Health Benefits and Pharmacological Properties of Carvone
Abstract
1. Introduction
2. Research Methodology
3. Results and Discussion
3.1. Natural Sources of Carvone
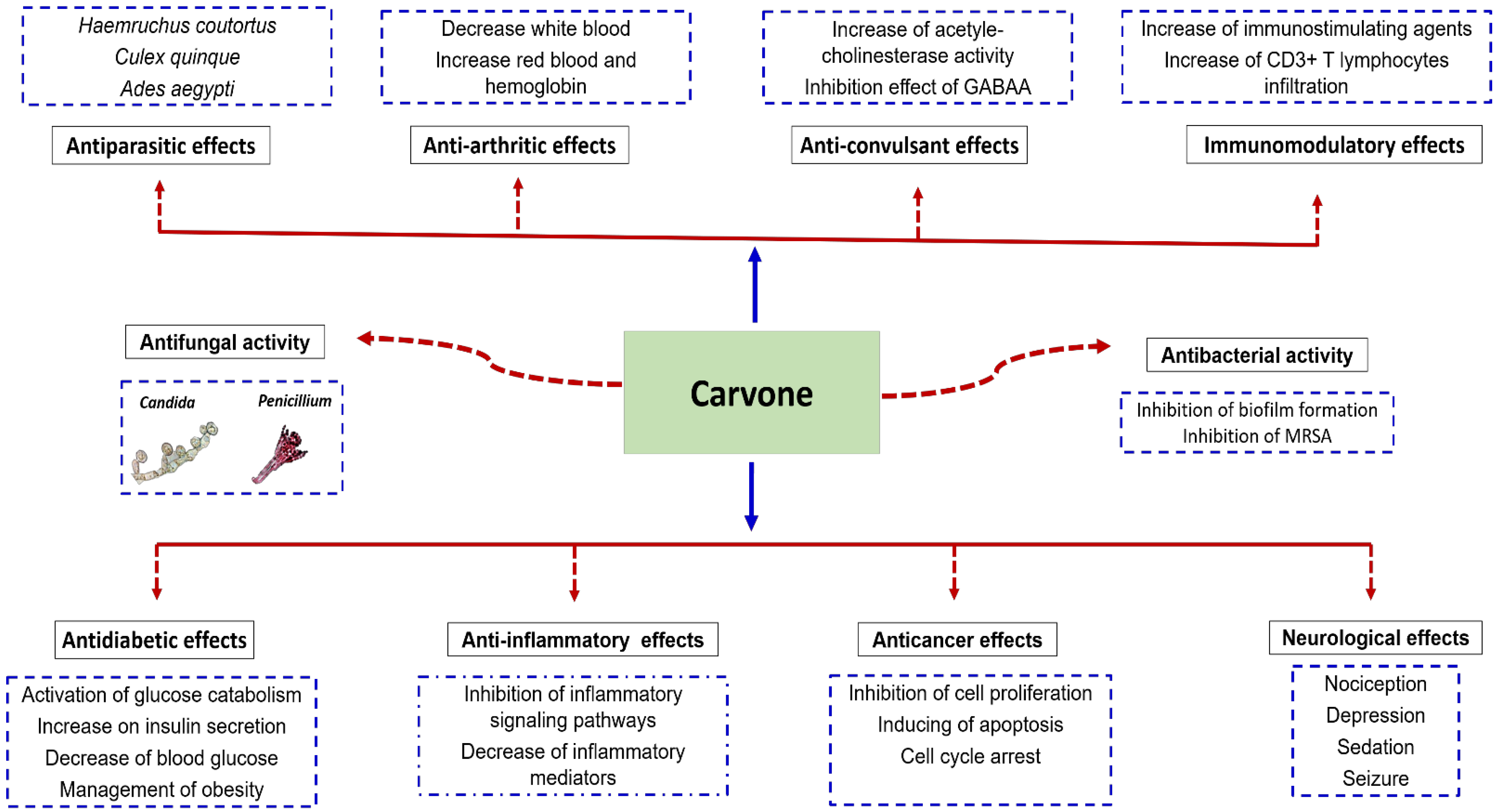
3.2. Pharmacological Properties of Carvone
3.2.1. Neurological Activity
3.2.2. Antidiabetic Activity
3.2.3. Antifungal Activity
3.2.4. Antibacterial Activity
3.2.5. Antibacterial and Antibiofilm Activities
3.2.6. Antiviral Activity
3.2.7. Antioxidant Activity
3.2.8. Anti-Inflammatory Activity
3.2.9. Anticancer Activity
3.2.10. Antiparasitic Activity
3.2.11. Anti-Arthritic Activity
3.2.12. Anticonvulsant Activity
3.2.13. Anxiolytic Activity
3.2.14. Immunomodulatory Activity
3.2.15. Antispasmodic Activity
3.2.16. Acaricidal Activity
3.2.17. Antimanic Activity
4. Conclusion and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bcl-2 | B-cell lymphoma 2 |
| BITC | Benzyl isothiocyanate |
| BZD | Benzodiazepines |
| CAP | Compound action potential |
| CDK1 | Cyclin-dependent kinase 1 |
| CNS | Central nervous system |
| CV | Crystal violet |
| DPPH | 2,2-dipenyl-1-picrylhydrazyl |
| EO | Essential oil |
| ESE | Evaporation solvent |
| FE-SEM | Field emission scanning electron microscope |
| GC-MS | Gas chromatography–mass spectrometry |
| GM-MIC | Geometric means–minimal inhibitory concentration |
| GTT | Glucose tolerance test |
| Hb | Hemoglobin |
| HbA1c | Glycosylated hemoglobin |
| IC50 | Half-maximal inhibitory concentration |
| IFN-γ | Interferon gamma |
| IL | Interleukin |
| ITT | Insulin tolerance test |
| LC50 | Lethal concentration 50% |
| LD50 | Medium lethal dose |
| LPS | Lipopolysaccharide |
| MBC | Minimum bactericidal concentration |
| MFC | Minimum fungicidal concentration |
| MIC | Minimum inhibitory concentration |
| MRSA | Methicillin-resistant staphylococcus aureus |
| MS | Mass spectrometry |
| NMR | Nuclear magnetic resonance |
| PARP | Poly adenosine diphosphate-ribose polymerase |
| PGE2 | Prostaglandin E2 |
| PLA | Poly (lactic acid) |
| PLGA | Poly (lactic-co-glycolic acid) |
| ROS | Reactive oxygen species |
| SIRT1 | Sirtuin-1 |
| STZ | Streptozotocin |
| TBARS | Thiobarbituric acid reactive species |
| TLC | Thin layer chromatography |
| TNF-α | Tumor necrosis factor-α |
References
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.-E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional Use, Phytochemistry, Toxicology, and Pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 265, 113318. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Et-Touys, A.; Abrini, J.; Talbaoui, A.; Fellah, H.; Bakri, Y.; Dakka, N. Lavandula stoechas Essential Oil from Morocco as Novel Source of Antileishmanial, Antibacterial and Antioxidant Activities. Biocatal. Agric. Biotechnol. 2017, 12, 179–184. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.-E.; Balahbib, A.; El-Shazly, M.; Chamkhi, I. Ethnomedicinal Use, Phytochemistry, Pharmacology, and Toxicology of Ajuga iva (L.) Schreb. J. Ethnopharmacol. 2020, 258, 112875. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; Guaouguaou, F.-E.; Benali, T.; Balahbib, A.; El Omari, N.; Taha, D.; El-Shazly, M.; El Menyiy, N. Ethnomedicinal Use, Phytochemistry, Pharmacology, and Food Benefits of Thymus capitatus. J. Ethnopharmacol. 2020, 259, 112925. [Google Scholar] [CrossRef]
- Bouyahya, A.; Lagrouh, F.; El Omari, N.; Bourais, I.; El Jemli, M.; Marmouzi, I.; Salhi, N.; Faouzi, M.E.A.; Belmehdi, O.; Dakka, N.; et al. Essential Oils of Mentha viridis Rich Phenolic Compounds Show Important Antioxidant, Antidiabetic, Dermatoprotective, Antidermatophyte and Antibacterial Properties. Biocatal. Agric. Biotechnol. 2020, 23, 101471. [Google Scholar] [CrossRef]
- Bouyahya, A.; Dakka, N.; Talbaoui, A.; Et-Touys, A.; El-Boury, H.; Abrini, J.; Bakri, Y. Correlation between Phenological Changes, Chemical Composition and Biological Activities of the Essential Oil from Moroccan Endemic Oregano (Origanum compactum Benth). Ind. Crops Prod. 2017, 108, 729–737. [Google Scholar] [CrossRef]
- Bouyahya, A.; Belmehdi, O.; El Jemli, M.; Marmouzi, I.; Bourais, I.; Abrini, J.; Faouzi, M.E.A.; Dakka, N.; Bakri, Y. Chemical Variability of Centaurium erythraea Essential Oils at Three Developmental Stages and Investigation of Their in Vitro Antioxidant, Antidiabetic, Dermatoprotective and Antibacterial Activities. Ind. Crops Prod. 2019, 132, 111–117. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical Composition of Mentha pulegium and Rosmarinus officinalis Essential Oils and Their Antileishmanial, Antibacterial and Antioxidant Activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef]
- De Sousa, D.P.; De Farias Nóbrega, F.F.; De Almeida, R.N. Influence of the Chirality of (R)-(−)- and (S)-(+)-Carvone in the Central Nervous System: A Comparative Study. Chirality 2007, 19, 264–268. [Google Scholar] [CrossRef]
- Faliagkas, L.; Vokou, D.; Theophilidis, G. Local Anaesthetic Properties vs. Neurotoxicity for (+)-and (−)-Carvone: An Ex Vivo Electrophysiological Study. Planta Med. Lett. 2015, 2, e6–e9. [Google Scholar] [CrossRef][Green Version]
- Gonçalves, J.C.R.; de Alves, A.M.H.; de Araújo, A.E.V.; Cruz, J.S.; Araújo, D.A.M. Distinct Effects of Carvone Analogues on the Isolated Nerve of Rats. Eur. J. Pharmacol. 2010, 645, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Borzone, M.; Delgado-Marín, L.; García, D.A. Inhibitory Effects of Carvone Isomers on the GABA A Receptor in Primary Cultures of Rat Cortical Neurons: Effects of Carvone Isomers on Gaba A Receptor. Chirality 2014, 26, 368–372. [Google Scholar] [CrossRef]
- Alsanea, S.; Liu, D. BITC and S-Carvone Restrain High-Fat Diet-Induced Obesity and Ameliorate Hepatic Steatosis and Insulin Resistance. Pharm. Res. 2017, 34, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Muruganathan, U.; Srinivasan, S.; Indumathi, D. Antihyperglycemic Effect of Carvone: Effect on the Levels of Glycoprotein Components in Streptozotocin-Induced Diabetic Rats. J. Acute Dis. 2013, 2, 310–315. [Google Scholar] [CrossRef]
- Muruganathan, U.; Srinivasan, S. Beneficial Effect of Carvone, a Dietary Monoterpene Ameliorates Hyperglycemia by Regulating the Key Enzymes Activities of Carbohydrate Metabolism in Streptozotocin-Induced Diabetic Rats. Biomed. Pharmacother. 2016, 84, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Boonruang, K.; Chinsirikul, W.; Hararak, B.; Kerddonfag, N.; Chonhenchob, V. Antifungal Poly (Lactic Acid) Films Containing Thymol and Carvone. MATEC Web Conf. 2016, 67, 06107. [Google Scholar] [CrossRef]
- Giovana, C.B.; de Simone, N.B.F.; de Priscilla, L.S.; Paula, C.A.; Marcelo, F.G.B.; Marcelle, M.B.-R.; Janaina, P.B.; de Thais, R.O.; Jose, F.H. Antifungal and Cytotoxic Activity of Purified Biocomponents as Carvone, Menthone, Menthofuran and Pulegone from Mentha spp. Afr. J. Plant Sci. 2016, 10, 203–210. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Montiel-Ramos, J.; Zapata, B.; Durán, C.; Betancur-Galvis, L.; Stashenko, E. Citral and Carvone Chemotypes from the Essential Oils of Colombian Lippia alba (Mill.) NE Brown: Composition, Cytotoxicity and Antifungal Activity. Mem. Inst. Oswaldo Cruz 2009, 104, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Morcia, C.; Malnati, M.; Terzi, V. In Vitro Antifungal Activity of Terpinen-4-Ol, Eugenol, Carvone, 1,8-Cineole (Eucalyptol) and Thymol against Mycotoxigenic Plant Pathogens. Food Addit. Contam. Part A 2011, 5, 1–8. [Google Scholar] [CrossRef][Green Version]
- Moro, I.J.; Gondo, G.D.G.A.; Pierri, E.G.; Pietro, R.C.L.R.; Soares, C.P.; de Sousa, D.P.; dos Santos, A.G. Evaluation of Antimicrobial, Cytotoxic and Chemopreventive Activities of Carvone and Its Derivatives. Braz. J. Pharm. Sci. 2018, 53. [Google Scholar] [CrossRef]
- Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Antifungal Activity of Essential Oil from Mentha spicata L. and Mentha pulegium L. Growing Wild in Sardinia Island (Italy). Nat. Prod. Res. 2021, 35, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.K.; Khanuja, S.P.S.; Ahmad, A.; Santha Kumar, T.R.; Gupta, V.K.; Kumar, S. Antimicrobial Activity Profiles of the Two Enantiomers of Limonene and Carvone Isolated from the Oils of Mentha spicata and Anethum sowa. Flavour Fragr. J. 2002, 17, 59–63. [Google Scholar] [CrossRef]
- Chan, Y.W.; Siow, K.S.; Ng, P.Y.; Gires, U.; Yeop Majlis, B. Plasma Polymerized Carvone as an Antibacterial and Biocompatible Coating. Mater. Sci. Eng. C 2016, 68, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Demirci, F.; Noma, Y.; Başera, K.H.C. Microbial Transformation of (−)-Carvone. Z. Nat. C 2004, 59, 389–392. [Google Scholar] [CrossRef]
- Esfandyari-Manesh, M.; Ghaedi, Z.; Asemi, M.; Khanavi, M.; Manayi, A.; Jamalifar, H.; Atyabi, F.; Dinarvand, R. Study of Antimicrobial Activity of Anethole and Carvone Loaded PLGA Nanoparticles. J. Pharm. Res. 2013, 7, 290–295. [Google Scholar] [CrossRef]
- Fatondji, H.R.; Gbaguidi, F.; Kpoviessi, S.; Sonounameto, E.; Lagnika, L.; Ambaliou, S.; Moudachirou, M.; Poupaert, J.; Accrombessi, G. Synthèse, Caractérisation et Étude de Propriétés Antimicrobiennes de La Semicarbazone et de La Thiosemicarbazone de La Carvone. J. Soc. Ouest Afr. Chim. 2010, 30, 11–17. [Google Scholar]
- Mun, S.-H.; Kang, O.-H.; Joung, D.-K.; Kim, S.-B.; Choi, J.-G.; Shin, D.-W.; Kwon, D.-Y. In Vitro Anti-MRSA Activity of Carvone with Gentamicin. Exp. Ther. Med. 2014, 7, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Porfírio, E.M.; Melo, H.M.; Pereira, A.M.G.; Cavalcante, T.T.A.; Gomes, G.A.; de Carvalho, M.G.; Costa, R.A.; Júnior, F.E.A.C. In Vitro Antibacterial and Antibiofilm Activity of Lippia alba Essential Oil, Citral, and Carvone against Staphylococcus aureus. Sci. World J. 2017, 2017, 4962707. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Chen, H. Anticancer Effects of Carvone in Myeloma Cells Is Mediated through the Inhibition of P38 MAPK Signalling Pathway, Apoptosis Induction and Inhibition of Cell Invasion. J. Buon. 2018, 5, 747–751. [Google Scholar]
- Gopalakrishnan, T.; Ganapathy, S.; Veeran, V.; Namasivayam, N. Preventive Effect of D-Carvone during DMBA Induced Mouse Skin Tumorigenesis by Modulating Xenobiotic Metabolism and Induction of Apoptotic Events. Biomed. Pharmacother. 2019, 111, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Choi, D.; Park, S.; Park, T. Carvone Decreases Melanin Content by Inhibiting Melanoma Cell Proliferation via the Cyclic Adenosine Monophosphate (CAMP) Pathway. Molecules 2020, 25, 5191. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.B.; Thakkar, V.R. L-Carvone Induces P53, Caspase 3 Mediated Apoptosis and Inhibits the Migration of Breast Cancer Cell Lines. Nutr. Cancer 2014, 66, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, M.L.; Oliveira, L.E.G.; Patrício Santos, C.C.M.; de Sousa, D.P.; de Almeida, R.N.; Araújo, D.A.M. Antinociceptive and Anti-Inflammatory Effects of the Monoterpene α,β-Epoxy-Carvone in Mice. J. Nat. Med. 2013, 67, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.H.C.; Marques, M.L.B.G.C.; Medeiros, J.-V.R.; Silva, R.O.; dos Reis Barbosa, A.L.; Lima, T.C.; de Sousa, D.P.; de Freitas, R.M. Cyane-Carvone, a Synthetic Derivative of Carvone, Inhibits Inflammatory Response by Reducing Cytokine Production and Oxidative Stress and Shows Antinociceptive Effect in Mice. Inflammation 2014, 37, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Du, J. Anti-Inflammatory and Protective Effects of D-Carvone on Lipopolysaccharide (LPS)-Induced Acute Lung Injury in Mice. J. King Saud. Univ. Sci. 2020, 32, 1592–1596. [Google Scholar] [CrossRef]
- Sousa, C.; Neves, B.M.; Leitão, A.J.; Mendes, A.F. Elucidation of the Mechanism Underlying the Anti-Inflammatory Properties of (S)-(+)-Carvone Identifies a Novel Class of Sirtuin-1 Activators in a Murine Macrophage Cell Line. Biomedicines 2021, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.M.; Singh, D.; Rocha, J.B.T. In Vitro Antioxidant Activity of S-Carvone Isolated from Zanthoxylum alatum. Pharm. Chem. J. 2015, 49, 187–191. [Google Scholar] [CrossRef]
- Jusoh, N.; Zainal, H.; Abdul Hamid, A.A.; Bunnori, N.M.; Abd Halim, K.B.; Abd Hamid, S. In Silico Study of Carvone Derivatives as Potential Neuraminidase Inhibitors. J. Mol. Model. 2018, 24, 93. [Google Scholar] [CrossRef]
- Pavela, R. Acute Toxicity and Synergistic and Antagonistic Effects of the Aromatic Compounds of Some Essential Oils against Culex quinquefasciatus Say Larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef]
- Michaelakis, A.; Papachristos, D.; Kimbaris, A.; Polissiou, M. Larvicidal Evaluation of Three Mentha Species Essential Oils and Their Isolated Major Components against the West Nile Virus Mosquito. Hell. Plant Prot. J. 2011, 4, 35–43. [Google Scholar]
- Katiki, L.; Evangelista, A.; Canova, E.; Piza, A.; Fornazari, B.; Araujo, R.; Louvandini, H.; Amarante, A.; Costa, R.; Bueno, M. Anthelmintic Activity of Anethole, Carvone, Carvacrol, Thymol, Linalool, Limonene, Eucalyptol, Vanillin, Cinnamaldehyde and Eugenol in in Vitro Tests. Planta Med. 2014, 80, P1L14. [Google Scholar] [CrossRef]
- Lima, T.C.; da Silva, T.K.M.; Silva, F.L.; Barbosa-Filho, J.M.; Marques, M.O.M.; Santos, R.L.C.; de Holanda Cavalcanti, S.C.; de Sousa, D.P. Larvicidal Activity of Mentha × Villosa hudson Essential Oil, Rotundifolone and Derivatives. Chemosphere 2014, 104, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kokkini, S.; Karousou, R.; Lanaras, T. Essential Oils of Spearmint (Carvone-Rich) Plants from the Island of Crete (Greece). Biochem. Syst. Ecol. 1995, 23, 425–430. [Google Scholar] [CrossRef]
- Younis, Y.M.; Beshir, S.M. Carvone-Rich Essential Oils from Mentha longifolia (L.) Huds. Ssp. Schimperi Briq. and Mentha spicata L. Grown in Sudan. J. Essent. Oil Res. 2004, 16, 539–541. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.; Omer, E.A. Effect of Essential Oils of Some Medicinal Plants on Phytonematodes. Anz. Schädl. Pflanz. Umw. 1995, 68, 82–84. [Google Scholar] [CrossRef]
- Monfared, A.; Nabid, M.R.; Rustaiyan, A. Composition of a Carvone Chemotype of Mentha longifolia (L.) Huds. from Iran. J. Essent. Oil Res. 2002, 14, 51–52. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D. Ultrasound-Assisted Extraction Coupled with under Vacuum Distillation of Flavour Compounds from Spearmint (Carvone-Rich) Plants: Comparison with Conventional Hydrodistillation. Ultrason. Sonochem. 2009, 16, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Barba, C.; Toledano, R.M.; Santa-María, G.; Herraiz, M.; Martínez, R.M. Enantiomeric Analysis of Limonene and Carvone by Direct Introduction of Aromatic Plants into Multidimensional Gas Chromatography. Talanta 2013, 106, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Terzi, V. Carvone (Mentha spicata L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 309–316. [Google Scholar]
- De Carvalho, C.C.; Da Fonseca, M.M.R. Carvone: Why and How Should One Bother to Produce This Terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- De Gonçalves, N.D.; Pena, F.L.; Sartoratto, A.; Derlamelina, C.; Duarte, M.C.T.; Antunes, A.E.C.; Prata, A.S. Encapsulated Thyme (Thymus vulgaris) Essential Oil Used as a Natural Preservative in Bakery Product. Food Res. Int. 2017, 96, 154–160. [Google Scholar] [CrossRef]
- Aydın, E.; Türkez, H.; Keleş, M.S. Potential Anticancer Activity of Carvone in N2a Neuroblastoma Cell Line. Toxicol. Ind. Health 2015, 31, 764–772. [Google Scholar] [CrossRef]
- Rafii, F.; Shahverdi, A.R. Comparison of Essential Oils from Three Plants for Enhancement of Antimicrobial Activity of Nitrofurantoin against Enterobacteria. Chemotherapy 2007, 53, 21–25. [Google Scholar] [CrossRef]
- Chauhan, R.S.; Kaul, M.K.; Shahi, A.K.; Kumar, A.; Ram, G.; Tawa, A. Chemical Composition of Essential Oils in Mentha spicata L. Accession [IIIM (J) 26] from North-West Himalayan Region, India. Ind. Crops Prod. 2009, 29, 654–656. [Google Scholar] [CrossRef]
- Elmastaş, M.; Dermirtas, I.; Isildak, O.; Aboul-Enein, H.Y. Antioxidant Activity of S-Carvone Isolated from Spearmint (Mentha spicata L. Fam. Lamiaceae). J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1465–1475. [Google Scholar] [CrossRef]
- Telci, I.; Sahbaz, N.I.; Yilmaz, G.; Tugay, M.E. Agronomical and Chemical Characterization of Spearmint (Mentha spicata L.) Originating in Turkey. Econ. Bot. 2004, 58, 721–728. [Google Scholar] [CrossRef]
- Tisserat, B.; Vaughn, S.F. Growth, Morphogenesis, and Essential Oil Production in Mentha spicata L. Plantlets In Vitro. Vitro Cell. Dev. Biol. Plant 2008, 44, 40–50. [Google Scholar] [CrossRef]
- Taghavi Nezhad, M.; Alipour, D.; Torabi Goudarzi, M.; Zamani, P.; Khodakaramian, G. Dose Response to Carvone Rich Essential Oils of Spearmint (Mentha spicata L.): In Vitro Ruminal Fermentation Kinetics and Digestibility. J. Agric. Sci. Technol. 2011, 13, 1013–1020. [Google Scholar]
- Khan, N.I.; Tisserat, B.; Berhow, M.; Vaughn, S.F. Influence of Autoclaved Fungal Materials on Spearmint (Mentha spicata L.) Growth, Morphogenesis, and Secondary Metabolism. J. Chem. Ecol. 2005, 31, 1579–1593. [Google Scholar] [CrossRef]
- Chowdhury, J.U.; Nandi, N.C.; Uddin, M.; Rahman, M. Chemical Constituents of Essential Oils from Two Types of Spearmint (Mentha spicata L. and M. cardiaca L.) Introduced in Bangladesh. Bangladesh J. Sci. Ind. Res. 2007, 42, 79–82. [Google Scholar] [CrossRef]
- Gherman, C.; Culea, M.; Cozar, O. Comparative Analysis of Some Active Principles of Herb Plants by GC/MS. Talanta 2000, 53, 253–262. [Google Scholar] [CrossRef]
- Murray, M.J.; Reitsema, R.H. The Genetic Basis of the Ketones, Carvone, and Menthone in Mentha Crispa L. J. Am. Pharm. Assoc. Sci. Ed. 1954, 43, 612–613. [Google Scholar] [CrossRef]
- Zheng, G.; Kenney, P.M.; Lam, L.K. Anethofuran, Carvone, and Limonene: Potential Cancer Chemoprotective Agents from Dill Weed Oil and Caraway Oil. Planta Med. 1992, 58, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, E.P.; Liang, T.T.; Schulz, K.R.; Schnoes, H.K.; Carter, G.T. Insecticidal and Synergistic Components Isolated from Dill Plants. J. Agric. Food Chem. 1974, 22, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Callan, N.W.; Johnson, D.L.; Westcott, M.P.; Welty, L.E. Herb and Oil Composition of Dill (Anethum graveolens L.): Effects of Crop Maturity and Plant Density. Ind. Crops Prod. 2007, 25, 282–287. [Google Scholar] [CrossRef]
- Dhalwal, K.; Shinde, V.M.; Mahadik, K.R. Efficient and Sensitive Method for Quantitative Determination and Validation of Umbelliferone, Carvone and Myristicin in Anethum graveolens and Carum carvi Seed. Chromatographia 2008, 67, 163–167. [Google Scholar] [CrossRef]
- Betts, T.J. Carvone in the Developing Fruits of Anethum graveolens L. and Carum carvi L. J. Pharm. Pharmacol. 1965, 17, 41S–43S. [Google Scholar] [CrossRef]
- Chemat, S.; Esveld, E.D. Contribution of Microwaves or Ultrasonics on Carvone and Limonene Recovery from Dill Fruits (Anethum graveolens L.). Innov. Food Sci. Emerg. Technol. 2013, 17, 114–119. [Google Scholar] [CrossRef]
- Toxopeus, H.; Lubberts, J.H.; Neervoort, W.; Folkers, W.; Huisjes, G. Breeding Research and in Vitro Propagation to Improve Carvone Production of Caraway (Carum carvi L.). Ind. Crops Prod. 1995, 4, 33–38. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Davies, J.A.; Smid, H.G.; Welten, R.S. Physiological Limitations to Carvone Yield in Caraway (Carum carvi L.). Ind. Crops Prod. 1995, 4, 39–51. [Google Scholar] [CrossRef]
- Ravid, U.; Putievsky, E.; Katzir, I.; Weinstein, V.; Ikan, R. Chiral GC Analysis of (S)(+)-and (R)(−)-Carvone with High Enantiomeric Purity in Caraway, Dill and Spearmint Oils. Flavour Fragr. J. 1992, 7, 289–292. [Google Scholar] [CrossRef]
- Toxopeus, H.; Lubberts, H.J. Effect of Genotype and Environment on Carvone Yield and Yield Components of Winter-Caraway in the Netherlands. Ind. Crops Prod. 1994, 3, 37–42. [Google Scholar] [CrossRef]
- András, C.D.; Salamon, R.V.; Barabas, I.; Volf, I.; Szep, A. Influence of Extraction Methods on Caraway (Carum carvi L.) Essential Oil Yield and Carvone/Limonene Ratio. Environ. Eng. Manag. J. EEMJ 2015, 14, 277. [Google Scholar] [CrossRef]
- Şanlı, A.; Karadoğan, T. Carvone Containing Essential Oils as Sprout Suppressants in Potato (Solanum tuberosum L.) Tubers at Different Storage Temperatures. Potato Res. 2019, 62, 345–360. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Hartmans, K.J.; Huizing, H.J. Inhibition of Potato (Solanum tuberosum) Sprout Growth by the Monoterpene S-Carvone: Reduction of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Activity without Effect on Its MRNA Level. J. Plant Physiol. 1993, 141, 463–469. [Google Scholar] [CrossRef]
- Whitten, W.M.; Williams, N.H.; Armbruster, W.S.; Battiste, M.A.; Strekowski, L.; Lindquist, N. Carvone Oxide: An Example of Convergent Evolution in Euglossine Pollinated Plants. Syst. Bot. 1986, 5, 222–228. [Google Scholar] [CrossRef]
- Peixoto, M.G.; Costa-Júnior, L.M.; Blank, A.F.; da Silva Lima, A.; Menezes, T.S.A.; de Alexandria Santos, D.; Alves, P.B.; de Holanda Cavalcanti, S.C.; Bacci, L.; de Fátima Arrigoni-Blank, M. Acaricidal Activity of Essential Oils from Lippia alba Genotypes and Its Major Components Carvone, Limonene, and Citral against Rhipicephalus microplus. Vet. Parasitol. 2015, 210, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Hatano, V.Y.; Torricelli, A.S.; Giassi, A.C.C.; Coslope, L.A.; Viana, M. de B. Anxiolytic Effects of Repeated Treatment with an Essential Oil from Lippia alba and (R)-(−)-Carvone in the Elevated T-Maze. Braz. J. Med. Biol. Res. 2012, 45, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Das Zoghbi, M.G.; Andrade, E.H.; Santos, A.S.; Silva, M.H.L.; Maia, J.G.S. Essential Oils of Lippia alba (Mill.) NE Br Growing Wild in the Brazilian Amazon. Flavour Fragr. J. 1998, 13, 47–48. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; González-Cervera, T.; Güette-Fernandez, J.; Jaramillo-Colorado, B.; Stashenko, E. Chemical Composition and Antioxidant Activity of Essential Oils Isolated from Colombian Plants. Rev. Bras. Farmacogn. 2010, 20, 568–574. [Google Scholar] [CrossRef]
- Ehlert, P.A.D.; Chaves, F.C.M.; Ming, L.C.; da SILVA, M.A.S. Effect of Substrata on the Development of Stem Cuttings of Lippia alba (Mill.) NE BR.-Limonene-Carvone Chemotype. In Proceedings of the International Conference on Medicinal and Aromatic Plants, Budapest, Hungary, 8–11 July 2001; pp. 259–262. [Google Scholar]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.-E.; Balahbib, A.; Belmehdi, O.; Salhi, N.; Imtara, H.; Mrabti, H.N.; El-Shazly, M. Moroccan Antidiabetic Medicinal Plants: Ethnobotanical Studies, Phytochemical Bioactive Compounds, Preclinical Investigations, Toxicological Validations and Clinical Evidences; Challenges, Guidance and Perspectives for Future Management of Diabetes Worldwide. Trends Food Sci. Technol. 2021, 28, 2652. [Google Scholar]
- Benali, T.; Habbadi, K.; Khabbach, A.; Marmouzi, I.; Zengin, G.; Bouyahya, A.; Chamkhi, I.; Chtibi, H.; Aanniz, T.; Achbani, E.H. GC–MS Analysis, Antioxidant and Antimicrobial Activities of Achillea odorata Subsp. Pectinata and Ruta montana Essential Oils and Their Potential Use as Food Preservatives. Foods 2020, 9, 668. [Google Scholar]
- Galstyan, A.S.; Martiryan, A.I.; Grigoryan, K.R.; Ghazaryan, A.G.; Samvelyan, M.A.; Ghochikyan, T.V.; Nenajdenko, V.G. Synthesis of Carvone-Derived 1, 2, 3-Triazoles Study of Their Antioxidant Properties and Interaction with Bovine Serum Albumin. Molecules 2018, 23, 2991. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Guaouguaou, F.-E.; El Omari, N.; El Menyiy, N.; Balahbib, A.; El-Shazly, M.; Bakri, Y. Anti-Inflammatory and Analgesic Properties of Moroccan Medicinal Plants: Phytochemistry, in Vitro and in Vivo Investigations, Mechanism Insights, Clinical Evidences and Perspectives. J. Pharm. Anal. 2021, 7, 266. [Google Scholar] [CrossRef]
- Mogosan, C.; Vostinaru, O.; Oprean, R.; Heghes, C.; Filip, L.; Balica, G.; Moldovan, R. A Comparative Analysis of the Chemical Composition, Anti-Inflammatory, and Antinociceptive Effects of the Essential Oils from Three Species of Mentha Cultivated in Romania. Molecules 2017, 22, 263. [Google Scholar] [CrossRef] [PubMed]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health Beneficial and Pharmacological Properties of p-Cymene. Food Chem. Toxicol. 2021, 11, 2259. [Google Scholar] [CrossRef]
- Bouyahya, A.; Belmehdi, O.; Benjouad, A.; El Hassani, R.A.; Amzazi, S.; Dakka, N.; Bakri, Y. Pharmacological Properties and Mechanism Insights of Moroccan Anticancer Medicinal Plants: What Are the next Steps? Ind. Crops Prod. 2020, 147, 112198. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Lakhdar, F.; Khouchlaa, A.; Bakrim, S.; El Omari, N.; Balahbib, A.; Shariati, M.A.; Zengin, G.; Fikri-Benbrahim, K.; Orlando, G. Health Benefits and Pharmacological Properties of Hinokitiol. Processes 2021, 9, 1680. [Google Scholar] [CrossRef]
- El Omari, N.; El Menyiy, N.; Zengin, G.; Goh, B.H.; Gallo, M.; Montesano, D.; Naviglio, D.; Bouyahya, A. Anticancer and Anti-Inflammatory Effects of Tomentosin: Cellular and Molecular Mechanisms. Separations 2021, 8, 207. [Google Scholar] [CrossRef]
- El Omari, N.; Bakha, M.; Imtara, H.; Guaouguaoua, F.-E.; Balahbib, A.; Zengin, G.; Bouyahya, A. Anticancer Mechanisms of Phytochemical Compounds: Focusing on Epigenetic Targets. Environ. Sci. Pollut. Res. 2021, 6, 47869–47903. [Google Scholar] [CrossRef]
- Hachlafi, N.E.; Aanniz, T.; Menyiy, N.E.; Baaboua, A.E.; Omari, N.E.; Balahbib, A.; Shariati, M.A.; Zengin, G.; Fikri-Benbrahim, K.; Bouyahya, A. In Vitro and in Vivo Biological Investigations of Camphene and Its Mechanism Insights: A Review. Food Rev. Int. 2021, 51, 1–28. [Google Scholar] [CrossRef]
- Omari, N.E.; Bakrim, S.; Bakha, M.; Lorenzo, J.M.; Rebezov, M.; Shariati, M.A.; Aboulaghras, S.; Balahbib, A.; Khayrullin, M.; Bouyahya, A. Natural Bioactive Compounds Targeting Epigenetic Pathways in Cancer: A Review on Alkaloids, Terpenoids, Quinones, and Isothiocyanates. Nutrients 2021, 13, 3714. [Google Scholar] [CrossRef]
- Villeneuve, A.; Polley, L.; Jenkins, E.; Schurer, J.; Gilleard, J.; Kutz, S.; Conboy, G.; Benoit, D.; Seewald, W.; Gagné, F. Parasite Prevalence in Fecal Samples from Shelter Dogs and Cats across the Canadian Provinces. Parasit. Vectors 2015, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Gushulak, B.D.; MacPherson, D.W. Globalization of Infectious Diseases: The Impact of Migration. Clin. Infect. Dis. 2004, 38, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.; Pasvol, G. Atlas of Tropical Medicine and Parasitology: Text with CD-ROM; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Nussbaum, K.; Honek, J.; Cadmus, C.; Efferth, T. Trypanosomatid Parasites Causing Neglected Diseases. Curr. Med. Chem. 2010, 17, 1594–1617. [Google Scholar] [CrossRef]
- Dhorda, M. Molecular Parasitology and Diagnosis of Malaria in Pregnancy. Ph.D. Thesis, University Paris, Paris, France, June 2010. [Google Scholar]
- Gehrig, S.; Efferth, T. Development of Drug Resistance in Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense. Treatment of Human African Trypanosomiasis with Natural Products. Int. J. Mol. Med. 2008, 22, 411–419. [Google Scholar]
- Hammond, J.A.; Fielding, D.; Bishop, S.C. Prospects for Plant Anthelmintics in Tropical Veterinary Medicine. Vet. Res. Commun. 1997, 21, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Barros, J.; Munekata, P.E.; de Carvalho, F.A.L.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Use of Tiger Nut (Cyperus esculentus L.) Oil Emulsion as Animal Fat Replacement in Beef Burgers. Foods 2020, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.; Hildreth, M.B.; Reese, R.N. In Vitro Screening of Forty Medicinal Plant Extracts from the United States Northern Great Plains for Anthelmintic Activity against Haemonchus contortus. Vet. Parasitol. 2014, 201, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Sergiev, V.; Morozova, L.; Turbabina, N.; Morozov, E. Imported Plasmodium vivax Malaria in the Russian Federation from Western Sub-Saharan Africa. J. Trop. Med. 2019, 2019, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Barrau, E.; Fabre, N.; Fouraste, I.; Hoste, H. Effect of Bioactive Compounds from Sainfoin (Onobrychis viciifolia Scop.) on the in Vitro Larval Migration of Haemonchus contortus: Role of Tannins and Flavonol Glycosides. Parasitology 2005, 131, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Akendengué, B.; Champy, P.; Nzamba, J.; Roblot, F.; Loiseau, P.M.; Bories, C. Antifungal and Anthelmintic Activities of Cleistopholis patens (Annonaceae). Planta Med. 2009, 75, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- Stepek, G.; Buttle, D.J.; Duce, I.R.; Lowe, A.; Behnke, J.M. Assessment of the Anthelmintic Effect of Natural Plant Cysteine Proteinases against the Gastrointestinal Nematode, Heligmosomoides polygyrus, In Vitro. Parasitology 2005, 130, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Song, Y.; Ma, F.; Ma, Y. Anti-Arthritic Activity of D-Carvone against Complete Freund’s Adjuvant-Induced Arthritis in Rats through Modulation of Inflammatory Cytokines. Korean J. Physiol. Pharmacol. 2020, 24, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.G.; Bertolucci, S.K.V.; Chagas, J.H.; Ferraz, E.O.; Pinto, J.E.B.P. Biomass Production, Yield and Chemical Composition of Peppermint Essential Oil Using Different Organic Fertilizer Sources. Ciênc. Agrotecnol. 2013, 37, 202–210. [Google Scholar] [CrossRef]
- Marques, T.H.C.; Branco, M.L.B.G.C.; Medeiros, J.-V.R.; Lima, T.C.; de Sousa, D.P.; de Freitas, R.M. Anticonvulsant Effects of Acute Treatment with Cyane-Carvone at Repeated Oral Doses in Epilepsy Models. Pharmacol. Biochem. Behav. 2014, 124, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Lasarte-Cia, A.; Lozano, T.; Pérez-González, M.; Gorraiz, M.; Iribarren, K.; Hervás-Stubbs, S.; Sarobe, P.; Rabal, O.; Cuadrado-Tejedor, M.; García-Osta, A.; et al. Immunomodulatory Properties of Carvone Inhalation and Its Effects on Contextual Fear Memory in Mice. Front. Immunol. 2018, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.V.M.; da Rocha, M.B.; de Souza, D.P.; Marçal, R.M. (−)-Carvone: Antispasmodic Effect and Mode of Action. Fitoterapia 2013, 85, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Nogoceke, F.P.; Barcaro, I.M.; de Sousa, D.P.; Andreatini, R. Antimanic-like Effects of (R)-(−)-Carvone and (S)-(+)-Carvone in Mice. Neurosci. Lett. 2016, 619, 43–48. [Google Scholar] [CrossRef] [PubMed]
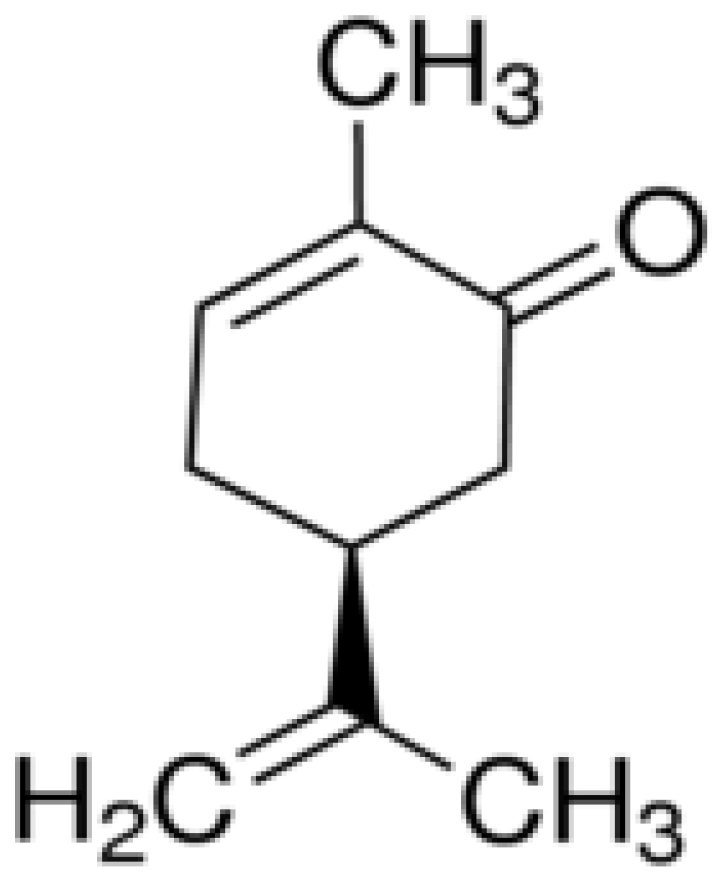

| Molecules | Origins | Models Used | Experimental Approaches | Key Results | References |
|---|---|---|---|---|---|
| (S)-(+)-Carvone and (R)-(−)-carvone | Purchased | Male Swiss mice | Pentobarbital-induced sleeping time Locomotor activity assessed in an activity cage PTZ-induced convulsions Pentobarbital-induced hypnosis PTZ-induced seizure PIC-induced seizure | LD50 = 484.2 mg/kg for (S)-(+)-carvone LD50 = 426.6 mg/kg for (R)-(−)-carvone Both enantiomers induced depressive effects Both enantiomers significantly reduced ambulation At 100 mg/kg, (R)-(−)-carvone was more effective than (S)-(+)-carvone in increasing pentobarbital sleeping duration At 200 mg/kg, (S)-(+)-carvone improved the latency of convulsions produced by PTZ and PIC (S)-(+)-carvone and (R)-(−)-carvone have depressant effects in the CNS (S)-(+)-carvone has anticonvulsant-like activity | [9] |
| (+)-carvone, (−)-carvone | Not reported | The sciatic nerve of the frog (Rana ridibunda) from both sex | Three-chambered recording bath for the assessment of local anesthetic activity | Both carvone enantiomers elicited comparable responses The action potential of the evoked compound was abolished in 6 to 7 min and had an immediate recovery of 83% to 87% Both carvones acted in the same way as lidocaine (10 mM) No recovery of the action potential of the elicited compound, when nerves have been exposed to carvones for more than 6–7 min The unusual neurotoxic effect of C+ and C− may be a disadvantage for their use in clinical practice | [10] |
| (+)-carvone, (−)-carvone | Purchased | Adult male Wistar rats | Sucrose-gap apparatus (ex vivo assay) for CAP-inhibitory effect | C- was less potent (IC50 = 10.7 ± 0.07 mM) in reducing nerve excitability than C+ (IC50 = 8.7 ± 0.1 mM) Both enantiomers acted in a similar manner The structure–function relationship of the enantiomers was linked to the CAP inhibitory action | [11] |
| (R)-(−)- carvone and (S)-(+)-carvone | Purchased | Cultures of cortical neurons prepared from the cerebral cortices of fetal rats | [3H] Flunitrazepam Binding Cell viability assay | Both isomers blocked GABA-induced activation of [3H] Flunitrazepam binding The doses required to produce negative receptor modulation were not lethal The insecticidal effect of carvones can be explained by their interaction with the GABAA receptor at its non-competitive blocker region | [12] |
| Molecules | Origins | Models Used | Experimental Approaches | Key Results | References |
|---|---|---|---|---|---|
| S-carvone | Purchased | C57BL/6 mice (male, ten weeks old) | GTT and ITT Histological examination Determination of hepatic triglyceride and serum lipid levels Determination of insulin resistance Gene expression analysis | Prevented weight gain, fat buildup in the liver, and insulin resistance Increased expression of macrophage marker genes in white adipose tissue, including F4/80, Cd11b, Cd11c, Cd206, and Tnf-α Decreased expression of genes involved for lipid production and transport in the liver (Ppar2, Scd1, Cd36) Inhibited high-fat diet-induced obesity and metabolic problems | [13] |
| Carvone | Purchased | Male Wistar rats weighing approximately 180–200 g | STZ-induced diabetes Estimation of blood glucose and plasma insulin levels Extraction and determination of glycoproteins | Improved glycemic status in a dose-dependent manner, in diabetic rats (30 mg/kg b.w.) Increased plasma insulin levels Reduced plasma glucose levels Restored the altered plasma and tissue glycoprotein levels Restored the abnormal levels of plasma and tissue glycoprotein components | [14] |
| Carvone | Purchased | Male Wistar rats (160–190 g) | STZ-induced diabetic rats Biochemical analysis Histopathological study of liver and pancreas Immunohistochemical examination of the pancreas | Decreased plasma glucose and HbA1c levels (50 mg/kg b.w.) Improved Hb and insulin levels Restored the reversed activity of carbohydrate metabolic enzymes, enzymic antioxidants, and hepatic marker enzymes Decreased STZ-induced damage to hepatic and pancreatic cells Controlled glucose metabolism by enhancing important enzymes in the hepatic tissues of diabetic rats | [15] |
| Molecules | Origins | Strains Used | Experimental Approaches | Key Results | References |
|---|---|---|---|---|---|
| R-(−)-carvone | Purchased | Poly (lactic acid) (PLA) films for food packaging applications | Inclusion of R-(−)-carvone in the polymer matrix Preparation and determination of film thickness Determination of remaining content Determination of thermal, mechanical and barrier properties | Lower Tg and Tm Higher gas permeability Lower tensile strength Higher elongation at break of antifungal PLA films Homogeneous and transparent antifungal films | [16] |
| Carvone | Purchased | Candida rugosa, Candida lusitaniae, Candida glabrata, Candida utilis, Candida krusei, Candida guilliermondii, Candida tropicalis, Candida albicans, Candida parapsilosis, and Candida dubliniensis | Planktonic anti-candida assay Evaluation of the inhibitory power of germ tube formation Evaluation of the anti-biofilm effect | MIC = 0.5 mg/mL The concentration of 0.5 mg/mL inhibited at least 50% of the biofilm Inhibited the polymorphism up to 86% Changes in yeast cell envelope and cell viability were greater than 50% Induced important antifungal activities | [17] |
| Carvone chemotype | Naturel | Candida parapsilosis, Candida krusei, Aspergillus flavus, and Aspergillus fumigatus Broth macro-dilution method AFST-EUCAST method CLSI M38-A method MIC determination | Determination of GM-MIC | GM-MIC > 500 μg/mL against the different strains studied No activity against selected clinical strains | [18] |
| Carvone | Purchased | Fusarium subglutinans, Fusarium cerealis, Fusarium verticillioides, Fusarium proliferatum, Fusarium oxysporum, Fusarium sporotrichioides, Aspergillus tubingensis, Aspergillus carbonarius, Alternaria alternata, and Penicillium sp. | In vitro antifungal activity Evaluation of deoxynivalenol production Evaluation of inhibitory effects on plant seed germination | Induced toxic effects on the growth of the mycelium of all fungal species | [19] |
| Carvone | Naturel (Mentha spicata) | Cryptococcus neoformans, dermatophytes (Trichophyton spp., Epidermophyton floccosum, and Microsporum spp.), and Aspergillus strains | In vitro antifungal activity Evaluation of the inhibitory activity of germ tube formation | Mentha spicata EO was effective against Cryptococcus neoformans, as well as the dermatophytes Trichophyton rubrum and Trichophyton verrucosum (0.32 μL/mL) Inhibited the germ tube development of Candida albicans, at concentrations below the MIC (0.16 μL/mL) | [21] |
| (+)-carvone (C+) (−)-carvone (C−) α,β-epoxycarvone (EP) (+)-hydroxy-dihydrocarvone (HC+) (−)-hydroxy-dihydrocarvone (HC−) | Purchased | Candida parapsilosis, Candida tropicalis, Candida krusei, and Candida albicans | Determination of MIC by microplate dilution method and MFC | Low antifungal activity against Candida tropicalis and Candida parapsilosis EP and C+ showed moderate activity against Candida krusei similar to C+ and C− against Candida albicans All the molecules tested showed fungistatic and fungicidal activity against Candida yeasts, and the most significant result was recorded with C+, C−, and EP | [20] |
| Molecules | Origins | Model Used | Experimental Approaches | Key Results | References |
|---|---|---|---|---|---|
| (S)-(−)-carvone (R)-(+)-carvone | Naturel (Mentha spicata and Anethum sowa Roxb.) | Bacillus subtilis, Enterobacter aerogenes, Enterococcus Faecalis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus mutans, Yersinia enterocolitica, Salmonella typhi, Escherichia coli, Staphylococcus epidermidis, and Mycobacterium smegmatis | Disk diffusion assay Broth dilution assay | The activity of carvone was comparable with the bioactivity of their original oils Active against a broad spectrum of human pathogenic bacteria (R)-(+)-limonene showed comparable bioactivity profile over the (S)-(−)-isomer | [22] |
| Carvone | Purchased | Staphylococcus aureus | Single-step plasma polymerization Plasma polymerization of carvone Surface characterization Antibacterial activity Live-dead fluorescence assay Crystal violet assay Morphology of bacteria by field emission scanning electron microscope (FE-SEM) | Polymerization provided a hydrophobic antibacterial coating (ppCar) with an average roughness < 1nm ppCar had a static water contact angle of 78° Reduced effectively Escherichia coli (86%) and Staphylococcus aureus (84%) Broken bacterial membrane | [23] |
| (−)-Carvone (+)-Carvone | Purchased | Absidia glauca, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Enterobacter aerogenes, Proteus vulgaris, and Salmonella typhimurium | Biotransformation Semi-preparative scale biotransformation and isolation GC-MS Antimicrobial assay | Biotransformation of carvone into diol 10-hydroxy-(+)-neodihydrocarveol by Absidia glauca Both molecules showed antimicrobial activity against all strains tested | [24] |
| Semicarbazone and thiosemicarbazone of R-(−) carvone | Synthetized | Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Enterococcus faecalis | Determination of MIC | Inhibitory activity on Pseudomonas aeruginosa for thiosemicarbazone (MIC = 78.1 μg/mL) and for semicarbazone (MIC = 312.5 μg/mL) Thiosemicarbazone was active on Staphylococcus aureus (MIC = 39 μg/mL) Thiosemicarbazone exerted interesting inhibitory activity on Staphylococcus aureus and Pseudomonas aeruginosa | [26] |
| Carvone | Purchased | Staphylococcus aureus and Enterococcus coli | Nanoparticles preparation Determination of drug loading and entrapment efficiency In vitro carvone release from nanoparticles Antibacterial properties of the carvone-loaded nanoparticles | Production of small nanoparticles (126 nm), with high drug loading (12.32%) and good inhibition of microbial growth Carvone-loaded nanoparticles inhibited Staphylococcus aureus (MIC = 182 mg/mL) and Enterococcus coli (MIC = 374 mg/mL) | [25] |
| (+)-carvone (−)-carvone (+)-hydroxy-dihydrocarvone (−)-hydroxyl-dihydrocarvone α,β-epoxycarvone | Synthesized/purchased | Escherichia coli and Staphylococcus aureus | Determination of MIC by microplate dilution method and MBC | C- and HC- showed low activity against Escherichia coli EP, C+, and HC+ did not inhibit the growth of the bacterial strains tested | [20] |
| R-carvone S-carvone | Purchased | Methicillin-resistant Staphylococcus aureus (MRSA) | Broth micro-dilution method Time-kill assay | MIC values for R- and S-carvone against six different strains of Staphylococcus aureus ranged between 500 and 1000 µg/mL R-carvone + gentamicin and S-carvone + gentamicin exhibited significant synergistic activity against MRSA The combined treatment improved the effectiveness of carvone | [27] |
| Carvone | Naturel (Lippia alba) | Staphylococcus aureus ATCC 6538 | Determination of MIC and MBC by the microdilution method Anti-biofilm Activity | Elimination of biofilm cells was confirmed at concentrations between 0.5 and 2 mg/mL No elimination of biofilm cells was observed with the use of carvone | [28] |
| Molecules | Models Used | Experimental Approaches | Key Results | References |
|---|---|---|---|---|
| Two analogues of carvone | In silico study | Molecular docking Molecular dynamics simulation | All ligands showed strong binding affinity against active neuraminidase sites, ranging from −4.77 to −8.30 kcal/mol Carvone derivatives could serve as potent neuraminidase inhibitors against the influenza virus | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouyahya, A.; Mechchate, H.; Benali, T.; Ghchime, R.; Charfi, S.; Balahbib, A.; Burkov, P.; Shariati, M.A.; Lorenzo, J.M.; Omari, N.E. Health Benefits and Pharmacological Properties of Carvone. Biomolecules 2021, 11, 1803. https://doi.org/10.3390/biom11121803
Bouyahya A, Mechchate H, Benali T, Ghchime R, Charfi S, Balahbib A, Burkov P, Shariati MA, Lorenzo JM, Omari NE. Health Benefits and Pharmacological Properties of Carvone. Biomolecules. 2021; 11(12):1803. https://doi.org/10.3390/biom11121803
Chicago/Turabian StyleBouyahya, Abdelhakim, Hamza Mechchate, Taoufiq Benali, Rokia Ghchime, Saoulajan Charfi, Abdelaali Balahbib, Pavel Burkov, Mohammad Ali Shariati, Jose M. Lorenzo, and Nasreddine El Omari. 2021. "Health Benefits and Pharmacological Properties of Carvone" Biomolecules 11, no. 12: 1803. https://doi.org/10.3390/biom11121803
APA StyleBouyahya, A., Mechchate, H., Benali, T., Ghchime, R., Charfi, S., Balahbib, A., Burkov, P., Shariati, M. A., Lorenzo, J. M., & Omari, N. E. (2021). Health Benefits and Pharmacological Properties of Carvone. Biomolecules, 11(12), 1803. https://doi.org/10.3390/biom11121803








