Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution
Abstract
1. Introduction
2. SARS-CoV-2 and COVID-19
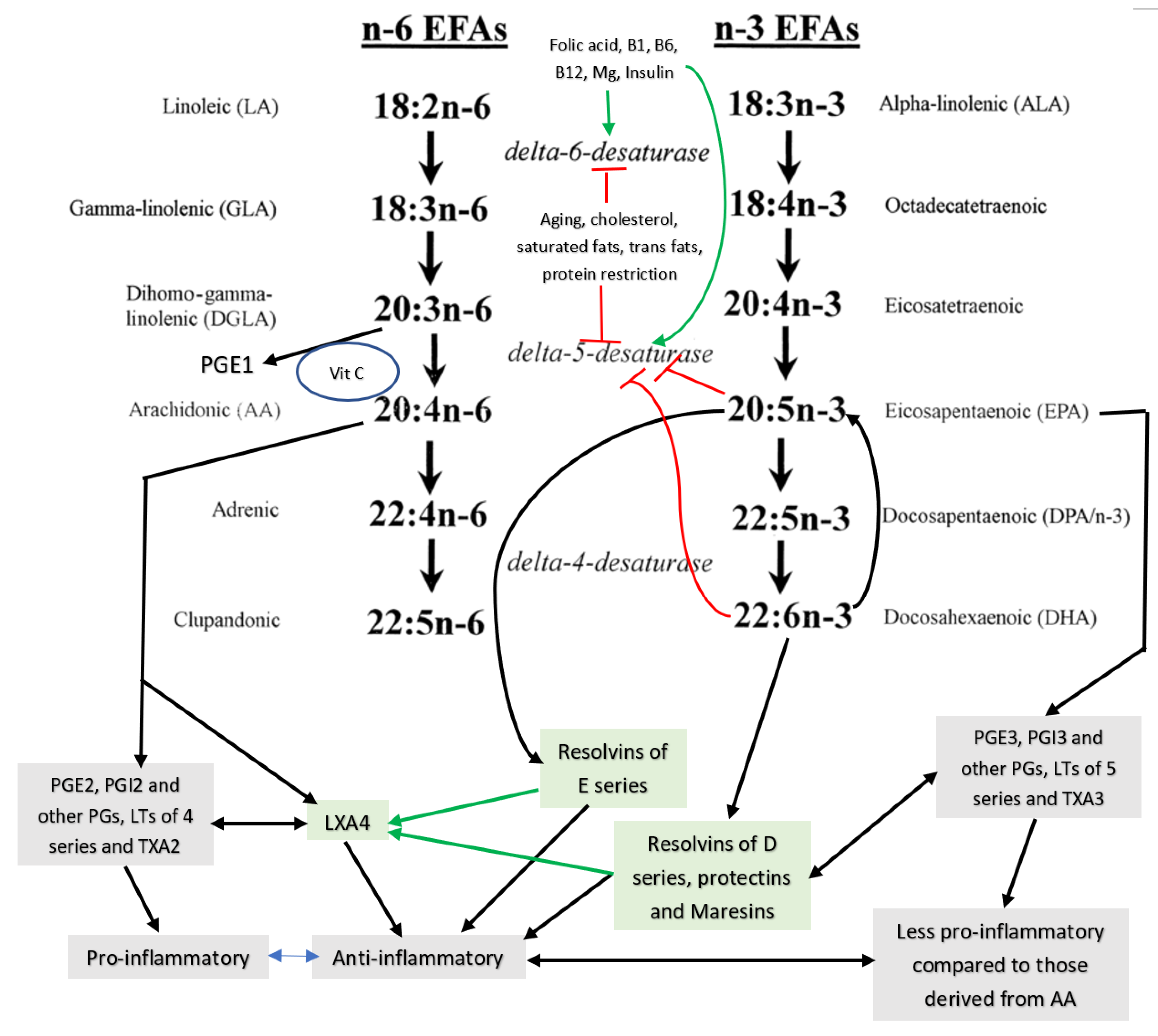
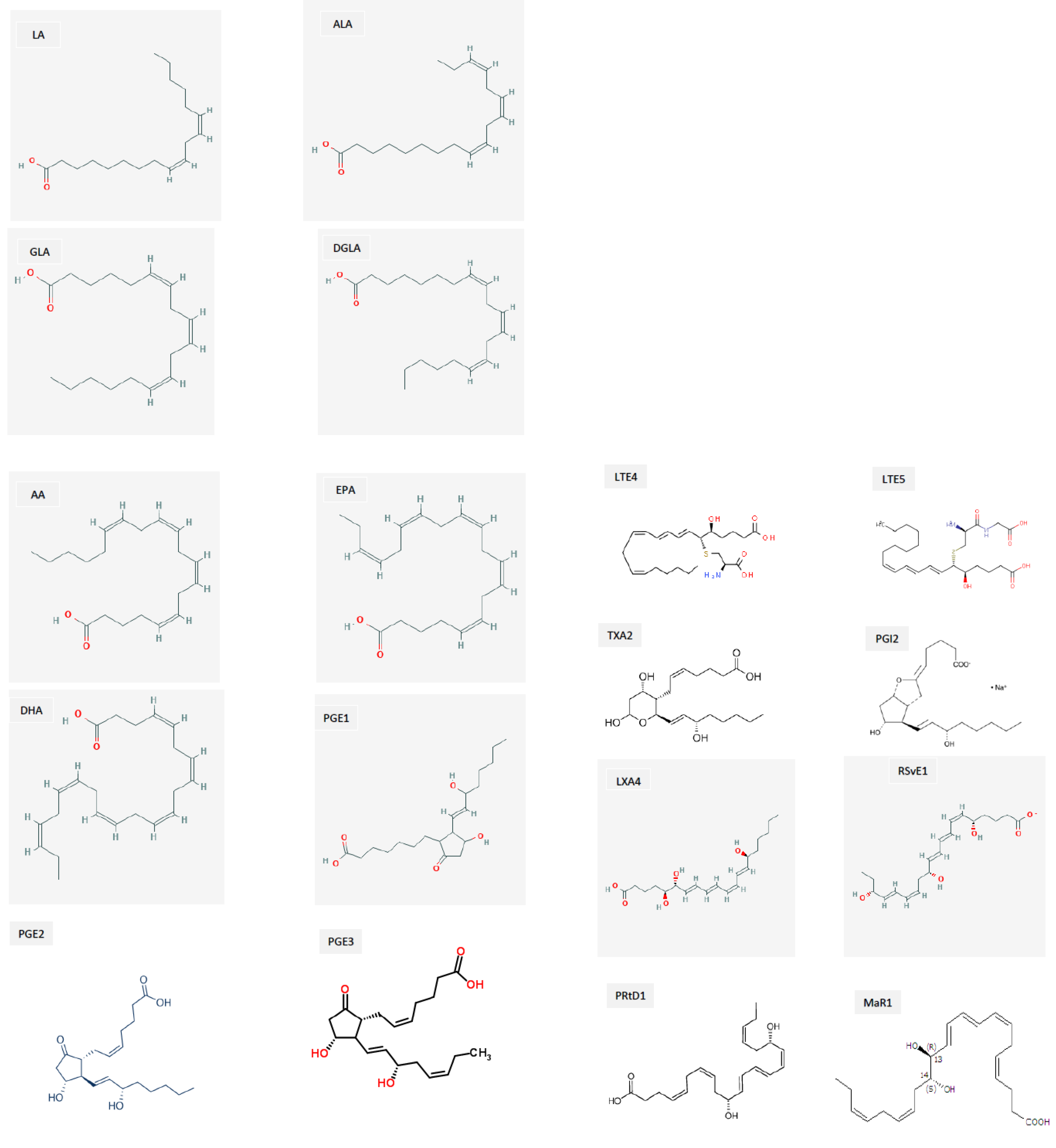
3. EFAs’ (Essential Fatty Acids’) Metabolism
4. Interaction among Various EFAs and Their Pro- and Anti-Inflammatory Metabolites
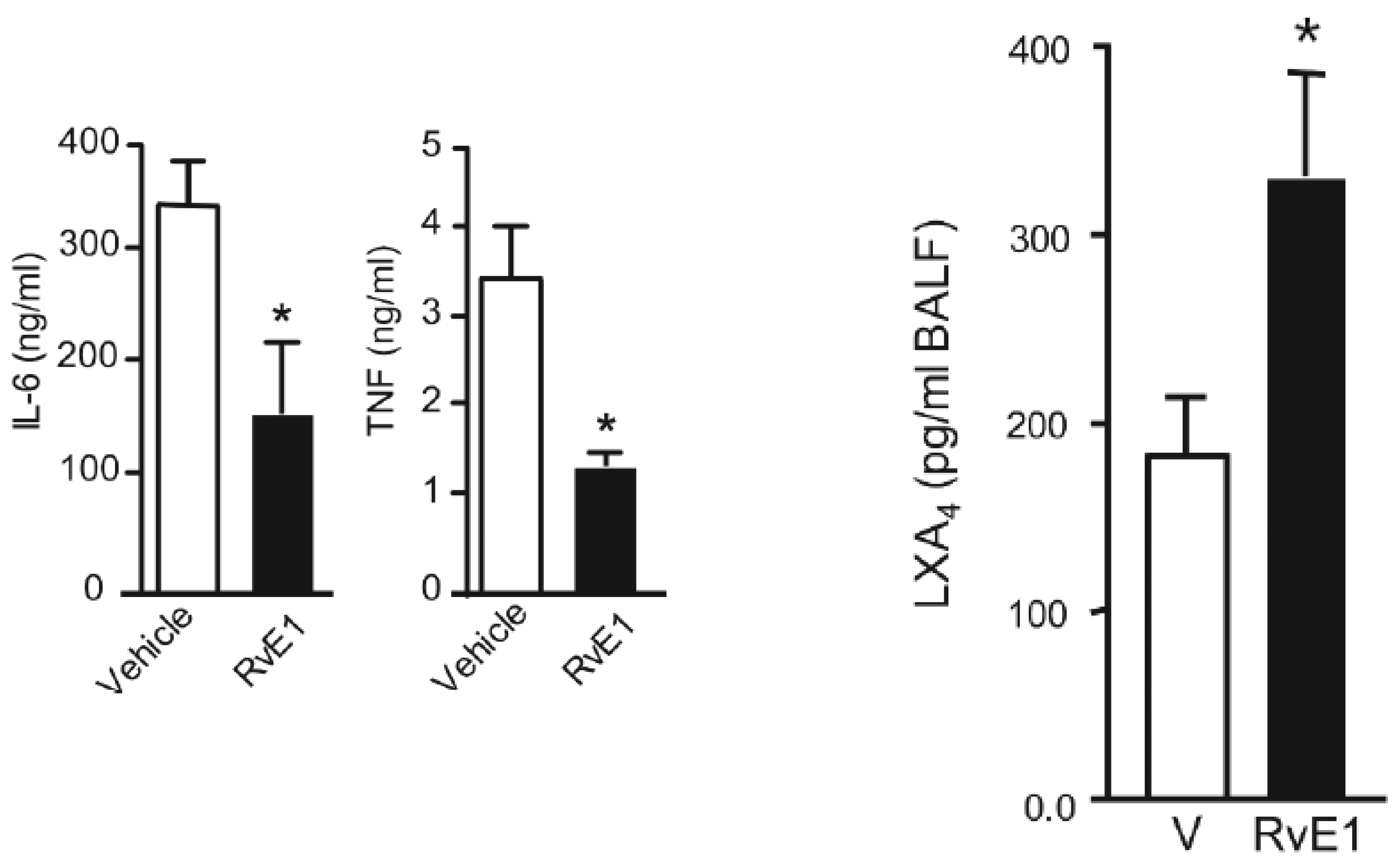
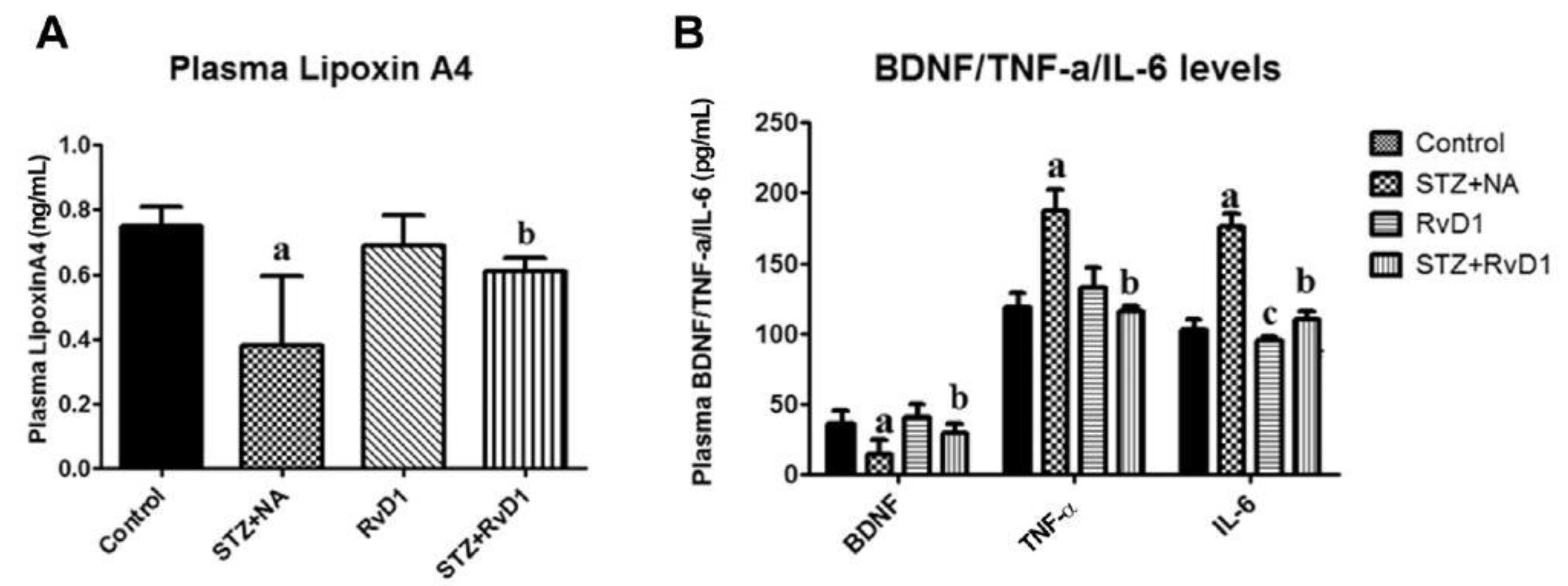
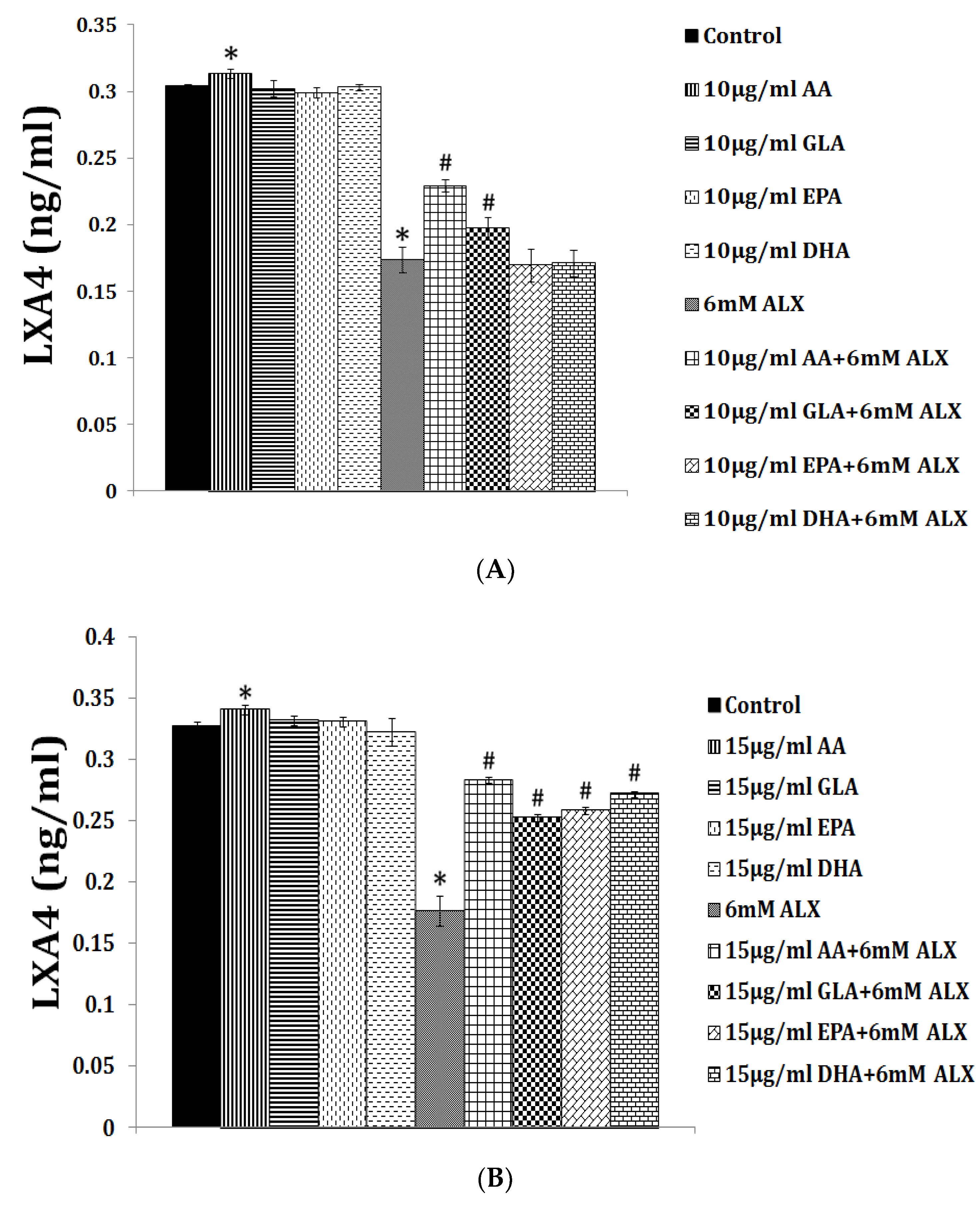
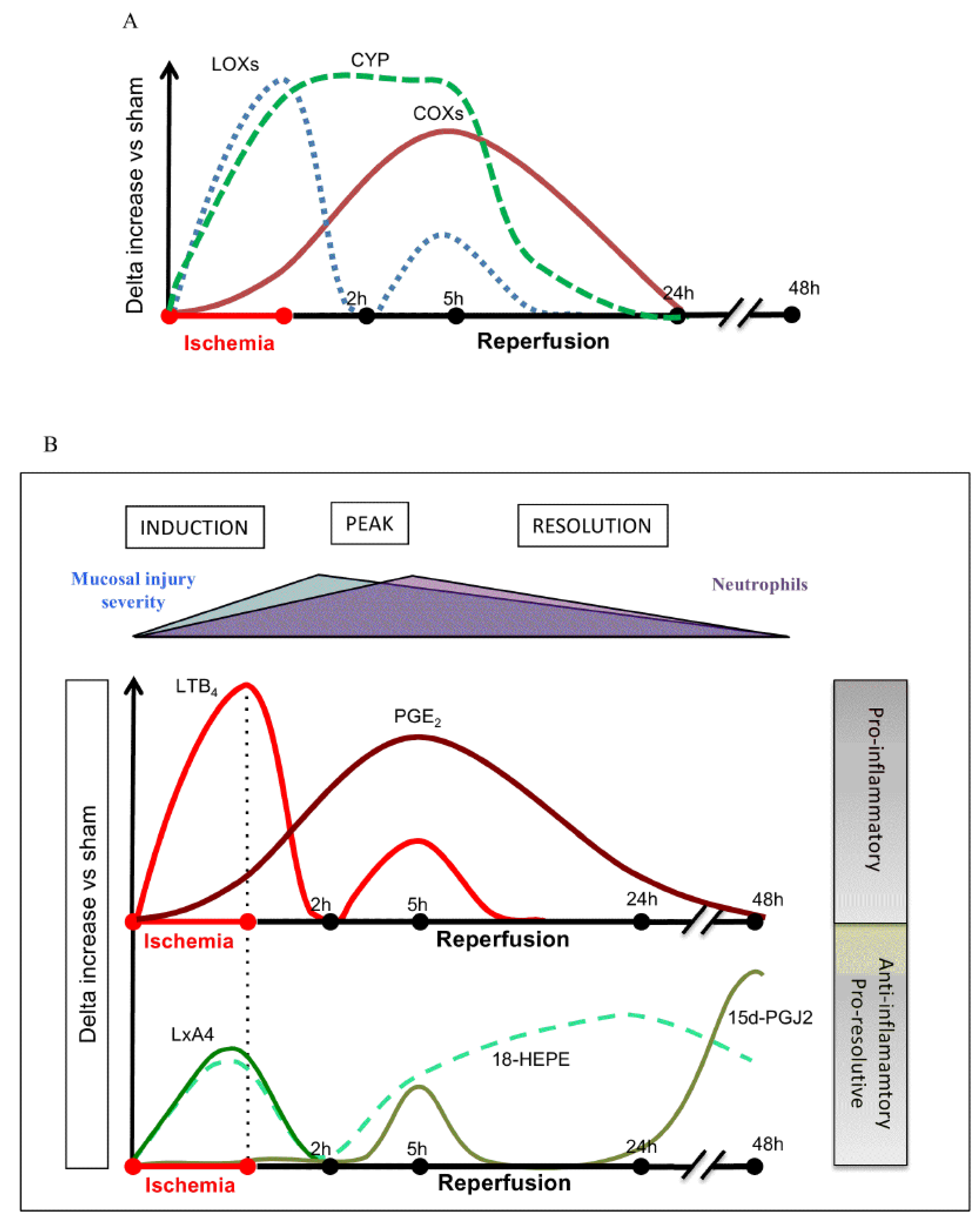
5. EFAs and Their Metabolites in Inflammation
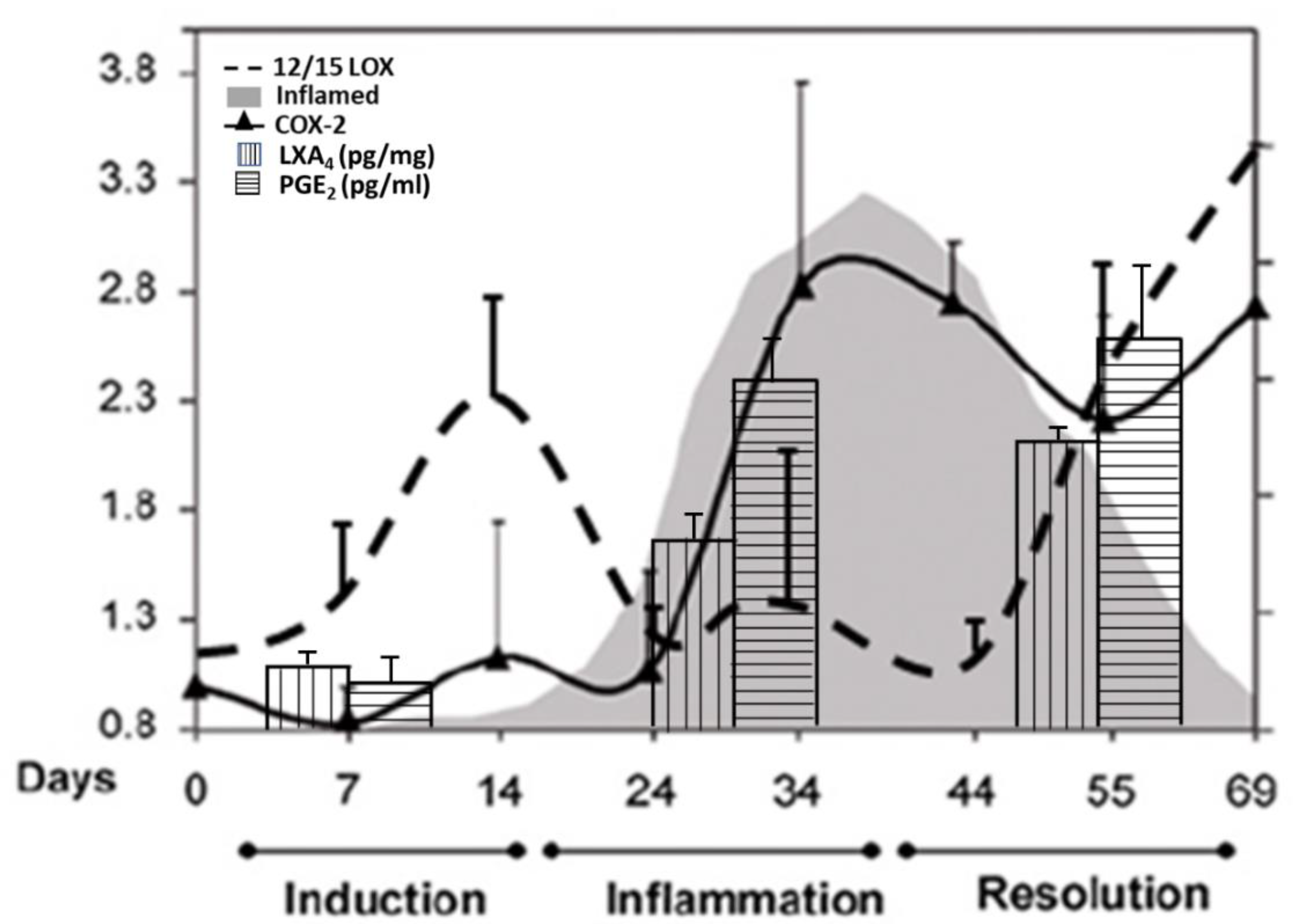
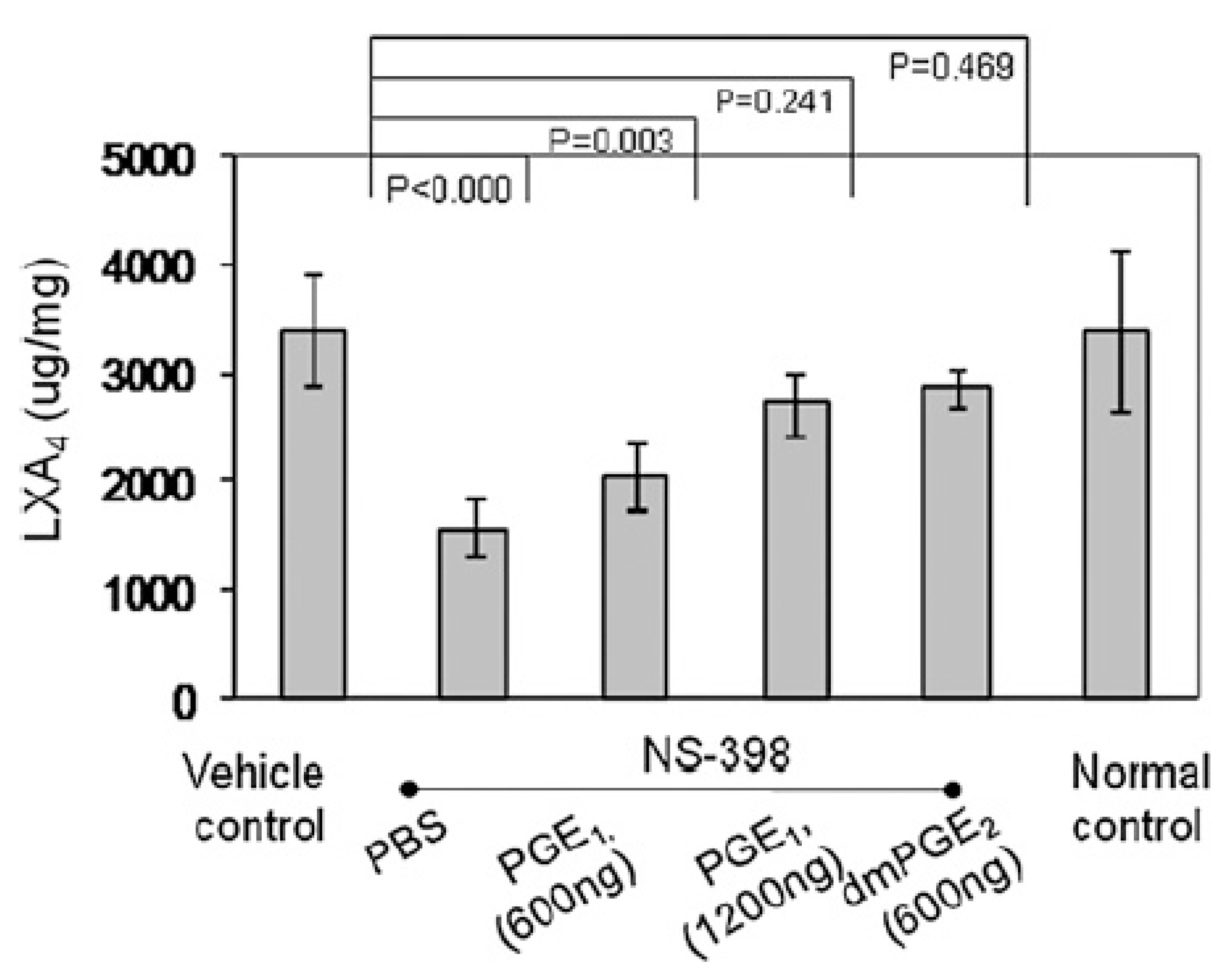
6. PGE2 and LXA4 Regulate Inflammation and Its Resolution
7. Critical Role of AA in Inflammation Resolution
8. AA and Other Bioactive Lipids Possess Antimicrobial Action
9. BALs Prevent Microbial Infection Including SARS-CoV-2
10. Conclusions and Therapeutic Implications
Funding
Conflicts of Interest
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Van De Veerdonk, F.L.; Netea, M.G.; Van Deuren, M.; Van Der Meer, J.W.; De Mast, Q.; Brüggemann, R.J.; Van Der Hoeven, H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife 2020, 9, 57555. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kuo, H.-H.; Boucau, J.; Farmer, J.R.; Allard-Chamard, H.; Mahajan, V.S.; Piechocka-Trocha, A.; Lefteri, K.; Osborn, M.; Bals, J.; et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 2020, 183, 143–157. [Google Scholar] [CrossRef]
- Kox, M.; Waalders, N.J.B.; Kooistra, E.J.; Gerretsen, J.; Pickkers, P. Cytokine Levels in Critically Ill Patients With COVID-19 and Other Conditions. JAMA 2020, 324, 1565–1567. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.A.P.M.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tsang, O.T.-Y.; et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, 8511. [Google Scholar] [CrossRef]
- Das, U. “Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and their Therapeutic Implications. Biomolecules 2021, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Das, U. Essential fatty acid metabolism in patients with essential hypertension, diabetes mellitus and coronary heart disease. Prostaglandins Leukot. Essent. Fat. Acids 1995, 52, 387–391. [Google Scholar] [CrossRef]
- Poorani, R.; Bhatt, A.N.; Dwarakanath, B.; Das, U.N. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur. J. Pharmacol. 2016, 785, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008, 7, 37. [Google Scholar] [CrossRef]
- Das, U.N. Bioactive Lipids in Age-Related Disorders. Adv. Exp. Med. Biol. 2020, 1260, 33–83. [Google Scholar] [CrossRef] [PubMed]
- Gundala, N.K.V.; Naidu, V.G.M.; Das, U.N. Arachidonic acid and lipoxinA4 attenuate streptozotocin-induced cytotoxicity to RIN5 F cells in vitro and type 1 and type 2 diabetes mellitus in vivo. Nutrition 2017, 35, 61–80. [Google Scholar] [CrossRef]
- Bathina, S.; Gundala, N.K.; Rhenghachar, P.; Polavarapu, S.; Hari, A.D.; Sadananda, M.; Das, U.N. Resolvin D1 Ameliorates Nicotinamide-streptozotocin-induced Type 2 Diabetes Mellitus by its Anti-inflammatory Action and Modulating PI3K/Akt/mTOR Pathway in the Brain. Arch. Med. Res. 2020, 51, 492–503. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U. Resolvin D1 Decreases Severity of Streptozotocin-Induced Type 1 Diabetes Mellitus by Enhancing BDNF Levels, Reducing Oxidative Stress, and Suppressing Inflammation. Int. J. Mol. Sci. 2021, 22, 1516. [Google Scholar] [CrossRef] [PubMed]
- Haworth, O.; Cernadas, M.; Yang, R.; Serhan, C.N.; Levy, B.D. Resolvin E1 regulates interleukin 23, interferon-γ and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008, 9, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Gundala, N.K.V.; Naidu, V.G.M.; Das, U.N. Arachidonic acid and lipoxin A4 attenuate alloxan-induced cytotoxicity to RIN5F cellsin vitroand type 1 diabetes mellitusin vivo. BioFactors 2017, 43, 251–271. [Google Scholar] [CrossRef]
- Riemersma, R.A.; Wood, D.A.; Butler, S.; Elton, R.A.; Oliver, M.; Salo, M.; Nikkari, T.; Vartiainen, E.; Puska, P.; Gey, F. Linoleic acid content in adipose tissue and coronary heart disease. BMJ 1986, 292, 1423–1427. [Google Scholar] [CrossRef]
- Wood, D.; Riemersma, R.; Butler, S.; Thomson, M.; Oliver, M.; Fulton, M.; Birtwhistle, A.; Elton, R. Adipose tissue and platelet fatty acids and coronary heart disease in Scottish men. Lancet 1984, 324, 117–121. [Google Scholar] [CrossRef]
- Wood, D.; Butler, S.; Macintyre, C.; Riemersma, R.; Thomson, M.; Elton, R.; Oliver, M. Linoleic and eicosapentaenoic acids in adipose tissue and platelets and risk of coronary heart disease. Lancet 1987, 329, 177–183. [Google Scholar] [CrossRef]
- Wood, D.A.; Butler, S.M.; Fulton, M.; Birtwhistle, A.; Riemersma, R.A.; Oliver, M.F.; Elton, R. Does low dietary and serum linoleic acid predispose to myocardial infarction? BMJ 1982, 285, 1738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salo, M.K.; Vartiainen, E.; Puska, P.; Nikkari, T. Platelet Aggregation in Finnish Men and Its Relation to Fatty Acids in Platelets, Plasma and Adipose Tissue. Thromb. Haemost. 1985, 54, 563–569. [Google Scholar] [CrossRef]
- Das, U.N. Biological significance of arachidonic acid. Med. Sci. Res. 1987, 15, 1485–1490. [Google Scholar]
- Willis, A. Dihomo-γ-Linolenic acid as the endogenous protective agent for myocardial infarction. Lancet 1984, 324, 697. [Google Scholar] [CrossRef]
- Juan, H.; Sametz, W. Dihomo-δ-linolenic acid increases the metabolism of eicosapentaenoic acid in perfused vascular tissue. Prostaglandins Leukot. Med. 1985, 19, 79–86. [Google Scholar] [CrossRef]
- Dooper, M.M.B.W.; Van Riel, B.; Graus, Y.M.F.; M’Rabet, L. Dihomo-γ-linolenic acid inhibits tumour necrosis factor-α production by human leucocytes independently of cyclooxygenase activity. Immunology 2003, 110, 348–357. [Google Scholar] [CrossRef]
- Cao, D.; Luo, J.; Zang, W.; Chen, D.; Xu, H.; Shi, H.; Jing, X. Gamma-Linolenic Acid Suppresses NF-κΒ Signaling via CD36 in the Lipopolysaccharide-Induced Inflammatory Response in Primary Goat Mammary Gland Epithelial Cells. Inflammation 2016, 39, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-S.; Sun, H.-L.; Lii, C.-K.; Chen, H.-W.; Chen, P.-Y.; Liu, K.-L. Gamma-Linolenic Acid Inhibits Inflammatory Responses by Regulating NF-κB and AP-1 Activation in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Inflammation 2010, 33, 46–57. [Google Scholar] [CrossRef]
- Kapoor, R. Gamma Linolenic Acid: An Antiinflammatory Omega-6 Fatty Acid. Curr. Pharm. Biotechnol. 2006, 7, 531–534. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Wang, J.; Lin, Y.-C.; Li, C.-C.; Tsai, C.-W.; Liu, T.-C.; Chen, H.-W.; Huang, C.-S.; Lii, C.-K.; Liu, K.-L. 18-Carbon polyunsaturated fatty acids ameliorate palmitate-induced inflammation and insulin resistance in mouse C2C12 myotubes. J. Nutr. Biochem. 2015, 26, 521–531. [Google Scholar] [CrossRef]
- Novak, F.; Vecka, M.; Meisnerova, E.; Sevela, S.; Vavrova, L.; Rychlikova, J.; Dolezalova, L.; Myslivcova, D.; Zak, A.; Vitek, L.; et al. Fish oil supplementation with various lipid emulsions suppresses in vitro cytokine release in home parenteral nutrition patients: A crossover study. Nutr. Res. 2019, 72, 70–79. [Google Scholar] [CrossRef]
- Hao, W.; Wong, O.Y.; Liu, X.; Lee, P.; Chen, Y.; Wong, K.K. ω-3 fatty acids suppress inflammatory cytokine production by macrophages and hepatocytes. J. Pediatr. Surg. 2010, 45, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Current and emerging strategies for the treatment and management of systemic lupus erythematosus based on mo-lecular signatures of acute and chronic inflammation. J. Inflamm. Res. 2010, 3, 143–170. [Google Scholar] [CrossRef]
- Loynes, C.A.; Lee, J.A.; Robertson, A.L.; Steel, M.J.; Ellett, F.; Feng, Y.; Levy, B.D.; Whyte, M.K.; Renshaw, S.A. PGE 2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci. Adv. 2018, 4, eaar8320. [Google Scholar] [CrossRef]
- Chan, M.M.-Y.; Moore, A.R. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibi-tion and restored by prostaglandin E2-mediated lipoxin A4 production. J. Immunol. 2010, 184, 6418–6426. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, D.; Colvillenash, P.R.; Willis, D.K.; Chivers, J.; Paulclark, M.J.; Willoughby, D. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999, 5, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, T.; Le Faouder, P.; Bertrand, J.; Dubourdeau, M.; Barocelli, E.; Cenac, N.; Vergnolle, N. Polyunsaturated Fatty Acid Metabolism Signature in Ischemia Differs from Reperfusion in Mouse Intestine. PLoS ONE 2013, 8, e75581. [Google Scholar] [CrossRef]
- Gobbetti, T.; Ducheix, S.; Le Faouder, P.; Perez-Berezo, T.; Riols, F.; Boue, J.; Bertrand-Michel, J.; Dubourdeau, M.; Guillou, H.; Perretti, M.; et al. Protective effects of n-6 fatty acids-enriched diet on intestinal ischaemia/reperfusion injury involve lipoxin A4and its receptor. Br. J. Pharmacol. 2014, 172, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Duffin, R.; O’Connor, R.A.; Crittenden, S.; Forster, T.; Yu, C.; Zheng, X.; Smyth, D.; Robb, C.T.; Rossi, F.; Skouras, C.; et al. Prostaglandin E 2 constrains systemic inflammation through an innate lymphoid cell–IL-22 axis. Science 2016, 351, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.P.; Colas, R.A.; George, M.J.; Flint, J.D.; Dalli, J.; Richard-Loendt, A.; De Maeyer, R.; Serhan, C.N.; Gilroy, D.W. Pro-resolving mediators promote resolution in a human skin model of UV-killed Escherichia coli–driven acute inflammation. JCI Insight 2018, 3, 94463. [Google Scholar] [CrossRef]
- Zhang, Y.; Desai, A.; Yang, S.Y.; Bae, K.B.; Antczak, M.I.; Fink, S.P.; Tiwari, S.; Willis, J.E.; Williams, N.S.; Dawson, D.M.; et al. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 2015, 348, aaa2340. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.F.; Li, N.; Lee, H.J.; Adamo, L.; Evans, S.M.; Willey, H.; Arora, N.; Torisawa, Y.-S.; Vickers, D.A.; Morris, S.A.; et al. Biomechanical forces promote blood development through prostaglandin E2 and the cAMP–PKA signaling axis. J. Exp. Med. 2015, 212, 665–680. [Google Scholar] [CrossRef] [PubMed]
- North, T.E.; Goessling, W.; Walkley, C.; Lengerke, C.; Kopani, K.R.; Lord, A.M.; Weber, G.J.; Bowman, T.V.; Jang, I.-H.; Grosser, T.; et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nat. Cell Biol. 2007, 447, 1007–1011. [Google Scholar] [CrossRef]
- Hoggatt, J.; Mohammad, K.S.; Singh, P.; Hoggatt, A.F.; Chitteti, B.R.; Speth, J.; Hu, P.; Poteat, B.A.; Stilger, K.N.; Ferraro, F.; et al. Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nat. Cell Biol. 2013, 495, 365–369. [Google Scholar] [CrossRef]
- Hoggatt, J.; Singh, P.; Sampath, J.; Pelus, L.M. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood 2009, 113, 5444–5455. [Google Scholar] [CrossRef]
- Ikushima, Y.M.; Arai, F.; Hosokawa, K.; Toyama, H.; Takubo, K.; Furuyashiki, T.; Narumiya, S.; Suda, T. Prostaglandin E2 regulates murine hematopoietic stem/progenitor cells directly via EP4 receptor and indirectly through mesenchymal progenitor cells. Blood 2013, 121, 1995–2007. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.L.; De-Brito, N.M.; Cunha-Costa, H.; Morandi, V.; Fierro, I.M.; Roitt, I.M.; Barja-Fidalgo, C. Lipoxin A4selectively programs the profile of M2 tumor-associated macrophages which favour control of tumor progression. Int. J. Cancer 2016, 140, 346–357. [Google Scholar] [CrossRef]
- Bi, C.; Fu, Y.; Zhang, Z.; Li, B. Prostaglandin E2 confers protection against diabetic coronary atherosclerosis by stimulating M2 macrophage polarization via the activation of the CREB/BDNF/TrkB signaling pathway. FASEB J. 2020, 34, 7360–7371. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Zhao, Y.; Li, S.; Zhao, T.; Tai, Y.; Zhang, B.; Wang, X.; Wang, C.; Chen, J.; et al. GRK2 Mediated Abnormal Transduction of PGE2-EP4-cAMP-CREB Signaling Induces the Imbalance of Macrophages Polarization in Collagen-Induced Arthritis Mice. Cells 2019, 8, 1596. [Google Scholar] [CrossRef]
- Luan, B.; Yoon, Y.-S.; Le Lay, J.; Kaestner, K.H.; Hedrick, S.; Montminy, M. CREB pathway links PGE2 signaling with macrophage polarization. Proc. Natl. Acad. Sci. USA 2015, 112, 15642–15647. [Google Scholar] [CrossRef]
- Lu, L.Y.; Loi, F.; Nathan, K.; Lin, T.-H.; Pajarinen, J.; Gibon, E.; Nabeshima, A.; Cordova, L.; Jämsen, E.; Yao, Z.; et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J. Orthop. Res. 2017, 35, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Zhang, X.; Zhu, D.; Qi, X.; Cao, X.; Fang, Y.; Che, Y.; Han, Z.-C.; He, Z.-X.; et al. Prostaglandin E2 hydrogel improves cutaneous wound healing via M2 macrophages polarization. Theranostics 2018, 8, 5348–5361. [Google Scholar] [CrossRef]
- Dalli, J.; Zhu, M.; Vlasenko, N.A.; Deng, B.; Haeggström, J.Z.; Petasis, N.A.; Serhan, C.N. The novel 13 S, 14 S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A 4 hydrolase (LTA 4 H), and shifts macrophage phenotype. FASEB J. 2013, 27, 2573–2583. [Google Scholar] [CrossRef]
- Nelson, A.J.; Stephenson, D.J.; Cardona, C.; Lei, X.; Almutairi, A.; White, T.D.; Tusing, Y.G.; Park, M.A.; Barbour, S.E.; Chalfant, C.E.; et al. Macrophage polarization is linked to Ca2+-independent phospholipase A2β-derived lipids and cross-cell signaling in mice. J. Lipid Res. 2020, 61, 143–158. [Google Scholar] [CrossRef]
- Naveen, K.V.G.; Naidu, V.G.M.; Das, U.N. Amelioration of streptozotocin-induced type 2 diabetes mellitus in Wistar rats by ara-chidonic acid. Biochem. Biophys. Res. Commun. 2018, 496, 105–113. [Google Scholar]
- Das, U.N. Molecular Biochemical Aspects of Cancer; Humana Press: New York, NY, USA, 2020. [Google Scholar]
- Das, U.N. Molecular Basis of Health and Disease; Springer: New York, NY, USA, 2011. [Google Scholar]
- Serhan, C.N.; Hamberg, M.; Samuelsson, B. Lipoxins: Novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. USA 1984, 81, 5335–5339. [Google Scholar] [CrossRef]
- Serhan, C.N.; Samuelsson, B. Lipoxins: A New Series of Eicosanoids (Biosynthesis, Stereochemistry, and Biological Activities). Lipoxins 1988, 229, 1–14. [Google Scholar] [CrossRef]
- Harris, W.S.; Mozaffarian, D.; Rimm, E.; Kris-Etherton, P.; Rudel, L.L.; Appel, L.J.; Engler, M.M.; Engler, M.B.; Sacks, F. Omega-6 fatty acids and risk for cardiovascular disease: A science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009, 119, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.; Mouktaroudi, M.; Adamis, T.; Koussoulas, V.; Baziaka, F.; Perrea, D.; Karayannacos, P.E.; Giamarellou, H. n-6 Polyunsaturated Fatty Acids Enhance the Activities of Ceftazidime and Amikacin in Experimental Sepsis Caused by Multidrug-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2004, 48, 4713–4717. [Google Scholar] [CrossRef]
- Tateishi, N.; Kakutani, S.; Kawashima, H.; Shibata, H.; Morita, I. Dietary supplementation of arachidonic acid increases arachidonic acid and lipoxin A4 contents in colon, but does not affect severity or prostaglandin E2 content in murine colitis model. Lipids Health Dis. 2014, 13, 30. [Google Scholar] [CrossRef]
- Tateishi, N.; Kaneda, Y.; Kakutani, S.; Kawashima, H.; Shibata, H.; Morita, I. Dietary supplementation with arachidonic acid increases arachidonic acid content in paw, but does not affect arthritis severity or prostaglandin E2 content in rat adjuvant-induced arthritis model. Lipids Health Dis. 2015, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, S.; Ishikura, Y.; Tateishi, N.; Horikawa, C.; Tokuda, H.; Kontani, M.; Kawashima, H.; Sakakibara, Y.; Kiso, Y.; Shibata, H.; et al. Supplementation of arachidonic acid-enriched oil increases arachidonic acid contents in plasma phospholipids, but does not increase their metabolites and clinical parameters in Japanese healthy elderly individuals: A randomized controlled study. Lipids Health Dis. 2011, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Ortsäter, H. Arachidonic acid fights palmitate: New insights into fatty acid toxicity in β-cells. Clin. Sci. 2010, 120, 179–181. [Google Scholar] [CrossRef]
- Keane, D.C.; Takahashi, H.K.; Dhayal, S.; Morgan, N.G.; Curi, R.; Newsholme, P. Arachidonic acid actions on functional in-tegrity and attenuation of the negative effects of palmitic acid in a clonal pancreatic β-cell line. Clin. Sci. 2011, 120, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Kim, C.H.; Kim, K.Y.; Cheon, H.G. Protective effects of arachidonic acid against palmitic acid-mediated lipotoxicity in HIT-T15 cells. Mol. Cell. Biochem. 2012, 364, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Naveen, G.V.; Das, U.N. Arachidonic acid rich ARASCO oil has anti-inflammatory and anti-diabetic actions against high fat diet-induced type 2 diabetes mellitus in Wistar rats. Nutrition 2019, 66, 203–218. [Google Scholar]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 2016, 6, 38308. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, J.; Saima, F.T.; Rhee, K.-J.; Hwang, S.; Kim, M.Y.; Baik, S.K.; Eom, Y.W.; Kim, H.-S. Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2. Biochem. Biophys. Res. Commun. 2018, 498, 988–995. [Google Scholar] [CrossRef]
- An, J.-H.; Song, W.-J.; Li, Q.; Kim, S.-M.; Yang, J.-I.; Ryu, M.-O.; Nam, A.R.; Bhang, D.H.; Jung, Y.-C.; Youn, H.-Y. Prostaglandin E2 secreted from feline adipose tissue-derived mesenchymal stem cells alleviate DSS-induced colitis by increasing regulatory T cells in mice. BMC Vet.-Res. 2018, 14, 354. [Google Scholar] [CrossRef]
- Rozenberg, A.; Rezk, A.; Boivin, M.-N.; Darlington, P.; Nyirenda, M.; Li, R.; Jalili, F.; Winer, R.; Artsy, E.A.; Uccelli, A.; et al. Human Mesenchymal Stem Cells Impact Th17 and Th1 Responses Through a Prostaglandin E2 and Myeloid-Dependent Mechanism. Stem Cells Transl. Med. 2016, 5, 1506–1514. [Google Scholar] [CrossRef]
- Phipps, R.P.; Stein, S.H.; Roper, R. A new view of prostaglandin E regulation of the immune response. Immunol. Today 1991, 12, 349–352. [Google Scholar] [CrossRef]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2008, 15, 42–49. [Google Scholar] [CrossRef]
- Ylöstalo, J.H.; Bartosh, T.J.; Coble, K.; Prockop, D.J. Human Mesenchymal Stem/Stromal Cells Cultured as Spheroids are Self-activated to Produce Prostaglandin E2 that Directs Stimulated Macrophages into an Anti-inflammatory Phenotype. Stem Cells 2012, 30, 2283–2296. [Google Scholar] [CrossRef]
- Poloso, N.J.; Urquhart, P.; Nicolaou, A.; Wang, J.; Woodward, D.F. PGE2 differentially regulates monocyte-derived dendritic cell cytokine responses depending on receptor usage (EP2/EP4). Mol. Immunol. 2013, 54, 284–295. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jung, Y.W.; Jeong, I.; Joh, J.-S.; Sim, S.Y.; Choi, B.; Jee, H.-G.; Lim, D.-G. Immune parameters differentiating active from latent tuberculosis infection in humans. Tuberculosis 2015, 95, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Perera, R.; Tan, D.; Fernandez, S.; Seddiki, N.; Waring, J.; French, M. Circulating mycobacterial-reactive CD4+ T cells with an immunosuppressive phenotype are higher in active tuberculosis than latent tuberculosis infection. Tuberculosis 2014, 94, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Marin, N.D.; París, S.C.; Vélez, V.M.; Rojas, C.A.; Rojas, M.; García, L.F. Regulatory T cell frequency and modulation of IFN-gamma and IL-17 in active and latent tuberculosis. Tuberculosis 2010, 90, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Gerosa, F.; Nisii, C.; Righetti, S.; Micciolo, R.; Marchesini, M.; Cazzadori, A.; Trinchieri, G. CD4(+) T cell clones producing both inter-feron-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin. Immunol. 1999, 92, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Berner, B.; Akça, D.; Jung, T.; Muller, G.A.; Reuss-Borst, M.A. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J. Rheumatol. 2000, 27, 1128–1135. [Google Scholar] [PubMed]
- Khader, S.A.; Cooper, A.M. IL-23 and IL-17 in tuberculosis. Cytokine 2008, 41, 79–83. [Google Scholar] [CrossRef]
- Wei, L.; Liu, M.; Xiong, H.; Peng, B. Up-regulation of IL-23 expression in human dental pulp fibroblasts by IL-17 via activation of the NF-κB and MAPK pathways. Int. Endod. J. 2018, 51, 622–631. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, Y.; Wei, H.; Xing, X.; Zhan, N.; Xiong, H.; Peng, B. IL-17R activation of human periodontal ligament fibroblasts induces IL-23 p19 production: Differential involvement of NF-κB versus JNK/AP-1 pathways. Mol. Immunol. 2011, 48, 647–656. [Google Scholar] [CrossRef]
- Liu, F.L.; Chen, C.H.; Chu, S.J.; Chen, J.H.; Lai, J.H.; Sytwu, H.K.; Chang, D.M. Interleukin (IL)-23 p19 expression induced by IL-1beta in human fibroblast-like synoviocytes with rheumatoid arthritis via active nuclear factor-kappaB and AP-1 dependent pathway. Rheumatology 2007, 46, 1266–1273. [Google Scholar] [CrossRef]
- Kim, H.-R.; Cho, M.-L.; Kim, K.-W.; Juhn, J.-Y.; Hwang, S.-Y.; Yoon, C.-H.; Park, S.-H.; Lee, S.-H. Up-regulation of IL-23p19 expression in rheumatoid arthritis synovial fibroblasts by IL-17 through PI3-kinase-, NF- B- and p38 MAPK-dependent signalling pathways. Rheumatology 2007, 46, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Domingo, E.; Municio, C.; Alvarez, Y.; Hugo, E.; Fernández, M.N.; Crespo, M.S. Polarization of the Innate Immune Response by Prostaglandin E2: A Puzzle of Receptors and Signals. Mol. Pharmacol. 2013, 85, 187–197. [Google Scholar] [CrossRef]
- Das, U.N. Molecular pathobiology of scleritis and its therapeutic implications. Int. J. Ophthalmol. 2020, 13, 163–175. [Google Scholar] [CrossRef]
- Das, U.N. Bioactive lipids in intervertebral disc degeneration and its therapeutic implications. Biosci. Rep. 2019, 39, 20192117. [Google Scholar] [CrossRef]
- Shibabaw, T. Inflammatory Cytokine: IL-17A Signaling Pathway in Patients Present with COVID-19 and Current Treatment Strategy. J. Inflamm. Res. 2020, 13, 673–680. [Google Scholar] [CrossRef]
- Schett, G.; Sticherling, M.; Neurath, M.F. COVID-19: Risk for cytokine targeting in chronic inflammatory diseases? Nat. Rev. Immunol. 2020, 20, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Monroy, J.C.; Parra-Medina, R.; Garavito, E.; Rojas-Villarraga, A. T Helper 17 Response to Severe Acute Respiratory Syndrome Coronavirus 2: A Type of Immune Response with Possible Therapeutic Implications. Viral Immunol. 2021, 34, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Orlov, M.; Wander, P.L.; Morrell, E.D.; Mikacenic, C.; Wurfel, M.M. A Case for Targeting Th17 Cells and IL-17A in SARS-CoV-2 Infections. J. Immunol. 2020, 205, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020, 53, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, G.; Zhang, L.; Li, F.; Liu, K.; Wang, Y.; Shi, Y.; Cao, K. Interleukin-17 promotes nitric oxide-dependent expression of PD-L1 in mesenchymal stem cells. Cell Biosci. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, D.; Trajkovic, V. Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev. 2004, 15, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, D.; Cvetkovic, I.; Vuckovic, O.; Stosic-Grujicic, S.; Mostarica Stojkovic, M.; Trajkovic, V. The role of interleukin-17 in inducible nitric oxide synthase-mediated nitric oxide production in endothelial cells. Cell Mol. Life Sci. 2003, 60, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, Q.; Lin, L.; Xu, C.; Zheng, C.; Chen, X.; Han, Y.; Li, M.; Cao, W.; Cao, K.; et al. Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 2014, 21, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Duan, X.; Hu, F.; Poorun, D.; Liu, X.; Wang, X.; Zhang, S.; Gan, L.; He, M.; Zhu, K.; et al. Resolvin D1 attenuates imiquimod-induced mice psoriasiform dermatitis through MAPKs and NF-κB pathways. J. Dermatol. Sci. 2018, 89, 127–135. [Google Scholar] [CrossRef]
- Oner, F.; Alvarez, C.; Yaghmoor, W.; Stephens, D.; Hasturk, H.; Firatli, E.; Kantarci, A. Resolvin E1 Regulates Th17 Function and T Cell Activation. Front. Immunol. 2021, 12, 637983. [Google Scholar] [CrossRef]
- Funaki, Y.; Hasegawa, Y.; Okazaki, R.; Yamasaki, A.; Sueda, Y.; Yamamoto, A.; Yanai, M.; Fukushima, T.; Harada, T.; Makino, H.; et al. Resolvin E1 Inhibits Osteoclastogenesis and Bone Resorption by Suppressing IL-17-induced RANKL Expression in Osteo-blasts and RANKL-induced Osteoclast Differentiation. Yonago Acta Med. 2018, 61, 8–18. [Google Scholar] [CrossRef]
- Wang, H.; Shi, P.; Huang, C.; Liu, Q. Maresin 1 ameliorates iron-deficient anemia in IL-10(−/−) mice with spontaneous colitis by the inhibition of hepcidin expression though the IL-6/STAT3 pathway. Am. J. Transl. Res. 2016, 8, 2758–2766. [Google Scholar]
- Kurtoğlu, E.L.; Kayhan, B.; Gül, M.; Kayhan, B.; Kayhan, M.A.; Karaca, Z.M.; Yeşilada, E.; Yılmaz, S. A bioactive product lipoxin A4 at-tenuates liver fibrosis in an experimental model by regulating immune response and modulating the expression of regeneration genes. Turk. J. Gastroenterol. 2019, 30, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Abbott, J.; Cheng, L.; Colby, J.K.; Lee, J.W.; Levy, B.D.; Matthay, M.A. Human Mesenchymal Stem (Stromal) Cells Promote the Resolution of Acute Lung Injury in Part through Lipoxin A4. J. Immunol. 2015, 195, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; He, S.; Yuan, J.; Miao, S.; Gao, H.; Zhang, J.; Li, Y.; Peng, W.; Wu, P. Lipoxin A4 attenuates LPS-induced mouse acute lung injury via Nrf2-mediated E-cadherin expression in airway epithelial cells. Free Radic. Biol. Med. 2016, 93, 52–66. [Google Scholar] [CrossRef]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and thera-peutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Abdelmawgoud, H.; Saleh, A. Anti-inflammatory and antioxidant effects of mesenchymal and hematopoietic stem cells in a rheumatoid arthritis rat model. Adv. Clin. Exp. Med. 2018, 27, 873–880. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, S.; Wu, S.; Qi, J.; Li, W.; Liu, S.; Cong, Y.; Chen, H.; Lu, L.; Shi, S.; et al. Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2. EBioMedicine 2019, 45, 341–350. [Google Scholar] [CrossRef]
- Holopainen, M.; Colas, R.A.; Valkonen, S.; Tigistu-Sahle, F.; Hyvärinen, K.; Mazzacuva, F.; Lehenkari, P.; Käkelä, R.; Dalli, J.; Kerkelä, E.; et al. Polyunsaturated fatty acids modify the extracellular vesicle membranes and increase the production of proresolving lipid mediators of human mesenchymal stromal cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 1350–1362. [Google Scholar] [CrossRef]
- Hyvärinen, K.; Holopainen, M.; Skirdenko, V.; Ruhanen, H.; Lehenkari, P.; Korhonen, M.; Käkelä, R.; Laitinen, S.; Kerkelä, E. Mesen-chymal Stromal Cells and Their Extracellular Vesicles Enhance the Anti-Inflammatory Phenotype of Regulatory Macrophages by Downregulating the Production of Interleukin (IL)-23 and IL-22. Front. Immunol. 2018, 8, 771. [Google Scholar] [CrossRef]
- Yang, F.Y.; Chen, R.; Zhang, X.; Huang, B.; Tsang, L.L.; Li, X.; Jiang, X. Preconditioning Enhances the Therapeutic Effects of Mesenchymal Stem Cells on Colitis Through PGE2-Mediated T-Cell Modulation. Cell Transplant. 2018, 27, 1352–1367. [Google Scholar] [CrossRef]
- Yang, H.M.; Song, W.J.; Li, Q.; Kim, S.Y.; Kim, H.J.; Ryu, M.O.; Ahn, J.O.; Youn, H.Y. Canine mesenchymal stem cells treated with TNF-α and IFN-γ enhance anti-inflammatory effects through the COX-2/PGE2 pathway. Res. Vet. Sci. 2018, 119, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lin, Y.; Hu, Y.; Shan, W.; Liu, S.; Xu, Y.; Zhang, H.; Cai, S.; Yu, X.; Cai, Z.; et al. mTOR inhibition improves the immunomodulatory properties of human bone marrow mesenchymal stem cells by inducing COX-2 and PGE2. Stem Cell Res. Ther. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, W.H.; Lee, M.W.; Park, H.J.; Jang, I.K.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Involvement of TLR3-Dependent PGES Expression in Immunosuppression by Human Bone Marrow Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2018, 14, 286–293. [Google Scholar] [CrossRef]
- Das, U.N. Bioactive Lipids as Mediators of the Beneficial Action(s) of Mesenchymal Stem Cells in COVID-19. Aging Dis. 2020, 11, 746–755. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, J.; He, Z.; Yang, M.; Li, L.; Jiang, H. Mesenchymal stem cells reverse diabetic nephropathy disease via lipoxin A4 by targeting transforming growth factor β (TGF-β)/smad pathway and pro-Inflammatory cytokines. Med. Sci. Monit. 2019, 25, 3069–3076. [Google Scholar] [CrossRef]
- Speert, D.P.; Wannamaker, L.W.; Gray, E.D.; Clawson, C.C. Bactericidal Effect of Oleic Acid on Group A Streptococci: Mechanism of Action. Infect. Immun. 1979, 26, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Juers, J.A.; Rogers, R.M.; McCurdy, J.B.; Cook, W.W. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J. Clin. Investig. 1976, 58, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Nieman, C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol. Rev. 1954, 18, 147–162. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Norén, B.; Odham, G. Antimicrobial Effect of Simple Lipids with Different Branches at the Methyl End Group. Antimicrob. Agents Chemother. 1975, 8, 742–750. [Google Scholar] [CrossRef]
- Heczko, P.B.; Lütticken, R.; Hryniewicz, W.; Neugebauer, M.; Pulverer, G. Susceptibility of Staphylococcus aureus and group A, B, C, and G streptococci to free fatty acids. J. Clin. Microbiol. 1979, 9, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Wyss, O.; Ludwig, B.J.; Joiner, R.R. The fungistatic and fungicidal action of fatty acids and related compounds. Arch. Biochem. 1945, 7, 415–424. [Google Scholar]
- Stock, C.C.; Francis, T., Jr. The inactivation of the virus of epidemic influenza by soaps. J. Exp. Med. 1940, 71, 661–681. [Google Scholar] [CrossRef]
- Sands, J.; Auperin, D.; Snipes, W. Extreme Sensitivity of Enveloped Viruses, Including Herpes Simplex, to Long-Chain Unsaturated Monoglycerides and Alcohols. Antimicrob. Agents Chemother. 1979, 15, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kohn, A.; Gitelman, J.; Inbar, M. Unsaturated free fatty acids inactivate animal enveloped viruses. Arch. Virol. 1980, 66, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, B.; Piët, M.P.; Prince, A.M.; Edwards, C.A.; Lippin, A.; Walakovits, L.A. Inactivation of lipid-enveloped viruses in labile blood derivatives by unsaturated fatty acids. Vox Sang. 1988, 54, 14–20. [Google Scholar] [CrossRef]
- Das, U.N. Anti-biotic-like action of essential fatty acids. Can. Med. Assoc. J. 1985, 132, 1350. [Google Scholar]
- Das, U.N. Do unsaturated fatty acids function as endogenous anti-bacterial and anti-viral molecules? Am. J. Clin. Nutr. 2006, 83, 390–391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Das, U. Can essential fatty acid deficiency predispose to AIDS? Can. Med. Assoc. J. 1985, 132, 900–902. [Google Scholar]
- Das, U.N. Essential fatty acids and acquired immunodeficiency syndrome. Med. Sci. Monit. 2005, 11, RA206–RA211. [Google Scholar]
- Das, U.N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimi-crobial molecules: A review. J. Adv. Res. 2018, 11, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.W.; Konings, W.N.; Freese, E. Effects of Acetate and Other Short-Chain Fatty Acids on Sugar and Amino Acid Uptake of Bacillus subtilis. J. Bacteriol. 1972, 111, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Ingram, L.O.; Eaton, L.C.; Erdos, G.W.; Tedder, T.F.; Vreeland, N.L. Unsaturated fatty acid requirement in Escherichia coli. Mechanism of palmitate-induced inhibition of growth by strain WNl. J. Membr. Biol. 1982, 1982, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Fay, J.P.; Farías, R.N. Inhibitory action of a non-metabolizable fatty acid on the growth of Escherichia coli: Role of metabolism and outer membrane integrity. J. Bacteriol. 1977, 132, 790–795. [Google Scholar] [CrossRef]
- Norris, P.; Arnardottir, H.; Sanger, J.M.; Fichtner, D.; Keyes, G.S.; Serhan, C.N. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot. Essent. Fat. Acids 2018, 138, 81–89. [Google Scholar] [CrossRef]
- Das, U.N. Can Bioactive Lipids Inactivate Coronavirus (COVID-19)? Arch. Med. Res. 2020, 51, 282–286. [Google Scholar] [CrossRef]
- Das, U.N. Can Bioactive Lipid Arachidonic Acid Prevent and Ameliorate COVID-19? Medicina (Kaunas) 2020, 56, 418. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Bioactive Lipids in COVID-19-Further Evidence. Arch. Med. Res. 2021, 52, 107–120. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids and their metabolites in the pathobiology of (coronavirus disease 2019) COVID-19. Nutrition 2020, 82, 111052. [Google Scholar] [CrossRef]
- Toelzer, C.; Gupta, K.; Yadav, S.K.N.; Borucu, U.; Davidson, A.D.; Kavanagh Williamson, M.; Shoemark, D.K.; Garzoni, F.; Staufer, O.; Milligan, R.; et al. Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science 2020, 370, 725–730. [Google Scholar] [CrossRef]
- Goc, A.; Niedzwiecki, A.; Rath, M. Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry. Sci. Rep. 2021, 11, 5207. [Google Scholar] [CrossRef]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 59–72. [Google Scholar] [CrossRef]
- Yan, B.; Chu, H.; Yang, D.; Sze, K.-H.; Lai, P.-M.; Yuan, S.; Shuai, H.; Wang, Y.; Kao, R.Y.-T.; Chan, J.F.-W.; et al. Characterization of the Lipidomic Profile of Human Coronavirus-Infected Cells: Implications for Lipid Metabolism Remodeling upon Coronavirus Replication. Viruses 2019, 11, 73. [Google Scholar] [CrossRef]
- Liu, T.; Yang, Q.; Cao, Y.-J.; Yuan, W.-M.; Lei, A.-H.; Zhou, P.; Zhou, W.; Liu, Y.-D.; Shi, M.-H.; Yang, Q.; et al. Cyclooxygenase-1 Regulates the Development of Follicular Th Cells via Prostaglandin E2. J. Immunol. 2019, 203, 864–872. [Google Scholar] [CrossRef]
- Duan, Y.-Q.; Xia, M.-H.; Ren, L.; Zhang, Y.-F.; Ao, Q.-L.; Xu, S.-P.; Kuang, D.; Liu, Q.; Yan, B.; Zhou, Y.-W.; et al. Deficiency of Tfh Cells and Germinal Center in Deceased COVID-19 Patients. Curr. Med. Sci. 2020, 40, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Garvin, M.R.; Alvarez, C.; Miller, J.I.; Prates, E.T.; Walker, A.M.; Amos, B.K.; Mast, A.E.; Justice, A.; Aronow, B.; Jacobson, D.A. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. eLife 2020, 9, e59177. [Google Scholar] [CrossRef] [PubMed]
- Candelario, J.; Chachisvilis, M. Activity of Bradykinin B2 Receptor Is Regulated by Long-Chain Polyunsaturated Fatty Acids. PLoS ONE 2013, 8, e68151. [Google Scholar] [CrossRef]
- Mann, E.R.; Menon, M.; Knight, S.B.; Konkel, J.E.; Jagger, C.; Shaw, T.N.; Krishnan, S.; Rattray, M.; Ustianowski, A.; Bakerly, N.D.; et al. Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci. Immunol. 2020, 5, 6197. [Google Scholar] [CrossRef]
- Arnardottir, H.H.; Dalli, J.; Colas, R.A.; Shinohara, M.; Serhan, C.N. Aging delays resolution of acute inflammation in mice: Repro-gramming the host response with novel nano-proresolving medicines. J. Immunol. 2014, 193, 4235–4244. [Google Scholar] [CrossRef] [PubMed]
- Chiscano-Camón, L.; Ruiz-Rodriguez, J.C.; Ruiz-Sanmartin, A.; Roca, O.; Ferrer, R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit. Care 2020, 24, 522. [Google Scholar] [CrossRef]
- Gardiner, N.; Duncan, J. Enhanced prostaglandin synthesis as a mechanism for inhibition of melanoma cell growth by ascorbic acid. Prostaglandins Leukot. Essent. Fat. Acids 1988, 34, 119–126. [Google Scholar] [CrossRef]
- Srivastava, K. Ascorbic acid enhances the formation of prostaglandin E1 in washed human platelets and prostacyclin in rat aortic rings. Prostaglandins Leukot. Med. 1985, 18, 227–233. [Google Scholar] [CrossRef]
- Kotani, N.; Hashimoto, H.; Kushikata, T.; Yoshida, H.; Muraoka, M.; Takahashi, S.; Matsuki, A. Intraoperative prostaglandin E1 improves antimicrobial and inflammatory responses in alveolar immune cells. Crit. Care Med. 2001, 29, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Is insulin an anti-inflammatory molecule? Nutrition 2001, 17, 409–413. [Google Scholar] [CrossRef]
- Das, U.N. Possible beneficial action(s) of glucose-insulin-potassium regimen in acute myocardial infarction and inflammatory conditions: A hypothesis. Diabetologia 2000, 43, 1081–1082. [Google Scholar] [PubMed]
- Li, J.; Zhang, H.; Wu, F.; Nan, Y.; Ma, H.; Guo, W.; Wang, H.; Ren, J.; Das, U.N.; Gao, F. Insulin inhibits tumor necrosis factor-alpha induction in myocardial ische-mia/reperfusion: Role of Akt and endothelial nitric oxide synthase phosphorylation. Crit. Care Med. 2008, 36, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Bellenger, J.; Bataille, A.; Massey, K.A.; Nicolaou, A.; Rialland, M.; Tessier, C.; Kang, J.X.; Narce, M. High Pancreatic n-3 Fatty Acids Prevent STZ-Induced Diabetes in Fat-1 Mice: Inflammatory Pathway Inhibition. Diabetes 2011, 60, 1090–1099. [Google Scholar] [CrossRef]

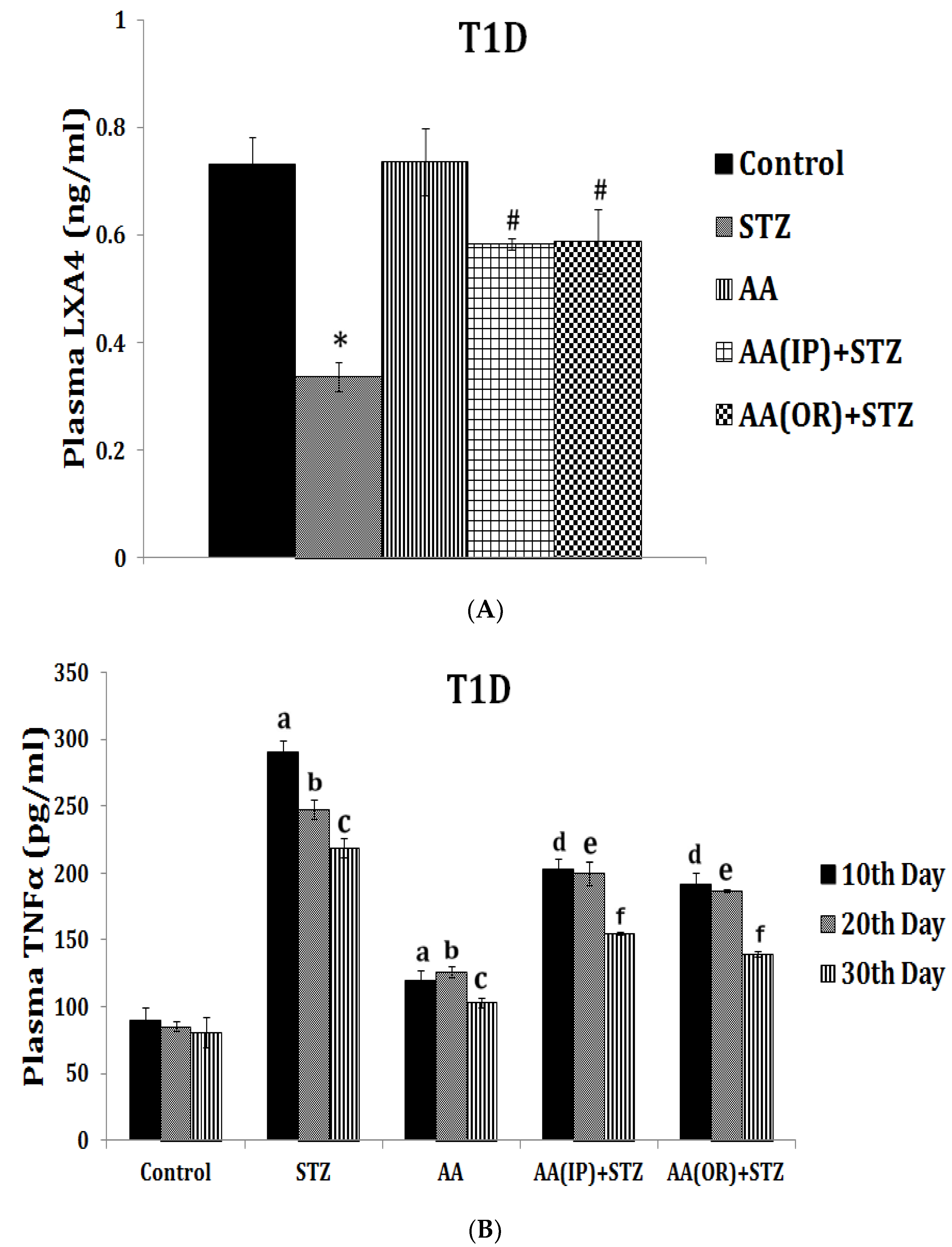
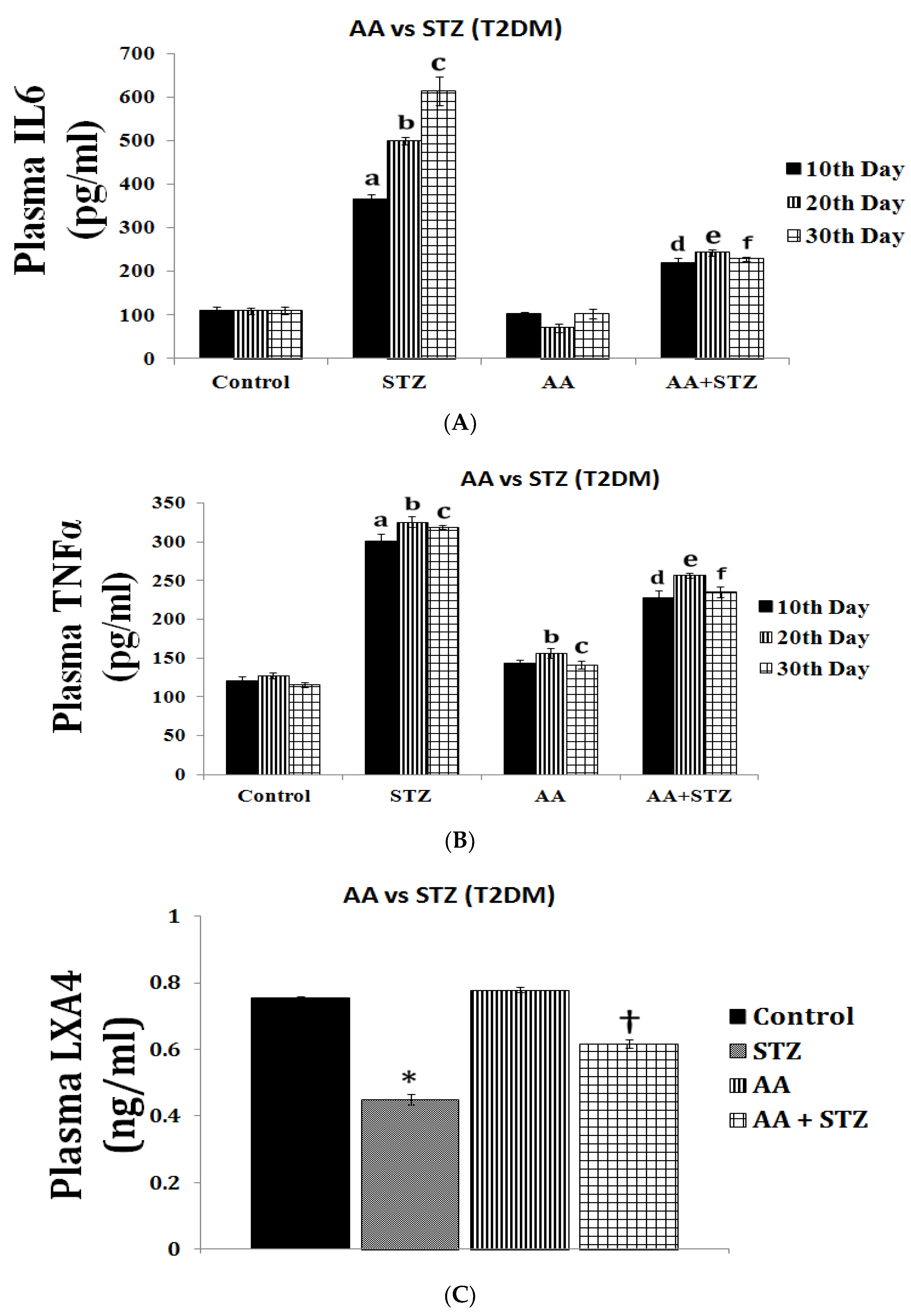
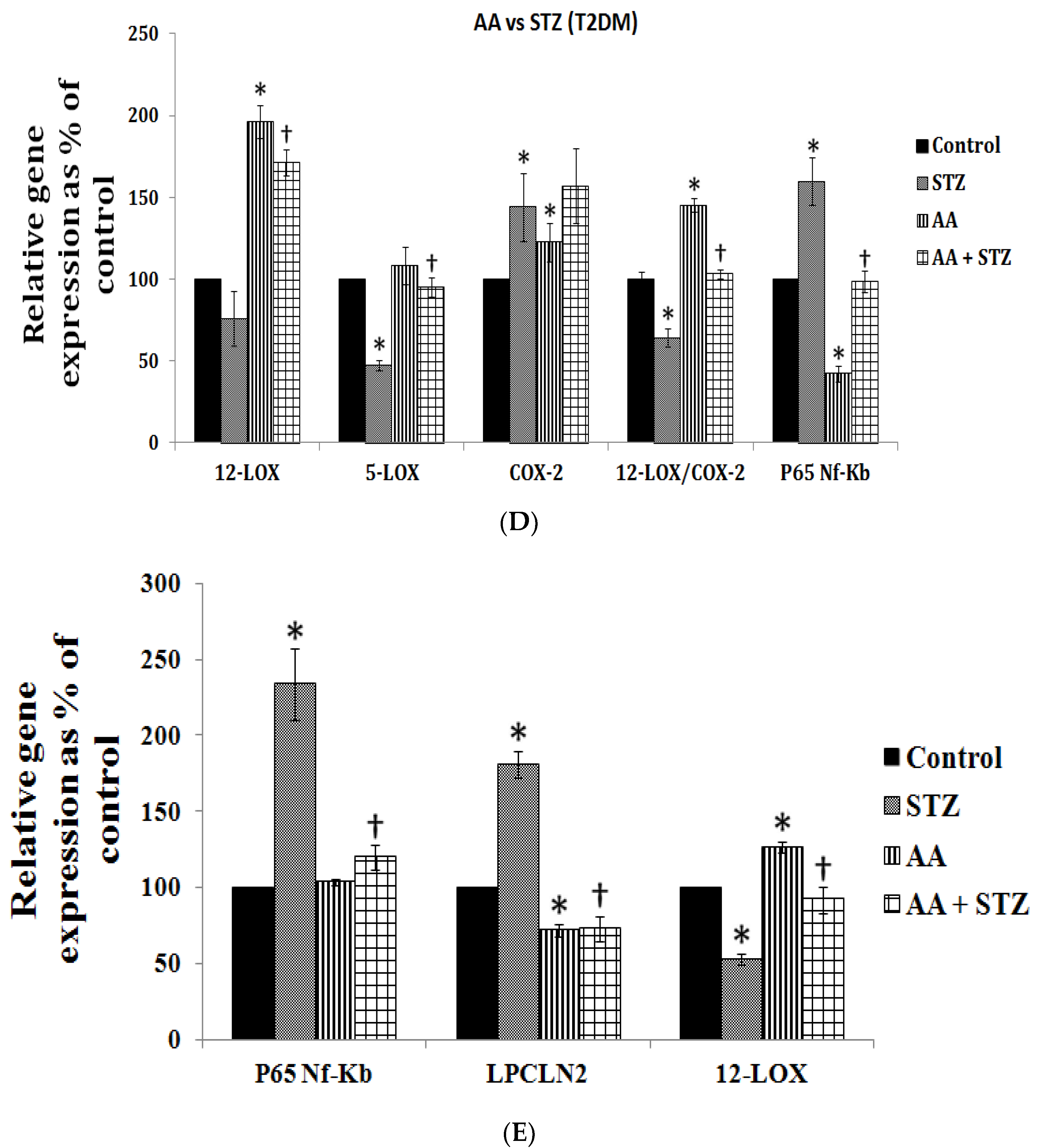
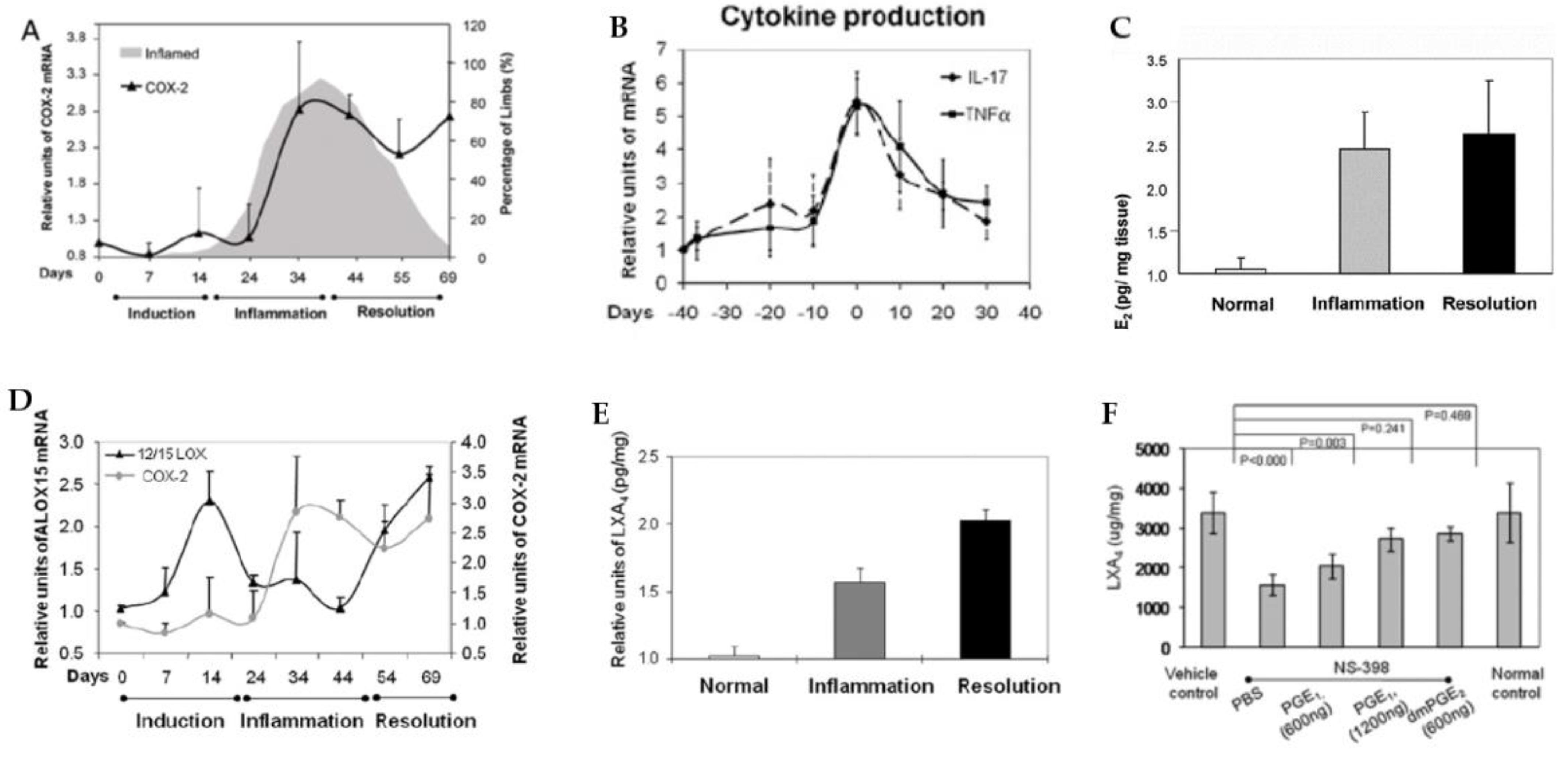



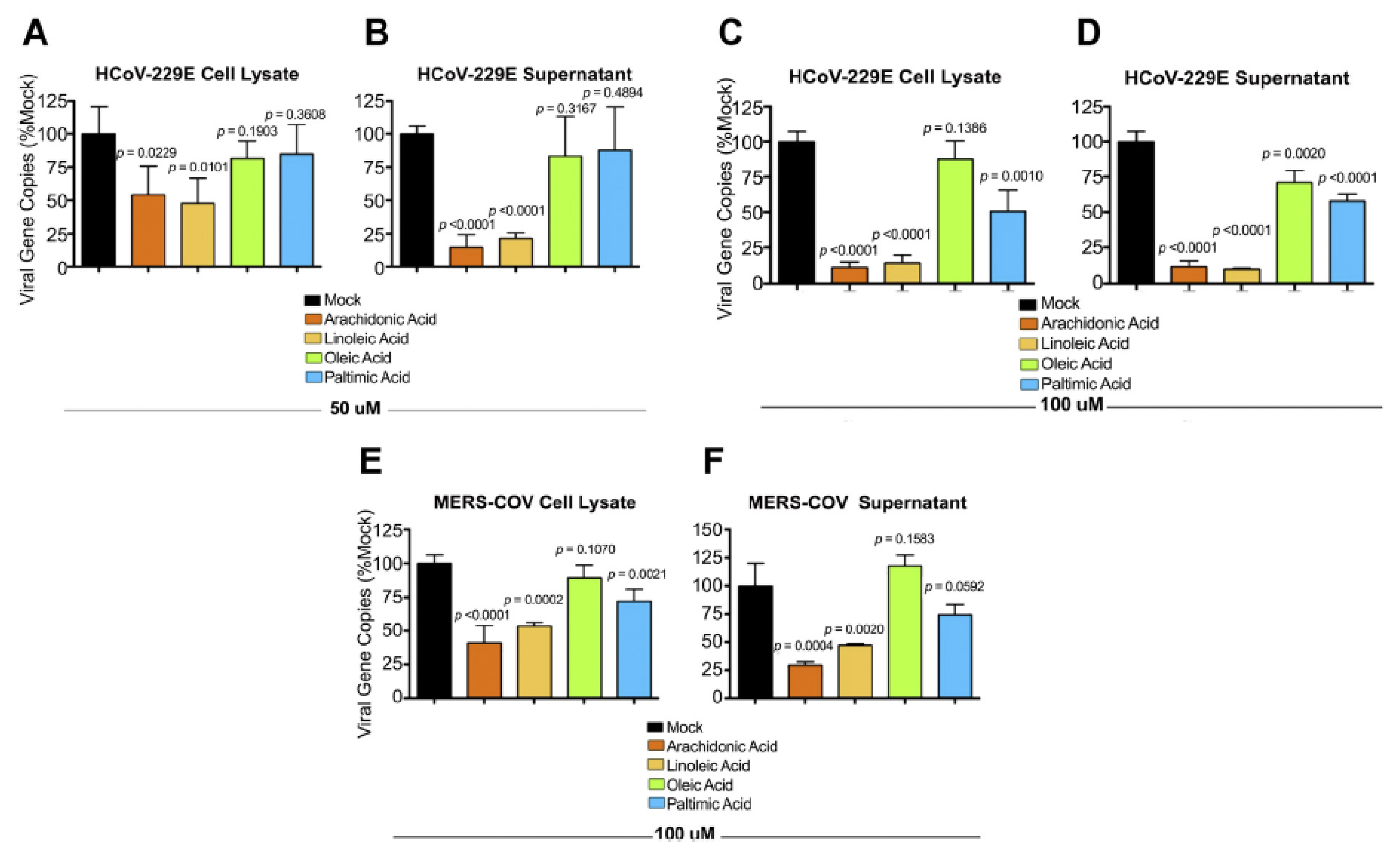
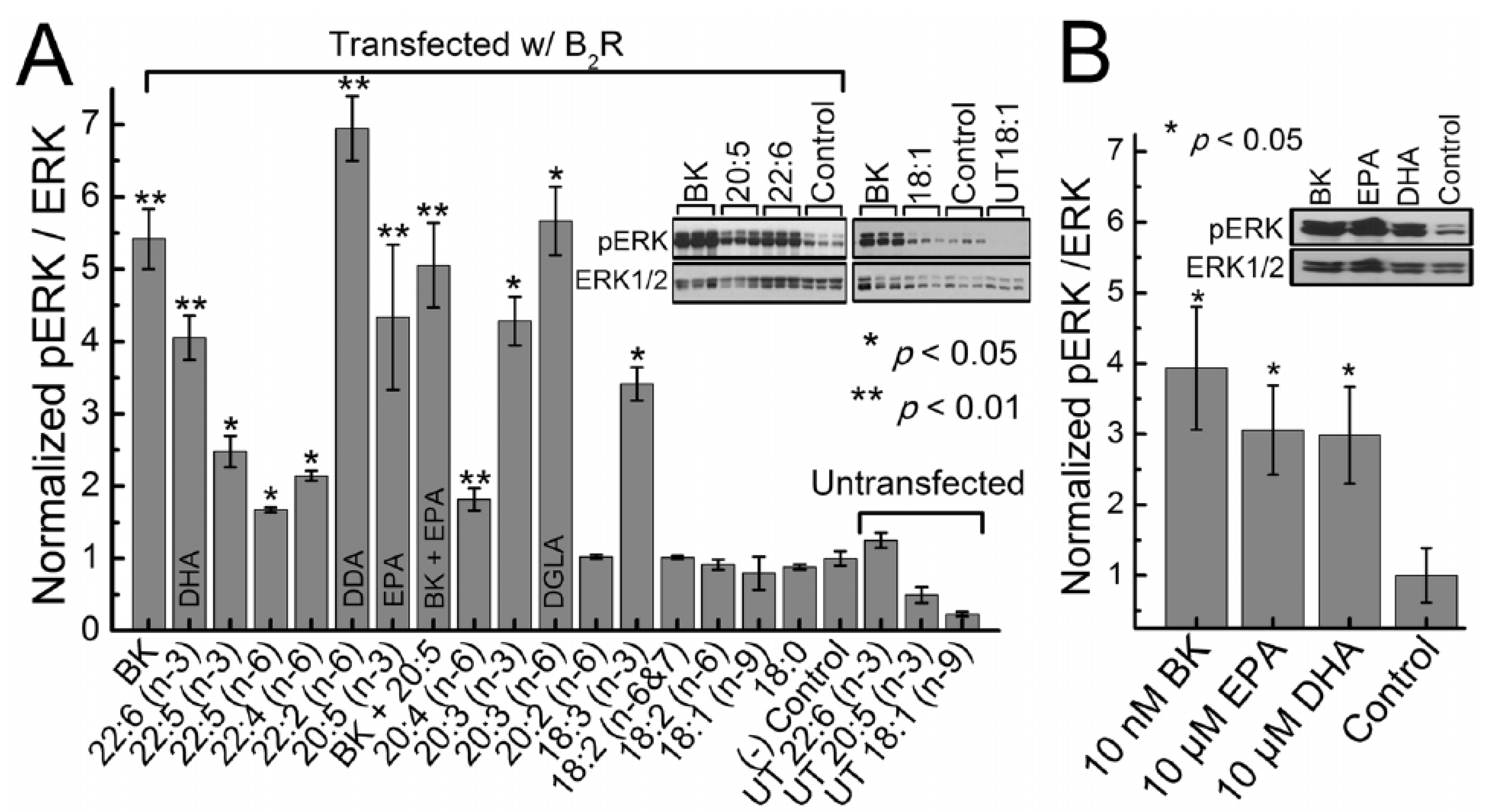
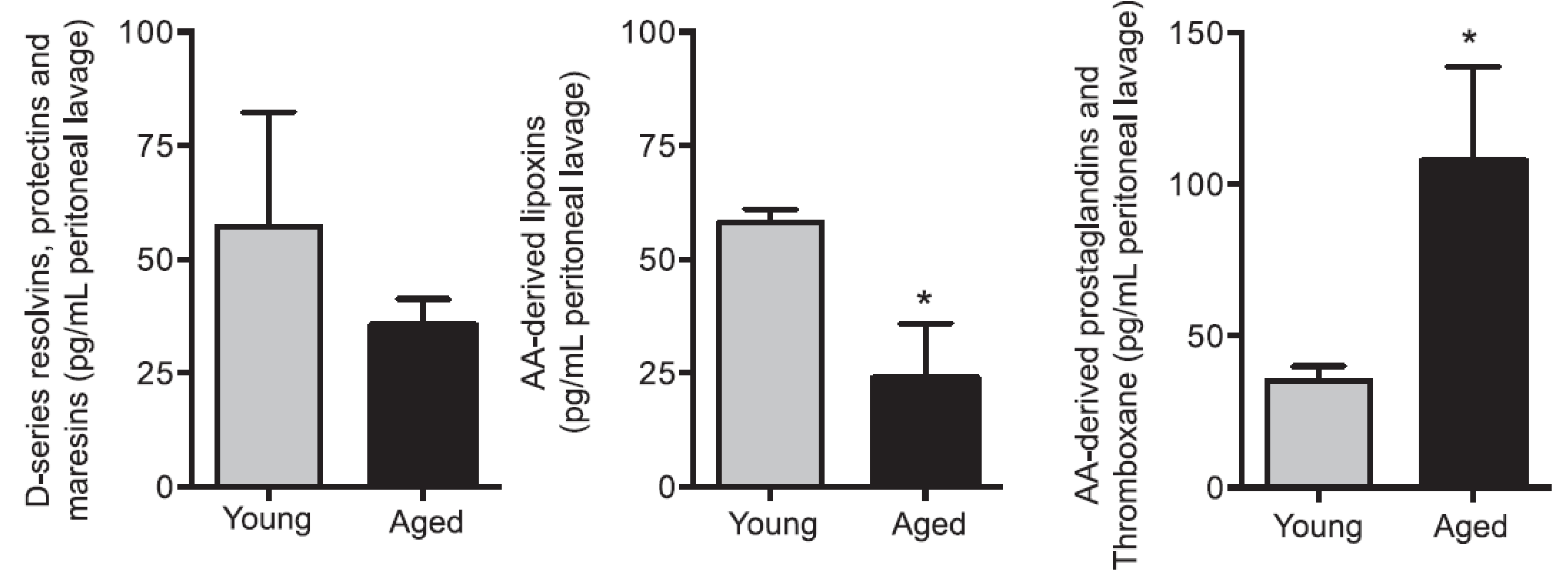
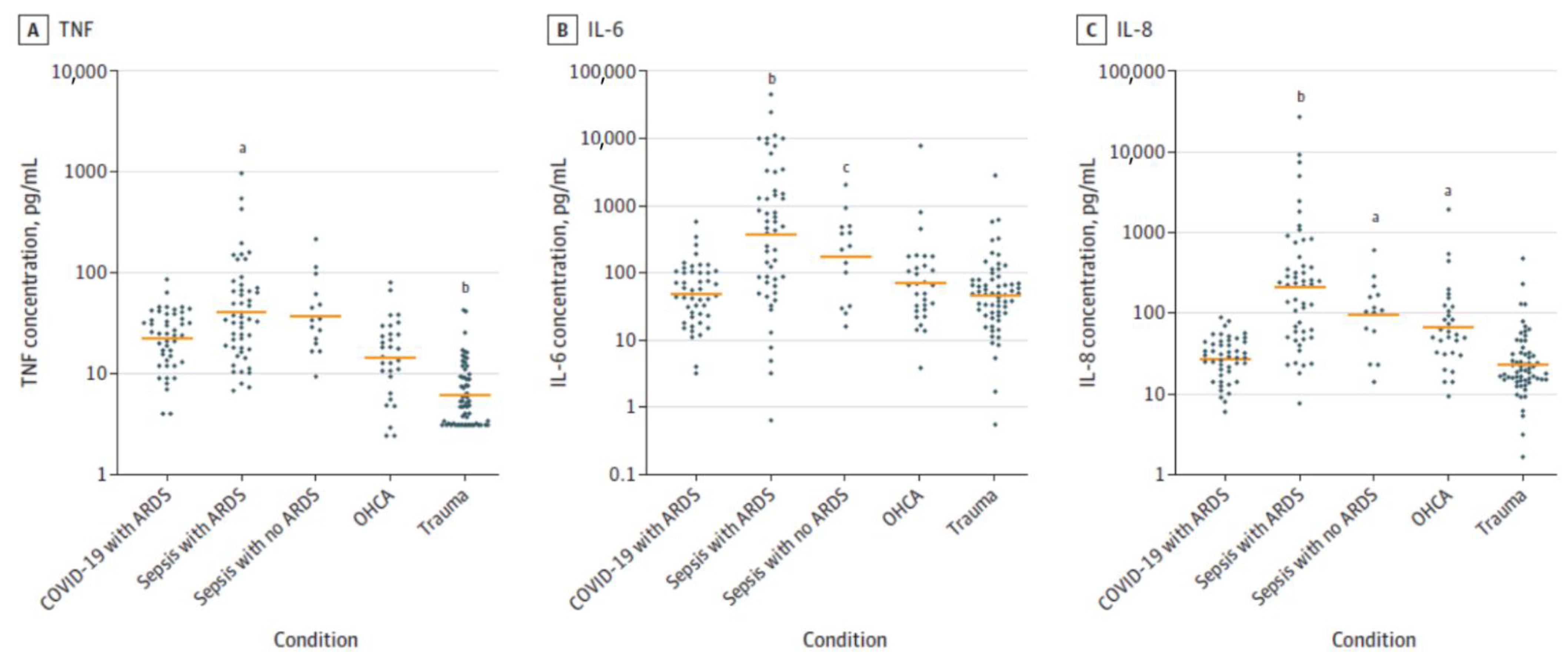
| Measurement (Fatty Acid) | Normal Intact Liver | Intact Yoshida Cells | Normal Liver Microsomes | Yoshida Microsomes |
|---|---|---|---|---|
| 20:4 (AA) | 16.7 ± 2.4 | 8.7 ± 0.7 | 19.1 ± 2.4 | 9.6 ± 0.8 |
| 22:6 (DHA) | 6.3 ± 0.2 | 5.2 ± 0.6 | 6.1 ± 0.3 | 5.3 ± 0.4 |
| Fatty Acid | Control | HTN | CHD | Type 2 DM | Diabetic Nephropathy |
|---|---|---|---|---|---|
| 18:2 n-6 (LA) | 18.6 ± 3.1 | 14.5 ± 3.1 * | 17.8 ± 5.0 | 13.9 ± 5.3 | 15.1 ± 3.1 |
| 18:3 n-6 (GLA) | 0.14 ± 0.1 | 0.4 ± 0.3 * | 0.1 ± 0.1 * | 0.2 ± 0.3 | 0.1 ± 0.2 |
| 20:3 n-6 (DGLA) | 3.4 ± 1.0 | 3.1 ± 0.9 | 2.7 ± 1.1 | 1.7 ± 1.0 * | 2.0 ± 0.8 * |
| 20:4 n-6 (AA) | 9.4 ± 1.8 | 7.8 ± 2.0 * | 7.0 ± 2.1 * | 4.6 ± 1.8 * | 6.6 ± 2.6 * |
| 22:5 n-6 | 0.7 ± 0.4 | 0.4 ± 0.4 * | 1.0 ± 0.9 | 2.1 ± 0.6 * | 1.3 ± 0.5 * |
| 20:4 n-6/18:2 n-6 | 0.51 | 0.54 | 0.39 | 0.33 | 0.43 |
| 18:3 n-3 (ALA) | 0.2 ± 0.1 | 0.4 ± 0.2 * | 0.3 ± 0.5 | 0.1 ± 0.2 * | 0.1 ± 0.1 * |
| 20:5 n-3 (EPA) | 0.4 ± 0.4 | 0.6 ± 0.6 | 0.1 ± 0.2 * | 0.3 ± 0.3 | 0.2 ± 0.3 |
| 22:5 n-3 | 0.5 ± 0.2 | 0.4 ± 0.5 | 0.3 ± 0.3 * | 1.6 ± 1.3 | 1.7 ± 1.1 |
| 22:6 n-3 (DHA) | 1.4 ± 0.5 | 1.2 ± 0.6 | 0.8 ± 0.4 * | 0.5 ± 0.4 * | 0.5 ± 0.3 * |
| 20:5 n-3/18:3 n-3 | 1.8 | 1.39 | 0.41 | 3.2 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, U.N. Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution. Biomolecules 2021, 11, 1873. https://doi.org/10.3390/biom11121873
Das UN. Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution. Biomolecules. 2021; 11(12):1873. https://doi.org/10.3390/biom11121873
Chicago/Turabian StyleDas, Undurti N. 2021. "Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution" Biomolecules 11, no. 12: 1873. https://doi.org/10.3390/biom11121873
APA StyleDas, U. N. (2021). Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution. Biomolecules, 11(12), 1873. https://doi.org/10.3390/biom11121873






