Andrographolide Attenuates Established Pulmonary Hypertension via Rescue of Vascular Remodeling
Abstract
:1. Introduction
2. Results
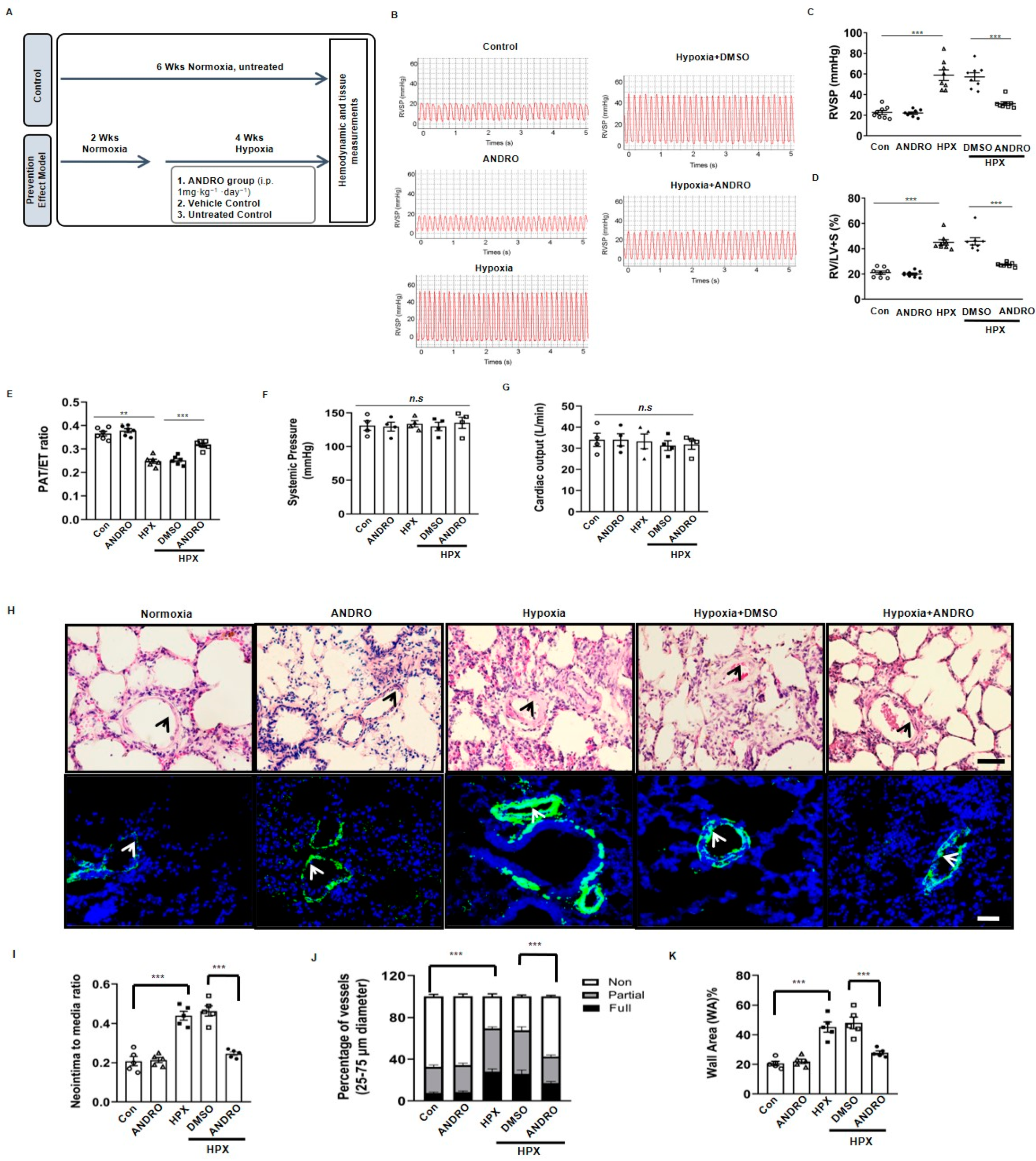
2.1. ANDRO Prevents the Development of PH in Hypoxia Mice
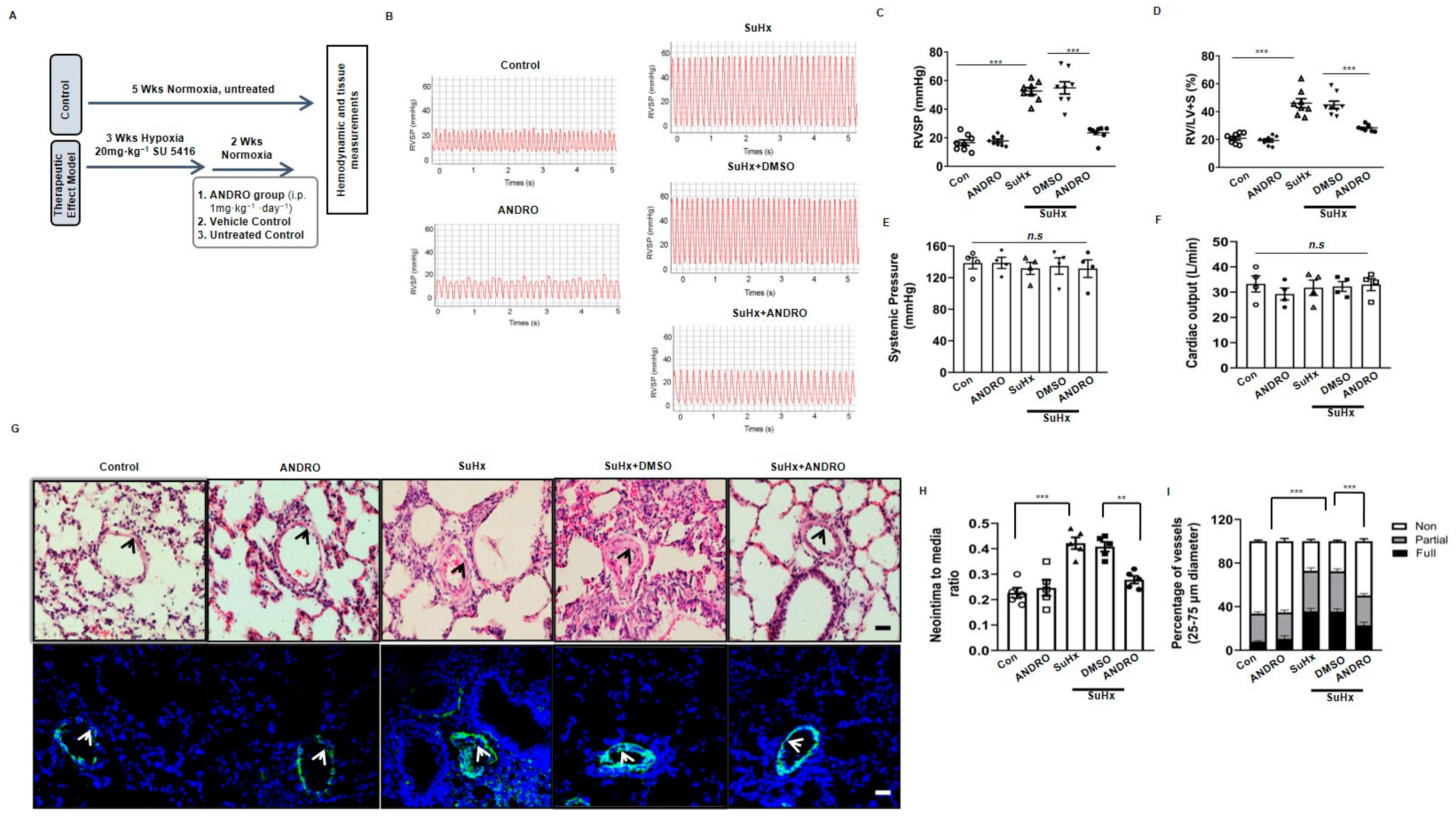
2.2. ANDRO Produces Therapeutic Effect on PH in Sugen-Hypoxia (SuHx) Mice
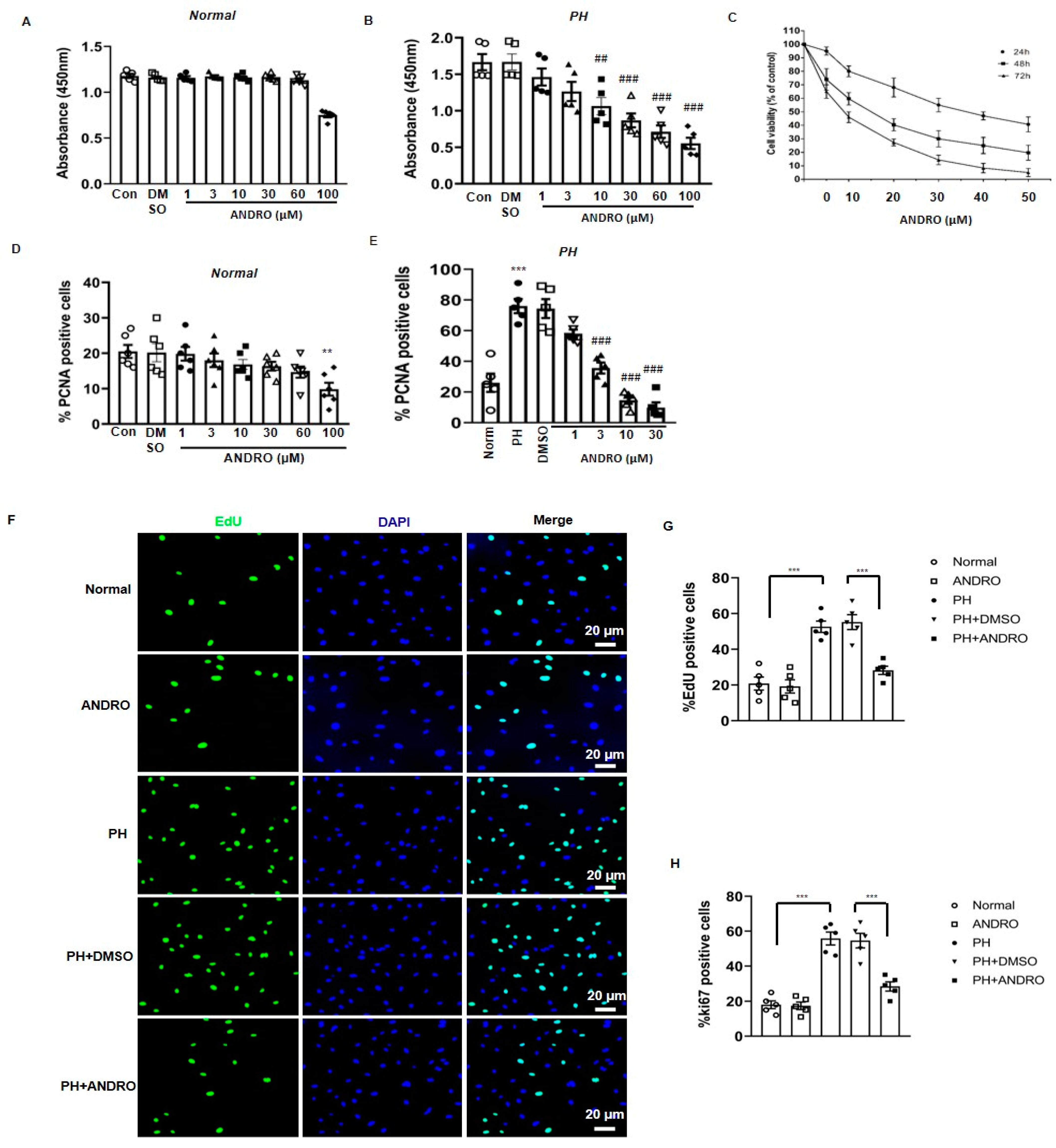
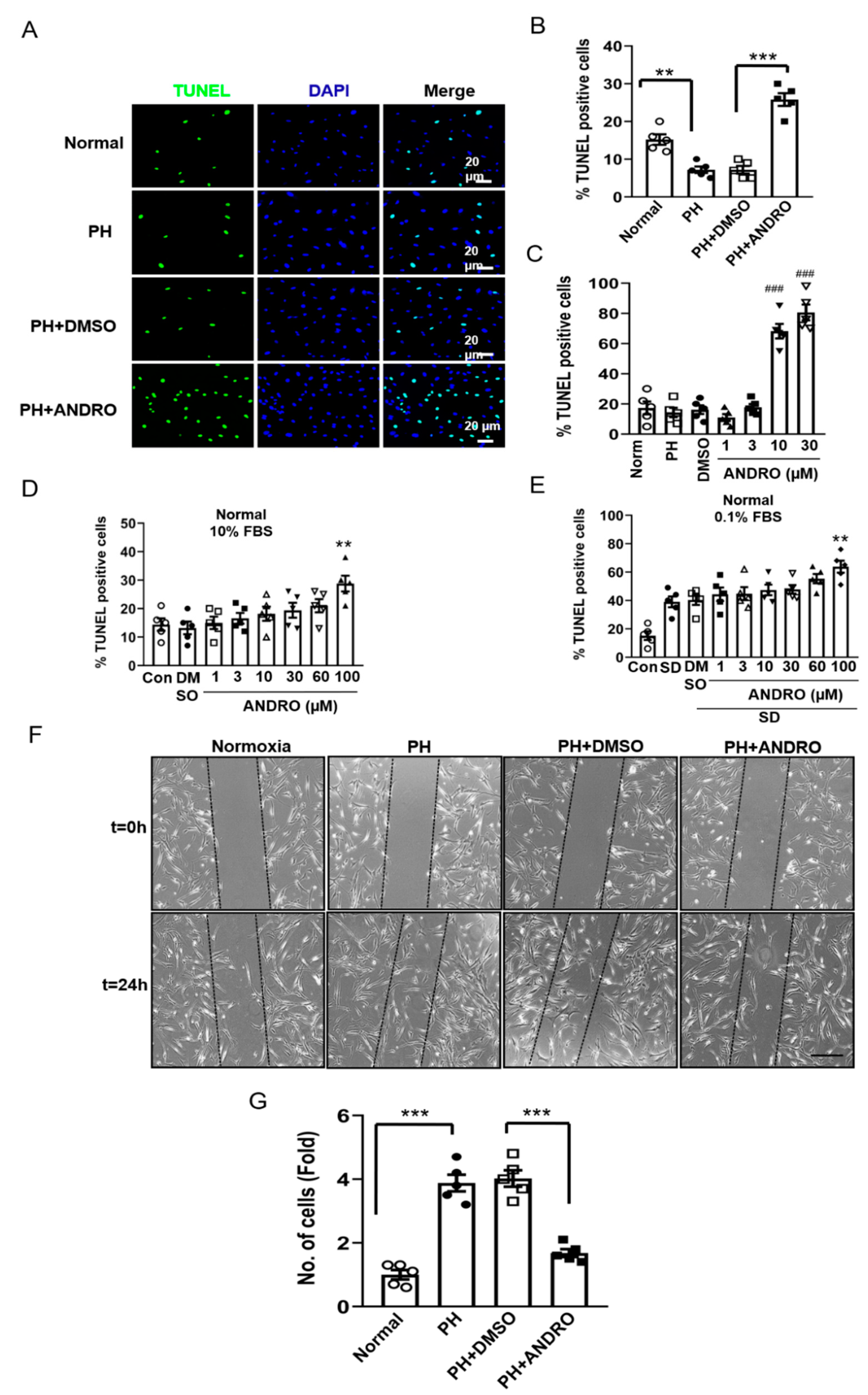
2.3. ANDRO Significantly Reduces Cell Proliferation and Increases Cell Apoptosis in PASMCs in SuHx-Induced PH Mice
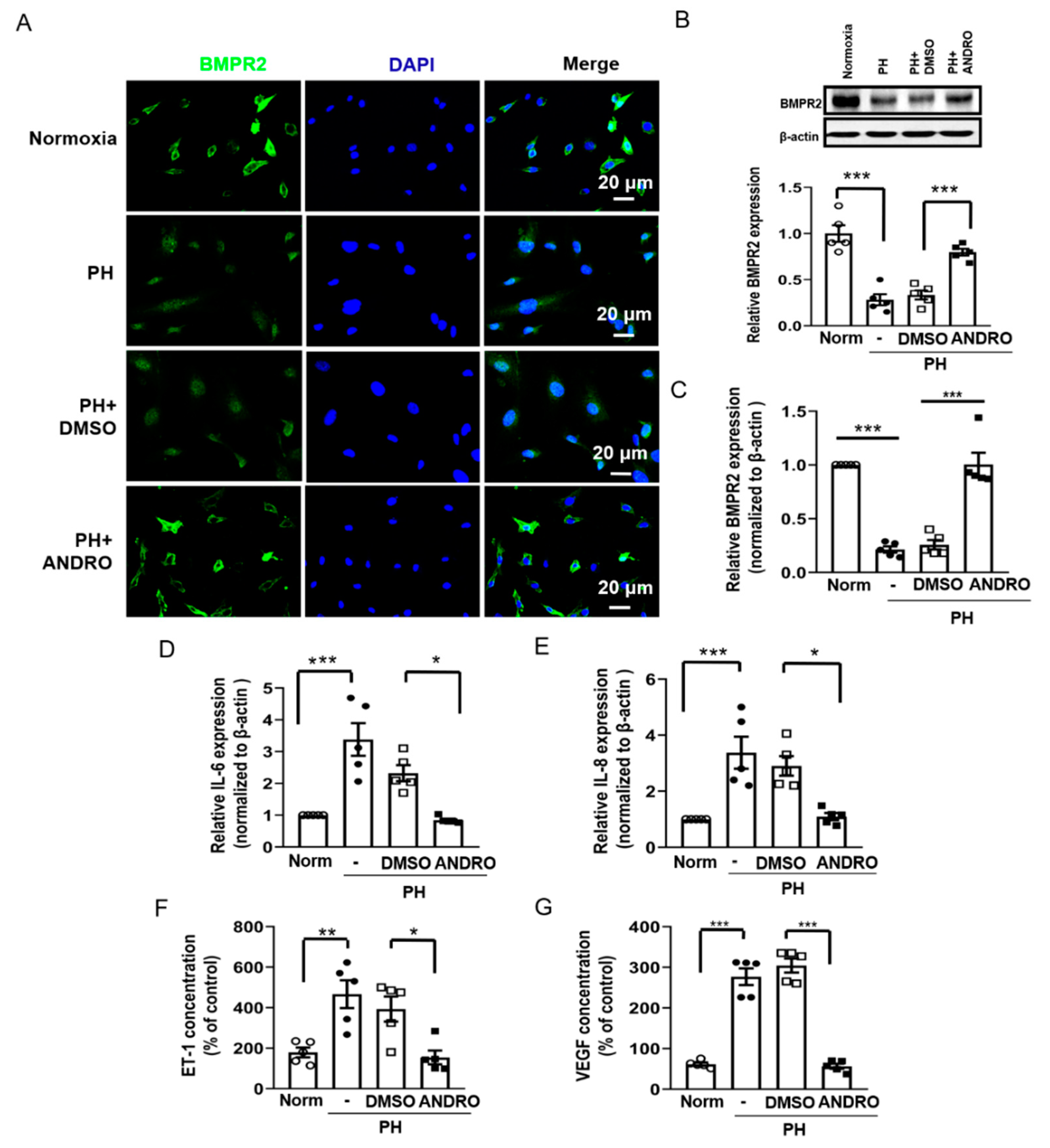
2.4. Effect of ANDRO on PASMCs Isolated from PH Patients (PH-PASMCs)
2.5. ANDRO Inhibits the TLR4/NF-κB Signaling Pathway in Human PH-PASMCs or PAs of PH Mice
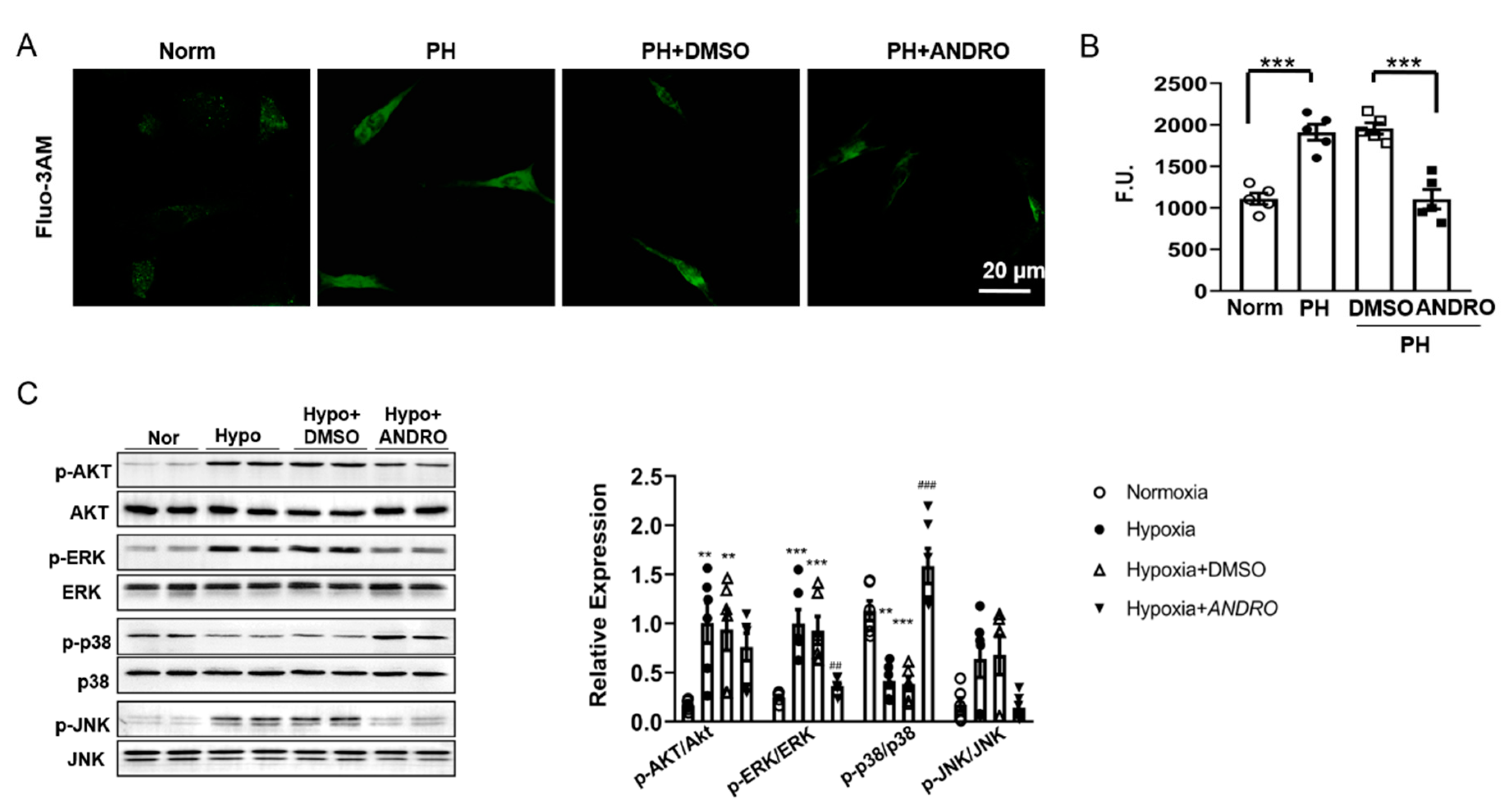
2.6. ANDRO Resumes NF-κB-Related Signaling Cascade in PH-PASMCs
2.7. ANDRO Regulates Intracellular [Ca2+]i, ROS Production and the Related Signaling in PH-PASMCs
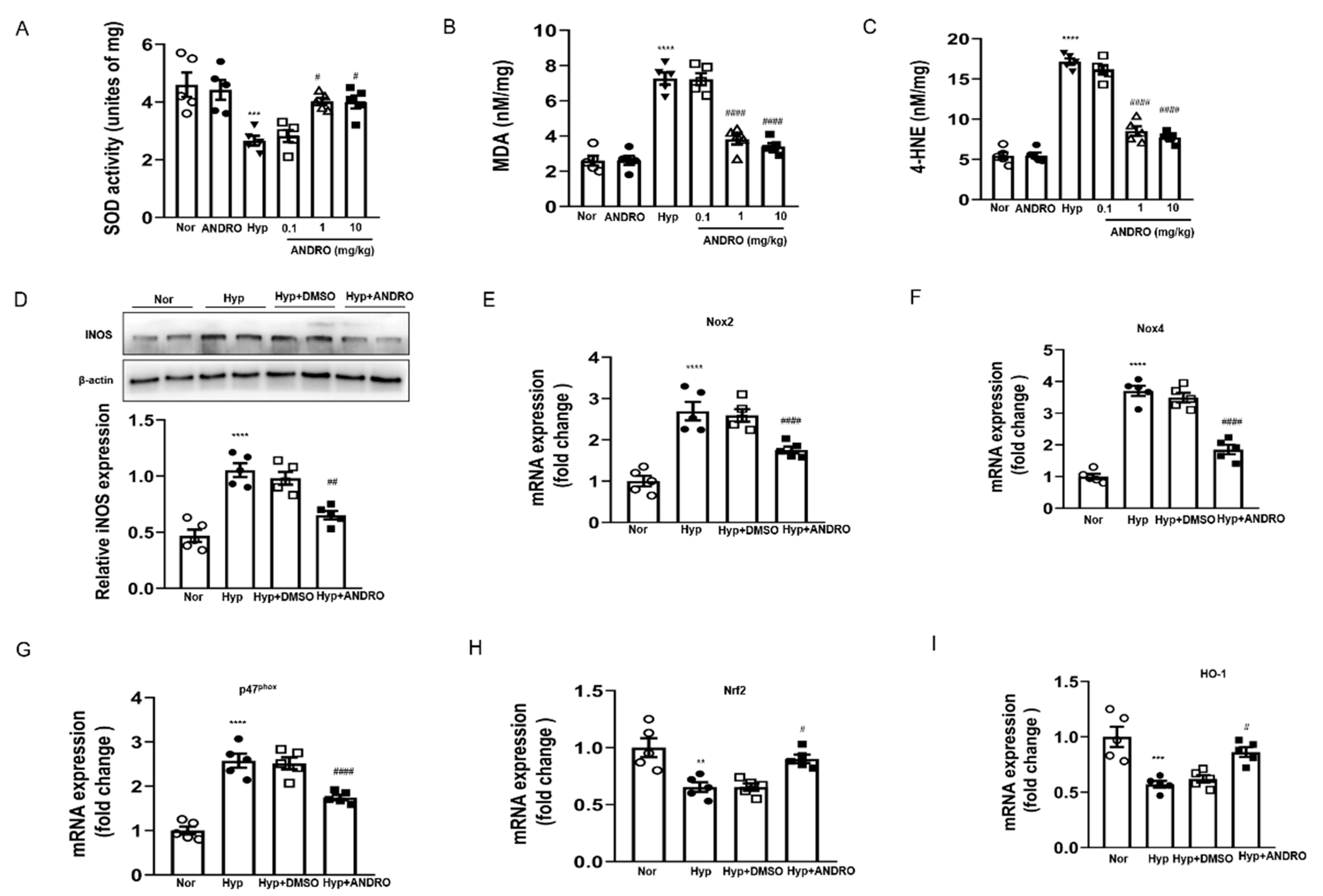
2.8. ANDRO Treatment Attenuates Hypoxia-Induced Pulmonary Oxidative and Nitrative Stress
2.9. ANDRO Treatment Blocks ROS Generation via Regulating NOXs/Nrf2 Redox Imbalance in Human PH-PASMCs
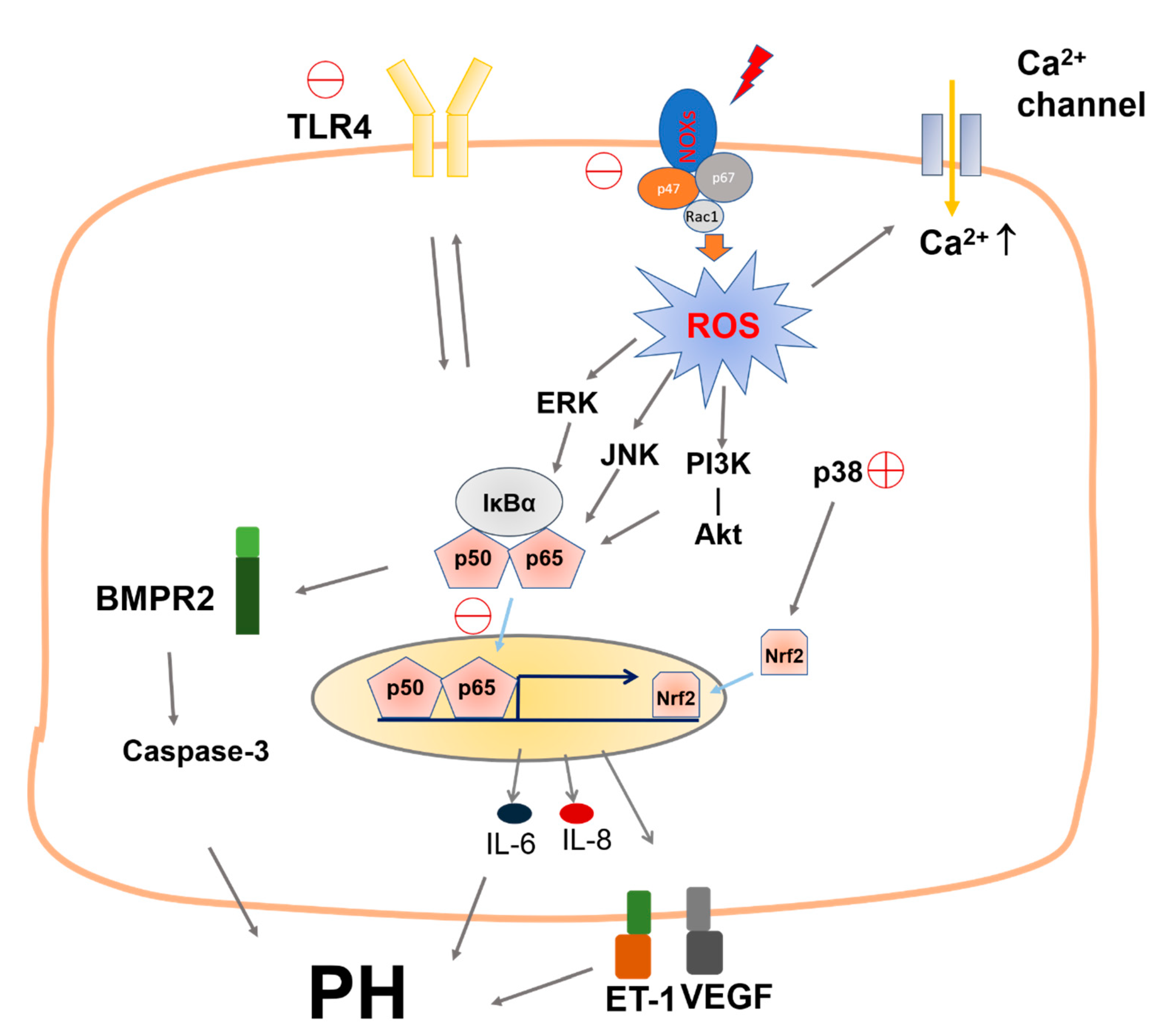
3. Discussion
4. Innovation
5. Materials and Methods
5.1. Materials
5.2. Study Design
5.3. Clinical Samples Collection
5.4. Animals and Experimental Protocols
5.4.1. Chronic Hypoxia Model of PH
5.4.2. SuHx-Induced PH Murine Model
5.5. Hemodynamic Studies and Evaluation of Right Ventricular Hypertrophy
5.6. Immunohistochemical Staining
5.7. Cell Culture
5.8. Cytotoxicity Assay
5.9. Cell Proliferation
5.10. Detection of Reactive Oxygen Species (ROS)
5.11. Enzyme-Linked Immunosorbent Assay (ELISA)
5.12. Cell Migration Assay
5.13. Immunoblotting Assay
5.14. Quantitative RT-PCR Analysis
5.15. Immunofluorescence Assay
5.16. Data and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANDRO | andrographolide |
| [Ca2+]i | intracellular free Ca2+ concentration |
| PH | pulmonary hypertension |
| PA | pulmonary artery |
| hPASMCs | human pulmonary artery smooth muscle cells |
| RV/(LV+S) | ratio of the weight of right ventricle to the weight of left ventricle and septum (also referred to as Fulton index) |
| RVSP | right ventricular systolic pressure |
| BMPR2 | bone morphogenetic protein receptor type-2 |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| qRT-PCR | quantitative real-time PCR |
| EdU | 5-ethynyl-2′-deoxyuridine |
| H&E | hematoxylin-eosin staining |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMSO | dimethylsulfoxide |
| siRNA | small interfering RNA |
| Fluo-3AM | fluo-3-pentaacetoxymethylester |
| ROS | reactive oxygen species |
| NOX | NADPH oxidase |
| Nrf2 | nuclear factor (erythroid-derived 2)-like 2 |
| HO-1 | heme oxygenase-1 |
References
- Austin, E.D.; Loyd, J.E. The genetics of pulmonary arterial hypertension. Circ. Res. 2014, 115, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Grant, J.S.; White, K.; MacLean, M.R.; Baker, A.H. MicroRNAs in pulmonary arterial remodeling. Cell. Mol. Life Sci. 2013, 70, 4479–4494. [Google Scholar] [CrossRef] [Green Version]
- Guignabert, C.; Tu, L.; Le Hiress, M.; Ricard, N.; Sattler, C.; Seferian, A.; Huertas, A.; Humbert, M.; Montani, D. Pathogenesis of pulmonary arterial hypertension: Lessons from cancer. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2013, 22, 543–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomberg-Maitland, M.; Maitland, M.L.; Barst, R.J.; Sugeng, L.; Coslet, S.; Perrino, T.J.; Bond, L.; Lacouture, M.E.; Archer, S.L.; Ratain, M.J. A dosing/cross-development study of the multikinase inhibitor sorafenib in patients with pulmonary arterial hypertension. Clin. Pharmacol. Ther. 2010, 87, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.L.; Ma, Y.M. Pharmacokinetic herb-drug interactions with traditional Chinese medicine: Progress, causes of conflicting results and suggestions for future research. Drug Metab. Rev. 2016, 48, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Preet, R.; Chakraborty, B.; Siddharth, S.; Mohapatra, P.; Das, D.; Satapathy, S.R.; Das, S.; Maiti, N.C.; Maulik, P.R.; Kundu, C.N.; et al. Synthesis and biological evaluation of andrographolide analogues as anti-cancer agents. Eur. J. Med. Chem. 2014, 85, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhao, J.; Wang, Y.; Xu, H.; Wu, Z.; Hu, Y.; Jiang, K.; Shen, P.; Ma, C.; Guan, Z.; et al. In vivo inhibitory activity of andrographolide derivative ADN-9 against liver cancer and its mechanisms involved in inhibition of tumor angiogenesis. Toxicol. Appl. Pharmacol. 2017, 327, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, C.; Jiang, H.; Cheng, J. Andrographolide inhibits hypoxia-inducible factor-1 through phosphatidylinositol 3-kinase/AKT pathway and suppresses breast cancer growth. Onco Targets Ther. 2015, 8, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, M.A.; Romero, A.; Figueroa, J.; Cortes, P.; Concha, I.I.; Hancke, J.L.; Burgos, R.A. Andrographolide interferes with binding of nuclear factor-kappaB to DNA in HL-60-derived neutrophilic cells. Br. J. Pharmcol. 2005, 144, 680–686. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yan, H.; Zhang, Z.; Zhang, G.; Sun, Y.; Yu, P.; Wang, Y.; Xu, L. Andrographolide derivative AL-1 improves insulin resistance through down-regulation of NF-kappaB signalling pathway. Br. J. Pharmacol. 2015, 172, 3151–3158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, R.K.; Potteti, H.R.; Tamatam, C.R.; Elangovan, I.; Reddy, S.P. c-Jun Is Required for Nuclear Factor-kappaB-Dependent, LPS-Stimulated Fos-Related Antigen-1 Transcription in Alveolar Macrophages. Am. J. Respir. Cell Mol. Biol. 2016, 55, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Crawley, C.D.; Raleigh, D.R.; Kang, S.; Voce, D.J.; Schmitt, A.M.; Weichselbaum, R.R.; Yamini, B. DNA damage-induced cytotoxicity is mediated by the cooperative interaction of phospho-NF-kappaB p50 and a single nucleotide in the kappaB-site. Nucleic Acids Res. 2013, 41, 764–774. [Google Scholar] [CrossRef] [Green Version]
- Sen, R.; Baltimore, D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 1986, 47, 921–928. [Google Scholar] [CrossRef]
- Nyati, K.K.; Masuda, K.; Zaman, M.M.; Dubey, P.K.; Millrine, D.; Chalise, J.P.; Higa, M.; Li, S.; Standley, D.M.; Saito, K.; et al. TLR4-induced NF-kappaB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. Nucleic Acids Res. 2017, 45, 2687–2703. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Chang, M.M.; Velichko, S.; Thai, P.; Hung, L.Y.; Huang, F.; Phuong, N.; Chen, Y.; Wu, R. NF-kappaB mediates IL-1beta- and IL-17A-induced MUC5B expression in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 246–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagoya, Y.; Yoshimi, A.; Kataoka, K.; Nakagawa, M.; Kumano, K.; Arai, S.; Kobayashi, H.; Saito, T.; Iwakura, Y.; Kurokawa, M. Positive feedback between NF-kappaB and TNF-alpha promotes leukemia-initiating cell capacity. J. Clin. Investig. 2014, 124, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, C.; Kim, I.K.; Janssen-Heininger, Y.; Gupta, S. Inhibition of nuclear factor-kappaB in the lungs prevents monocrotaline-induced pulmonary hypertension in mice. Hypertension 2014, 63, 1260–1269. [Google Scholar] [CrossRef] [Green Version]
- Kracun, D.; Klop, M.; Knirsch, A.; Petry, A.; Kanchev, I.; Chalupsky, K.; Wolf, C.M.; Gorlach, A. NADPH oxidases and HIF1 promote cardiac dysfunction and pulmonary hypertension in response to glucocorticoid excess. Redox Biol. 2020, 34, 101536. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, X.; Zhao, M.; Zhou, B.; Han, Y. Angiotensin-(1–7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci. Rep. 2016, 6, 34621. [Google Scholar] [CrossRef]
- Lai, Y.C.; Potoka, K.C.; Champion, H.C.; Mora, A.L.; Gladwin, M.T. Pulmonary arterial hypertension: The clinical syndrome. Circ. Res. 2014, 115, 115–130. [Google Scholar] [CrossRef] [Green Version]
- Thistlethwaite, P.A. Linking Vascular Remodeling and Inflammation in Pulmonary Arterial Hypertension: Is There a Common Root Cause? Am. J. Respir. Cell Mol. Biol. 2017, 57, 15–17. [Google Scholar] [CrossRef]
- Zamanian, R.T.; Kudelko, K.T.; Sung, Y.K.; Perez, V.J.; Liu, J.; Spiekerkoetter, E. Current clinical management of pulmonary arterial hypertension. Circ. Res. 2014, 115, 131–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeper, M.M.; Kramer, T.; Pan, Z.; Eichstaedt, C.A.; Spiesshoefer, J.; Benjamin, N.; Olsson, K.M.; Meyer, K.; Vizza, C.D.; Vonk-Noordegraaf, A.; et al. Mortality in pulmonary arterial hypertension: Prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur. Respir. J. 2017, 50, 1700740. [Google Scholar] [CrossRef] [Green Version]
- Vetvicka, V.; Vannucci, L. Biological properties of andrographolide, an active ingredient of Andrographis Paniculata: A narrative review. Ann. Transl. Med. 2021, 9, 1186. [Google Scholar] [CrossRef]
- Chowdhury, A.; Aich, A.; Jain, G.; Wozny, K.; Luchtenborg, C.; Hartmann, M.; Bernhard, O.; Balleiniger, M.; Alfar, E.A.; Zieseniss, A.; et al. Defective Mitochondrial Cardiolipin Remodeling Dampens HIF-1alpha Expression in Hypoxia. Cell Rep. 2018, 25, 561–570.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, T.; Galougahi, K.K.; Celermajer, D.; Rasko, N.; Tang, O.; Bubb, K.J.; Figtree, G. Oxidative and nitrosative signalling in pulmonary arterial hypertension—Implications for development of novel therapies. Pharmacol. Ther. 2016, 165, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, T.; Sun, H.; Pinchuk, I.V.; Milewicz, D.M.; Tilton, R.G.; Brasier, A.R. Deletion of NF-kappaB/RelA in Angiotensin II-Sensitive Mesenchymal Cells Blocks Aortic Vascular Inflammation and Abdominal Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1881–1890. [Google Scholar] [CrossRef] [Green Version]
- Farkas, D.; Alhussaini, A.A.; Kraskauskas, D.; Kraskauskiene, V.; Cool, C.D.; Nicolls, M.R.; Natarajan, R.; Farkas, L. Nuclear factor kappaB inhibition reduces lung vascular lumen obliteration in severe pulmonary hypertension in rats. Am. J. Respir. Cell Mol. Biol. 2014, 51, 413–425. [Google Scholar] [CrossRef]
- Hiram, R.; Rizcallah, E.; Marouan, S.; Sirois, C.; Sirois, M.; Morin, C.; Fortin, S.; Rousseau, E. Resolvin E1 normalizes contractility, Ca2+ sensitivity and smooth muscle cell migration rate in TNF-alpha- and IL-6-pretreated human pulmonary arteries. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 309, L776–L788. [Google Scholar] [CrossRef]
- Liang, Y.; Li, X.; Zhang, X.; Li, Z.; Wang, L.; Sun, Y.; Liu, Z.; Ma, X. Elevated levels of plasma TNF-alpha are associated with microvascular endothelial dysfunction in patients with sepsis through activating the NF-kappaB and p38 mitogen-activated protein kinase in endothelial cells. Shock 2014, 41, 275–281. [Google Scholar] [CrossRef]
- Huang, T.H.; Chung, S.Y.; Chua, S.; Chai, H.T.; Sheu, J.J.; Chen, Y.L.; Chen, C.H.; Chang, H.W.; Tong, M.S.; Sung, P.H.; et al. Effect of early administration of lower dose versus high dose of fresh mitochondria on reducing monocrotaline-induced pulmonary artery hypertension in rat. Am. J. Transl. Res. 2016, 8, 5151–5168. [Google Scholar]
- Bellofiore, A.; Dinges, E.; Naeije, R.; Mkrdichian, H.; Beussink-Nelson, L.; Bailey, M.; Cuttica, M.J.; Sweis, R.; Runo, J.R.; Keevil, J.G.; et al. Reduced haemodynamic coupling and exercise are associated with vascular stiffening in pulmonary arterial hypertension. Heart 2017, 103, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.R.; Yang, L.M.; Tsai, W.J.; Chiou, W.F. Andrographolide acts through inhibition of ERK1/2 and Akt phosphorylation to suppress chemotactic migration. Eur. J. Pharmacol. 2004, 498, 45–52. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, C.; Liang, H.; Wang, X.; Liu, Y.; Li, X.; Ji, K.; Xu, H.; Yang, M.; Liu, K.; et al. Andrographolide derivative CX-10 ameliorates dextran sulphate sodium-induced ulcerative colitis in mice: Involvement of NF-kappaB and MAPK signalling pathways. Int. Immunopharmacol. 2018, 57, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.W.; Liao, S.Q.; Zhang, M.J.; Liu, Y.; Li, B.H.; Zhou, Y.; Chen, L.; Gao, C.Y.; Li, J.C.; Zhang, L.L. TLR4-mediated inflammation promotes foam cell formation of vascular smooth muscle cell by upregulating ACAT1 expression. Cell Death Dis. 2014, 5, e1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Peters, A.; Papke, C.L.; Villamizar, C.; Ringuette, L.J.; Cao, J.; Wang, S.; Ma, S.; Gong, L.; Byanova, K.L.; et al. Loss of Smooth Muscle alpha-Actin Leads to NF-kappaB-Dependent Increased Sensitivity to Angiotensin II in Smooth Muscle Cells and Aortic Enlargement. Circ. Res. 2017, 120, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Wynants, M.; Vengethasamy, L.; Ronisz, A.; Meyns, B.; Delcroix, M.; Quarck, R. NF-kappaB pathway is involved in CRP-induced effects on pulmonary arterial endothelial cells in chronic thromboembolic pulmonary hypertension. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2013, 305, L934–L942. [Google Scholar] [CrossRef] [Green Version]
- Weng, X.; Yu, L.; Liang, P.; Li, L.; Dai, X.; Zhou, B.; Wu, X.; Xu, H.; Fang, M.; Chen, Q.; et al. A crosstalk between chromatin remodeling and histone H3K4 methyltransferase complexes in endothelial cells regulates angiotensin II-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 2015, 82, 48–58. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Gomez-Arroyo, J. The role of vascular endothelial growth factor in pulmonary arterial hypertension. The angiogenesis paradox. Am. J. Respir. Cell Mol. Biol. 2014, 51, 474–484. [Google Scholar] [CrossRef]
- Hwangbo, C.; Lee, H.W.; Kang, H.; Ju, H.; Wiley, D.S.; Papangeli, I.; Han, J.; Kim, J.D.; Dunworth, W.P.; Hu, X.; et al. Modulation of Endothelial Bone Morphogenetic Protein Receptor Type 2 Activity by Vascular Endothelial Growth Factor Receptor 3 in Pulmonary Arterial Hypertension. Circulation 2017, 135, 2288–2298. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Holmes, M.D.; Danilov, S.M.; Reynolds, P.N. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur. Respir. J. 2012, 39, 329–343. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Kim, I.K.; Chiasson, V.; Chatterjee, P.; Gupta, S. NF-kappaB mediated miR-130a modulation in lung microvascular cell remodeling: Implication in pulmonary hypertension. Exp. Cell Res. 2017, 359, 235–242. [Google Scholar] [CrossRef]
- Chen, P.I.; Cao, A.; Miyagawa, K.; Tojais, N.F.; Hennigs, J.K.; Li, C.G.; Sweeney, N.M.; Inglis, A.S.; Wang, L.; Li, D.; et al. Amphetamines promote mitochondrial dysfunction and DNA damage in pulmonary hypertension. JCI Insight 2017, 2, e90427. [Google Scholar] [CrossRef] [Green Version]
- Salazar, G.; Huang, J.; Feresin, R.G.; Zhao, Y.; Griendling, K.K. Zinc regulates Nox1 expression through a NF-kappaB and mitochondrial ROS dependent mechanism to induce senescence of vascular smooth muscle cells. Free Radic. Biol. Med. 2017, 108, 225–235. [Google Scholar] [CrossRef]
- Yen, T.L.; Chen, R.J.; Jayakumar, T.; Lu, W.J.; Hsieh, C.Y.; Hsu, M.J.; Yang, C.H.; Chang, C.C.; Lin, Y.K.; Lin, K.H.; et al. Andrographolide stimulates p38 mitogen-activated protein kinase-nuclear factor erythroid-2-related factor 2-heme oxygenase 1 signaling in primary cerebral endothelial cells for definite protection against ischemic stroke in rats. Transl. Res. J. Lab. Clin. Med. 2016, 170, 57–72. [Google Scholar] [CrossRef]
- Mittal, S.P.K.; Khole, S.; Jagadish, N.; Ghosh, D.; Gadgil, V.; Sinkar, V.; Ghaskadbi, S.S. Andrographolide protects liver cells from H2O2 induced cell death by upregulation of Nrf-2/HO-1 mediated via adenosine A2a receptor signalling. Biochim. Biophys. Acta 2016, 1860, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.L.; Chen, M.F.; Liu, C.H.; Pang, C.Y.; Hsu, Y.H.; Lee, T.J. Induction of endothelium-dependent constriction of mesenteric arteries in endotoxemic hypotensive shock. Br. J. Pharmacol. 2016, 173, 1179–1195. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Yang, P.; Xu, K.; Peng, X.; Cai, W.; Zhao, S.; Hu, L.; Li, Z.; Cui, F.; et al. Andrographolide alleviates bleomycin-induced NLRP3 inflammasome activation and epithelial-mesenchymal transition in lung epithelial cells by suppressing AKT/mTOR signaling pathway. Ann. Transl. Med. 2021, 9, 764. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Xia, W.; Chen, J. Regulation of lung transplantation in China. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2012, 31, 1147–1148. [Google Scholar] [CrossRef]
- Huang, X.; Zou, L.; Yu, X.; Chen, M.; Guo, R.; Cai, H.; Yao, D.; Xu, X.; Chen, Y.; Ding, C.; et al. Salidroside attenuates chronic hypoxia-induced pulmonary hypertension via adenosine A2a receptor related mitochondria-dependent apoptosis pathway. J. Mol. Cell. Cardiol. 2015, 82, 153–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowburn, A.S.; Crosby, A.; Macias, D.; Branco, C.; Colaco, R.D.; Southwood, M.; Toshner, M.; Crotty Alexander, L.E.; Morrell, N.W.; Chilvers, E.R.; et al. HIF2alpha-arginase axis is essential for the development of pulmonary hypertension. Proc. Natl. Acad. Sci. USA 2016, 113, 8801–8806. [Google Scholar] [CrossRef] [Green Version]
- Aliotta, J.M.; Pereira, M.; Wen, S.; Dooner, M.S.; Del Tatto, M.; Papa, E.; Goldberg, L.R.; Baird, G.L.; Ventetuolo, C.E.; Quesenberry, P.J.; et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc. Res. 2016, 110, 319–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Xiao, R.; Zhang, X.; Lang, Y.; Liu, F.; Yu, Z.; Zhang, J.; Su, Y.; Lu, Y.; Wang, T.; et al. Spermine on Endothelial Extracellular Vesicles Mediates Smoking-Induced Pulmonary Hypertension Partially Through Calcium-Sensing Receptor. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 482–495. [Google Scholar] [CrossRef] [Green Version]
- Long, L.; Ormiston, M.L.; Yang, X.; Southwood, M.; Graf, S.; Machado, R.D.; Mueller, M.; Kinzel, B.; Yung, L.M.; Wilkinson, J.M.; et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat. Med. 2015, 21, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Marsboom, G.; Toth, P.T.; Ryan, J.J.; Hong, Z.; Wu, X.; Fang, Y.H.; Thenappan, T.; Piao, L.; Zhang, H.J.; Pogoriler, J.; et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ. Res. 2012, 110, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Chen, W.; Zhi, X.; Ma, T.; Xia, X.; Liu, H.; Zhang, Q.; Hu, Q.; Zhang, Y.; Bai, X.; et al. Serotonin promotes the proliferation of serum-deprived hepatocellular carcinoma cells via upregulation of FOXO3a. Mol. Cancer 2013, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Thakur, A.; Alam, M.J.; Ajayakumar, M.R.; Ghaskadbi, S.; Sharma, M.; Goswami, S.K. Norepinephrine-induced apoptotic and hypertrophic responses in H9c2 cardiac myoblasts are characterized by different repertoire of reactive oxygen species generation. Redox Biol. 2015, 5, 243–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Miyagawa, K.; Chen, S.Y.; Tamosiuniene, R.; Wang, L.; Sharpe, O.; Samayoa, E.; Harada, D.; Moonen, J.A.J.; Cao, A.; et al. Upregulation of Human Endogenous Retrovirus-K Is Linked to Immunity and Inflammation in Pulmonary Arterial Hypertension. Circulation 2017, 136, 1920–1935. [Google Scholar] [CrossRef]
- Adiguzel, E.; Hou, G.; Mulholland, D.; Hopfer, U.; Fukai, N.; Olsen, B.; Bendeck, M. Migration and growth are attenuated in vascular smooth muscle cells with type VIII collagen-null alleles. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Yeligar, S.M.; Kang, B.Y.; Bijli, K.M.; Kleinhenz, J.M.; Murphy, T.C.; Torres, G.; San Martin, A.; Sutliff, R.L.; Hart, C.M. PPARgamma Regulates Mitochondrial Structure and Function and Human Pulmonary Artery Smooth Muscle Cell Proliferation. Am. J. Respir. Cell Mol. Biol. 2018, 58, 648–657. [Google Scholar] [CrossRef]
- Song, S.; Carr, S.G.; McDermott, K.M.; Rodriguez, M.; Babicheva, A.; Balistrieri, A.; Ayon, R.J.; Wang, J.; Makino, A.; Yuan, J.X. STIM2 (Stromal Interaction Molecule 2)-Mediated Increase in Resting Cytosolic Free Ca2+ Concentration Stimulates PASMC Proliferation in Pulmonary Arterial Hypertension. Hypertension 2018, 71, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Sysol, J.R.; Dille, B.; Jones, N.; Chen, J.; Machado, R.F. Hemin Causes Lung Microvascular Endothelial Barrier Dysfunction by Necroptotic Cell Death. Am. J. Respir. Cell Mol. Biol. 2017, 57, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Peterlini, T.; Bourgeois, A.; Nadeau, V.; Breuils-Bonnet, S.; Boilet-Molez, S.; Potus, F.; Meloche, J.; Chabot, S.; Lambert, C.; et al. Mitochondrial HSP90 Accumulation Promotes Vascular Remodeling in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.J.; Alexander, S.; Cirino, G.; Docherty, J.R.; George, C.H.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. Br. J. Pharmacol. 2018, 175, 987–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.; Shen, C.; Tan, J.; Yang, X.; Wang, W.; Dai, Y.; Sun, H.; Wu, Z.; Chen, J. Andrographolide Attenuates Established Pulmonary Hypertension via Rescue of Vascular Remodeling. Biomolecules 2021, 11, 1801. https://doi.org/10.3390/biom11121801
Nie X, Shen C, Tan J, Yang X, Wang W, Dai Y, Sun H, Wu Z, Chen J. Andrographolide Attenuates Established Pulmonary Hypertension via Rescue of Vascular Remodeling. Biomolecules. 2021; 11(12):1801. https://doi.org/10.3390/biom11121801
Chicago/Turabian StyleNie, Xiaowei, Chenyou Shen, Jianxin Tan, Xusheng Yang, Wei Wang, Youai Dai, Haijian Sun, Zhiyuan Wu, and Jingyu Chen. 2021. "Andrographolide Attenuates Established Pulmonary Hypertension via Rescue of Vascular Remodeling" Biomolecules 11, no. 12: 1801. https://doi.org/10.3390/biom11121801
APA StyleNie, X., Shen, C., Tan, J., Yang, X., Wang, W., Dai, Y., Sun, H., Wu, Z., & Chen, J. (2021). Andrographolide Attenuates Established Pulmonary Hypertension via Rescue of Vascular Remodeling. Biomolecules, 11(12), 1801. https://doi.org/10.3390/biom11121801






