubMed]
- Gorla, M.; Bashaw, G.J. Molecular mechanisms regulating axon responsiveness at the midline. Dev. Biol. 2020, 466, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.; Tessier-Lavigne, M. Hierarchical organization of guidance receptors: Silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science 2001, 291, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Pignata, A.; Ducuing, H.; Boubakar, L.; Gardette, T.; Kindbeiter, K.; Bozon, M.; Tauszig-Delamasure, S.; Falk, J.; Thoumine, O.; Castellani, V. A Spatiotemporal Sequence of Sensitization to Slits and Semaphorins Orchestrates Commissural Axon Navigation. Cell Rep. 2019, 29, 347–362.e345. [Google Scholar] [CrossRef]
- Croteau, L.P.; Kao, T.J.; Kania, A. Ephrin-A5 potentiates netrin-1 axon guidance by enhancing Neogenin availability. Sci. Rep. 2019, 9, 12009. [Google Scholar] [CrossRef]
- Pasterkamp, R.J.; Burk, K. Axon guidance receptors: Endocytosis, trafficking and downstream signaling from endosomes. Prog. Neurobiol. 2020, 101916. [Google Scholar] [CrossRef]
- Winckler, B.; Yap, C.C. Endocytosis and endosomes at the crossroads of regulating trafficking of axon outgrowth-modifying receptors. Traffic 2011, 12, 1099–1108. [Google Scholar] [CrossRef]
- Szymanska, E.; Budick-Harmelin, N.; Miaczynska, M. Endosomal “sort” of signaling control: The role of ESCRT machinery in regulation of receptor-mediated signaling pathways. Semin. Cell Dev. Biol. 2018, 74, 11–20. [Google Scholar] [CrossRef]
- Boissier, P.; Chen, J.; Huynh-Do, U. EphA2 signaling following endocytosis: Role of Tiam1. Traffic 2013, 14, 1255–1271. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ohashi, R.; Nakamura, R.; Shinmura, K.; Kamo, T.; Sakai, R.; Sugimura, H. Tiam1 mediates neurite outgrowth induced by ephrin-B1 and EphA2. EMBO J. 2004, 23, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Keleman, K.; Rajagopalan, S.; Cleppien, D.; Teis, D.; Paiha, K.; Huber, L.A.; Technau, G.M.; Dickson, B.J. Comm sorts robo to control axon guidance at the Drosophila midline. Cell 2002, 110, 415–427. [Google Scholar] [CrossRef]
- Farias, G.G.; Guardia, C.M.; De Pace, R.; Britt, D.J.; Bonifacino, J.S. BORC/kinesin-1 ensemble drives polarized transport of lysosomes into the axon. Proc. Natl. Acad. Sci. USA 2017, 114, E2955–E2964. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.C.; Fernandopulle, M.S.; Wang, G.; Choi, H.; Hao, L.; Drerup, C.M.; Patel, R.; Qamar, S.; Nixon-Abell, J.; Shen, Y.; et al. RNA Granules Hitchhike on Lysosomes for Long-Distance Transport, Using Annexin A11 as a Molecular Tether. Cell 2019, 179, 147–164.e120. [Google Scholar] [CrossRef]
- Corradi, E.; Dalla Costa, I.; Gavoci, A.; Iyer, A.; Roccuzzo, M.; Otto, T.A.; Oliani, E.; Bridi, S.; Strohbuecker, S.; Santos-Rodriguez, G.; et al. Axonal precursor miRNAs hitchhike on endosomes and locally regulate the development of neural circuits. EMBO J. 2020, 39, e102513. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, G.; Schlehe, J.S.; LaVoie, M.J.; Schwarz, T.L. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J. Cell Biol. 2014, 206, 655–670. [Google Scholar] [CrossRef]
- Maday, S.; Wallace, K.E.; Holzbaur, E.L. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J. Cell Biol. 2012, 196, 407–417. [Google Scholar] [CrossRef]
- Canter, R.G.; Penney, J.; Tsai, L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef]
- Caligiore, D.; Helmich, R.C.; Hallett, M.; Moustafa, A.A.; Timmermann, L.; Toni, I.; Baldassarre, G. Parkinson’s disease as a system-level disorder. NPJ Parkinsons Dis. 2016, 2, 16025. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Konnerth, A. Impairments of neural circuit function in Alzheimer’s disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- McGregor, M.M.; Nelson, A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Blumenstock, S.; Dudanova, I. Cortical and Striatal Circuits in Huntington’s Disease. Front. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef]
- Harris, S.S.; Wolf, F.; De Strooper, B.; Busche, M.A. Tipping the Scales: Peptide-Dependent Dysregulation of Neural Circuit Dynamics in Alzheimer’s Disease. Neuron 2020, 107, 417–435. [Google Scholar] [CrossRef]
- Fenlon, L.R.; Richards, L.J. Contralateral targeting of the crpus callosum in normal and pathological brain function. Trends Neurosci. 2015, 38, 264–272. [Google Scholar] [CrossRef]
- Meneret, A.; Franz, E.A.; Trouillard, O.; Oliver, T.C.; Zagar, Y.; Robertson, S.P.; Welniarz, Q.; Gardner, R.J.M.; Gallea, C.; Srour, M.; et al. Mutations in the netrin-1 gene cause congenital mirror movements. J. Clin. Investig. 2017, 127, 3923–3936. [Google Scholar] [CrossRef]
- Srour, M.; Riviere, J.B.; Pham, J.M.; Dube, M.P.; Girard, S.; Morin, S.; Dion, P.A.; Asselin, G.; Rochefort, D.; Hince, P.; et al. Mutations in DCC cause congenital mirror movements. Science 2010, 328, 592. [Google Scholar] [CrossRef]
- Marsh, A.P.; Heron, D.; Edwards, T.J.; Quartier, A.; Galea, C.; Nava, C.; Rastetter, A.; Moutard, M.L.; Anderson, V.; Bitoun, P.; et al. Mutations in DCC cause isolated agenesis of the Corpus callosum with incomplete penetrance. Nat. Genet. 2017, 49, 511–514. [Google Scholar] [CrossRef]
- Marsh, A.P.L.; Edwards, T.J.; Galea, C.; Cooper, H.M.; Engle, E.C.; Jamuar, S.S.; Meneret, A.; Moutard, M.L.; Nava, C.; Rastetter, A.; et al. DCC mutation update: Congenital mirror movements, isolated agenesis of the Corpus callosum, and developmental split brain syndrome. Hum. Mutat. 2018, 39, 23–39. [Google Scholar] [CrossRef]
- Jamuar, S.S.; Schmitz-Abe, K.; D’Gama, A.M.; Drottar, M.; Chan, W.M.; Peeva, M.; Servattalab, S.; Lam, A.N.; Delgado, M.R.; Clegg, N.J.; et al. Biallelic mutations in human DCC cause developmental split-brain syndrome. Nat. Genet. 2017, 49, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Jen, J.C.; Chan, W.M.; Bosley, T.M.; Wan, J.; Carr, J.R.; Rub, U.; Shattuck, D.; Salamon, G.; Kudo, L.C.; Ou, J.; et al. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science 2004, 304, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.M.; Traboulsi, E.I.; Arthur, B.; Friedman, N.; Andrews, C.; Engle, E.C. Horizontal gaze palsy with progressive scoliosis can result from compound heterozygous mutations in ROBO3. J. Med. Genet. 2006, 43, e11. [Google Scholar] [CrossRef] [PubMed]
- Volk, A.E.; Carter, O.; Fricke, J.; Herkenrath, P.; Poggenborg, J.; Borck, G.; Demant, A.W.; Ivo, R.; Eysel, P.; Kubisch, C.; et al. Horizontal gaze palsy with progressive scoliosis: Three novel ROBO3 mutations and descriptions of the phenotypes of four patients. Mol. Vis. 2011, 17, 1978–1986. [Google Scholar] [PubMed]
- Xiu, Y.; Lv, Z.; Wang, D.; Chen, X.; Huang, S.; Pan, M. Introducing and Reviewing a Novel Mutation of ROBO3 in Horizontal Gaze Palsy with Progressive Scoliosis from a Chinese Family. J. Mol. Neurosci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.V.; Kenwrick, S.; Willems, P.; Lemmon, V. Mutations in the cell adhesion molecule L1 cause mental retardation. Trends Neurosci. 1995, 18, 168–172. [Google Scholar] [CrossRef]
- Fransen, E.; Van Camp, G.; Vits, L.; Willems, P.J. L1-associated diseases: Clinical geneticists divide, molecular geneticists unite. Hum. Mol. Genet. 1997, 6, 1625–1632. [Google Scholar] [CrossRef]
- Engle, E.C. Human genetic disorders of axon guidance. Cold Spring Harb. Perspect. Biol. 2010, 2, a001784. [Google Scholar] [CrossRef]
- Dode, C.; Hardelin, J.P. Clinical genetics of Kallmann syndrome. Ann. Endocrinol. (Paris) 2010, 71, 149–157. [Google Scholar] [CrossRef]
- Soussi-Yanicostas, N.; de Castro, F.; Julliard, A.K.; Perfettini, I.; Chedotal, A.; Petit, C. Anosmin-1, defective in the X-linked form of Kallmann syndrome, promotes axonal branch formation from olfactory bulb output neurons. Cell 2002, 109, 217–228. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, D.; Kim, S.H.; Hu, Y.; Guimond, S.; Schofield, J.; Winyard, P.; Vannelli, G.B.; Turnbull, J.; Bouloux, P.M. Anosmin-1 modulates fibroblast growth factor receptor 1 signaling in human gonadotropin-releasing hormone olfactory neuroblasts through a heparan sulfate-dependent mechanism. J. Neurosci. 2004, 24, 10384–10392. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Otis, J.M.; Suciu, S.K.; Catalano, C.; Xing, L.; Constable, S.; Wachten, D.; Gupton, S.; Lee, J.; Lee, A.; et al. Primary Cilia Signaling Promotes Axonal Tract Development and Is Disrupted in Joubert Syndrome-Related Disorders Models. Dev. Cell. 2019, 51, 759–774.e755. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.A. The molecular genetics of Joubert syndrome and related ciliopathies: The challenges of genetic and phenotypic heterogeneity. Transl. Sci. Rare Dis. 2019, 4, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Ferent, J.; Constable, S.; Gigante, E.D.; Yam, P.T.; Mariani, L.E.; Legue, E.; Liem, K.F., Jr.; Caspary, T.; Charron, F. The Ciliary Protein Arl13b Functions Outside of the Primary Cilium in Shh-Mediated Axon Guidance. Cell Rep. 2019, 29, 3356–3366.e3353. [Google Scholar] [CrossRef] [PubMed]
- Miyake, N.; Chilton, J.; Psatha, M.; Cheng, L.; Andrews, C.; Chan, W.M.; Law, K.; Crosier, M.; Lindsay, S.; Cheung, M.; et al. Human CHN1 mutations hyperactivate alpha2-chimaerin and cause Duane’s retraction syndrome. Science 2008, 321, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Nugent, A.A.; Park, J.G.; Wei, Y.; Tenney, A.P.; Gilette, N.M.; DeLisle, M.M.; Chan, W.M.; Cheng, L.; Engle, E.C. Mutant alpha2-chimaerin signals via bidirectional ephrin pathways in Duane retraction syndrome. J. Clin. Investig. 2017, 127, 1664–1682. [Google Scholar] [CrossRef]
- Ferrario, J.E.; Baskaran, P.; Clark, C.; Hendry, A.; Lerner, O.; Hintze, M.; Allen, J.; Chilton, J.K.; Guthrie, S. Axon guidance in the developing ocular motor system and Duane retraction syndrome depends on Semaphorin signaling via alpha2-chimaerin. Proc. Natl. Acad. Sci. USA 2012, 109, 14669–14674. [Google Scholar] [CrossRef]
- Clark, C.; Austen, O.; Poparic, I.; Guthrie, S. Alpha2-Chimaerin regulates a key axon guidance transition during development of the oculomotor projection. J. Neurosci. 2013, 33, 16540–16551. [Google Scholar] [CrossRef]
- Poirier, K.; Saillour, Y.; Bahi-Buisson, N.; Jaglin, X.H.; Fallet-Bianco, C.; Nabbout, R.; Castelnau-Ptakhine, L.; Roubertie, A.; Attie-Bitach, T.; Desguerre, I.; et al. Mutations in the neuronal ss-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum. Mol. Genet. 2010, 19, 4462–4473. [Google Scholar] [CrossRef]
- Tischfield, M.A.; Baris, H.N.; Wu, C.; Rudolph, G.; Van Maldergem, L.; He, W.; Chan, W.M.; Andrews, C.; Demer, J.L.; Robertson, R.L.; et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell 2010, 140, 74–87. [Google Scholar] [CrossRef]
- Twigg, S.R.F.; Kan, R.; Babbs, C.; Bochukova, E.G.; Robertson, S.P.; Wall, S.A.; Morriss-Kay, G.M.; Wilkie, A.O.M. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc. Natl. Acad. Sci. USA 2004, 101, 8652–8657. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, T.; Blanchard, S.; Toli, D.; Roy, E.; Bigou, S.; Froissart, R.; Rouvet, I.; Vitry, S.; Heard, J.M.; Bohl, D. Modeling neuronal defects associated with a lysosomal disorder using patient-derived induced pluripotent stem cells. Hum. Mol. Genet. 2011, 20, 3653–3666. [Google Scholar] [CrossRef] [PubMed]
- Salvalaio, M.; D’Avanzo, F.; Rigon, L.; Zanetti, A.; D’Angelo, M.; Valle, G.; Scarpa, M.; Tomanin, R. Brain RNA-Seq Profiling of the Mucopolysaccharidosis Type II Mouse Model. Int. J. Mol. Sci. 2017, 18, 1072. [Google Scholar] [CrossRef] [PubMed]
- Parente, M.K.; Rozen, R.; Cearley, C.N.; Wolfe, J.H. Dysregulation of gene expression in a lysosomal storage disease varies between brain regions implicating unexpected mechanisms of neuropathology. PLoS ONE 2012, 7, e32419. [Google Scholar] [CrossRef] [PubMed]
| Disorder | Human Gene | Axon Guidance-Related Defect | Symptoms | Reference |
|---|---|---|---|---|
| Congenital mirror movements (CMM) and partial or complete agenesis of the corpus callosum | NETRIN1 (NTN1) | Corticospinal tract (CST) abnormality | Involuntary movements of one hand that mirror intentional movements of the opposite hand | [77,78] |
| Congenital mirror movements (CMM) and/or isolated agenesis of the corpus callosum | Deleted in colorectal cancer (DCC) | Decreased crossing of descending corticospinal tract projections | Variable range of intellectual disabilities, cognitive impairment, language delay, and visual and spatial deficits | [79,80] |
| Developmental split-brain syndrome (gaze palsy, familial horizontal, with progressive scoliosis 2, with impaired intellectual development) | Deleted in colorectal cancer (DCC) | Agenesis of the corpus callosum and absence of the anterior and hippocampal commissures | Neurological abnormalities, horizontal gaze palsy, intellectual disability, and progressive scoliosis | [81] |
| Horizontal gaze palsy with progressive scoliosis (HGPPS) | Roundabout guidance receptor 3 (ROBO3) | Abnormal flattening of the basis pontis and hypoplasia in the pontine tegmentum; anomalous innervations of the lateral rectus muscle of the eye by the abducens supranuclear nerve | Horizontal gaze palsy, intellectual disability and progressive scoliosis | [82,83,84,85] |
| CRASH syndrome (Corpus callosum agenesis, Retardation, Adducted thumbs, Shuffling gait, and Hydrocephalus) | L1CAM | Agenesis of the corpus callosum and corticospinal tract | Microcephaly, mental retardation, spastic paraparesis | [86,87] |
| Kallman syndrome (X-linked) | ANOS1 (KAL1) | Defective olfactory axon guidance and migration | Congenital anosmia, hypogonadotropic hypogonadism, mirror movements, and aberrant corticospinal tract | [89,90] |
| Joubert syndrome and related disorders (JSRD) | Multiple genes (AHI1, NPHP1, CEP290, TMEM67, RPGRIP1, ARL13B, CC2D2A) | Hypotonia, ataxia, mental retardation, altered respiratory patterns, social disabilities, and synkinetic mirror movements | Cerebellar vermian hypoplasia, reduction in pontine neurons, and reduced decussation of the superior cerebellar peduncles. | [92,93] |
| Duane retraction syndrome (DRS) | α2-CHIMERIN | Absence of abducens motor neurons and nerves; aberrant innervation of the lateral rectus muscle by the oculomotor nerve | Restricted horizontal gaze and ocular synkinesis | [95,96] |
| Cortical dysplasia, complex, with other brain malformations 1 (CDCBM1) | Beta tubulin protein family member TUBB3 | Thin corpus callosum, hypoplastic brainstem, and dysplastic cerebellar vermis | Severe mental retardation, strabismus, axial hypotonia, and spasticity | [99] |
| Congenital fibrosis of the extraocular muscles 3 (CFEOM3) | Beta tubulin protein family member TUBB3 | Dysgenesis of the corpus callosum and anterior commissure (AC), and internal capsule; generalized loss of white matter; basal ganglia dysmorphisms | Aberrant eye movements, facial weakness, axonal peripheral neuropathy, contractures of the wrist and fingers, delayed development, and learning disabilities | [100] |
| Craniofrontonasal syndrome (CFNS) | Ephrin B1(EFNB1) | Dysgenesis or agenesis of the corpus callosum | Variable difficulties in speech and language, limited or no intellectual disabilities, facial asymmetry, skeletal and dermatological abnormalities | [101] |
| Mucopolysaccharidosis type II (Hunter syndrome) | Iduronate sulfatase (IDS) | Indirect experimental observation | Mental retardation, language delay, cognitive impairment | [103] |
| Mucopolysaccharidosis type IIIb (Sanfilippo Syndrome) | α-N-acetylglucosaminidase (NAGLU) | Indirect experimental observation | Mental retardation, cognitive decline, dysphagia, sleep problems, seizures | [102] |
| Mucopolysaccharidosis type VII (Sly syndrome) | β-glucuronidase (GUSB) | Indirect experimental observation | developmental Delay, speech delay, intellectual disability of variable degree | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzoli, R.; Badenetti, L.; Rubin, M.; Moro, E. Lysosomal Function and Axon Guidance: Is There a Meaningful Liaison? Biomolecules 2021, 11, 191. https://doi.org/10.3390/biom11020191
Manzoli R, Badenetti L, Rubin M, Moro E. Lysosomal Function and Axon Guidance: Is There a Meaningful Liaison? Biomolecules. 2021; 11(2):191. https://doi.org/10.3390/biom11020191
Chicago/Turabian StyleManzoli, Rosa, Lorenzo Badenetti, Michela Rubin, and Enrico Moro. 2021. "Lysosomal Function and Axon Guidance: Is There a Meaningful Liaison?" Biomolecules 11, no. 2: 191. https://doi.org/10.3390/biom11020191
APA StyleManzoli, R., Badenetti, L., Rubin, M., & Moro, E. (2021). Lysosomal Function and Axon Guidance: Is There a Meaningful Liaison? Biomolecules, 11(2), 191. https://doi.org/10.3390/biom11020191







