Characterization of SLC34A2 as a Potential Prognostic Marker of Oncological Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Preparation
2.2. Prediction of the Functional Impact of Mutation
2.3. Analysis of the Expression Levels in Various Tumors
2.4. Survival Analysis
3. Results
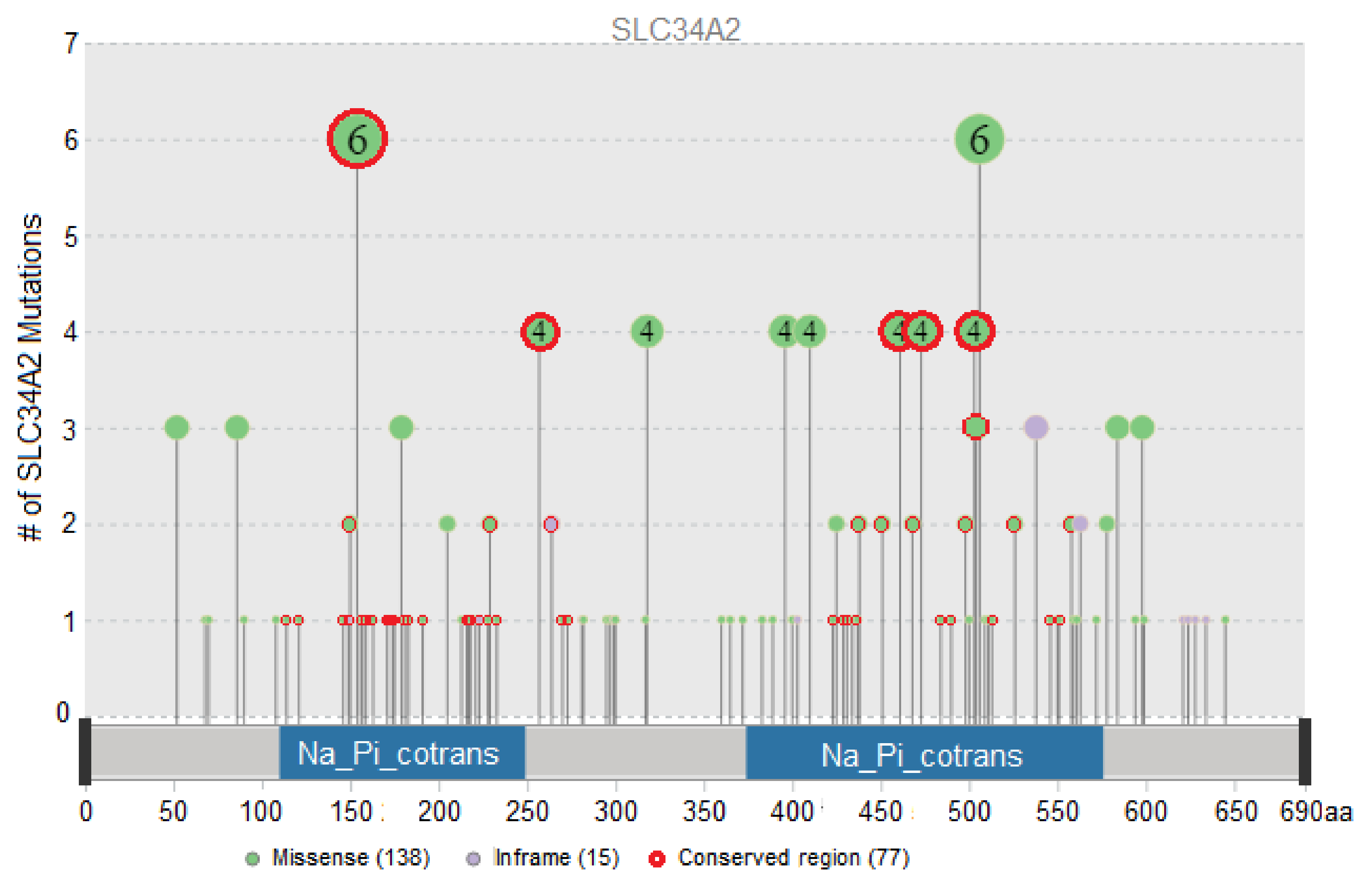
3.1. Evaluation of the Functional Impact of Missense and Indel Mutations in SLC34A2
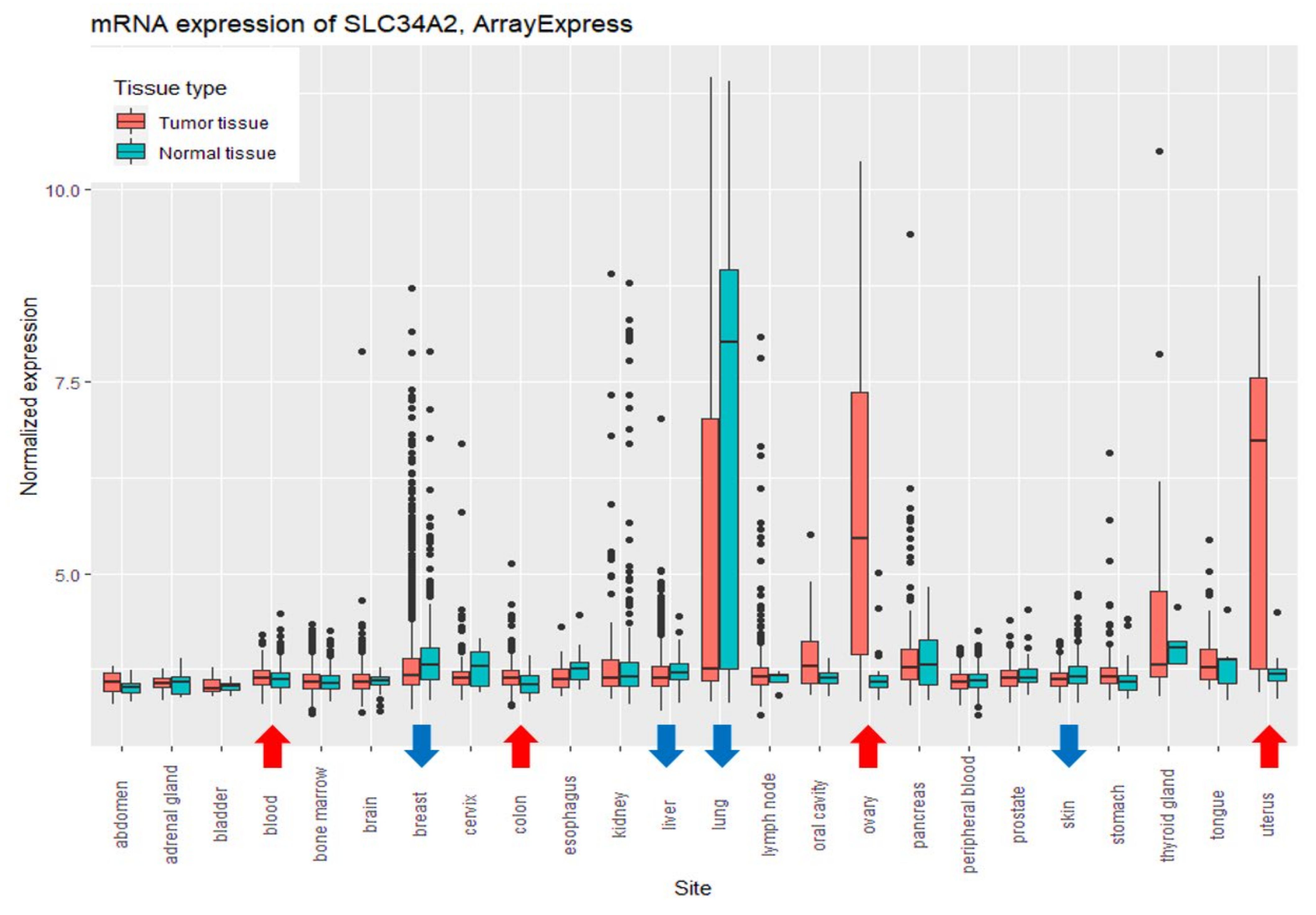
3.2. Comparison of the Expression Levels of SLC34A2 between Relatively Healthy and Tumor Tissues
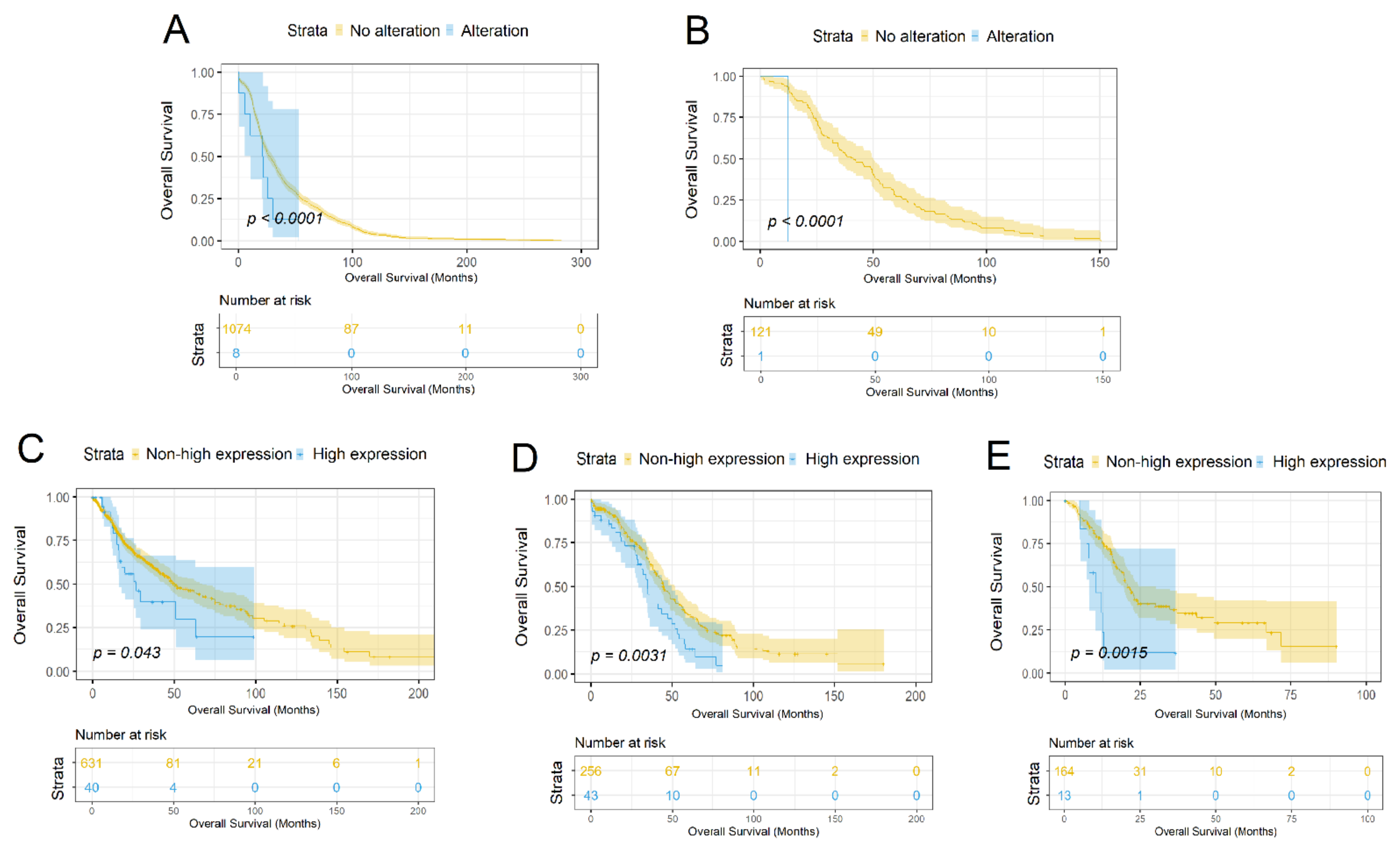
3.3. Characterization of the SLC34A2 Gene as a Prognostic Marker
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boonstra, M.C.; De Geus, S.W.; Prevoo, H.A.; Hawinkels, L.J.; Van De Velde, C.J.; Kuppen, P.J.; Vahrmeijer, A.L.; Sier, C.F. Selecting Targets for Tumor Imaging: An Overview of Cancer-Associated Membrane Proteins. Biomark. Cancer 2016, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bai, L.; Collins, J.F.; Ghishan, F.K. Molecular Cloning, Functional Characterization, Tissue Distribution, and Chromosomal Localization of a Human, Small Intestinal Sodium-Phosphate (Na+-Pi) Transporter (SLC34A2). Genomics 1999, 62, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Rangel, L.B.; Sherman-Baust, C.A.; Wernyj, R.P.; Schwartz, D.R.; Cho, K.R.; Morin, P.J. Characterization of Novel Human Ovarian Cancer-Specific Transcripts (HOSTs) Identified by Serial Analysis of Gene Expression. Oncogene 2003, 22, 7225–7232. [Google Scholar] [CrossRef]
- Murer, H.; Forster, I.; Biber, J. The Sodium Phosphate Cotransporter Family SLC34. Pflug. Arch. Eur. J. Physiol. 2004, 447, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Tenenhouse, H.S.; Hoag, H.M.; Wang, D.; Khadeer, M.A.; Namba, N.; Feng, X.; Hruska, K.A. Identification of the Type II Na(+)-Pi Cotransporter (Npt2) in the Osteoclast and the Skeletal Phenotype of Npt2-/- Mice. Bone 2001, 29, 467–476. [Google Scholar] [CrossRef]
- Sabbagh, Y.; O’Brien, S.P.; Song, W.; Boulanger, J.H.; Stockmann, A.; Arbeeny, C.; Schiavi, S.C. Intestinal Npt2b Plays a Major Role in Phosphate Absorption and Homeostasis. J. Am. Soc. Nephrol. JASN 2009, 20, 2348–2358. [Google Scholar] [CrossRef]
- Kopantzev, E.P.; Monastyrskaya, G.S.; Vinogradova, T.V.; Zinovyeva, M.V.; Kostina, M.B.; Filyukova, O.B.; Tonevitsky, A.G.; Sukhikh, G.T.; Sverdlov, E.D. Differences in Gene Expression Levels between Early and Later Stages of Human Lung Development Are Opposite to Those between Normal Lung Tissue and Non-Small Lung Cell Carcinoma. Lung Cancer 2008, 62, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, Y.; Pu, Q.; Ye, S.; Ma, Q.; Ren, J.; Zhong, G.; Liu, L.; Zhu, W. Elevated Expression of SLC34A2 Inhibits the Viability and Invasion of A549 Cells. Mol. Med. Rep. 2014, 10, 1205. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Z.; Shi, S.; Wang, J.; Li, N. Long Non-Coding RNA MEG3 Regulates Migration and Invasion of Lung Cancer Stem Cells via MiR-650/SLC34A2 Axis. Biomed. Pharmacother. 2019, 120, 109457. [Google Scholar] [CrossRef]
- Jiang, Z.; Hao, Y.; Ding, X.; Zhang, Z.; Liu, P.; Wei, X.; Xi, J. The Effects and Mechanisms of SLC34A2 on Tumorigenicity in Human Non-Small Cell Lung Cancer Stem Cells. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 10383–10392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, X.; Zhang, L.; Yang, F.; Qin, L.; Zhang, D.; Qin, Y. SLC34A2 Regulates MiR-25-Gsk3β Signaling Pathway to Affect Tumor Progression in Gastric Cancer Stem Cell-like Cells. Mol. Carcinog. 2018, 57, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, H.; Xiao, J.; Shao, C.; Zhou, Y.; Cong, W.; Gong, M.; Sun, J.; Shan, L.; Hao, Z.; et al. SLC34A2 Up-Regulation And SLC4A4 Down-Regulation Correlates With Invasion, Metastasis, And The MAPK Signaling Pathway In Papillary Thyroid Carcinomas. Int. Publ. J. Cancer 2021, 12, 5439–5453. [Google Scholar] [CrossRef] [PubMed]
- Kiyamova, R.; Shyian, M.; Lyzogubov, V.V.; Usenko, V.S.; Gout, T.; Filonenko, V. Immunohistochemical Analysis of NaPi2b Protein (MX35 Antigen) Expression and subcellular Localization in Human Normal and Cancer Tissues. Exp. Oncol. 2011, 33, 157–161. [Google Scholar] [PubMed]
- Yin, B.; Kiyamova, R.; Chua, R.; Caballero, O.L.; Gout, I.; Gryshkova, V.; Bhaskaran, N.; Souchelnytskyi, S.; Hellman, U.; Filonenko, V.; et al. Monoclonal Antibody MX35 Detects the membrane Transporter NaPi2b (SLC34A2) in Human Carcinomas. Cancer Immun. J. Acad. Cancer Immunol. 2008, 8, 3. [Google Scholar]
- Kiyamova, R.G.; Gryshkova, V.S.; Usenko, V.S.; Khozaenko, Y.S.; Gurtovyy, V.A.; Yin, B.; Ritter, G.; Old, L.; Gout, I.T.; Filonenko, V.V. Identification of Phosphate Transporter Napi2b as MX35 Cancer Antigen by Modified SEREX Approach. Biopolym. Cell 2008, 24, 218–224. [Google Scholar] [CrossRef]
- Bodyak, N.D.; Mosher, R.; Yurkovetskiy, A.V.; Yin, M.; Bu, C.; Conlon, P.R.; Demady, D.R.; DeVit, M.J.; Gumerov, D.R.; Gurijala, V.R.; et al. The Dolaflexin-Based Antibody–Drug Conjugate XMT-1536 Targets the Solid Tumor Lineage Antigen SLC34A2/NaPi2b. Mol. Cancer Ther. 2021, 20, 896–905. [Google Scholar] [CrossRef]
- Dos Santos, M.L.; Yeda, F.P.; Tsuruta, L.R.; Horta, B.B.; Pimenta, A.A.; Degaki, T.L.; Soares, I.C.; Tuma, M.C.; Okamoto, O.K.; Alves, V.A.F.; et al. Rebmab200, a Humanized Monoclonal Antibody Targeting the Sodium Phosphate Transporter NaPi2b Displays Strong Immune Mediated Cytotoxicity against Cancer: A Novel Reagent for Targeted Antibody Therapy of Cancer. PLoS ONE 2013, 8, e70332. [Google Scholar] [CrossRef]
- Kato, Y.; Ninomiya, K.; Ohashi, K.; Tomida, S.; Makimoto, G.; Watanabe, H.; Kudo, K.; Matsumoto, S.; Umemura, S.; Goto, K.; et al. Combined Effect of Cabozantinib and Gefitinib in Crizotinib-Resistant Lung Tumors Harboring ROS1 Fusions. Cancer Sci. 2018, 109, 3149–3158. [Google Scholar] [CrossRef]
- Yin, X.; Wang, H.; Wu, D.; Zhao, G.; Shao, J.; Dai, Y. SLC34A2 Gene Mutation of Pulmonary Alveolar Microlithiasis: Report of Four Cases and Review of Literatures. Respir. Med. 2013, 107, 217–222. [Google Scholar] [CrossRef][Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- AACR Project GENIE AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [CrossRef]
- Hudson, T.J.; Anderson, W.; Artez, A.; Barker, A.D.; Bell, C.; Bernabé, R.R.; Bhan, M.K.; Calvo, F.; Eerola, I.; Gerhard, D.S.; et al. International Network of Cancer Genome Projects. Nature 2010, 464, 993–998. [Google Scholar] [CrossRef]
- Athar, A.; Füllgrabe, A.; George, N.; Iqbal, H.; Huerta, L.; Ali, A.; Snow, C.; Fonseca, N.A.; Petryszak, R.; Papatheodorou, I.; et al. ArrayExpress Update—From Bulk to Single-Cell Expression Data. Nucleic Acids Res. 2019, 47, D711–D715. [Google Scholar] [CrossRef] [PubMed]
- Torrente, A.; Lukk, M.; Xue, V.; Parkinson, H.; Rung, J.; Brazma, A. Identification of Cancer Related Genes Using a Comprehensive Map of Human Gene Expression. PLoS ONE 2016, 11, e0157484. [Google Scholar] [CrossRef]
- Choi, Y.; Sims, G.E.; Murphy, S.; Miller, J.R.; Chan, A.P. Predicting the Functional Effect of Amino Acid Substitutions and Indels. PLoS ONE 2012, 7, e46688. [Google Scholar] [CrossRef]
- Sim, N.L.; Kumar, P.; Hu, J.; Henikoff, S.; Schneider, G.; Ng, P.C. SIFT Web Server: Predicting Effects of Amino Acid Substitutions on Proteins. Nucleic Acids Res. 2012, 40, W452–W457. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A Method and Server for Predicting Damaging Missense Mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Thomas, P.D. PANTHER in 2013: Modeling the Evolution of Gene Function, and Other Gene Attributes, in the Context of Phylogenetic Trees. Nucleic Acids Res. 2013, 41, D377–D386. [Google Scholar] [CrossRef] [PubMed]
- Shihab, H.A.; Gough, J.; Cooper, D.N.; Stenson, P.D.; Barker, G.L.; Edwards, K.J.; Day, I.N.; Gaunt, T.R. Predicting the Functional, Molecular, and Phenotypic Consequences of Amino Acid Substitutions Using Hidden Markov Models. Hum. Mutat. 2013, 34, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Reva, B.; Antipin, Y.; Sander, C. Predicting the Functional Impact of Protein Mutations: Application to Cancer Genomics. Nucleic Acids Res. 2011, 39, e118. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The Conserved Domain Database in 2020. Nucleic Acids Res. 2020, 48, D265. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.E.; Kang, S.Y.; Do, I.G.; Lee, S.; Ha, S.Y.; Cho, J.; Kang, W.K.; Jang, J.; Ou, S.H.; et al. Identification of ROS1 Rearrangement in Gastric Adenocarcinoma. Cancer 2013, 119, 1627–1635. [Google Scholar] [CrossRef]
- Li, X.; Xing, J.; Wang, H.; Yu, E. The SLC34A2-ROS-HIF-1-Induced up-Regulation of EZH2 Expression Promotes Proliferation and Chemo-Resistance to Apoptosis in Colorectal Cancer. Biosci. Rep. 2019, 39, BSR20180268. [Google Scholar] [CrossRef]
- Lin, K.; Rubinfeld, B.; Zhang, C.; Firestein, R.; Harstad, E.; Roth, L.; Tsai, S.P.; Schutten, M.; Xu, K.; Hristopoulos, M.; et al. Preclinical Development of an Anti-NaPi2b (SLC34A2) Antibody-Drug Conjugate as a Therapeutic for Non-Small Cell Lung and Ovarian Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 5139–5150. [Google Scholar] [CrossRef]
- Davis, L.; Mills, K.I.; Orchard, K.H.; Guinn, B.-A. Identification of Genes Whose Expression Overlaps Age Boundaries and Correlates with Risk Groups in Paediatric and Adult Acute Myeloid Leukaemia. Cancers 2020, 12, 2769. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Y.; Zhou, X.; Yan, X.; Wu, Z. Solute Carrier Family 34 Member 2 Overexpression Contributes to Tumor Growth and Poor Patient Survival in Colorectal Cancer. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 99, 645–654. [Google Scholar] [CrossRef]
- Shyian, M.; Gryshkova, V.; Kostianets, O.; Gorshkov, V.; Gogolev, Y.; Goncharuk, I.; Nespryadko, S.; Vorobjova, L.; Filonenko, V.; Kiyamova, R. Quantitative Analysis of SLC34A2 Expression in Different Types of Ovarian Tumors—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21716206/ (accessed on 28 July 2021).
- Kiyamova, R.; Gryshkova, V.; Ovcharenko, G.; Lituyev, D.; Malyuchik, S.; Usenko, V.; Khozhayenko, Y.; Gurtovyy, V.; Yin, B.; Ritter, G.; et al. Development of Monoclonal Antibodies Specific for the Human Sodium-Dependent Phosphate Co-Transporter NaPi2b. Hybridoma 2008, 27, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.R.; Chien, S.Y.; Kuo, S.J.; Teng, Y.H.; Tsai, H.T.; Kuo, J.H.; Chung, J.G. SLC34A2 as a Novel Marker for Diagnosis and Targeted Therapy of Breast Cancer—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21036732/ (accessed on 28 July 2021).
- Li, Y.; Chen, X.; Lu, H. Knockdown of SLC34A2 Inhibits Hepatocellular Carcinoma Cell Proliferation and Invasion. Oncol. Res. 2016, 24, 511. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, S.; Zhang, M.; Wu, J.; Yan, H.; Li, X.; He, J. High Expression of SLC34A2 Is a Favorable Prognostic Marker in Lung Adenocarcinoma Patients. Tumor Biol. 2017, 39, 1010428317720212. [Google Scholar] [CrossRef]
- Ge, G.; Zhou, C.; Ren, Y.; Tang, X.; Wang, K.; Zhang, W.; Niu, L.; Zhou, Y.; Yan, Y.; He, J. Enhanced SLC34A2 in Breast Cancer Stem Cell-like Cells Induces Chemotherapeutic Resistance to Doxorubicin via SLC34A2-Bmi1-ABCC5 Signaling. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 5049–5062. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wang, T.; Fan, J.; Zhang, Z.; Zhang, J.; Xu, C.; Li, Y.; Zhao, G.; He, C.; Meng, H.; et al. The Effects and Mechanisms of SLC34A2 on Maintaining Stem Cell-like Phenotypes in CD147 + Breast Cancer Stem Cells. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2017, 39, 1010428317695927. [Google Scholar] [CrossRef] [PubMed]
- Gryshkova, V.; Goncharuk, I.; Gurtovyy, V.; Khozhayenko, Y.; Nespryadko, S.; Vorobjova, L.; Usenko, V.; Gout, I.; Filonenko, V.; Kiyamova, R. The Study of Phosphate Transporter NAPI2B Expression in Different Histological Types of Epithelial Ovarian Cancer. Available online: https://pubmed.ncbi.nlm.nih.gov/19300415/ (accessed on 3 August 2021).
- Bao, Z.; Chen, L.; Guo, S. Knockdown of SLC34A2 Inhibits Cell Proliferation, Metastasis, and Elevates Chemosensitivity in Glioma. J. Cell. Biochem. 2019, 120, 10205–10214. [Google Scholar] [CrossRef] [PubMed]
| Location | cBioPortal, TCGA | ICGC | cBioPortal, Genie | ArrayExpress |
|---|---|---|---|---|
| Adrenal gland | 92 | - | - | 36 |
| Biliary Tract | 36 | - | - | - |
| Bladder | 411 | - | 2118 | 20 |
| Bone | - | - | 718 | 1987 |
| Bowel | 594 | 411 | 9682 | 947 |
| Breast | 1084 | 569 | 11,742 | 1505 |
| CNS and Brain | 1106 | - | 6609 | 791 |
| Cervix | 297 | - | - | 292 |
| Esophagus and Stomach | 622 | - | 4419 | 259 |
| Eye | 80 | - | - | - |
| Head and neck | 523 | - | 2223 | 120 |
| Kidney | 860 | - | 1665 | 293 |
| Liver | 372 | 773 | - | 636 |
| Lung | 1053 | 170 | 14,844 | 794 |
| Lymphoid | 48 | 274 | 2625 | 565 |
| Myeloid | 200 | - | 2820 | 2431 |
| Ovary | 585 | - | 3527 | 573 |
| Pancreas | 184 | - | 3613 | 235 |
| Pleura | 87 | - | 620 | - |
| Prostate | 494 | - | 3490 | 314 |
| Skin | 448 | - | 4537 | 647 |
| Soft Tissue | 433 | - | 2752 | - |
| Testis | 149 | - | - | - |
| Thymus | 123 | - | - | - |
| Thyroid | 500 | - | 1398 | 30 |
| Uterus | 586 | - | 2905 | 275 |
| Various tumors | - | - | 3065 | - |
| Sample Count | 10,967 | 2197 | 85,369 | 12,750 |
| Protein Consequence | Tumor Locations and Sample Count | Total Sample Count | Conserved Region |
|---|---|---|---|
| SLC34A2-ROS1 | Lung (10) | 10 | |
| p.T154A | Breast (1), Esophagus and Stomach (2), Lymphoid (1), Ovary (1), Pleura (1) | 6 | + |
| p.P506S/R/L | Bowel (1), Liver (2), Lung (1), Skin (2) | 6 | |
| p.G257A/E/R | Lung (1), Ovary (1), Skin (2) | 4 | + |
| p.S318W | Breast (2), Kidney (2) | 4 | |
| p.A396T | Lung (1), Thymus (1), Uterus (2) | 4 | |
| p.P410L/S/H | Skin (3), Uterus (1) | 4 | |
| p.S461C | Lung (4) | 4 | + |
| p.A473T/V | Esophagus and Stomach (1), Head and neck (1), Lung (1), Uterus (1) | 4 | + |
| p.Y503H/C/F | Bowel (2), Esophagus and Stomach (1), Head and neck (1) | 4 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasenkova, R.; Nurgalieva, A.; Akberova, N.; Bogdanov, M.; Kiyamova, R. Characterization of SLC34A2 as a Potential Prognostic Marker of Oncological Diseases. Biomolecules 2021, 11, 1878. https://doi.org/10.3390/biom11121878
Vlasenkova R, Nurgalieva A, Akberova N, Bogdanov M, Kiyamova R. Characterization of SLC34A2 as a Potential Prognostic Marker of Oncological Diseases. Biomolecules. 2021; 11(12):1878. https://doi.org/10.3390/biom11121878
Chicago/Turabian StyleVlasenkova, Ramilia, Alsina Nurgalieva, Natalia Akberova, Mikhail Bogdanov, and Ramziya Kiyamova. 2021. "Characterization of SLC34A2 as a Potential Prognostic Marker of Oncological Diseases" Biomolecules 11, no. 12: 1878. https://doi.org/10.3390/biom11121878
APA StyleVlasenkova, R., Nurgalieva, A., Akberova, N., Bogdanov, M., & Kiyamova, R. (2021). Characterization of SLC34A2 as a Potential Prognostic Marker of Oncological Diseases. Biomolecules, 11(12), 1878. https://doi.org/10.3390/biom11121878









