Tempol Alters Urinary Extracellular Vesicle Lipid Content and Release While Reducing Blood Pressure during the Development of Salt-Sensitive Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Osmotic Minipump Infusion of Tempol
2.3. Blood Pressure Measurements
2.4. Metabolic Cage Studies and Animal Diet
2.5. Kidney Homogenate Preparation
2.6. BCA Assay
2.7. SDS PAGE, Immunoblotting, and Densitometry
2.8. Electrolyte Measurements
2.9. Urinary EV Isolation
2.10. Nanoparticle Tracking Analysis
2.11. Extraction of Lipids from Urinary EVs
2.12. Liquid Chromatography Tandem Mass Spectrometry and Scanning Parameters
2.13. Statistical Analysis
3. Results
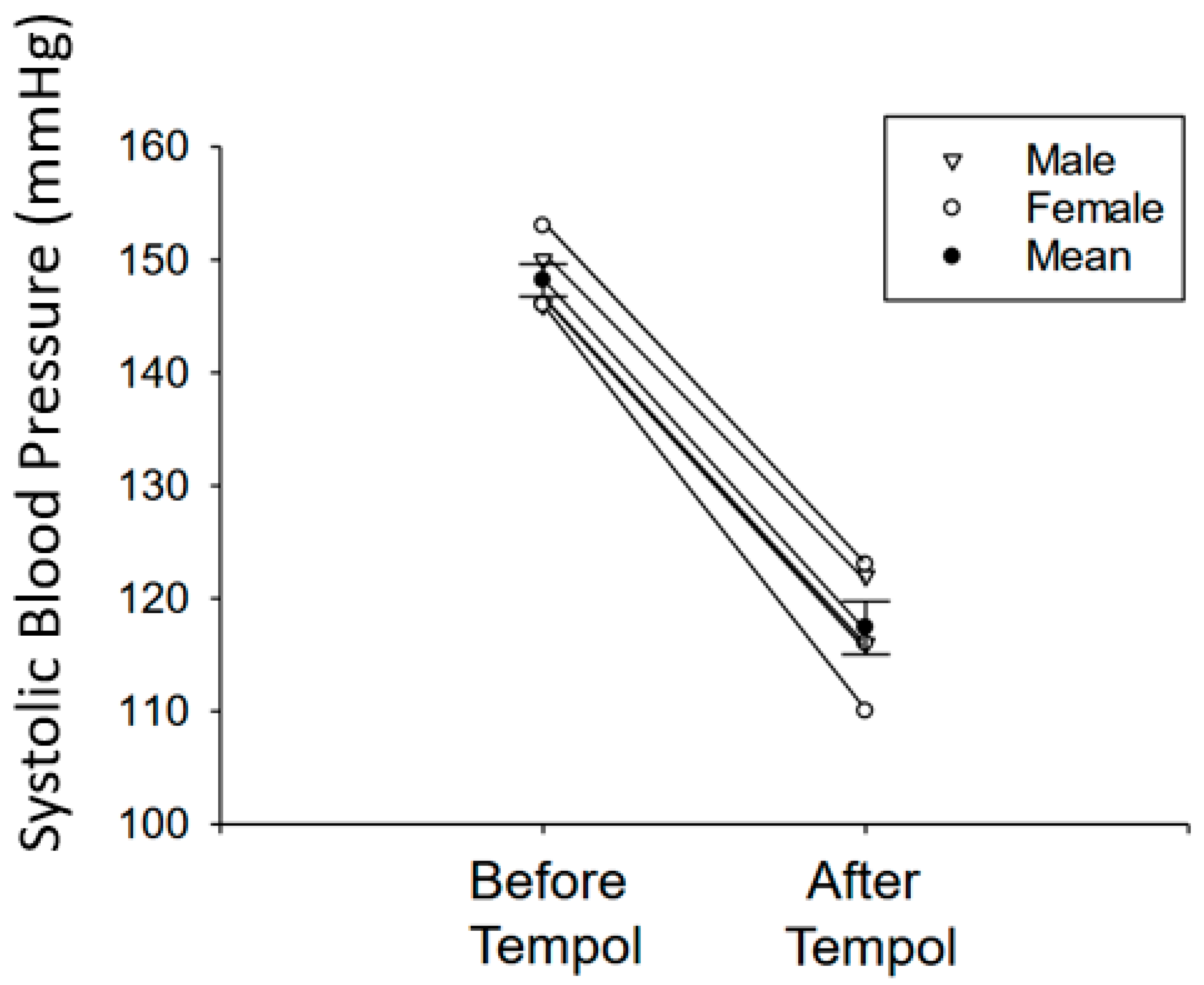
3.1. Tempol Treatment Reduces Systolic Blood Pressure in Salt-Loaded Hypertensive 129Sv Mice
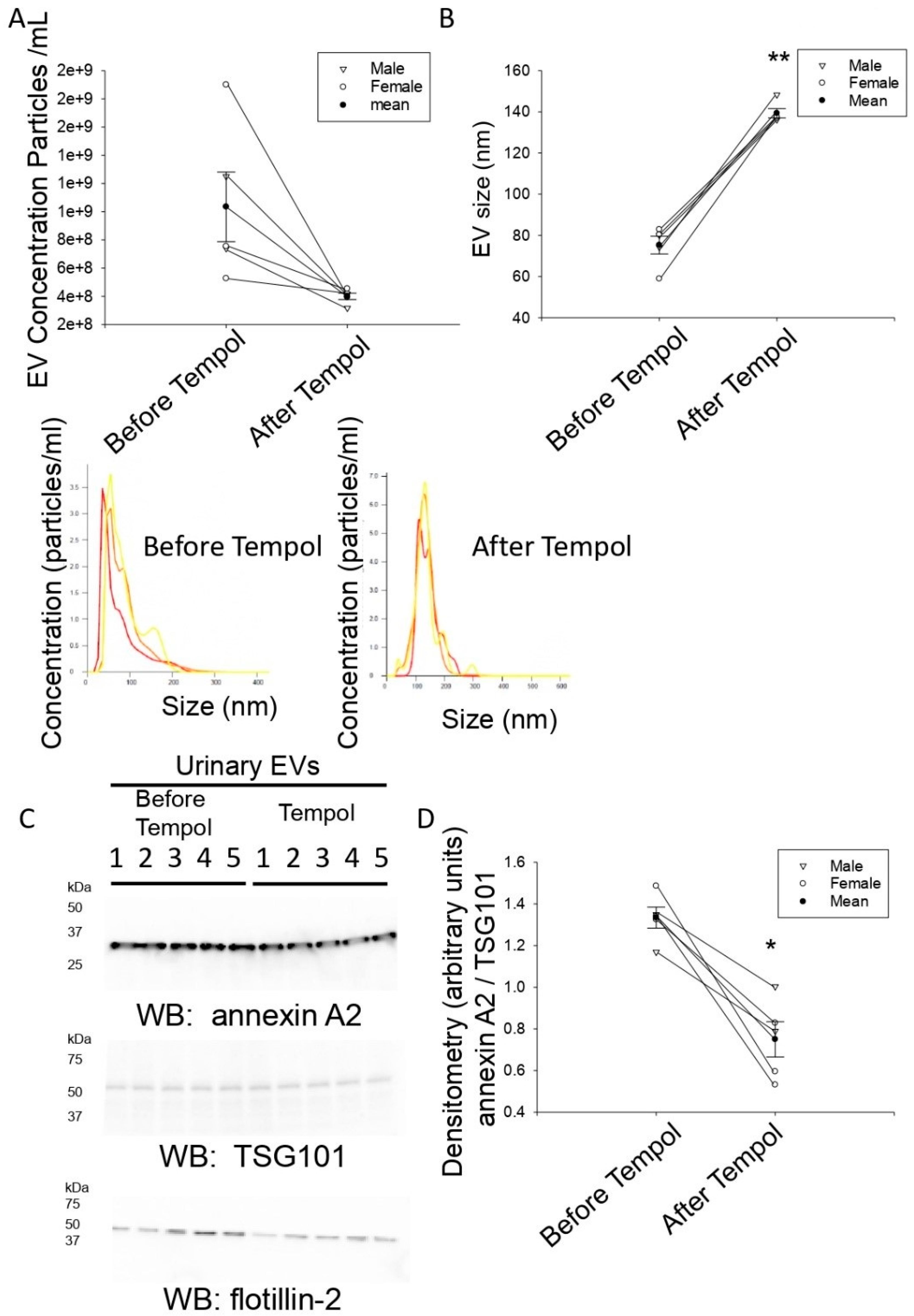
3.2. Tempol Infusion Attenuates the Excretion of EVs in the Urine of 129Sv Mice
3.3. Tempol Administration Increases the Size of Urinary EVs from 129Sv Mice
3.4. Reduced Annexin A2 in Urinary EVs after Tempol Treatment
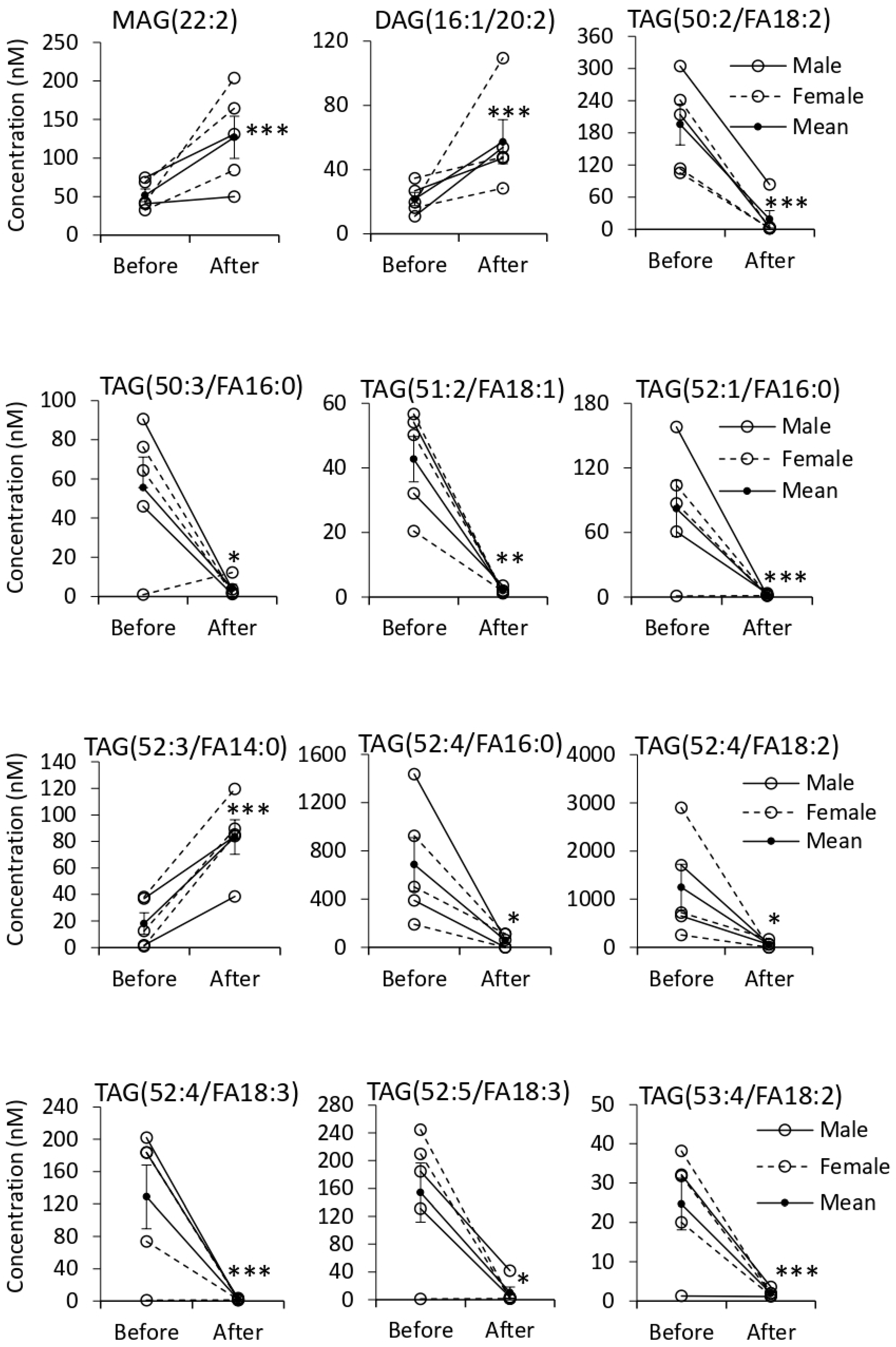
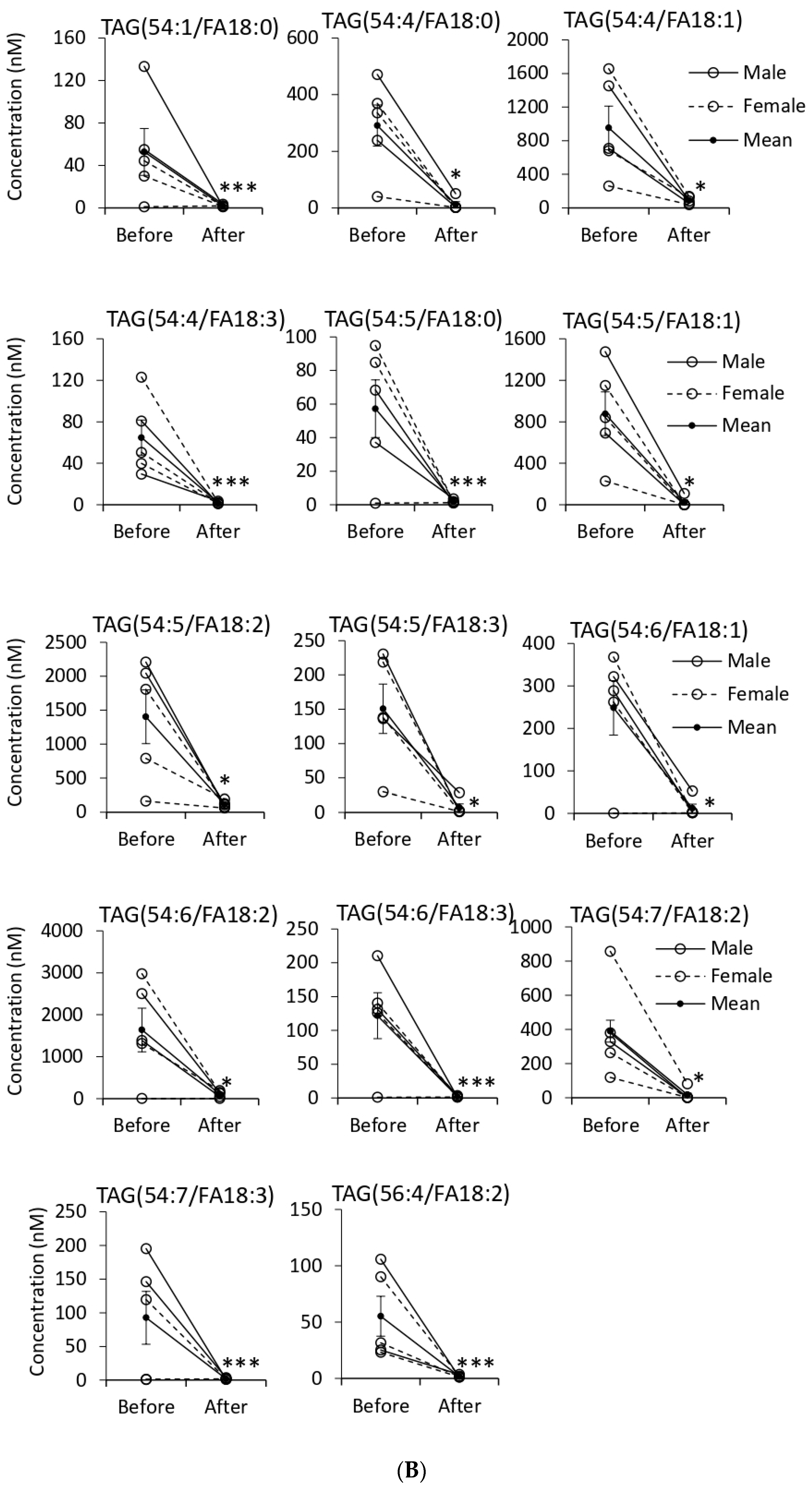
3.5. Quantification of EV Lipids before and after Tempol Infusion
3.6. Tempol Treatment Increases Urinary Sodium Concentrations but Does Not Affect Urine Osmolality in Hypertensive 129Sv Mice
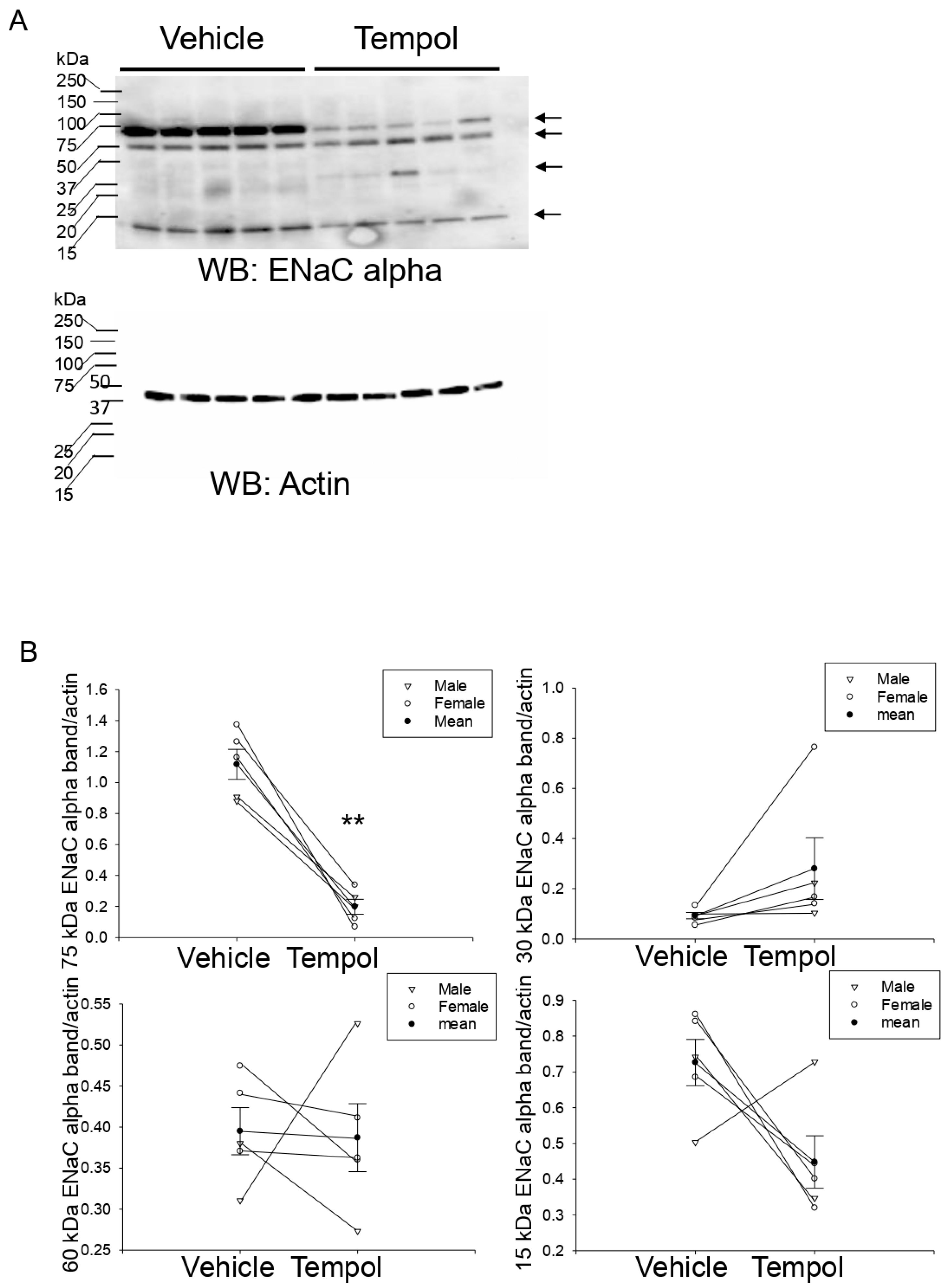
3.7. Tempol Treatment Decreases Renal ENaC Alpha Protein Expression and Proteolysis in Hypertensive 129Sv Mice
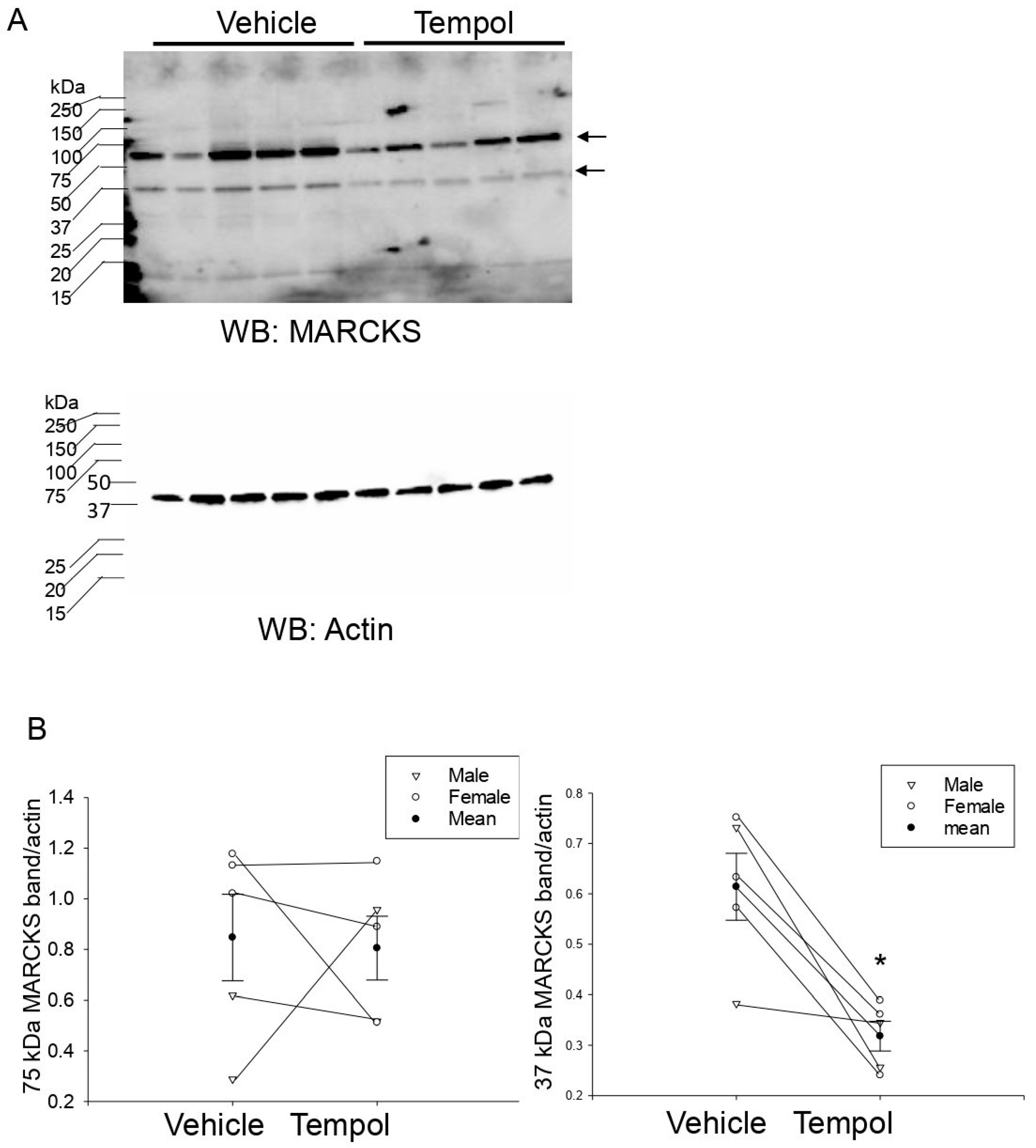
3.8. Tempol Treatment Decreases MLP1 Protein Expression in Hypertensive 129Sv Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CE | cholesteryl ester |
| CER | ceramide |
| DAG | diacylglycerol |
| DCER | dihydroceramide |
| EDTA | ethylenediaminetetraacetic acid |
| ENaC | epithelial sodium channel |
| hAoEC | human aortic endothelial cells |
| HCER | hexosylceramide |
| LCER | lactosylceramide |
| LPC | lysophosphatidylcholine |
| LPE | lysophosphatidylethanolamine |
| LPG | lysophosphatidylglycerol |
| LPI | lysophosphatidylinositol |
| LPS | lysophosphatidylserine |
| MAG | monoacylglycerol |
| NO | nitric oxide |
| PBS | phosphate buffered saline |
| PC | phosphatidylcholine, |
| PE | phosphatidylethanolamine, |
| PG | phosphatidylglycerol, |
| PI | phosphatidylinositol |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PS | phosphatidylserine |
| RIPA | radioimmunoprecipitation assay buffer |
| SM | sphingomyelin |
| TAG | triacylglycerol |
References
- Sánchez, G.B.; Bunn, K.E.; Pua, H.H.; Rafat, M. Extracellular vesicles: Mediators of intercellular communication in tissue injury and disease. Cell Commun. Signal. 2021, 19, 1–18. [Google Scholar] [CrossRef]
- Fang, S.-B.; Zhou, Z.-R.; Peng, Y.-Q.; Liu, X.-Q.; He, B.-X.; Chen, D.-H.; Chen, D.; Fu, Q.-L. Plasma EVs Display Antigen-Presenting Characteristics in Patients With Allergic Rhinitis and Promote Differentiation of Th2 Cells. Front. Immunol. 2021, 12, 710372. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chung, J.; Byun, Y.; Kim, K.H.; An, S.H.; Kwon, K. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Protect Cardiomyocytes from Doxorubicin-Induced Car-diomyopathy by Upregulating Survivin Expression via the miR-199a-3p-Akt-Sp1/p53 Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 7102. [Google Scholar] [CrossRef]
- Jella, K.K.; Yu, L.; Yue, Q.; Friedman, D.; Duke, B.J.; Alli, A.A. Exosomal GAPDH from Proximal Tubule Cells Regulate ENaC Activity. PLoS ONE 2016, 11, e0165763. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Snyder, P.M. The epithelial Na+ channel: Cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr. Rev. 2002, 23, 258–275. [Google Scholar] [CrossRef]
- Pavlov, T.S.; Staruschenko, A. Involvement of ENaC in the development of salt-sensitive hypertension. Am. J. Physiol. Physiol. 2017, 313, F135–F140. [Google Scholar] [CrossRef]
- Alli, A.A.; Bao, H.F.; Alli, A.A.; Aldrugh, Y.; Song, J.Z.; Ma, H.P.; Yu, L.; Al-Khalili, O.; Eaton, D.C. Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein. Am. J. Physiol. Renal Physiol. 2012, 303, F800–F811. [Google Scholar] [CrossRef]
- Alli, A.A.; Bao, H.-F.; Liu, B.-C.; Yu, L.; Aldrugh, S.; Montgomery, D.S.; Ma, H.-P.; Eaton, D.C. Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am. J. Physiol. Physiol. 2015, 309, F456–F463. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S.; Pearlman, A. Chemistry and Antihypertensive Effects of Tempol and Other Nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef]
- Bernardy, C.C.F.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Calixto-Campos, C.; de Carvalho, T.T.; Fattori, V.; Borghi, S.M.; Casagrande, R.; Verri, W.A. Tempol, a Superoxide Dismutase Mimetic Agent, Inhibits Superoxide Anion-Induced Inflammatory Pain in Mice. BioMed Res. Int. 2017, 2017, 9584819. [Google Scholar] [CrossRef] [PubMed]
- Laight, D.W.; Andrews, T.J.; Haj-Yehia, A.I.; Carrier, M.J.; Änggård, E.E. Microassay of superoxide anion scavenging activity in vitro. Environ. Toxicol. Pharmacol. 1997, 3, 65–68. [Google Scholar] [CrossRef]
- Nunes, D.V.; da Costa, C.A.; de Bem, G.; Cordeiro, V.S.; Santos, I.B.; Carvalho, L.C.; Jordão, A.K.; Cunha, A.; Ferreira, V.; Moura, R.S.; et al. Tempol, a superoxide dismutase-mimetic drug, prevents chronic ischemic renal injury in two-kidney, one-clip hypertensive rats. Clin. Exp. Hypertens. 2018, 40, 721–729. [Google Scholar] [CrossRef]
- Simonsen, U.; Christensen, F.H.; Buus, N.H. The effect of tempol on endothelium-dependent vasodilatation and blood pressure. Pharmacol. Ther. 2009, 122, 109–124. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Glover, S.C.; Nouri, M.; Tuna, K.M.; Alvarez, L.B.M.; Ryan, L.K.; Shirley, J.F.; Tang, Y.; Denslow, N.D.; Alli, A.A. Lipidomic analysis of urinary exosomes from hereditary α-tryptasemia patients and healthy volunteers. FASEB BioAdvances 2019, 1, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.-Z.; Yu, L.; Liu, L.P.; Chacko, K.M.; Denslow, N.D.; LaDisa, J.F., Jr.; Alli, A.A. Increased endothelial sodium channel activity by extracellular vesicles in human aortic endothelial cells: Putative role of MLP1 and bioactive lipids. Am. J. Physiol. Physiol. 2021, 321, C535–C548. [Google Scholar] [CrossRef]
- Hagiwara, K.; Katsuda, T.; Gailhouste, L.; Kosaka, N.; Ochiya, T. Commitment of Annexin A2 in recruitment of microRNAs into extracellular vesicles. FEBS Lett. 2015, 589, 4071–4078. [Google Scholar] [CrossRef]
- Koritzinsky, E.H.; Street, J.M.; Chari, R.R.; Glispie, D.M.; Bellomo, T.R.; Aponte, A.M.; Star, R.A.; Yuen, P.S.T. Circadian variation in the release of small extracellular vesicles can be normalized by vesicle number or TSG101. Am. J. Physiol. Physiol. 2019, 317, F1098–F1110. [Google Scholar] [CrossRef]
- Phuyal, S.; Hessvik, N.P.; Skotland, T.; Sandvig, K.; Llorente, A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014, 281, 2214–2227. [Google Scholar] [CrossRef]
- Drivas, T.G.; Holzbaur, E.L.; Bennett, J. Disruption of CEP290 microtubule/membrane-binding domains causes retinal degenera-tion. J. Clin. Investig. 2013, 123, 4525–4539. [Google Scholar] [CrossRef] [PubMed]
- Tuna, K.M.; Liu, B.C.; Yue, Q.; Ghazi, Z.M.; Ma, H.P.; Eaton, D.C.; Alli, A.A. Mal protein stabilizes luminal membrane PLC-beta3 and negatively regulates ENaC in mouse cortical collecting duct cells. Am. J. Physiol. Renal Physiol. 2019, 317, F986–F995. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, F.; Wang, Z.; Tang, L.; Zou, B.; Xu, P.; Yu, T. Hypoxic bone marrow mesenchymal cell-extracellular vesicles containing miR-328-3p promote lung cancer progression via the NF2-mediated Hippo axis. J. Cell. Mol. Med. 2021, 25, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.-F.; Song, J.Z.; Duke, B.J.; Ma, H.-P.; Denson, D.D.; Eaton, D.C. Ethanol stimulates epithelial sodium channels by elevating reactive oxygen species. Am. J. Physiol. Physiol. 2012, 303, C1129–C1138. [Google Scholar] [CrossRef][Green Version]
- Zou, L.; Linck, V.; Zhai, Y.-J.; Galarza-Paez, L.; Li, L.; Yue, Q.; Al-Khalili, O.; Bao, H.-F.; Ma, H.-P.; Thai, T.L.; et al. Knockout of mitochondrial voltage-dependent anion channel type 3 increases reactive oxygen species (ROS) levels and alters renal sodium transport. J. Biol. Chem. 2018, 293, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.; Pope, T.W.; Moore, S.A.; Campbell, C.L. The tragedy of TRIUMPH for nitric oxide synthesis inhibition in cardiogenic shock: Where do we go from here? Am. J. Cardiovasc. Drugs 2007, 7, 337–345. [Google Scholar] [CrossRef]
- Hisaki, R.; Fujita, H.; Saito, F.; Kushiro, T. Tempol Attenuates the Development of Hypertensive Renal Injury in Dahl Salt-Sensitive Rats. Am. J. Hypertens. 2005, 18, 707–713. [Google Scholar] [CrossRef]
- Bonny, O.; Chraibi, A.; Loffing, J.; Jaeger, N.F.; Gründer, S.; Horisberger, J.-D.; Rossier, B.C. Functional expression of a pseudohypoaldosteronism type I mutated epithelial Na+ channel lacking the pore-forming region of its α subunit. J. Clin. Investig. 1999, 104, 967–974. [Google Scholar] [CrossRef]
- Montgomery, D.S.; Yu, L.; Ghazi, Z.M.; Thai, T.L.; Al-Khalili, O.; Ma, H.-P.; Eaton, D.C.; Alli, A.A. ENaC activity is regulated by calpain-2 proteolysis of MARCKS proteins. Am. J. Physiol. Physiol. 2017, 313, C42–C53. [Google Scholar] [CrossRef]
- Ramsden, J.J. MARCKS: A case of molecular exaptation? Int. J. Biochem. Cell Biol. 2000, 32, 475–479. [Google Scholar] [CrossRef]
- Stumpo, D.J.; Eddy, R.L., Jr.; Haley, L.L.; Sait, S.; Shows, T.B.; Lai, W.S.; Scott Young, W., III; Speer, M.C.; Dehejia, A.; Polymeropoulos, M.; et al. Promoter sequence, expression, and fine chromosomal mapping of the human gene (MLP) encoding the MARCKS-like protein: Identification of neighboring and linked polymorphic loci for MLP and MACS and use in the evaluation of human neural tube defects. Genomics 1998, 49, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Hemmings, H.C., Jr.; Aderem, A. Stimulus-dependent phosphorylation of MacMARCKS, a protein kinase C substrate, in nerve termini and PC12 cells. J. Biol. Chem. 1996, 271, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, T.O.; Lass, A. DAG tales: The multiple faces of diacylglycerol—stereochemistry, metabolism, and signaling. Cell. Mol. Life Sci. 2015, 72, 3931–3952. [Google Scholar] [CrossRef] [PubMed]
- Borodzicz, S.; Czarzasta, K.; Kuch, M.; Cudnoch-Jedrzejewska, A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Heal. Dis. 2015, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Spijkers, L.J.A.; Akker, R.F.P.V.D.; Janssen, B.J.A.; Debets, J.J.; De Mey, J.G.R.; Stroes, E.S.G.; Born, B.-J.H.V.D.; Wijesinghe, D.S.; Chalfant, C.E.; MacAleese, L.; et al. Hypertension Is Associated with Marked Alterations in Sphingolipid Biology: A Potential Role for Ceramide. PLoS ONE 2011, 6, e21817. [Google Scholar] [CrossRef]
- Valapala, M.; Vishwanatha, J.K. Lipid Raft Endocytosis and Exosomal Transport Facilitate Extracellular Trafficking of Annexin A2. J. Biol. Chem. 2011, 286, 30911–30925. [Google Scholar] [CrossRef] [PubMed]



| Lipids (Category) | Scan Mode | Number of Scanned Lipids | Number of Identified Lipids * | Change in Concentration after Treatment |
|---|---|---|---|---|
| (Glycerophospholipids) | ||||
| Phosphatidylcholine | Neg. | 79 | 17(4) | 9↓ |
| phosphatidylethanolamine | Neg. | 66 | 46(7) | 18↓ |
| PE(O) | Neg. | 28 | 13(2) | 5↑4↓ |
| PE(P) | Neg. | 48 | 46(4) | 17↓ |
| Phosphatidylglycerol | Neg. | 78 | 44(5) | 9↑ 13↓ |
| Phosphatidylinositol | Neg. | 77 | 6(1) | 1↓ |
| Phosphatidylserine | Neg. | 78 | 13 | 1↓ |
| Lysophosphatidylcholine | Neg. | 16 | 7 | 5↑ |
| lysophosphatidylethanolamine | Neg. | 16 | 10 | 3↑ 2↓ |
| lysophosphatidylglycerol | Neg. | 16 | - | - |
| Lysophosphatidylinositol | Neg. | 16 | - | - |
| Lysophosphatidylserine | Neg. | 16 | - | - |
| (Sphingolipids) | ||||
| Sphingomyelin | Pos. | 12 | 12 | 5↓ |
| Ceramide | Pos. | 12 | 7 | 7↓ |
| Dihydroceramide | Pos. | 12 | 4(2) | 2↓ |
| Hexosylceramide | Pos. | 12 | 9 | - |
| Lactosylceramide | Pos. | 12 | - | - |
| (Glycerolipids) | ||||
| Triacylglycerol | Pos. | 445 | 79(15) | 2↑ 29↓ |
| Diacylglycerol | Pos. | 50 | 7 | 4↑ |
| Monoacylglycerol | Pos. | 17 | 12 | 5↑ |
| (Sterol lipids) | ||||
| cholesteryl ester | Pos. | 21 | 2 | - |
| Total | 1127 | 334 | 33↑ 108↓ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacko, K.M.; Nouri, M.-Z.; Schramm, W.C.; Malik, Z.; Liu, L.P.; Denslow, N.D.; Alli, A.A. Tempol Alters Urinary Extracellular Vesicle Lipid Content and Release While Reducing Blood Pressure during the Development of Salt-Sensitive Hypertension. Biomolecules 2021, 11, 1804. https://doi.org/10.3390/biom11121804
Chacko KM, Nouri M-Z, Schramm WC, Malik Z, Liu LP, Denslow ND, Alli AA. Tempol Alters Urinary Extracellular Vesicle Lipid Content and Release While Reducing Blood Pressure during the Development of Salt-Sensitive Hypertension. Biomolecules. 2021; 11(12):1804. https://doi.org/10.3390/biom11121804
Chicago/Turabian StyleChacko, Kevin M., Mohammad-Zaman Nouri, Whitney C. Schramm, Zeeshan Malik, Lauren P. Liu, Nancy D. Denslow, and Abdel A. Alli. 2021. "Tempol Alters Urinary Extracellular Vesicle Lipid Content and Release While Reducing Blood Pressure during the Development of Salt-Sensitive Hypertension" Biomolecules 11, no. 12: 1804. https://doi.org/10.3390/biom11121804
APA StyleChacko, K. M., Nouri, M.-Z., Schramm, W. C., Malik, Z., Liu, L. P., Denslow, N. D., & Alli, A. A. (2021). Tempol Alters Urinary Extracellular Vesicle Lipid Content and Release While Reducing Blood Pressure during the Development of Salt-Sensitive Hypertension. Biomolecules, 11(12), 1804. https://doi.org/10.3390/biom11121804







