1. Introduction
Fabry Disease (FD, OMIM #301500) is a genetic disorder caused by mutations in
GLA (NC_000023.1, Xq22) [
1], with an incidence estimated from 1:40,000 to 1:117,000. However, this value can reach up to 1:3000, according to newborn screening studies or including late-onset variants [
2,
3].
GLA encodes for α-Galactosidase A (α-GalA, EC3.2.1.22), a rate limiting enzyme in lysosomal metabolism. The enzymatic deficit leads to the progressive accumulation of glycosphingolipids, such as globotriaosylceramide (Gb3) and its deacylated form Lyso-Gb3, within the lysosome of a variety of cell types (endothelial cells, smooth muscle cells, renal podocytes, tubular cells, glomerular endothelial, mesangial and interstitial cells, cardiac cardiomyocytes fibroblasts, and nerve cells) [
4]. FD courses as a progressive disorder characterized by painful small fiber neuropathy, cardiac disease, chronic renal insufficiency, and a high predisposition for cerebrovascular strokes [
5]. Currently, FD is treated by enzyme replacement therapy (ERT), which consists in biweekly intravenous (i.v.) injections of recombinant human α-GalA (agalsidase alfa or agalsidase beta) or the orally administrated pharmacological chaperone (PC) Migalastat (also known as 1-deoxygalactonojirimycin, DGJ).
ERT has been the gold standard for the treatment since 2000 [
6], although it presents important limitations such as the low half-life and bioavailability of the recombinant enzyme, its incapability to cross the blood–brain barrier, and the frequent activation of the native immune system. DGJ, an iminosugar mimicking structure of D-galactose, is orally administrated and overcomes some of the limitations of ERT, since it is widely distributed, can cross the blood–brain barrier, and does not activate the immune system [
7].
Clinical trials demonstrated that this drug is generally well tolerated, stabilizes renal function, and significantly reduces the left ventricular mass index. Lyso-Gb3 levels in plasma and Gb3 levels in kidney interstitial capillary are significantly reduced in naïve patients treated with Migalastat, compared to placebo-treated patients. Gastrointestinal symptoms are also meliorated in treated patients [
8,
9].
DGJ acts like a PC, stabilizing the structure of the mutant enzyme and avoiding its degradation by the quality control mechanisms of the endoplasmic reticulum, which allows the enzyme to traffic to the lysosome. Once the complex has reached the target organelle the PC is released from the enzyme, upon acid hydrolysis, and the protein can catalyze substrate degradation, still in the presence of an amino acid substitution [
10].
Nevertheless, this mechanism of action is efficient only when the mutation is located in specific areas within the protein (i.e., in proximity of the active site), and partially affects the folding of the enzyme. Therefore, the use of Migalastat is only indicated for FD patients with amenable mutations in
GLA ([
11] consulted 31 October 2021).
Considering the relatively and increasingly high incidence of the disease, new molecular scaffolds are needed to provide therapeutic alternatives for treatment of this distressing disease. Therefore, the aim of this work was to assess the efficacy of additional galactose analogues, which stabilize α-GalA, and evaluate their potential use as PCs for the treatment of FD in patients with mutations that are not amenable for the treatment with Migalastat.
2. Materials and Methods
2.1. Synthesis of PBX Molecules
Chemical reagent 1,2:3,4-Di-O-isopropylidene-D-galactopyranose was purchased from Acros Organics (part of Thermofisher, Fair Lawn, NJ, USA) All other chemical reagents were obtained from Sigma-Aldrich (part of Merck, Darmstdt, Germany). Solvents were distilled and dried before use according to standard procedures. Reactions were monitored by analytical thin-layer chromatography (TLC) using silica gel precoated plates (Merck 60 F254, 0.25 mm, Merck, Darmstdt, Germany) and flash column chromatography was carried out using silica gel Merck 60 (230–400 mesh) (Merck, Darmstdt, Germany). NMR spectra were performed on a Bruker ARX400 spectrometer (Bruker, Fällanden, Swiss); chemical shifts (δ) are given in parts per million (ppm) downfield of TMS as internal standard and coupling constants (J) are reported in hertz (Hz). High-resolution mass spectra (HRMS) were recorded on a Bruker microTOF-Focus spectrofotometer.
2.2. Mutant Plasmids
Mutant plasmids were generated with QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent, Santa Clara, CA, USA) using vector pR-M10-αGalA [
12] as wild type template and following the manufacturer’s instructions. Specific primers (
Supplementary Table S1) were used to introduce selected mutations, which affect protein folding (p.Asp170Val, p.Pro205Ser, p.Gln279Arg, p.Arg301Gln), in
GLA.
PCR products were used to transform XL10-Gold ultracompetent cells (Stratagene, (part of Thermofisher, Fair Lawn, NJ, USA) and mutated plasmids were purified using the DNA extraction kit NZYMiniprep (Nzytech, Lisboa, Portugal) before sequencing by the Sanger method to confirm the presence of the introduced mutations.
Finally, mutated plasmid maxipreps were prepared from transformed E. Coli, using the HiSpeed Plasmid Maxi Kit (Qiagen, Hilden, Germany).
2.3. Cell Culture and Transfection
The 293T Hek cells (Human Embryonic Kidney 293T cells #CRL-3216, ATCC, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle’s medium (DMEM, #31966021, Thermofisher, Fair Lawn, NJ, USA) with Fetal Bovine Serum (FBS, #A4766801 Thermofischer) 10% (v/v), Penicillin-Streptomycin (#03-031-1C, Biological Industry, part of Thermofischer Fair Lawn, NJ, USA) 1% (v/v) and Amphotericin B 0.25 μg/mL (#A9598, Sigma-Aldrich, part of Merck, Darmstdt, Germany), at 37 °C and 5% CO2. Cells were transfected with Lipofectamine 3000 (#L3000015, Thermofisher), following the manufacturer’s instructions and using 0.75 µg of each plasmid.
The synthesized compound PBXs (PB48, PB49, PB51) were added to the medium at the concentration of 5 or 10 μM and incubated for 72 h at 37 °C and 5% CO2. Compounds DGJ, (1-Azido-β-deoxy-D-Galactopyranoside, #D9641), 1-Azido-1-deoxy-β-D-galactopyranoside (ADBGP, #513989); Allyl-α-D-Galactopyranoside (AaBGP, #48149-72-0) and galactose (#G0750) were ordered from Merk (Merck, Darmstdt, Germany). PB48 is also commercially available at Merck (6-Azido- 6-deoxy-D-Galactopyranoside, #712760).
Cells were harvested by scraping in Phosphate Buffered Saline (PBS) medium and lysed by 3 cycles of freeze and thaw, followed by sonication. Cytosolic fractions were collected by centrifugation at 10,000× g, 30 min at 4 °C, before measuring α-GalA activity.
2.4. FD Patients’ Lymphocytes Treatment
Upon written consent, we obtained blood samples from patients who were previously diagnosed with FD and are followed at Hospital Álvaro Cunqueiro (SERGAS), Vigo, Spain, as well as at other Spanish national centers. The study was approved by the Ethics Committee of Pontevedra-Vigo-Ourense, Galicia Autonomic Government, Spain (code: 2017/569; 21 December 2017). Enrolled patients present mutations in GLA (p.Gln279Arg, p.Leu131Gln, p.Met290Thr), which are involved in α-GalA protein folding. Peripheral Blood Mononuclear Cells (PBMCs) were purified from whole blood extracted in Heparin-Na+ by Ficoll-Paque-Plus (#GE17-1440-02 Healthcare, Chicago, IL, USA) density gradient, following blood dilution (1:1) with PBS. After centrifugation (35′ at 805× g withouth brake, r.t.), white cells were collected, washed twice with PBS and cultured with Roswell Park Memorial Institute medium (RPMI, #27016) with FBS 10% (v/v), Penicillin-Streptomycin 1% (v/v) and Amphotericin B 0.25 μg/mL at 37 °C and 5% CO2. Cells were seeded in 24 well plates at a concentration of 20,0000 cells/mL and treated with the PBX compounds at either 5 or 10 μM for 72 h. Finally, cells were harvested and lysed with the same protocol used for transfected cells.
2.5. α-GalA Activity
Activity of α-GalA was measured using the method of Chamoles et al. [
13]. Briefly, cell lysates activity was measured in the presence of the substrate, 4 mM 4-methylumbelliferyl-α-D-galactopyranoside (4-MU-α-Gal, Glycosynth, #44039, Warrington WA2 8QU, UK) and 50 mM N-acetyl-D-galactosamine (#A2795, Sigma-Aldrich, part of Merck, Darmstdt, Germany) in 0.15 M Phosphate-Citrate buffer (pH = 4.2). The reactions were incubated for 2 h at 37 °C and, at this point, the stopping solution (0.1 M ethylenediamine, pH = 11.4) was added to halt the activity. Then, sample fluorescence was measured, using Twinkle LB 970 Fluorometer (Berthold, Baden Württemberg, Germany), at 360 nm excitation and 450 nm emission wavelengths. Hydrolysed μmoles of substrate were calculated with reference to a 4-methylumbelliferone standard curve and divided for incubation time and total amount of protein in the cell lysate. Total protein concentration was measured with Pierce™ BCA Protein Assay Kit (Thermo-Fisher, Thermofisher, Fair Lawn, NJ, USA) following the manufacturer’s instructions.
2.6. α-GalA Kinetics
Recombinant human α-GalA (#6146-GH, R&D Systems, Minneapolis, MN, USA) was pre-incubated at either 37 °C, 39 °C, or 42 °C for 15 min with different concentrations of PB48 (150, 75, 50, 20, 10, 5, 2, 0.5, 0 μM) in Phosphate-Citrate buffer 0.15 M (pH = 4.2) with HALT protease inhibitor. After pre-incubation, the substrate 4-MU-α-Gal was added at different concentrations (4096, 3034, 2048, 1024, 512, 256, 128, 64, 32, 16, 0 μM) to each enzyme/compound solution and the product formation was assessed by measuring fluorescence at 37 °C (360/450 nm) after 30 min. The reaction was stopped with 0.1 M ethylenediamine pH = 11.4 and the product was quantified relative to a standard 4-methylumbelliferone curve. Another experiment was carried out pre-incubating the wild type enzyme with PB48 (150, 100, 75, 50, 10, 0 μM) in Phosphate-Citrate buffer 0.15 M at either pH 4.2 or pH 6.5 for 15 min and assessing substrate cleavage at 37 °C after the addition of 4-MU-α-Gal at the concentrations of 4096, 3034, 2048, 1024, 512, 256, 64, 0 μM. Interpolated Michaelis Menten curves of enzymatic reaction velocity versus substrate concentration and its reciprocal (Lineweaver–Burk plots) were obtained using Graph Pad Prism 9.1 (San Diego, CA, USA).
2.7. Molecular Modelling
The construction of a p.Asp170Val mutant was performed by using the mutagenesis toolkit in PyMol [
14] (
http://www.pymol.org/pymol, 20 October 2021) and a series of molecular docking experiments were performed to evaluate the affinity of the native ligand (galactose) and the PC Migalastat, using the Autodock-Vina suite of programs and their associated force field [
15,
16]. Clustering of the various conformations found for each ligand was performed via default root mean square deviation (RMSD) parameters and an exhaustiveness value of 20 [
17]. Relative energy analysis for each cluster and its associated affinity was then evaluated using the force field included in the Autodock-Vina suite.
2.8. Compound Cytotoxicity
Cytotoxicity of Hek cells treated with galactose analogues was real-time assessed, using RTCA DP XCelligence (Acea Biosciences, part of Agilent, Santa Clara, CA, USA). Briefly, cells were seeded, at the density of 7500 cells/well, in special plates equipped with electrodes to measure impedance (RTCA E-Palte 16 PET Acea, #00300600890, part of Agilent, Santa Clara, CA, USA) and grown at 37 °C and 5% CO2. Following 24 h, cells were treated with increasing concentrations of PB48, PB51, galactose, or DGJ (0 μM; 10 µM; 20 µM; 50 µM; 100 µM; 200 µM; 500 µM; 1 mM) and cell growth was followed up for an additional 72 h. Calculating the variation of impedance on plate surface, which is proportional to cell detachment and death, the RTCA software 2.0 (part of Agilent, Santa Clara, CA, USA) allowed to plot growth curves (cell index versus time) for each treatment and concentration.
2.9. Statistics
Normalized α-GalA activity data significance was assessd by ANOVA (one way non-parametric, Kruskal–Wallis test, multiple comparisons), using Graph Pad Prism v9.1 software. IC50 (molar) was calculated, using X-Celligence software, from a cell index at each concentration of galactose analogues and plotted versus time. IC50 at time 52 h (corresponding to linear growth phase) was extrapolated from these plots to compare compound effects on cell proliferation.
3. Results
3.1. PBXs Galactose Analogues Synthesis
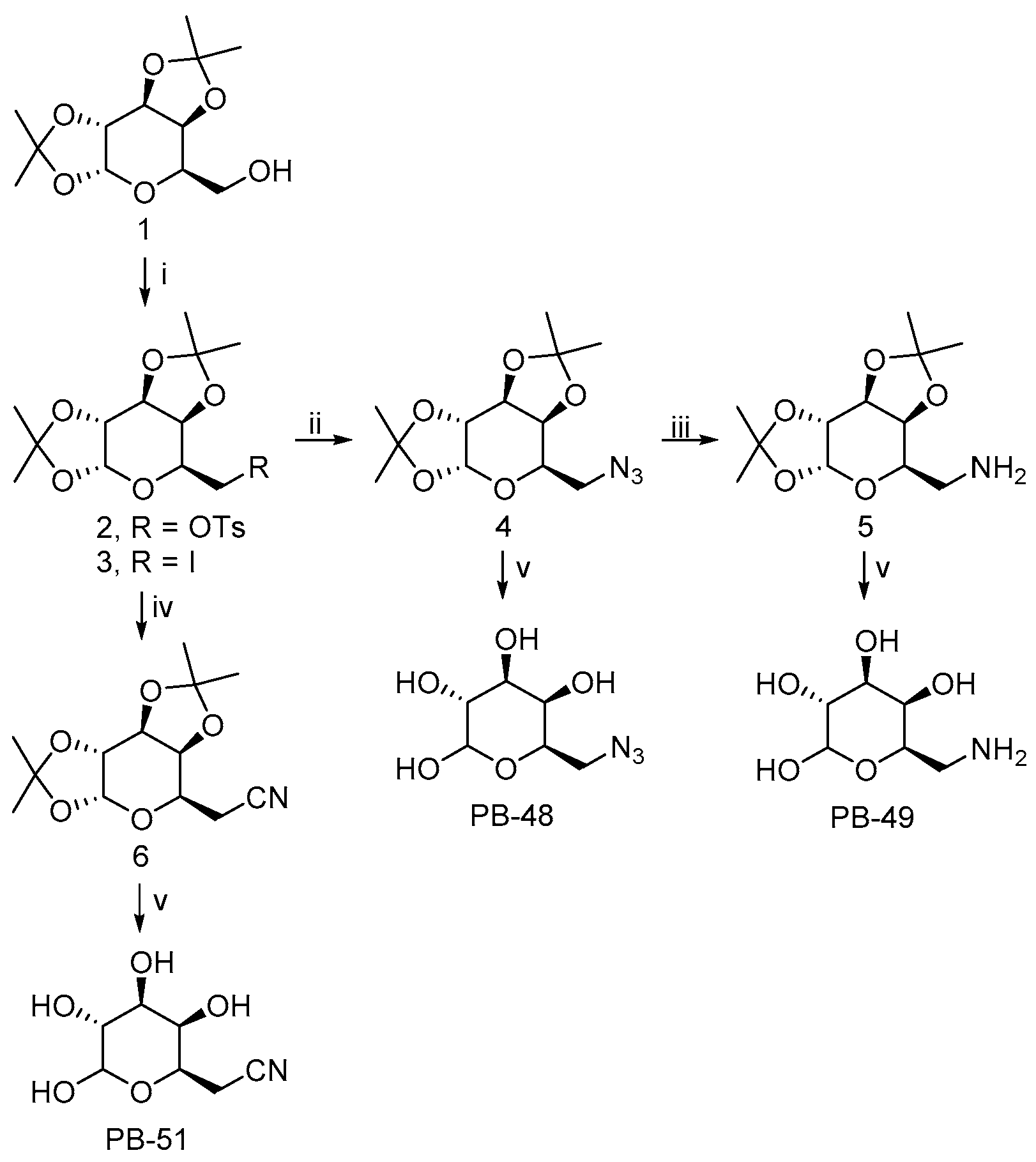
The 6-substituted galactose analogues PB48, PB49, and PB51 were prepared from 1,2:3,4-Di-
O-isopropylidene-D-galactopyranose (
1) as shown in
Figure 1, following or adapting previously described procedures [
18,
19,
20].
Synthesis and structural characterization of galactose analogues PB48, PB49, and PB51 are detailed below.
3.2. General Procedure for the Preparation of 6-Substituted-6-deoxy-α,β-D-galactopyranosides
The corresponding 6-substituted-1,2:3,4-di-O-isopropylidene-D-galactopyranose 4–6 (0.3 mmol) was treated with trifluoroacetic acid/water (4:1, 1 mL). The reaction mixture was stirred at room temperature for 1–4 h and the solvent was evaporated to dryness. The crude solid obtained was washed with H2O (3 × 2 mL) and EtOAc (3 × 2 mL). The residue was purified by column chromatography on silica gel (CH2Cl2/MeOH, 85:15) to afford the desired compound PB48 and PB51, or by reversed-phase SupelcleanTM LC-18 SPE tube (H2O/MeOH, 9:1) to obtain PB49.
3.2.1. PB48: 6-Azido-6-deoxy-α,β-D-galactopyranoside
PB48 (90% yield) was obtained as a white solid after stirring the reaction mixture for 1 h. PB48 occurred as a 1:1.6 α/β anomeric equilibrium mixture in D2O (H-1 integration); Rf = 0.35 (CH2Cl2/MeOH, 4:1); 1H NMR (D2O): δ = 5.14 (d, J = 3.8 Hz, 1H, H-1α), 4.48 (d, J = 8.5 Hz, 1H, H-1β), 4.10–4.04 (m, 1H, H-5α), 3.85–3.81 (m, 1H, H-4α), 3.79–3.76 (m, 1H, H-4β), 3.76–3.64 (m, 3H, H-5β, H-3α, H-2α), 3.55–3.50 (m, 1H, H-3β), 3.50–3.31 (m, 5H, H-2β, H-6α, H-6β); 13C NMR (D2O): δ = 96.4 (C1β), 92.3 (C1α), 73.4, 72.6, 71.6, 69.6, 69.0, 68.9, 68.8, 68.1, 50.8 (CH2), 50.7 (CH2); HRMS (ESI): m/z [M + Na]+ calculated for C6H11N3NaO5: 228.05909, found: 228.05963.
3.2.2. PB49: 6-Amino-6-deoxy-α,β-D-galactopyranoside
PB49 (85% yield) was obtained as a brown solid after stirring the reaction mixture for 2 h. PB49 occurred as a 1:1.5 α/β anomeric equilibrium mixture in D2O (H-1 integration); 1H NMR (D2O): δ = 5.17 (d, J = 3.8 Hz, 1H, H-1α), 4.48 (d, J = 7.9 Hz, 1H, H-1β), 4.18–4.12 (m, 1H, H-5α), 3.89–3.85 (m, 1H, H-4α), 3.83–3.72 (m, 3H, H-4β, H-5β, H-3α), 3.72–3.66 (m, 1H, H-2α), 3.57–3.52 (m, 1H, H-3β), 3.42–3.35 (m, 1H, H-2β), 3.21–3.09 (m, 4H, H-6α, H-6β); 13C NMR (D2O): δ = 96.3 (C1β), 92.2 (C1α), 72.4, 71.4, 70.7, 69.7, 69.2, 68.8, 67.9, 66.1, 40.2 (CH2), 40.1 (CH2); HRMS (ESI): m/z [M + Na]+ calculated for C6H13NNaO5: 202.06859, found: 202.06834.
3.2.3. PB51: 6-Cyano-6-deoxy-α,β-D-galactopyranoside
PB51 (88% yield) was obtained as a white solid after stirring the reaction mixture for 4 h. PB51 occurred as a 1:1.4 α/β anomeric equilibrium mixture in D2O (H-1 integration); Rf = 0.36 (CH2Cl2/MeOH, 4:1); 1H NMR (D2O): δ = 5.12 (d, J = 3.8 Hz, 1H, H-1α), 4.48 (d, J = 7.8 Hz, 1H, H-1β), 4.28–4.20 (m, 1H, H-5α), 3.92–3.85 (m, 1H, H-5β), 3.83–3.78 (m, 1H, H-4α), 3.77–3.70 (m, 2H, H-3α, H-4β), 3.68–3.61 (m, 1H, H-2α), 3.55–3.49 (m, 1H, H-3β), 3.38–3.30 (m, 1H, H-2β), 2.75–2.64 (m, 4H, H-6α, H-6β); 13C NMR (D2O): δ = 119.0 (CN), 96.4 (C1β), 92.4 (C1α), 72.4, 71.3, 70.2, 70.1, 69.5, 68.8, 67.8, 66.0, 19.4 (CH2); HRMS (ESI): m/z [M + Na]+ calculated for C7H11NNaO5: 212.05294, found: 212.05289.
3.3. Galactose Analogues PB48 and PB51 Increase α-GalA Activity in Cells Transfected with Wild Type Enzyme or Selected Variants Affecting Protein Folding
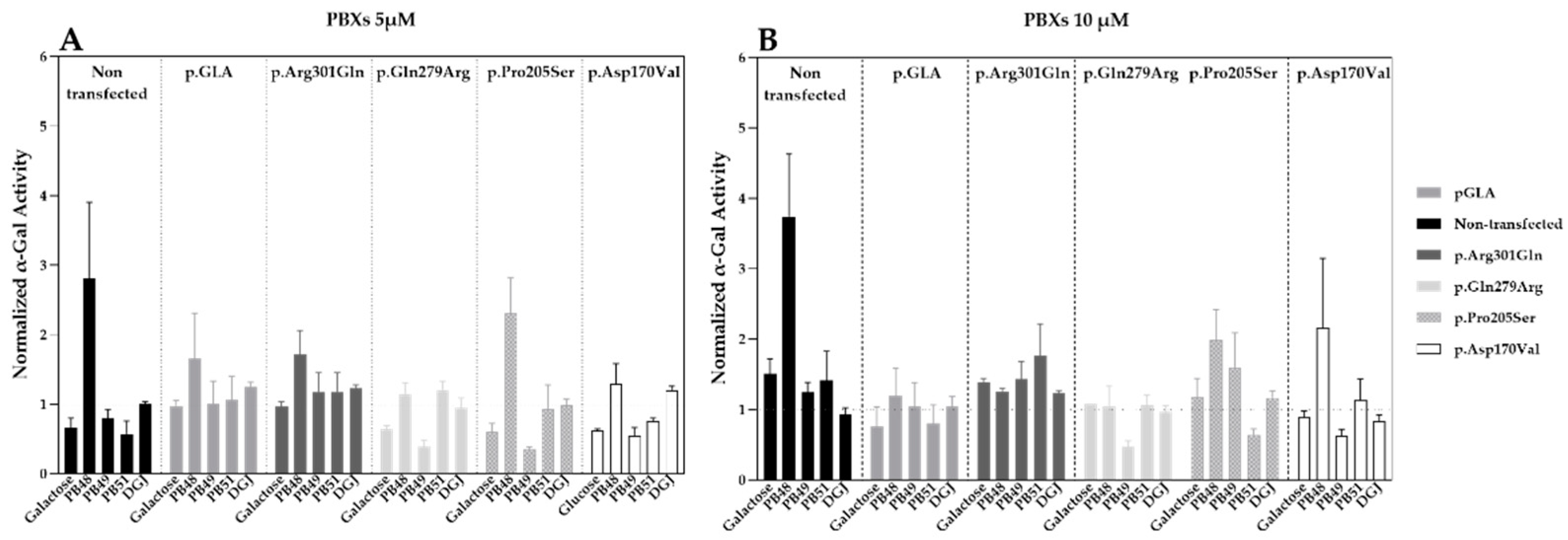
Hek cells were transfected with plasmids that encode wild type α-GalA (pGLA) or mutated isoforms of the enzyme (i.e., p.Asp170Val, p.Pro205Ser, p.Gln279Arg, p.Arg301Gln) with affected protein folding. Then, cells were treated with PBX compounds, galactose, or DGJ at concentrations of 5 or 10 µM for 72 h.
Treatment with PB48 increases the activity of α-GalA compared to baseline (non-transfected, non-treated cells) in all the analyzed variants at both tested concentrations (
Figure 2A,B). In particular, PB48 increases the activity of α-GalA more efficiently than galactose or DGJ.
PB51 also increased enzyme activity when applied at 5 µM on cells transfected with p.Arg301Gln, p.Gln279Arg, and p.Pro205Ser α-GalA mutants, while it stabilizes the p.Arg301Gln and p.Asp170Val variants at the concentration of 10 µM.
On the other hand, PB49 only partially increases the activity of wild type α-GalA or p.Arg301Gln isoform when applied at the concentration of 10 µM.
Treatment with galactose at the concentration of 5 µM had an inhibitory effect on the activity of p.Gln279Arg, p.Pro205Ser, and p.Asp170Val enzymes. Treatment with DGJ showed stabilizing effect on p.Arg301Gln and p.Pro205Ser variants at the concentration on 10 µM. However, the increment of enzyme activity is lower than the one observed for the treatment with PB48 at the same concentration.
None of the tested compounds determines a significant increase of α-GalA activity, as assessed by Kruskal–Wallis test, probably due to the overexpression of the transfected plasmids and the contribution of the endogenous enzyme to the measured activity values.
Transfection levels were similar in each sample, as assessed by WB (data not shown).
3.4. Galactose Analogues with Different Characteristics Compared to PBXs Inhibit α-GalA Activity
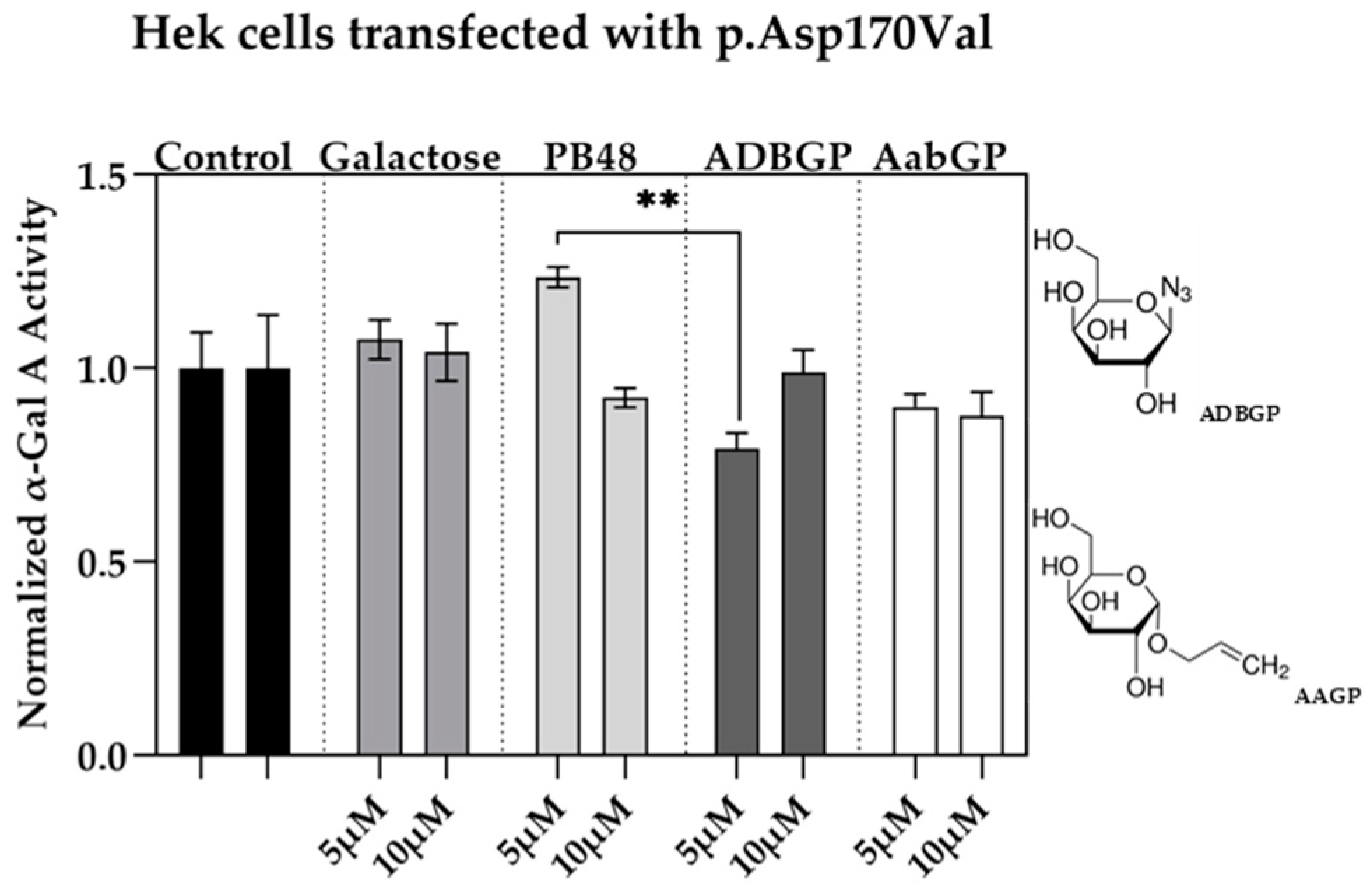
We assessed the relation between structure and activity of the compounds at studying by testing the effect of additional galactose analogues. We transfected Hek cells with p.Asp170Val mutant, which affects the interaction of galactose substrate with α-GalA, and therefore allows to understand the differential interactions of the PBX molecules with the active site of the enzyme. We treated the transfected cells with the galactose analogue ADBGP, which presents an N
3 radical in position 1 of galactose ring, or AaBGP which presents an allyl radical in the same position. Treatment with both compounds at 5 or 10 μM has an inhibitory effect on the p.Asp170Val variant activity. In particular, we found significant differences in normalized α-GalA activity, following treatment with PB48 in comparison with ADBGP at the concentration of 5 µM (
Figure 3).
These results show that substrates with different polarity, length of lateral chain, or substituents in different positions, may affect the stabilizing effect of the galactose analogue on α-GalA mutated enzyme.
3.5. PB48 Stabilizes α-GalA When Perturbating Temperature or pH of the Enzymatic Reaction
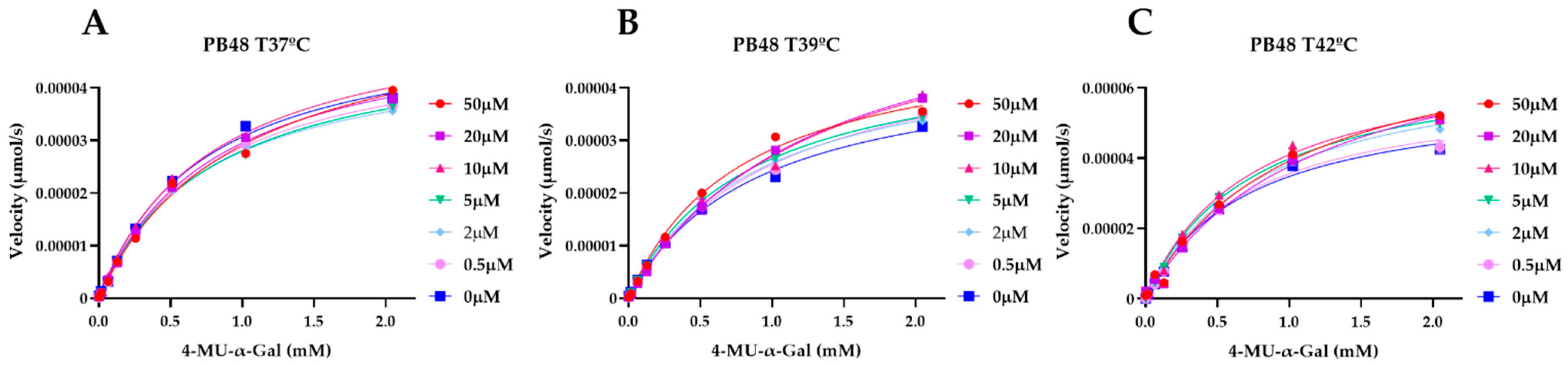
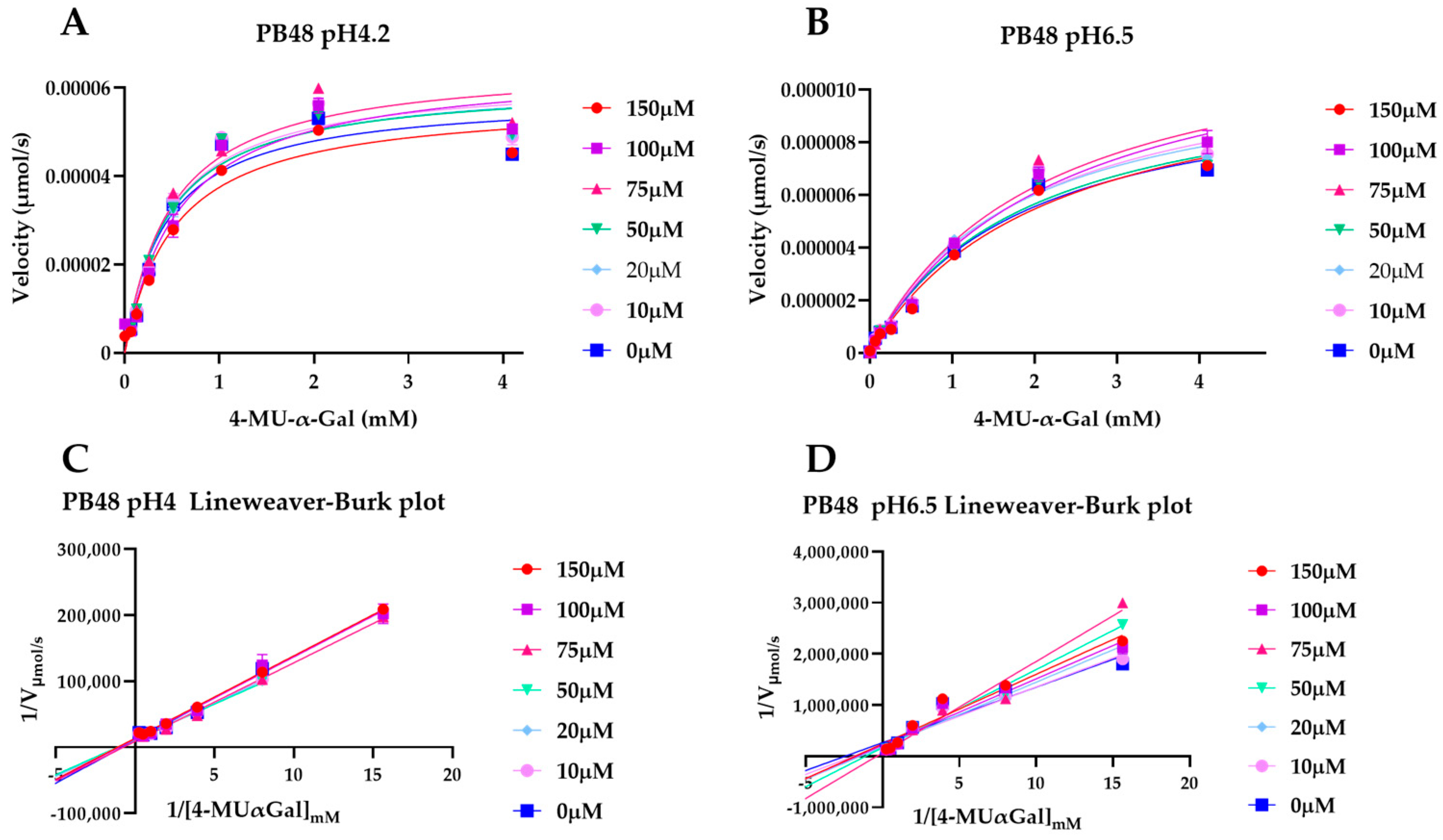
To explain the action mechanism of the new galactose analogues and confirm that PB48 stabilizes α-GalA when it binds to the active site of the enzyme, we followed the kinetics of purified wild type α-GalA at increasing concentrations of the substrate 4-MU-α-Gal (2048, 1024, 512, 256, 128, 64, 16, 0 μM), pre-treating the enzyme with PB48 (50, 20, 10, 5, 2, 0.5, 0 μM) at different temperatures (37–39–42 °C) (
Figure 4). When the enzyme is treated with PB48 in the concentration range used for the assays in cellular models, the compound does not significantly change the kinetic of the α-GalA; however, it clearly accelerates enzymatic reactions when pre-incubating the enzyme at high temperatures. This effect is reached for all tested substrate concentrations at the temperature of 42 °C and with substrate concentrations higher than 16 μM at the temperature of 39 °C for each PB48 concentration (
Supplementary Figure S1).
Treating α-GalA with up to 150 μM PB48 (150, 100, 75, 50, 20, 10, 0 μM), the 4-MU-α-Gal cleavage reaction is also accelerated by the treatment with the compound. The velocity of the enzymatic reaction increases, compared to the non-treated control, when the enzyme is pre-incubated at 39 °C and 42 °C (
Supplementary Figure S2) and also when the reaction is assessed at 37 °C at pH 6.5, at least for substrate concentrations higher than 128 μM (
Figure 5).
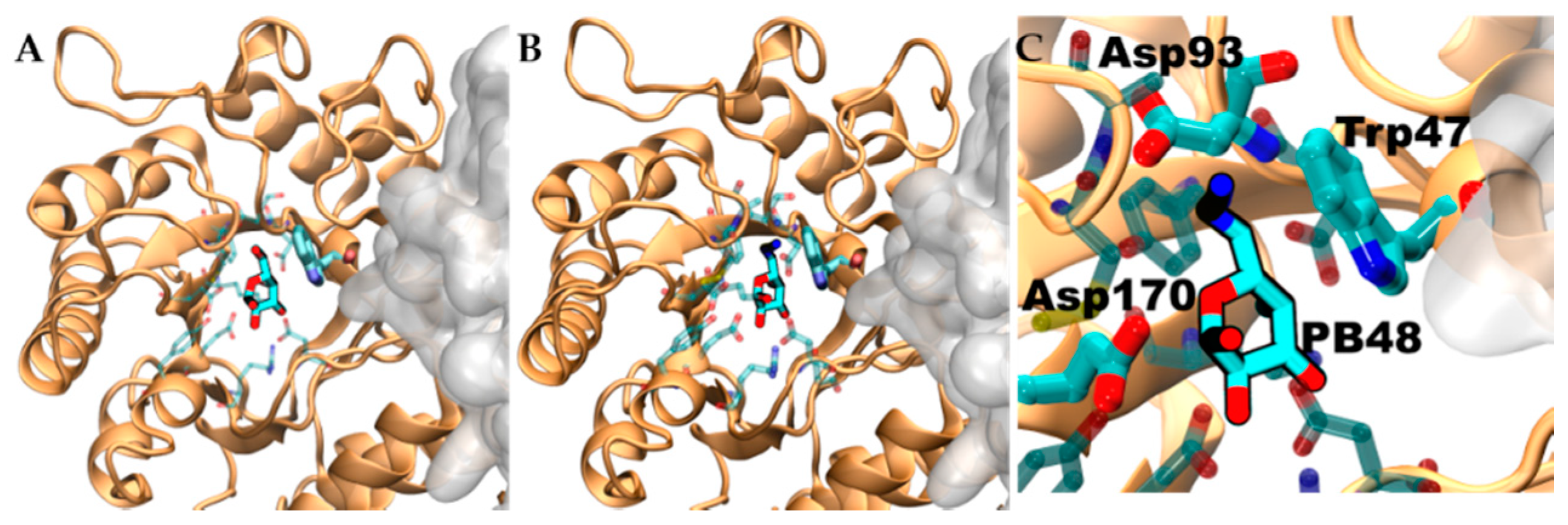
3.6. PB48 Establishes Additional Interaction Points within the α-GalA Binding Site Compared to Galactose or DGJ
To evaluate structural implication of the p.Asp170Val, we analyzed the structural changes of α-GalA (PDBid:3S5Z) and its active site upon mutation p.Asp170Ala (PDBid:3TV8). An RMSD fit between both crystallographic structures reveals negligible differences (
Supplementary Figure S3A,B), suggesting that also p.Asp170Val mutation shares the same topology. Docking experiments, using galactose and PB48, on the wild type protein, the variant p.Asp170A, and an in-silico modeled p.Asp170V variant showed comparable binding energies and poses (
Supplementary Table S2).
Aiming to rationalize the binding differences between galactose, DGJ, and PB48, we compared the docking poses of the different ligands using X-ray structures of acid-alpha-galactosidase A in complex with galactose (PDBid:3GXP) and DGJ (PDBid:3GXT).
Despite the similar binding poses of galactose and PB48 (
Figure 6A,B), the Azido group in PB48 adds a salt bridge with Asp93 and makes contact with the indol group of Trp47 (
Figure 5C). These highly stabilizing interactions provide a putative explanation for the potent stabilizing effect of PB48. Indeed, similar azido–indol interactions have been reported in other protein–ligand interactions (see, for instance, PDB structures 6HOC and 3O8G). The putative azido–indol interaction at the absolutely conserved Trp47 is relevant because this amino acid is close to the protein–protein interface with the second protomer in the homodimer (
Figure 6).
3.7. PB48 and PB51 Increase α-GalA Activity in Leukocytes from FD Patients with the p.Gln279Arg Mutation, Which Is Not Amenable to the Treatment with Migalastat
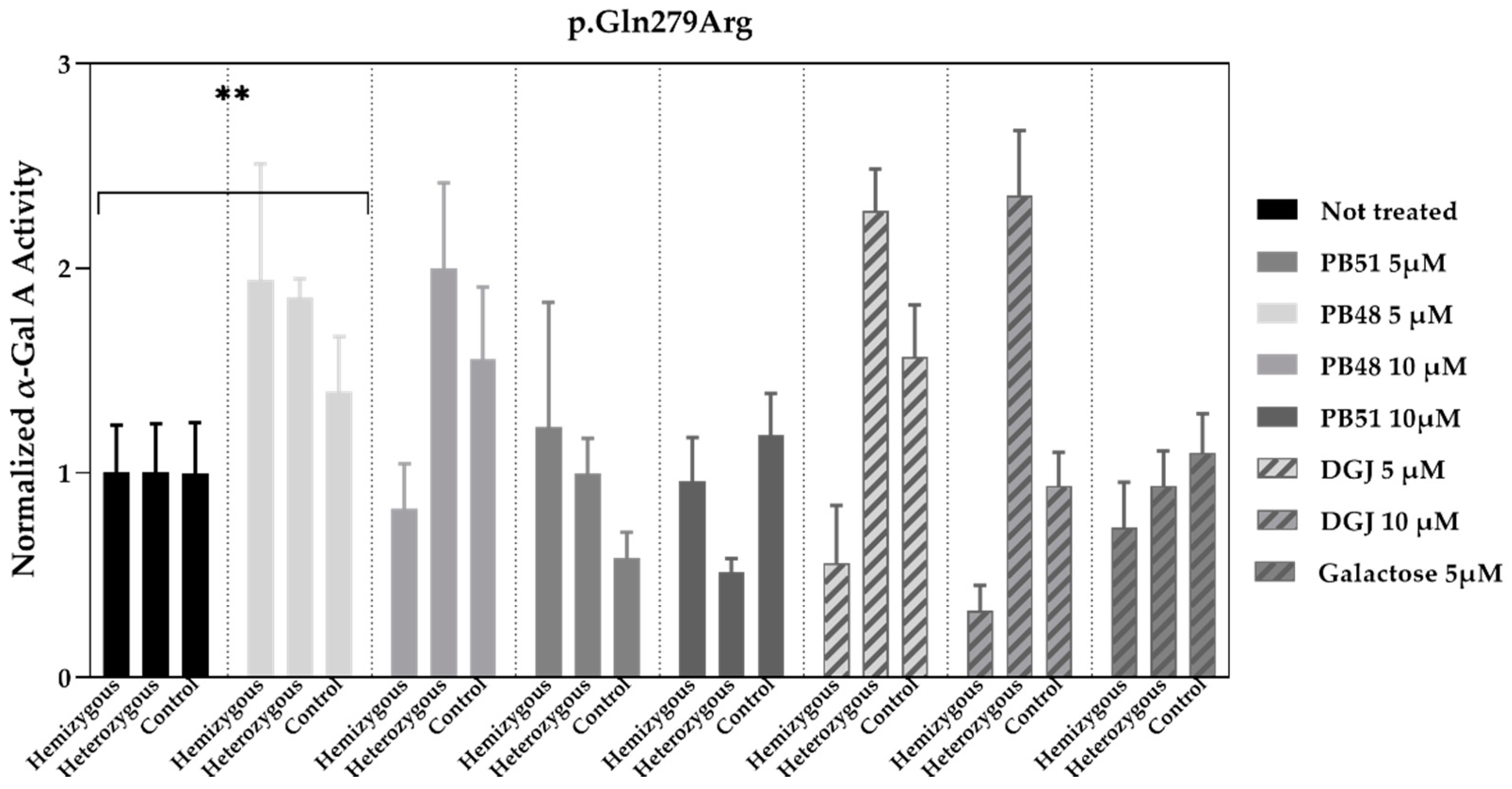
To validate the data obtained in transfected cells, similar studies were carried out in leukocytes extracted from peripheral blood of FD patients treated with PBX analogues.
We cultured cells from healthy subjects (
N = 2), as well as a hemizygous patient and a heterozygous patient with the p.Gln279Arg variant in presence of PB48, PB51, DGJ (5 or 10 μM), or galactose (5 μM,
Figure 7).
Normalized α-GalA activity was increased in all patients’ samples treated with PB48 at the concentration of 5 μM, while it decreases in the hemizygous patient’s cell treated with DGJ, thus confirming that the p.Gln279Arg variant is not amenable for the treatment with Migalastat [
11].
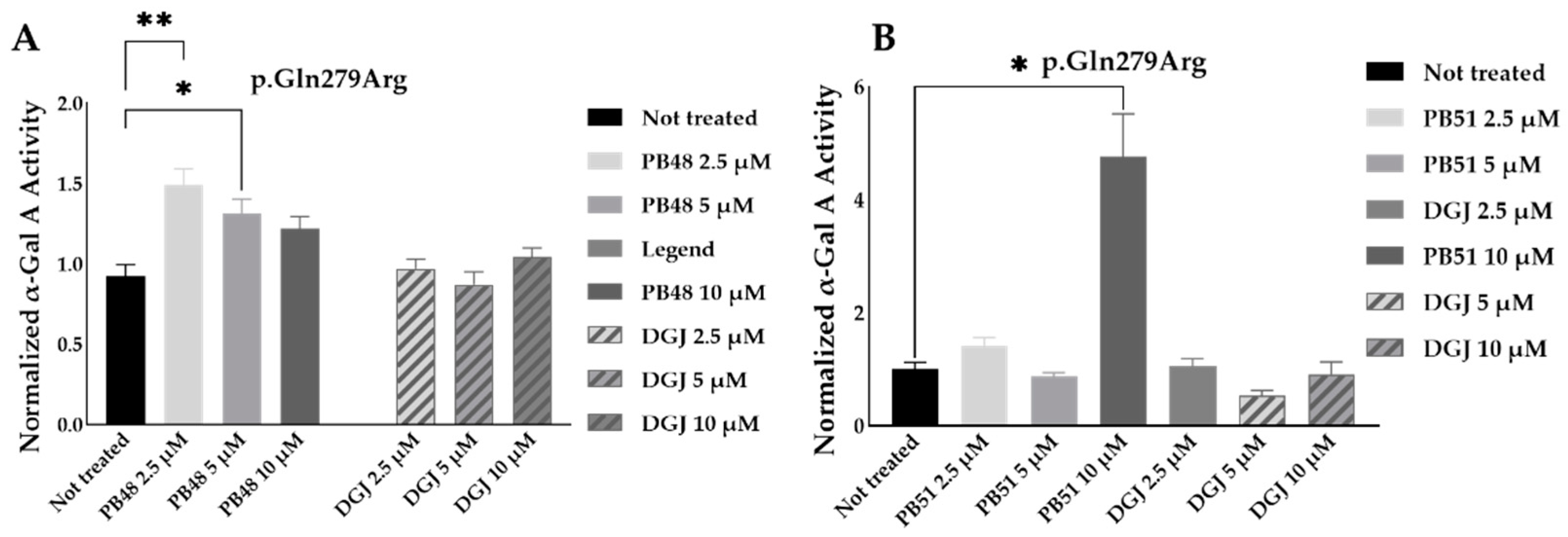
To further substantiate results, we assessed the effect of PB48 and PB51 on p.Gln279Arg variant from leukocytes from three different hemizygous patients belonging to the same family. In hemizygous patients’ leukocytes treated with either PB48, PB51, or DGJ (2.5 µM, 5 µM and 10 µM), the activity of α-GalA was significantly increased by treatment with PB48 at the concentrations of 2.5 µM and 5 µM and at higher rates compared to DGJ (
Figure 8A). The treatment with PB51 significantly increased normalized α-GalA activity more than DGJ, only when applied at 10 µM (
Figure 8B).
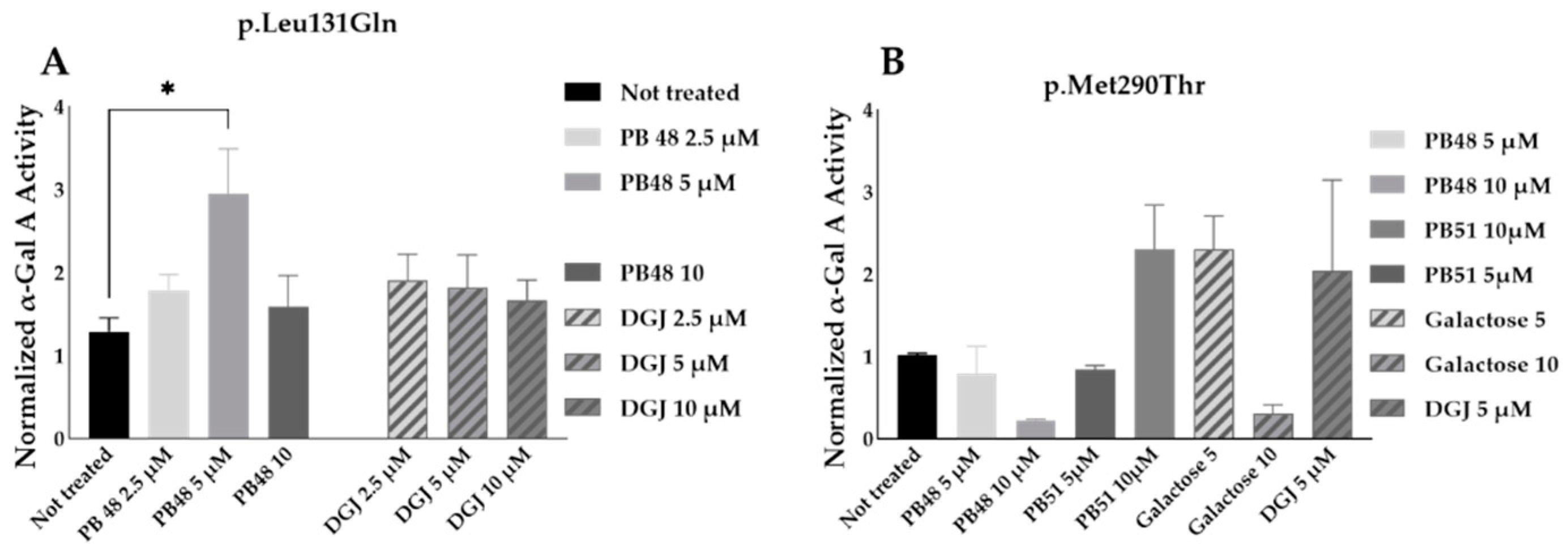
3.8. PB48 and PB51 Are Also Effective in Cells from FD Patients with the Mutations p.Leu131Gln or p.Met290Thr
PB48 and DGJ were also tested in leukocytes from hemizygous patients with, respectively, the p.Leu131Gln or the Met290Thr variant in GLA.
The normalized enzymatic activity of the α-GalA is significantly higher in p.Leu131Gln hemizygous patient cells treated with PB48 at the concentration of 5 µM when compared to untreated cells. It is worth noting that DGJ has no significant effect on this activity (
Figure 9A), which in fact is described as not amenable to the treatment [
11].
Analyzing samples from a single patient with the p.Met290Thr variant, we found increased normalized α-GalA, only in cells treated with 10 µM PB51 (
Figure 9B).
3.9. PBX Has No Significant Inhibitory Effect on Cell Growth
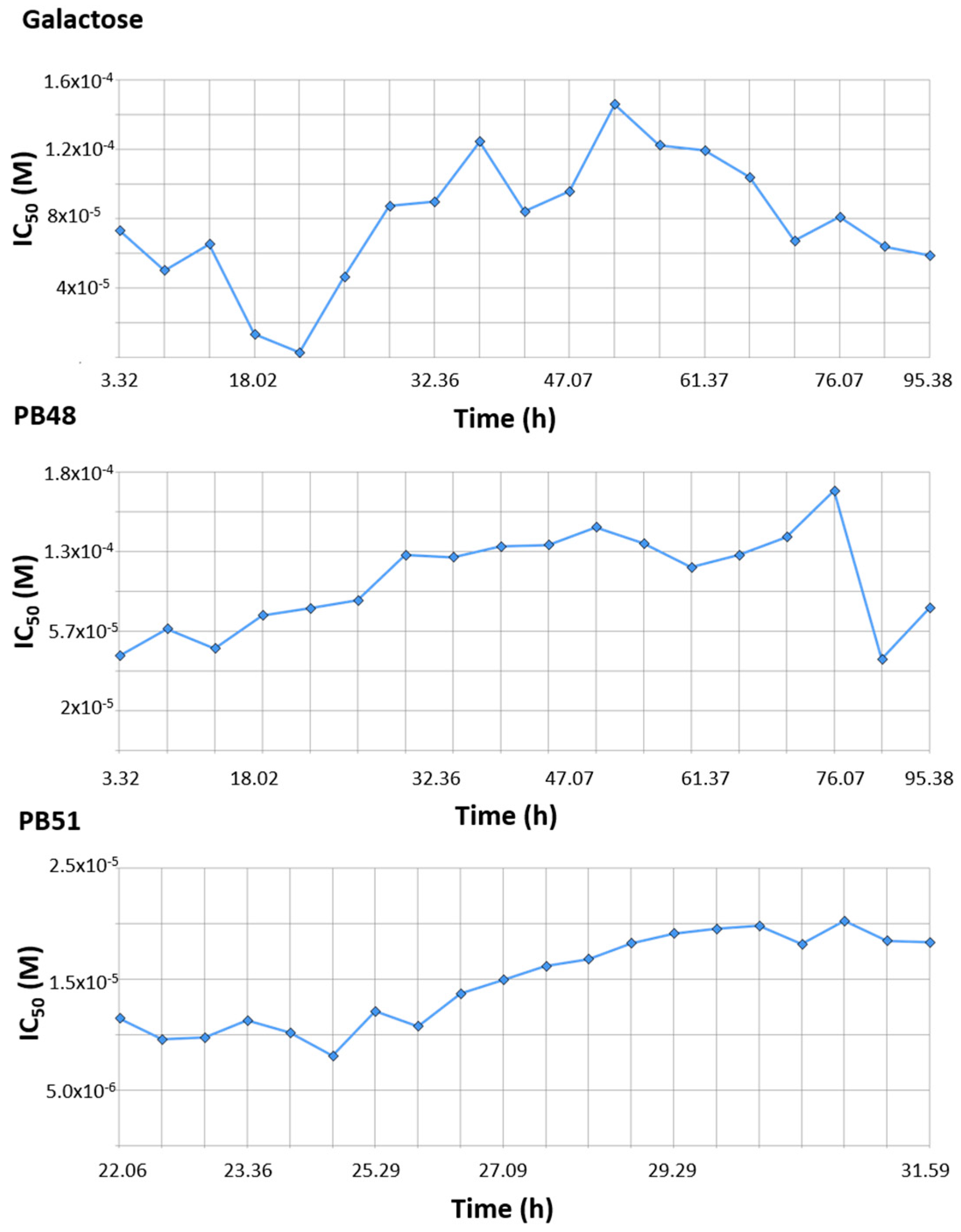
Finally, we tested the effect of PBX compounds and galactose on cell growth in the 10 μM to 1 mM range. We monitored real time cell growth by registering impedance of the cells on the plate surface for 72 h after treatment and demonstrated that there are no significant differences in cell growth curves from untreated cells compared to cells treated with PB48, PB51, or galactose (
Supplementary Figure S5). The IC
50 along the time of the experiment is also comparable among treatments and its value at time 52 h (linear cell growth phase) is, respectively, 8.43 × 10
−5, 1.4 × 10
−4, 1.4 × 10
−4 for galactose PB48 and PB51 (
Figure 10). We also assessed cell growth in cells treated with DGJ (0–500 μM range), obtaining comparable results to the PBXs and galactose (IC
50 = 2 × 10
−5 at 52 h,
Supplementary Figure S6).
4. Discussion
In the present article, we assessed the efficacy of the synthesized galactose analogues in increasing α-GalA activity and acting as possible PCs in the treatment of FD.
We were able to show that α-GalA activity values of wild type and mutated enzymes were increased upon treatment with PB48 or PB51 in different cellular models and experimental conditions (temperature and pH shifts), demonstrating that the enzyme is more stable in presence of PBX analogues.
The effect of PBXs was demonstrated in Hek cells transfected with clinically meaningful variants associated to FD (i.e., p.Arg301Gln; p.Gln279Arg; p.Pro205Ser; p.Asp170Val). This system is a relevant cellular model to show PC activity for α-GalA since a similar assay, carried out in GLP condition, has been approved to evaluate susceptibility of α-GalA variants to the treatment with Migalastat, before considering its administration in FD patients [
21]. Our assay was not performed under GLP conditions, but the experimental procedures were very similar to the ones of the approved test.
Moreover, treatment with PB48 of patients’ cells determines a significative increase in α-GalA activity of the p.Gln279Arg or p.Leu103Gln mutated enzymes, which are not amenable to the treatment with Migalastat, while PB51 may stabilize p.Met290Thr enzyme.
As shown by modeling studies, PB48 establishes an interaction with Trp47, which is located at the interface between monomers and, unlike galactose or Migalastat, it can stabilize mutants which are located at a certain distance from the active site (i.e., p.Gln279Arg) because it most likely facilitates monomer interactions and stabilizes the quaternary structure of the protein. Indeed, Try47 is highly conserved in α-GalA and several mutations of this residue have been related to FD classical presentation (i.e., p.Trp47Arg; p.Trp47Gly; p.Trp47Leu; p.Trp47*) [
22,
23,
24].
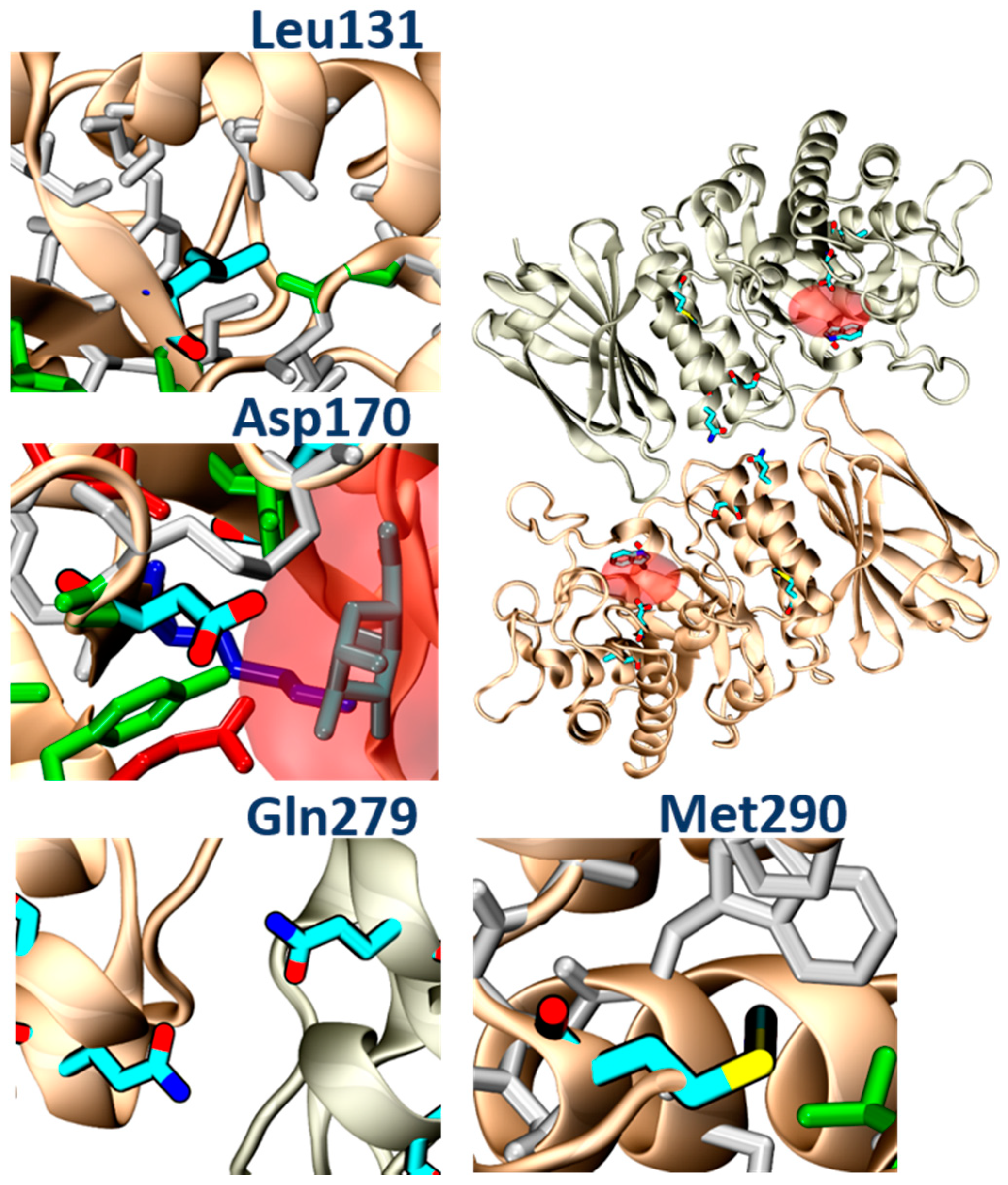
In addition, molecular analysis of the structures of α-GalA variants found in FD patients, and analyzed in the present study (p.Leu131, p.Gln279Arg, p.Met290Thr), suggested that all of these mutations impair protein folding. In particular, the p.Leu131Gln variant and the Met290Thr variant would place a polar amino acid within a hydrophobic environment. The p.Gln279Arg would place two positive charges one in front of the other, destabilizing the dimerization interface, while the p.Asp170Val, which was studied in transfected Hek cells, would remove a hydrogen bond between the protein and the ligand (
Figure 11). PB48 forms an additional salt bridge with Asp93, which further stabilizes the active site and explains the efficacy of PB48 to stabilize mutants where Asp170 residue is affected. On the contrary, both galactose and DGJ need to interact with Asp170 to bind to the active site.
The experimental data obtained upon treatment with galactose analogues confirm that PB48 increases α-GalA activity and therefore suffices to overcome the loss of a hydrogen bond in the p.Asp170Val mutant and stabilizes the protein–protein interface in the case of the p.Gln279Arg.
Thus, the binding and stabilization of different types of interactions highlight the relevance of the azido–indol interaction and the additional salt bridge established by PB48 and suggest that this compound could also be effective against additional mutations that affect protein folding, which were not directly assessed in the present work.
Based on the modeling results, we hypothesized that PBXs can stabilize α-GalA wild type and mutated enzyme structures, thus acting as PC and facilitating translocation of α-GalA to the lysosome for substrate cleavage.
Following the kinetic of 4-MU-α-Gal hydrolysis in presence of PB48 at different temperatures and pH values we were able to confirm that PB48 allows the enzyme to resist environmental changes, therefore confirming that it stabilizes the protein when bound to its active site.
Unlike DGJ, which is a strong α-GalA inhibitor [
25], PB48 does not significantly inhibit the enzymatic reaction at the tested concentrations (
Supplementary Figure S7), and therefore it can stabilize the protein without significantly blocking its function.
However, as it binds to the active site, we cannot exclude that PB48 can compete with the substrate when it is administered at higher concentrations than the ones tested in this study (≥75 mM).
Moreover, we have shown that PB48 stabilizes α-GalA at pH 6.5; therefore, it should favour the stabilization of the folding enzyme during its synthesis in the endoplasmic reticulum (≈pH 7). The complex enzyme/PB48 should easily translocate to the lysosome, where PB48 is possibly released due to the very high substrate concentration and the lower pH.
When mutations that affect protein folding are present in the enzyme structure, the stabilizing effect of PB48 should be even higher than the one assessed with the wild type protein, due to the unique interactions that this compound establishes with crucial residues that stabilize dimer formation, and as suggested by docking simulation results (
Supplementary Table S2).
ERT treatment for FD presents important limitations; therefore, new therapeutic alternatives are definitively needed and some possible strategy is under evaluation in clinical trials [
26].
Migalastat (DGJ) is currently the only available oral compound for the treatment of FD, which can overcome some of the ERT limitations (i.e., immunogenicity, bioavailability). However, the use of Migalastat is only allowed in patients with amenable mutations, which are generally variants that surround the active site of α-GalA. In fact, the interactions that this chaperone establishes can stabilize a limited area in proximity of its binding site. Moreover, DGJ has little effect in variants of the active site like the Asp170Val because the chaperoning action of DGJ strictly depends on the interaction of the iminosugar with the carboxylate of the Asp170, as shown by crystallography and other experimental data obtained with α-GalA mutants at the residue 170 [
25].
Like Migalastat, PBX compounds still present some limitations, since they can stabilize α-GalA only when the causing mutation does not affect protein synthesis. Therefore, PBXs are willing to be inactive in patients with large deletions, frame-shift mutation, or splicing variants, which may determine null protein production or largely truncated peptides. However, the galactose analogues that we synthesized present the advantage of establishing a wider interaction range with enzyme residues and therefore to better stabilize 3D structure of α-GalA (monomers interactions), even in variants that are currently excluded from the indications of the oral treatment.
There are more than 1000 mutations that have been related to FD (
www.hgmd.org, 20 October 2021) and the majority of them (about 70%) are point mutations, most likely related to protein stability defects; therefore, PBXs may represent a new oral option for a considerable number of patients, who present mutations that affect protein folding, including variants that are not amenable for the treatment with DGJ.
PBXs still have to demonstrate their efficacy in vivo in animal models, but given the similarity of the structure of the molecule with galactose and DGJ, it is assumable that pharmacokinetic properties of these compounds will be similar. In vivo studies with DGJ on FD transgenic mouse models showed significant increase of α-GalA activity in heart, kidney, spleen, and liver of transgenic mouse models with mutations affecting enzyme folding (i.e., p.Arg301Gln), as well as decreased levels of Gb3 and Lyso-Gb3 in the affected tissues [
27,
28,
29]. Furthermore, the efficacy of DGJ to stabilize disease progression was proved in clinical trials and open-label extension studies in patients with amenable mutations [
30,
31].
In addition, we have proven that treatment with PBXs is not cytotoxic since treated Hek cells present growth curves similar to the ones obtained by treatment with galactose or DGJ and comparable or higher IC50.
While we still have to confirm whether PBXs are effective in increasing α-GalA activity in vivo, we have to consider that a limited increase in enzyme activity may be sufficient to metabolize accumulated glycosphingolipids and to revert clinical phenotype. Indeed, in several LSDs, substrate accumulation that determines clinical manifestations occurs when residual enzyme activity decays below a certain threshold (about 10% of residual activity or lower), as demonstrated for β-hexosaminidase A and arylsulfatase A [
32]. This low threshold is probably justified by the fact that many LSDs are caused by impaired enzymes, which act as catalysts for biological reactions. Additionally, affected cells in most lysosomal disorders can benefit from the cross correction effect [
33]. In terms of treatment, these features support that a low improvement in enzyme activity could be highly effective to meliorate clinical signs.
Moreover, PBXs, like other small molecules, may have the advantage to potentiate the effect, the half-life, and the bioavailability of ERT in a possible combinational therapy, since the chaperone is also active on the wild type enzyme, which can also be stabilized.
More efforts also have to be spent on fine tuning the in vivo dose of the drug, since PBXs, binding to the active site of the target enzyme, may act as inhibitor when administered at high doses; however, we showed little or no inhibitory effect of PBXs on α-GalA activity at the tested concentrations. Turnover of the ligand is favored by high substrate concentrations and by binders that present a shorter half-life than the enzyme, as well as reduced binding affinity at lysosomal pH.
On the other hand, the option to inhibit α-GalA activity with PBXs at high concentrations can open new therapeutic possibilities. It is worth considering that lysosomal enzymes participate in a common pathway [
34] to cellular metabolism and α-GalA inhibition may help to restore substrate synthesis/cleavage balance in other LSDs or even in other more frequent conditions characterized by lysosomal dysfunction and autophagy blockage.