Extra Virgin Olive Oil Phenols Vasodilate Rat Mesenteric Resistance Artery via Phospholipase C (PLC)-Calcium Microdomains-Potassium Channels (BKCa) Signals
Abstract
1. Introduction
2. Materials and Methods
2.1. Extra Virgin Olive Oil Phenols
2.2. Animals
2.3. Rat Isolated Mesenteric Artery Preparation
2.4. Experimental Protocol
2.5. Drug and Solutions
2.6. Data Analysis and Statistics
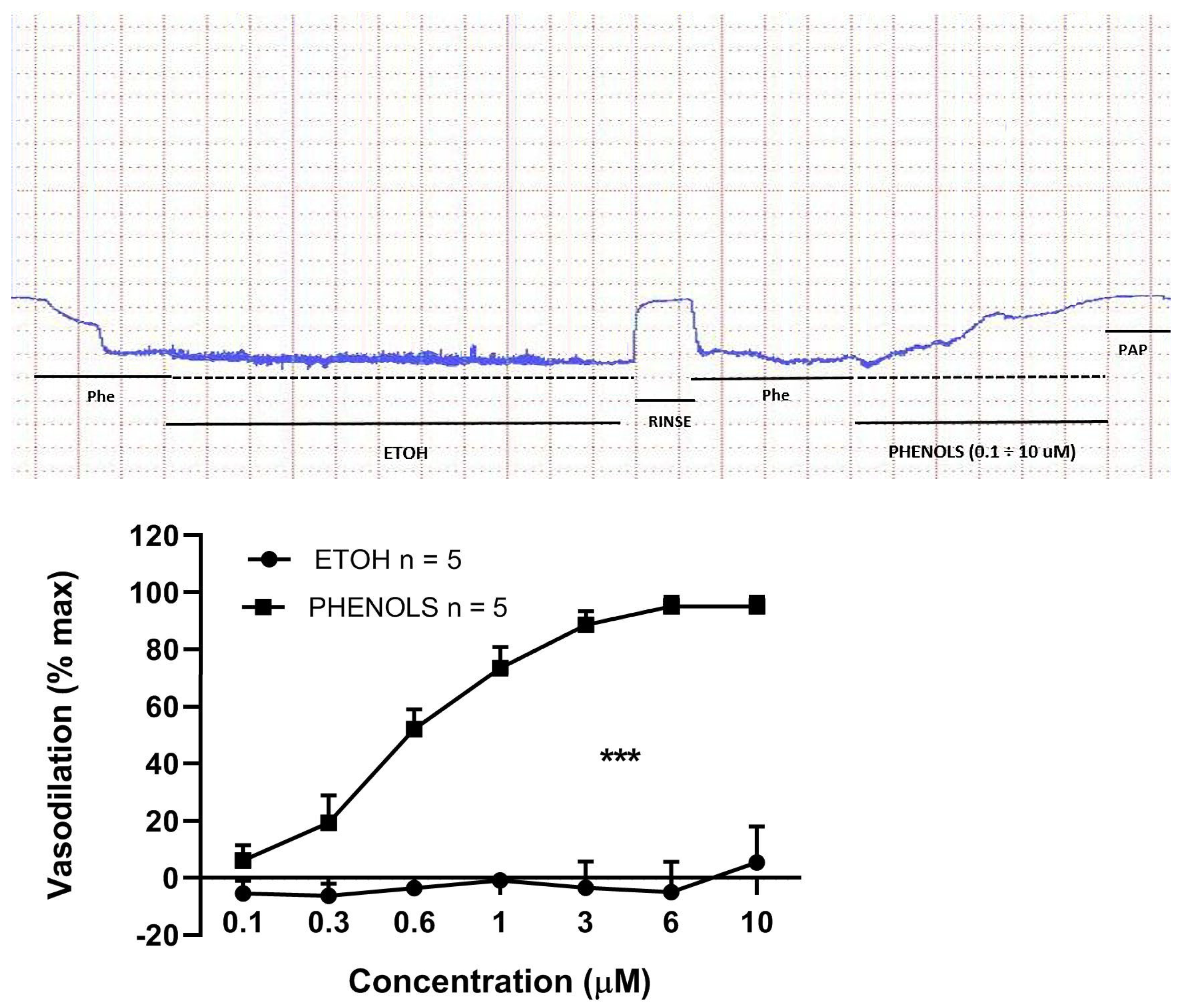
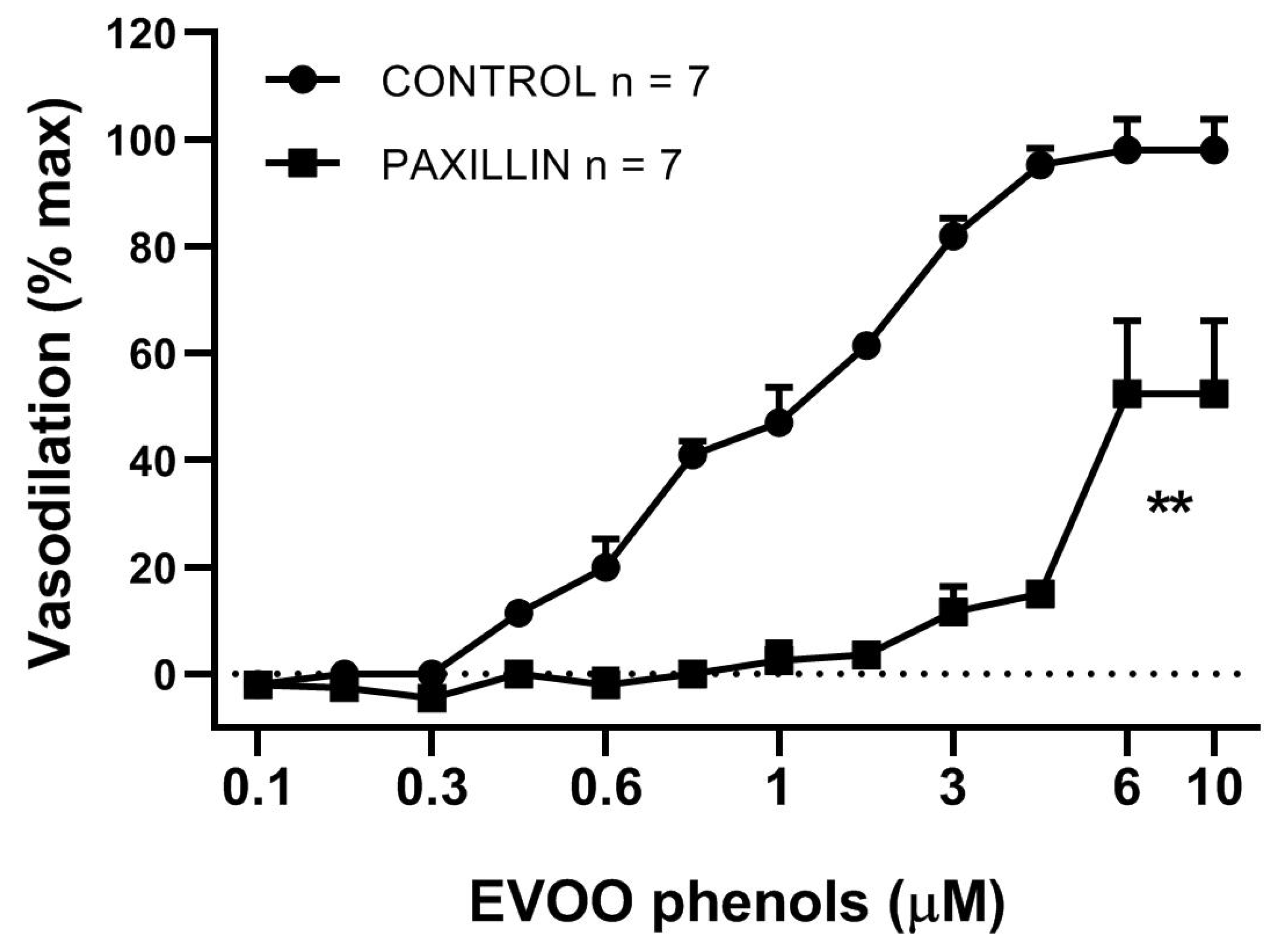
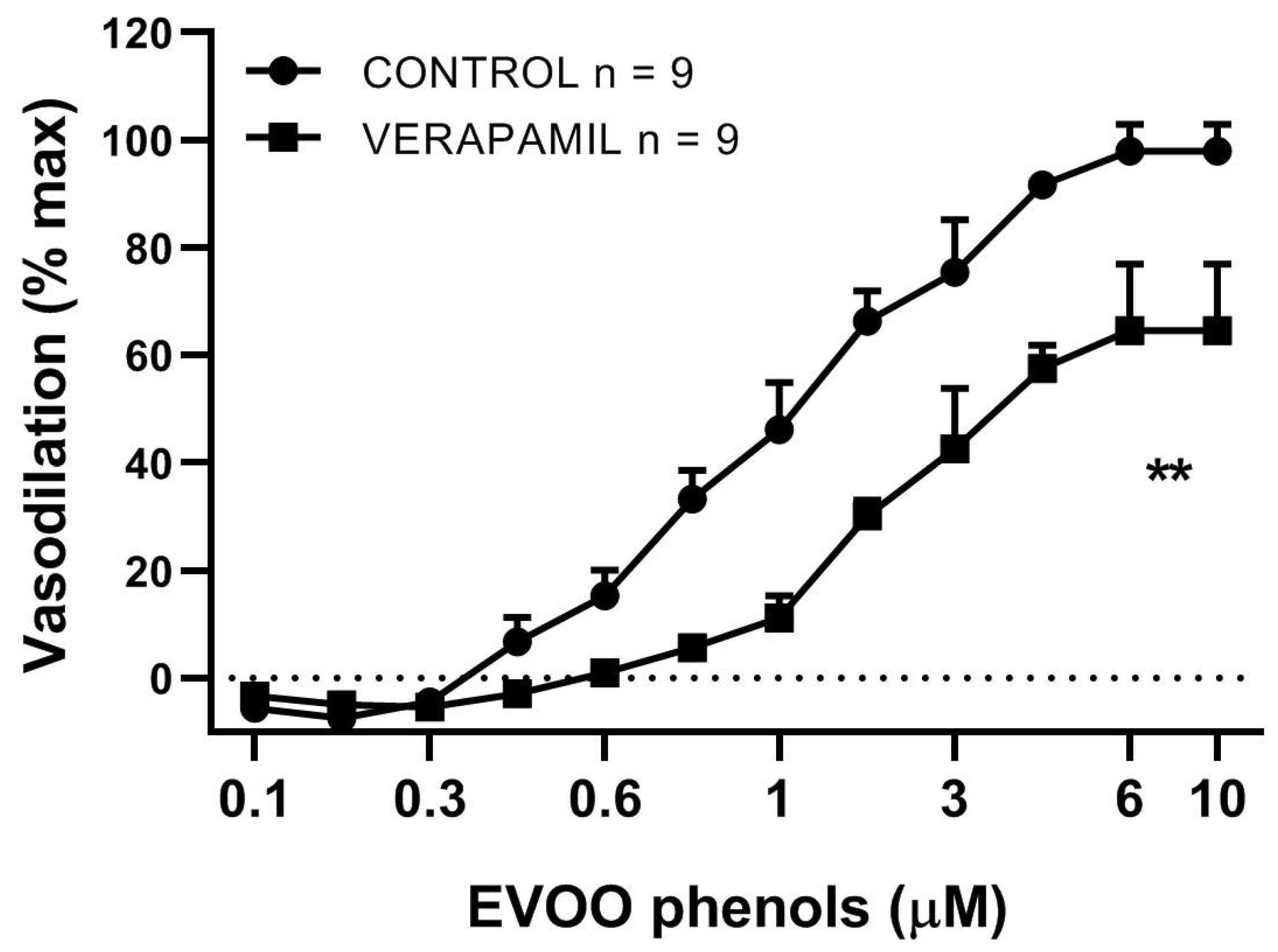
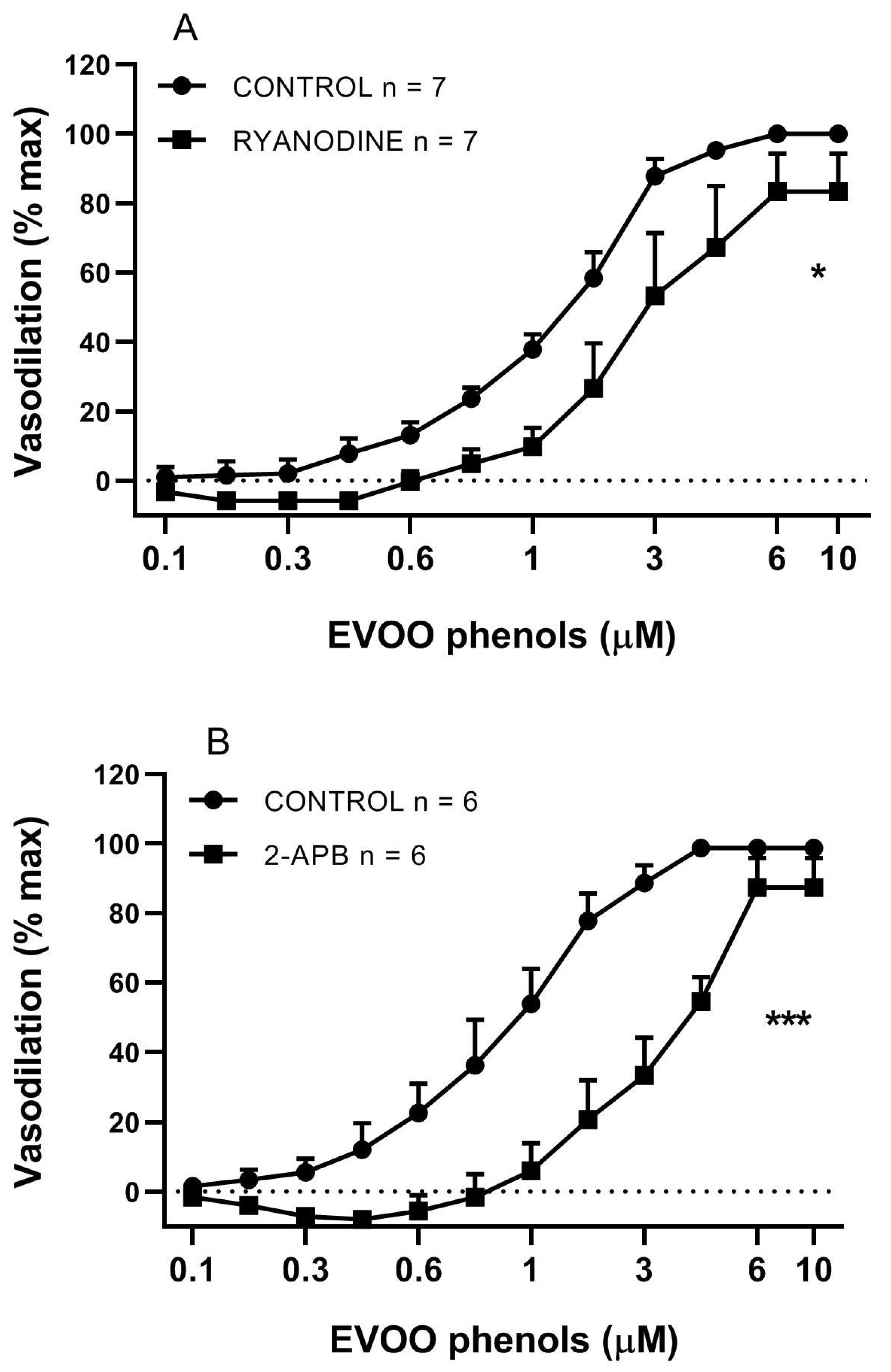
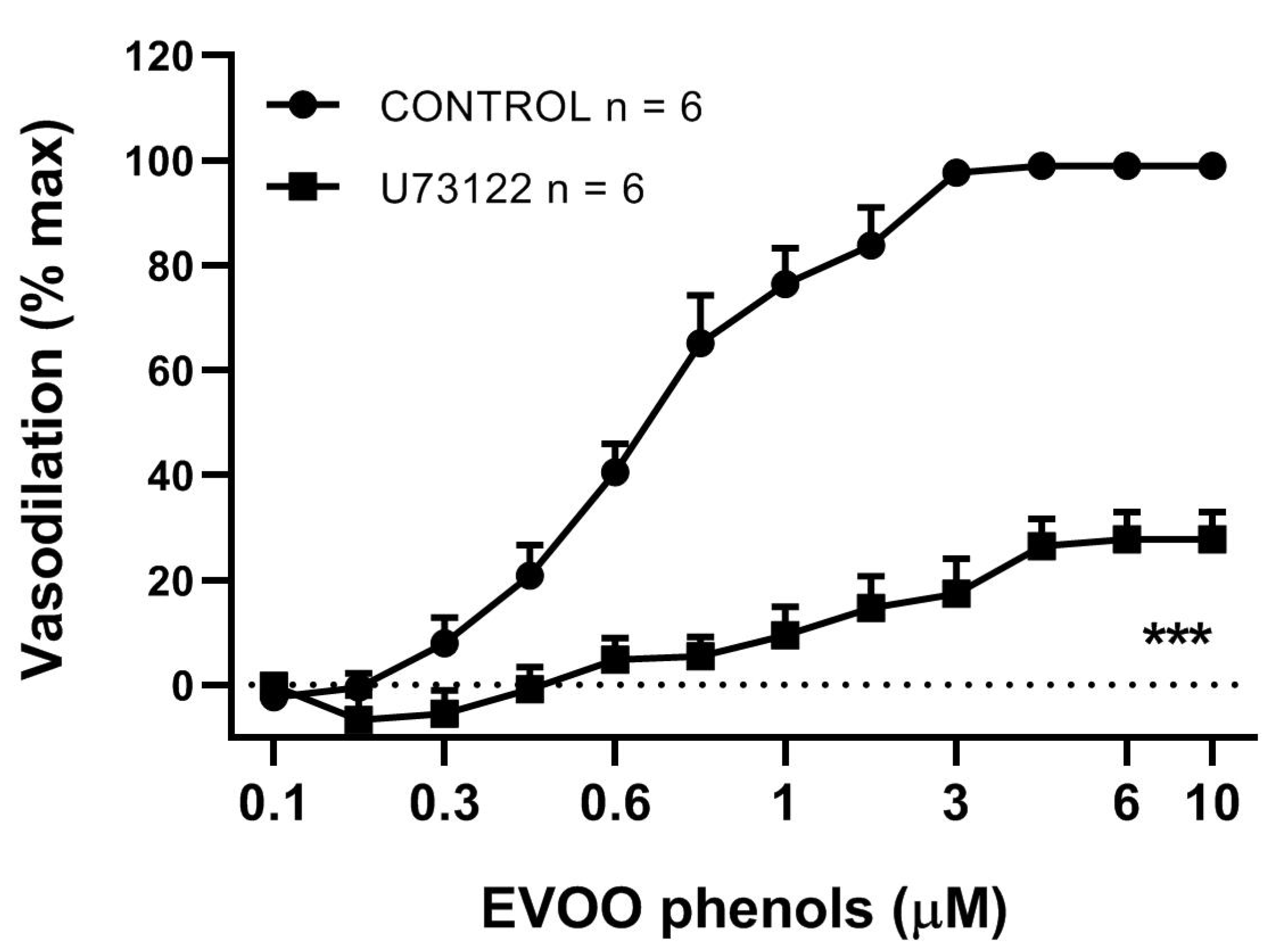
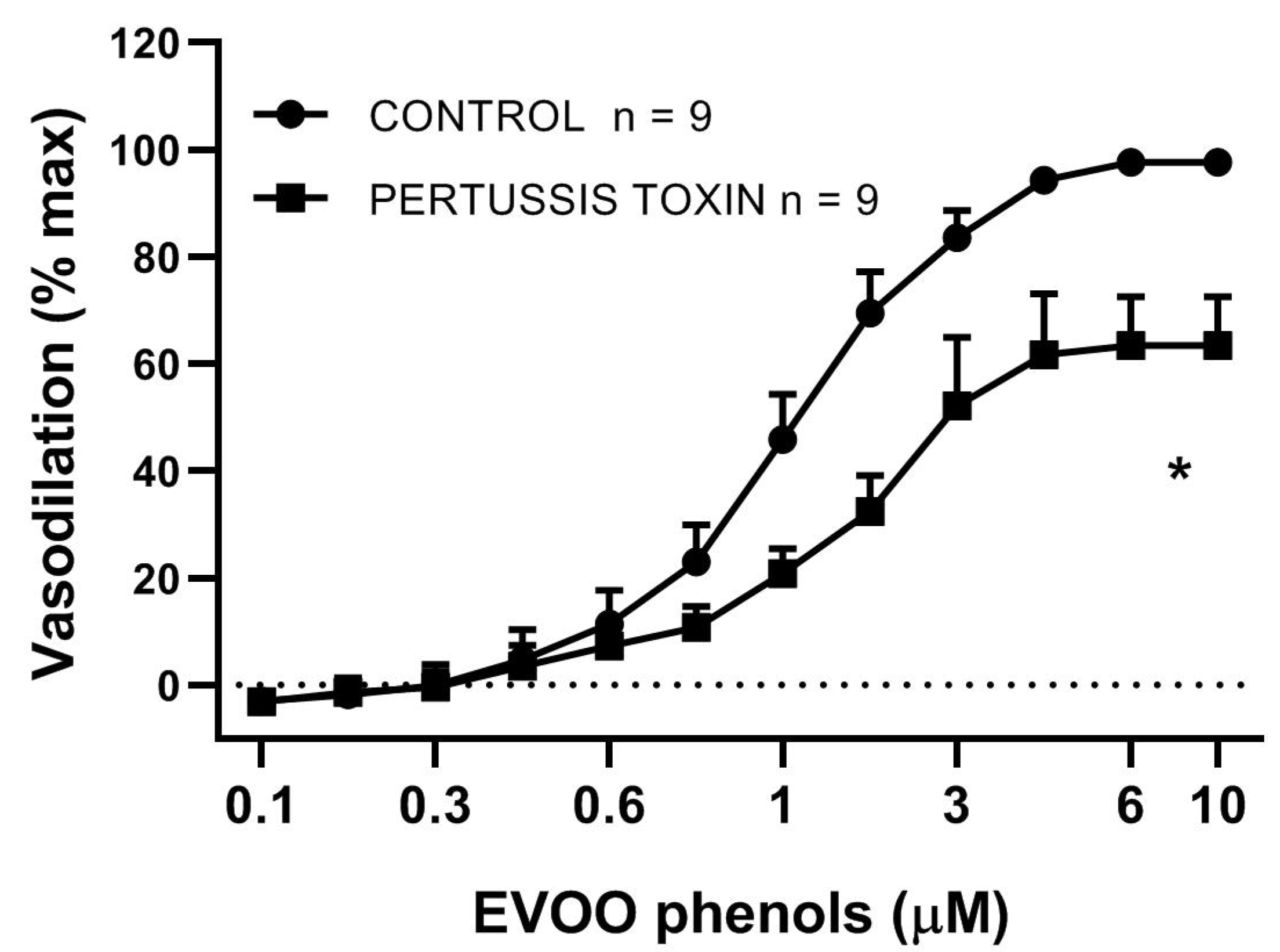
3. Results
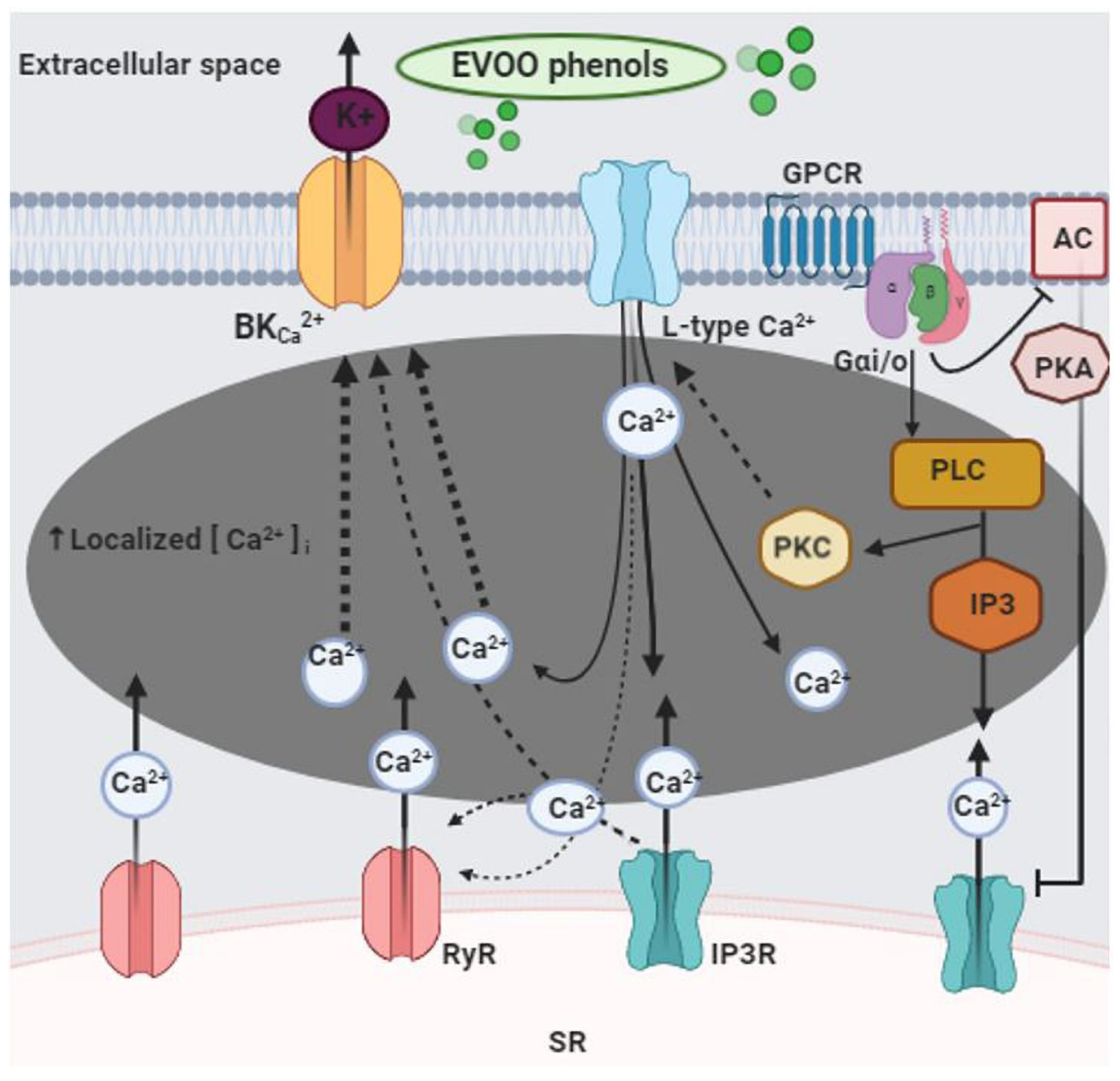
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis Primers 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J.; Hu, F.B.; Manson, J.E.; Rimm, E.B.; Willett, W.C. Primary prevention of coronary heartdisease in women through diet and lifestyle. N. Engl. J. Med. 2000, 343, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Knoops, K.T.; de Groot, L.C.; Kromhout, D.; Perrin, A.E.; Moreiras-Varela, O.; Menotti, A.; van Staveren, W.A. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: The HALE project. JAMA 2004, 292, 1433–1439. [Google Scholar] [CrossRef]
- Gaforio, J.J.; Visioli, F.; Alarcón-de-la-Lastra, C.; Castañer, O.; Delgado-Rodríguez, M.; Fitó, M.; Hernández, A.F.; Huertas, J.R.; Martínez-González, M.A.; Menendez, J.A. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019, 11, 2039. [Google Scholar] [CrossRef]
- Alarcon de la Lastra, G.; Barranco, M.D.; Motilva, V.; Herrerias, J.M. Mediterranean diet and health: Biological importance of olive oil. Curr. Pharm. Des. 2001, 7, 933–950. [Google Scholar] [CrossRef]
- Carluccio, M.A.; Massaro, M.; Scoditti, E.; De Caterina, R. Vasculoprotective potential of olive oil components. Mol. Nutr. Food Res. 2007, 51, 1225–1234. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra Virgin Olive Oil and Cardiovascular Diseases: Benefits for Human Health. Endocr. Metab. Immune Disord. Drug Targets. 2018, 18, 4–13. [Google Scholar] [CrossRef]
- Perona, J.S.; Canizares, J.; Montero, E.; Sanchez-Dominguez, J.M.; Catala, A.; Ruiz-Gutierrez, V. Virgin olive oil reduces blood pressure in hypertensive elderly subjects. Clin. Nutr. 2004, 23, 1113–1121. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Domenech, M.; Roman, P.; Lapetra, J.; Garcia de la Corte, F.J.; Sala-Vila, A.; de la Torre, R.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Lamuela-Raventos, R.M.; et al. Mediterranean diet reduces 24-hour ambulatory blood pressure, blood glucose, and lipids: One-year randomized, clinical trial. Hypertension 2014, 64, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D.; Tsimidou, M.; Blekas, G. Polar Phenolic Compounds. In Olive Oil: Chemistry and Technology, 2nd ed.; The American Oil Chemists’ Society: Urbana, IL, USA, 2006. [Google Scholar]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants 2013, 3, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Alemany, R.; Terés, S.; Baamonde, C.; Benet, M.; Vögler, O.; Escribá, P.V. 2-Hydroxyoleic Acid: A new Hypotensive Molecule. Hypertension 2004, 43, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Terés, S.; Barceló-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J.E.; Escriba, P.V. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl Acad. Sci. USA 2008, 105, 13811–13816. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.I.; Munoz-Aguayo, D.; Khymenets, O.; de la Torre, R.; Saez, G.; Tormos Mdel, C.; Toledo, E.; Marti, A.; Ruiz-Gutierrez, V.; et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: A randomized controlled trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef]
- Fito, M.; Cladellas, M.; de la Torre, R.; Marti, J.; Alcantara, M.; Pujadas-Bastardes, M.; Marrugat, J.; Bruguera, J.; Lopez-Sabater, M.C.; Vila, J.; et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomized, crossover, controlled, clinical trial. Atherosclerosis 2005, 181, 149–158. [Google Scholar] [CrossRef]
- Moreno-Luna, R.; Munoz-Hernandez, R.; Miranda, M.L.; Costa, A.F.; Jimenez-Jimenez, L.; Vallejo-Vaz, A.J.; Muriana, F.J.; Villar, J.; Stiefel, P. Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. Am. J. Hypertens. 2012, 25, 1299–1304. [Google Scholar] [CrossRef]
- D’Agostino, R.; Barberio, L.; Gatto, M.; Muzzalupo, I.; Mandalà, M. Extra Virgin Olive Oil Phenols Dilate the Rat Mesenteric Artery by Activation of BKCa2+ Channels in Smooth Muscle Cells. Molecules 2020, 25, 2601. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J. Agr. Food Chem. 1992, 40, 1571–1576. [Google Scholar] [CrossRef]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of phenols, flavones, and lignans in virgin olive oils by solid-phase extraction and high-performance liquid chromatography with diode array ultraviolet detection. J. Agr. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Colton, I.; Mandalà, M.; Morton, J.; Davidge, S.T.; Osol, G. Influence of Constriction, Wall Tension, Smooth Muscle Activation and Cellular Deformation on Rat Resistance Artery Vasodilator Reactivity. Cell Physiol. Biochem. 2012, 29, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Moreau, P.; Küng, C.F.; Nava, E.; Lüscher, T.F. Antihypertensive therapy prevents endothelial dysfunction in chronic nitric oxide deficiency. Effect of verapamil and trandolapril. Hypertension 1996, 27, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, G.; Sonkusare, S.K.; Heppner, T.J.; Nelson, M.T. Opposing roles of smooth muscle BK channels and ryanodine receptors in the regulation of nerve-evoked constriction of mesenteric resistance arteries. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H981–H988. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, K.Y.; Thugorka, O.M.; Bouryi, V.A.; Harhun, M.I.; Gordienko, D.V. Mechanisms of the sarcoplasmic reticulum Ca2+ release induced by P2X receptor activation in mesenteric artery myocytes. Pharmacol. Rep. 2014, 66, 363–372. [Google Scholar] [CrossRef]
- Wei, R.; Lunn, S.E.; Tam, R.; Gust, S.L.; Classen, B.; Kerr, P.M.; Plane, F. Vasoconstrictor stimulus determines the functional contribution of myoendothelial feedback to mesenteric arterial tone. J Physiol. 2018, 596, 1181–1197. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, X.; Zhang, Y.; Sun, M.; Li, L.; Gao, Q.; Tang, J.; Zhang, P.; Lv, J.; Zhou, X.; et al. Prenatal hypoxia inhibited propionate-evoked BK channels of mesenteric artery smooth muscle cells in offspring. J. Cell Mol. Med. 2020, 24, 3192–3202. [Google Scholar] [CrossRef]
- Benkhalti, F.; Legssyer, A.; Gómez, P.; Paz, E.; Lopez-Miranda, J.; Jiménez, F.P.; El Boustani, E.S. Effects of virgin olive oil phenolic compounds on LDL oxidation and vasorelaxation activity. Therapies 2003, 58, 133–137. [Google Scholar] [CrossRef]
- Segade, M.; Bermejo, R.; Monteiro-Silva, F.; Paiva-Martins, F.; Gil-Longo, J.; Campos-Toimil, M. Involvement of endothelium in the vasorelaxant effects of 3,4-DHPEA-EA and 3,4-DHPEA-EDA, two major functional bioactives in olive oil. J. Funct. Foods 2016, 23, 637–646. [Google Scholar] [CrossRef]
- Koide, M.; Nystoriak, M.A.; Krishnamoorthy, G.; O’Connor, K.P.; Bonev, A.D.; Nelson, M.T.; Wellman, G.C. Reduced Ca2+ Spark Activity after Subarachnoid Hemorrhage Disables BK Channel Control of Cerebral Artery Tone. Br. J. Pharmacol. 2010, 31, 3–16. [Google Scholar] [CrossRef]
- Fan, G.; Cui, Y.; Gollasch, M.; Kassmann, M. Elementary calcium signaling in arterial smooth muscle. Channels 2019, 13, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Khavandi, K.; Baylie, R.A.; Sugden, S.A.; Ahmed, M.; Csato, V.; Eaton, P.; Hill-Eubanks, D.C.; Bonev, A.D.; Nelson, M.T.; Greenstein, A.S. Pressure-induced oxidative activation of PKG enables vasoregulation by Ca2+ sparks and BK channels. Sci. Signal. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Dabertrand, F.; Nelson, M.T.; Brayden, J.E. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ. Res. 2012, 110, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Westcott, E.B.; Goodwin, E.L.; Segal, S.S.; Jackson, W.F. Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J. Physiol. 2012, 590, 1849–1869. [Google Scholar] [CrossRef]
- Westcott, E.B.; Jackson, W.F. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1616–H1630. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Xu, Z.; Wu, Y.; Zhang, Y.; Shi, L. Chronic exercise mediates epigenetic suppression of L-type Ca2+ channel and BKCa channel in mesenteric arteries of hypertensive rats. J. Hypertens. 2020, 38, 1763–1776. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yamamura, H.; Ohya, S.; Imaizumi, Y. Caveolin-1 facilitates the direct coupling between large conductance Ca2+-activated K+ (BKCa) and Cav1.2 Ca2+ channels and their clustering to regulate membrane excitability in vascular myocytes. J. Biol. Chem. 2013, 288, 36750–36761. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, H.; Mahavadi, S.; Sriwai, W.; Murthy, K.S. Inhibition of Gαq-dependent PLC-beta1 activity by PKG and PKA is mediated by phosphorylation of RGS4 and GRK2. Am. J. Physiol. Cell Physiol. 2007, 292, C200–C208. [Google Scholar] [CrossRef]
- Al Suleimani, Y.M.; Hiley, C.R. Mechanisms of vasorelaxation induced by oleoylethanolamide in the rat small mesenteric artery. Eur. J. Pharmacol. 2013, 702, 1–11. [Google Scholar] [CrossRef]
- Ruisanchez, É.; Dancs, P.; Kerék, M.; Németh, T.; Faragó, B.; Balogh, A.; Patil, R.; Jennings, B.L.; Liliom, K.; Malik, K.U.; et al. Lysophosphatidic acid induces vasodilation mediated by LPA1 receptors, phospholipase C, and endothelial nitric oxide synthase. FASEB J. 2014, 28, 880–890. [Google Scholar] [CrossRef]
- Heathcote, H.R.; Lee, M.D.; Zhang, X.; Saunter, C.D.; Wilson, C.; McCarron, J.G. Endothelial TRPV4 channels modulate vascular tone by Ca2+ -induced Ca2+ release at inositol 1,4,5-trisphosphate receptors. Br. J. Pharmacol. 2019, 176, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Yang, J.; Santulli, G.; Reiken, S.R.; Wronska, A.; Kim, M.M.; Osborne, B.W.; Lacampagne, A.; Yin, Y.; Marks, A.R. Maintenance of normal blood pressure is dependent on IP3R1-mediated regulation of eNOS. Proc. Natl. Acad. Sci. USA 2016, 113, 8532–8537. [Google Scholar] [CrossRef] [PubMed]
- Knot, H.J.; Standen, N.B.; Nelson, M.T. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J. Physiol. 1998, 508, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Strassheim, D.; Karoor, V.; Stenmark, K.; Verin, A.; Gerasimovskaya, E. A current view of G protein-coupled receptor - mediated signaling in pulmonary hypertension: Finding opportunities for therapeutic intervention. Vessel. Plus 2018, 2, 29. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Agostino, R.; Barberio, L.; Gatto, M.; Tropea, T.; De Luca, M.; Mandalà, M. Extra Virgin Olive Oil Phenols Vasodilate Rat Mesenteric Resistance Artery via Phospholipase C (PLC)-Calcium Microdomains-Potassium Channels (BKCa) Signals. Biomolecules 2021, 11, 137. https://doi.org/10.3390/biom11020137
D’Agostino R, Barberio L, Gatto M, Tropea T, De Luca M, Mandalà M. Extra Virgin Olive Oil Phenols Vasodilate Rat Mesenteric Resistance Artery via Phospholipase C (PLC)-Calcium Microdomains-Potassium Channels (BKCa) Signals. Biomolecules. 2021; 11(2):137. https://doi.org/10.3390/biom11020137
Chicago/Turabian StyleD’Agostino, Rossana, Laura Barberio, Mariacarmela Gatto, Teresa Tropea, Maria De Luca, and Maurizio Mandalà. 2021. "Extra Virgin Olive Oil Phenols Vasodilate Rat Mesenteric Resistance Artery via Phospholipase C (PLC)-Calcium Microdomains-Potassium Channels (BKCa) Signals" Biomolecules 11, no. 2: 137. https://doi.org/10.3390/biom11020137
APA StyleD’Agostino, R., Barberio, L., Gatto, M., Tropea, T., De Luca, M., & Mandalà, M. (2021). Extra Virgin Olive Oil Phenols Vasodilate Rat Mesenteric Resistance Artery via Phospholipase C (PLC)-Calcium Microdomains-Potassium Channels (BKCa) Signals. Biomolecules, 11(2), 137. https://doi.org/10.3390/biom11020137





