Opposite Effects of Chronic Central Leptin Infusion on Activation of Insulin Signaling Pathways in Adipose Tissue and Liver Are Related to Changes in the Inflammatory Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Experimental Design
2.4. Tissue Homogenization and Protein Quantification
2.5. Western Blotting
2.6. Phosphorylation of Insulin Receptor
2.7. Multiplexed Bead Immunoassays
2.8. Statistical Analysis
3. Results
3.1. General Characteristics of the Experimental Groups
3.2. Leptin Modifies the Activation of Inflammatory Targets and Cytokine Levels in Inguinal Fat and Liver in an Opposed Manner
3.3. Inguinal IRS1/PI3K Signaling Is Reduced in Leptin-Treated Rats
3.4. Chronic Leptin Exposure Increases Hepatic IRS1/PI3K Signaling
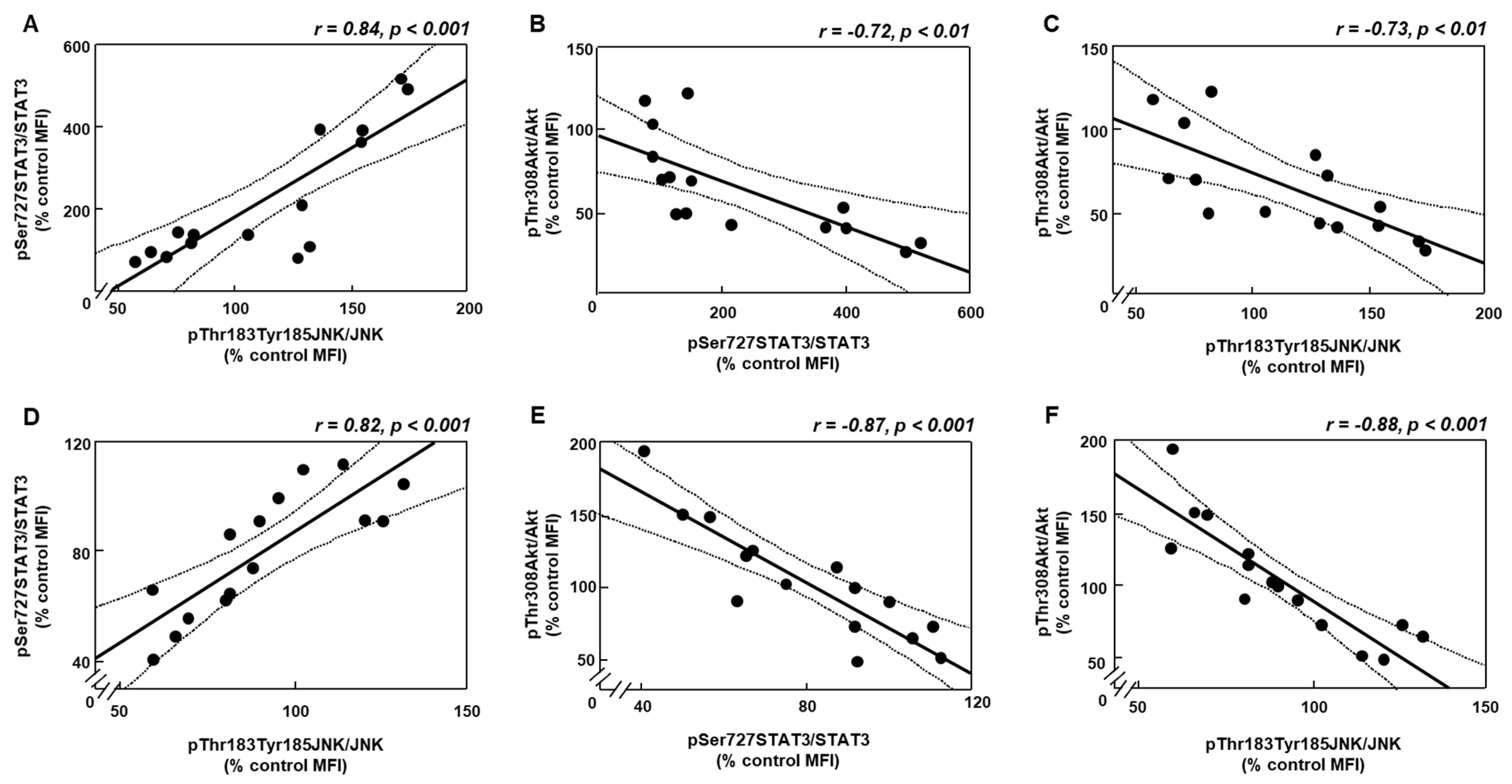
3.5. Changes in Akt Activation Are Inversely Related to STAT3 and JNK Phosphorylation
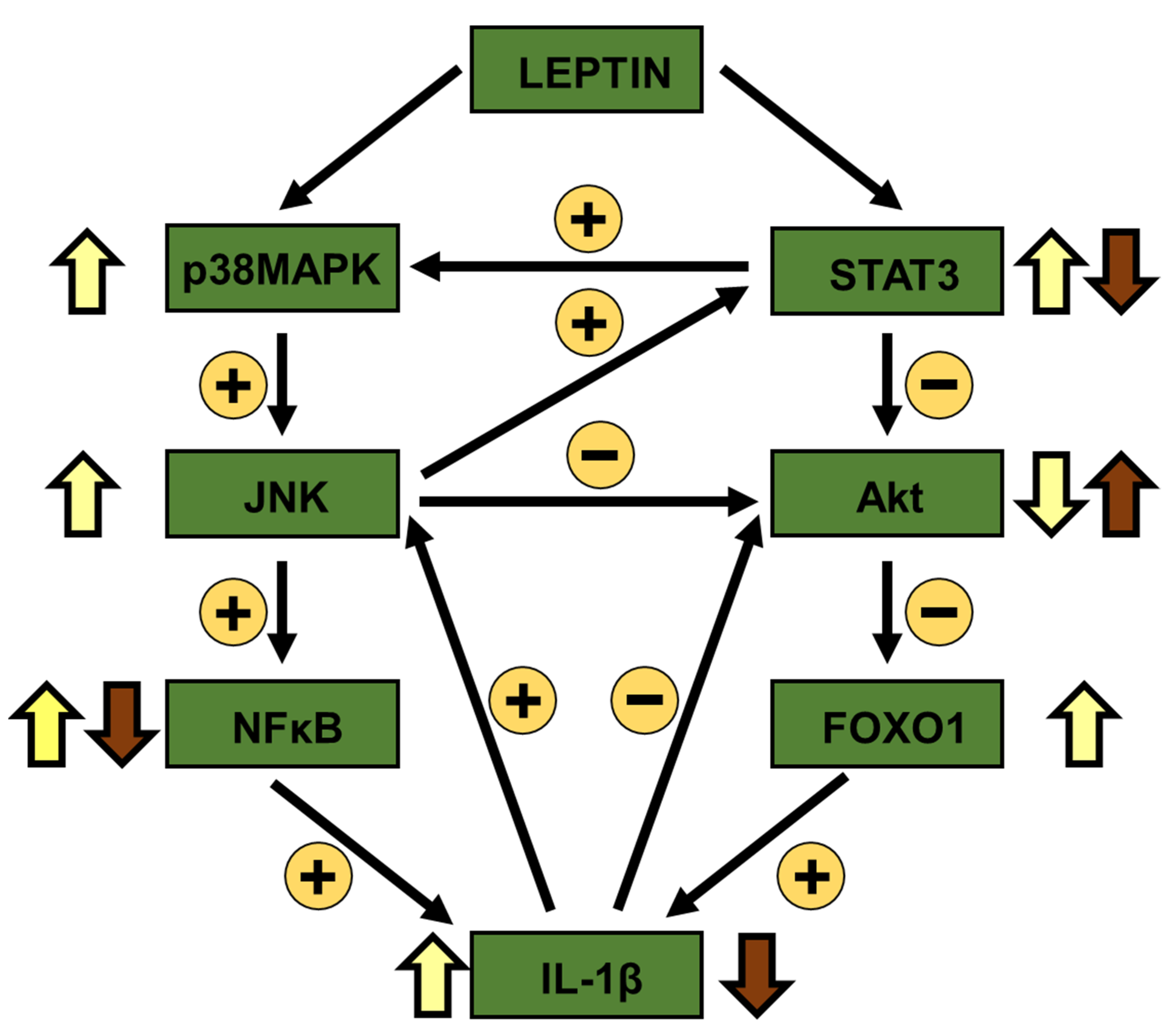
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, H.K.; Ahima, R.S. Leptin signaling. F1000Prime Rep. 2014, 6, 73. [Google Scholar] [CrossRef]
- Wu, C.W.; Storey, K.B. mTOR Signaling in Metabolic Stress Adaptation. Biomolecules 2021, 11, 681. [Google Scholar] [CrossRef]
- Borer, K.T. Counterregulation of insulin by leptin as key component of autonomic regulation of body weight. World J. Diabetes 2014, 5, 606–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Chinookoswong, N.; Scully, S.; Qi, M.; Shi, Z.Q. Differential effects of leptin in regulation of tissue glucose utilization in vivo. Endocrinology 1999, 140, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Kumariya, S.; Katekar, R.; Verma, S.; Goand, U.K.; Gayen, J.R. JNK signaling pathway in metabolic disorders: An emerging therapeutic target. Eur. J. Pharmacol. 2021, 901, 174079. [Google Scholar] [CrossRef]
- El-Zein, O.; Usta, J.; El Moussawi, L.; Kreydiyyeh, S.I. Leptin inhibits the Na(+)/K(+) ATPase in Caco-2 cells via PKC and p38MAPK. Cell. Signal. 2015, 27, 416–423. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.Y.; Son, E.J.; Heo, J.; Cheong, J. Oleic acid inhibits hepatic insulin signaling through deregulation of STAT3 activation and C/EBPalpha expression. Cell. Signal. 2009, 21, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Chen, P.H.; Yen, J.H. Inhibiting adipose tissue M1 cytokine expression decreases DPP4 activity and insulin resistance in a type 2 diabetes mellitus mouse model. PLoS ONE 2021, 16, e0252153. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Park, J.; Sun, J.L.; Ahn, S.H.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Kim, H.C.; Shim, J.H.; Shin, S.; Jeong, J.H. Administration of kynurenic acid reduces hyperlipidemia-induced inflammation and insulin resistance in skeletal muscle and adipocytes. Mol. Cell. Endocrinol. 2020, 518, 110928. [Google Scholar] [CrossRef]
- Dalmas, E.; Rouault, C.; Abdennour, M.; Rovere, C.; Rizkalla, S.; Bar-Hen, A.; Nahon, J.L.; Bouillot, J.L.; Guerre-Millo, M.; Clément, K.; et al. Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction. Am. J. Clin. Nutr. 2011, 94, 450–458. [Google Scholar] [CrossRef]
- Burgos-Ramos, E.; Canelles, S.; Rodríguez, A.; Frago, L.M.; Gómez-Ambrosi, J.; Chowen, J.A.; Frühbeck, G.; Argente, J.; Barrios, V. The increase in fiber size in male rat gastrocnemius after chronic central leptin infusion is related to activation of insulin signaling. Mol. Cell. Endocrinol. 2018, 470, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Perianes-Cachero, A.; Burgos-Ramos, E.; Puebla-Jiménez, L.; Canelles, S.; Viveros, M.P.; Mela, V.; Chowen, J.A.; Argente, J.; Arilla-Ferreiro, E.; Barrios, V. Leptin-induced downregulation of the rat hippocampal somatostatinergic system may potentiate its anorexigenic effects. Neurochem. Int. 2012, 61, 1385–1396. [Google Scholar] [CrossRef]
- García-Cáceres, C.; Fuente-Martín, E.; Burgos-Ramos, E.; Granado, M.; Frago, L.M.; Barrios, V.; Horvath, T.; Argente, J.; Chowen, J.A. Differential acute and chronic effects of leptin on hypothalamic astrocyte morphology and synaptic protein levels. Endocrinology 2011, 152, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Smith, M.S.; Reda, D.; Suffredini, A.F.; McCoy, J.P., Jr. Multiplex bead array assays for detection of soluble cytokines: Comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin. Cytom. 2004, 61, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Ramos, E.; Sackmann-Sala, L.; Baquedano, E.; Cruz-Topete, D.; Barrios, V.; Argente, J.; Kopchick, J.J. Central leptin and insulin administration modulates serum cytokine- and lipoprotein-related markers. Metabolism 2012, 61, 1646–1657. [Google Scholar] [CrossRef]
- Burgos-Ramos, E.; Canelles, S.; Rodríguez, A.; Gómez-Ambrosi, J.; Frago, L.M.; Chowen, J.A.; Frühbeck, G.; Argente, J.; Barrios, V. Chronic central leptin infusion modulates the glycemia response to insulin administration in male rats through regulation of hepatic glucose metabolism. Mol. Cell. Endocrinol. 2015, 415, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, Z.; Yin, J.; Quon, M.J.; Ye, J. S6K directly phosphorylates IRS-1 on Ser-270 to promote insulin resistance in response to TNF-(alpha) signaling through IKK2. J. Biol. Chem. 2008, 283, 35375–35382. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.G.; Jin, S.W.; Kim, Y.A.; Khanal, T.; Lee, G.H.; Kim, S.J.; Rhee, S.D.; Chung, Y.C.; Hwang, Y.J.; Jeong, T.C.; et al. Leptin induces CREB-dependent aromatase activation through COX-2 expression in breast cancer cells. Food Chem. Toxicol. 2017, 106, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Bugler-Lamb, A.R.; Hasib, A.; Weng, X.; Hennayake, C.K.; Lin, C.; McCrimmon, R.J.; Stimson, R.H.; Ashford, M.L.J.; Wasserman, D.H.; Kang, L. Adipocyte integrin-linked kinase plays a key role in the development of diet-induced adipose insulin resistance in male mice. Mol. Metab. 2021, 49, 101197. [Google Scholar] [CrossRef]
- Ceddia, R.B.; Koistinen, H.A.; Zierath, J.R.; Sweeney, G. Analysis of paradoxical observations on the association between leptin and insulin resistance. FASEB J. 2002, 16, 1163–1176. [Google Scholar] [CrossRef]
- Dreyer, M.G.; Juge-Aubry, C.E.; Gabay, C.; Lang, U.; Rohner-Jeanrenaud, F.; Dayer, J.M.; Meier, C.A. Leptin activates the promoter of the interleukin-1 receptor antagonist through p42/44 mitogen-activated protein kinase and a composite nuclear factor kappaB/PU.1. binding site. Biochem. J. 2003, 370, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Souza-Almeida, G.; Palhinha, L.; Liechocki, S.; da Silva Pereira, J.A.; Reis, P.A.; Dib, P.R.B.; Hottz, E.D.; Gameiro, J.; Vallochi, A.L.; de Almeida, C.J.; et al. Peripheral leptin signaling persists in innate immune cells during diet-induced obesity. J. Leukoc. Biol. 2021, 109, 1131–1138. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Metlakunta, A.; Huang, W.; Stefanovic-Racic, M.; Dedousis, N.; Sipula, I.; O’Doherty, R.M. Kupffer cells facilitate the acute effects of leptin on hepatic lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E11–E18. [Google Scholar] [CrossRef] [Green Version]
- de Meijer, V.E.; Sverdlov, D.Y.; Le, H.D.; Popov, Y.; Puder, M. Tissue-specific differences in inflammatory infiltrate and matrix metalloproteinase expression in adipose tissue and liver of mice with diet-induced obesity. Hepatol. Res. 2012, 42, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Chand, A.; Gough, D.; Ernst, M. Therapeutically exploiting STAT3 activity in cancer—Using tissue repair as a road map. Nat. Rev. Cancer 2019, 19, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Balic, J.J.; Albargy, H.; Luu, K.; Kirby, F.J.; Jayasekara, W.S.N.; Mansell, F.; Garama, D.J.; De Nardo, D.; Baschuk, N.; Louis, C.; et al. STAT3 serine phosphorylation is required for TLR4 metabolic reprogramming and IL-1β expression. Nat. Commun. 2020, 11, 3816. [Google Scholar] [CrossRef]
- Margină, D.; Ungurianu, A.; Purdel, C.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Tekos, F.; Mesnage, R.; Kouretas, D.; Tsatsakis, A. Chronic inflammation in the context of everyday life: Dietary changes as mitigating factors. Int. J. Environ. Res. Public Health 2020, 17, 4135. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations (*). Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Gang, X.; Yang, S.; Cui, M.; Sun, L.; Li, Z.; Wang, G. The alterations in and the role of the Th17/Treg balance in metabolic diseases. Front. Immunol. 2021, 12, 678355. [Google Scholar] [CrossRef]
- Soutto, M.; Bhat, N.; Khalafi, S.; Zhu, S.; Poveda, J.; Garcia-Buitrago, M.; Zaika, A.; El-Rifai, W. NF-kB-dependent activation of STAT3 by H. pylori is suppressed by TFF1. Cancer Cell Int. 2021, 21, 444. [Google Scholar] [CrossRef] [PubMed]
- Benomar, Y.; Wetzler, S.; Larue-Achagiotis, C.; Djiane, J.; Tomé, D.; Taouis, M. In vivo leptin infusion impairs insulin and leptin signalling in liver and hypothalamus. Mol. Cell. Endocrinol. 2005, 242, 59–66. [Google Scholar] [CrossRef]
- Perry, R.J.; Camporez, J.G.; Kursawe, R.; Titchenell, P.M.; Zhang, D.; Perry, C.J.; Jurczak, M.J.; Abudukadier, A.; Han, M.S.; Zhang, X.M.; et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 2015, 160, 745–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, A.; Fernández, Y.; Fernández, R.; Ordóñez, P.; Moreno, M.; Díaz, F.; Patterson, A.M.; González, C. Effect of food restriction on the insulin signalling pathway in rat skeletal muscle and adipose tissue. J. Nutr. Biochem. 2005, 16, 602–609. [Google Scholar] [CrossRef]
- Kobashi, C.; Asamizu, S.; Ishiki, M.; Iwata, M.; Usui, I.; Yamazaki, K.; Tobe, K.; Kobayashi, M.; Urakaze, M. Inhibitory effect of IL-8 on insulin action in human adipocytes via MAP kinase pathway. J. Inflamm. (Lond). 2009, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, C.; Zhao, J.; Guo, C. JNK downregulation improves olanzapine-induced insulin resistance by suppressing IRS1(Ser307) phosphorylation and reducing inflammation. Biomed. Pharmacother. 2021, 142, 112071. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoon, M.S.; Chen, J. Signal transducer and activator of transcription 3 (STAT3) mediates amino acid inhibition of insulin signaling through serine 727 phosphorylation. J. Biol. Chem. 2009, 284, 35425–35432. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Qian, D.W.; Jiang, S.; Shang, E.X.; Zhu, Z.H.; Duan, J.A. Scutellariae radix and coptidis rhizoma improve glucose and lipid metabolism in T2DM rats via regulation of the metabolic profiling and MAPK/PI3K/Akt signaling pathway. Int. J. Mol. Sci. 2018, 19, 3634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weichhart, T.; Säemann, M.D. The PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic applications. Ann. Rheum. Dis. 2008, 67 (Suppl 3), 70–74. [Google Scholar] [CrossRef] [PubMed]
- Ao, N.; Ma, Z.; Yang, J.; Jin, S.; Zhang, K.; Luo, E.; Du, J. Liraglutide ameliorates lipotoxicity-induced inflammation through the mTORC1 signalling pathway. Peptides 2020, 133, 170375. [Google Scholar] [CrossRef]
- Chen, L.; Gao, B.; Zhang, Y.; Lu, H.; Li, X.; Pan, L.; Yin, L.; Zhi, X. PAR2 promotes M1 macrophage polarization and inflammation via FOXO1 pathway. J. Cell. Biochem. 2019, 120, 9799–9809. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Inoue, M.; Kubota, N.; Takamoto, I.; Mineyama, T.; Iwayama, K.; Tokuyama, K.; Moroi, M.; Ueki, K.; Yamauchi, T.; et al. Downregulation of macrophage Irs2 by hyperinsulinemia impairs IL-4-indeuced M2a-subtype macrophage activation in obesity. Nat. Commun. 2018, 9, 4863. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Accili, D. All roads lead to FoxO. Cell Metab. 2005, 1, 215–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassouf, T.; Sumara, G. Impact of conventional and atypical MAPKs on the development of metabolic diseases. Biomolecules 2020, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Higginbotham, D.A.; Judd, R.L.; White, B.D. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1084–E1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonzón-Kulichenko, E.; Fernández-Agulló, T.; Moltó, E.; Serrano, R.; Fernández, A.; Ros, M.; Carrascosa, J.M.; Arribas, C.; Martínez, C.; Andrés, A.; et al. Regulation of insulin-stimulated glucose uptake in rat white adipose tissue upon chronic central leptin infusion: Effects on adiposity. Endocrinology 2011, 152, 1366–1377. [Google Scholar] [CrossRef]
- Sahu, A.; Metlakunta, A.S. Hypothalamic phosphatidylinositol 3-kinase-phosphodiesterase 3B-cyclic AMP pathway of leptin signalling is impaired following chronic central leptin infusion. J. Neuroendocrinol. 2005, 17, 720–726. [Google Scholar] [CrossRef]
- Sahu, A. Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. J. Neuroendocrinol. 2002, 14, 796–804. [Google Scholar] [CrossRef]
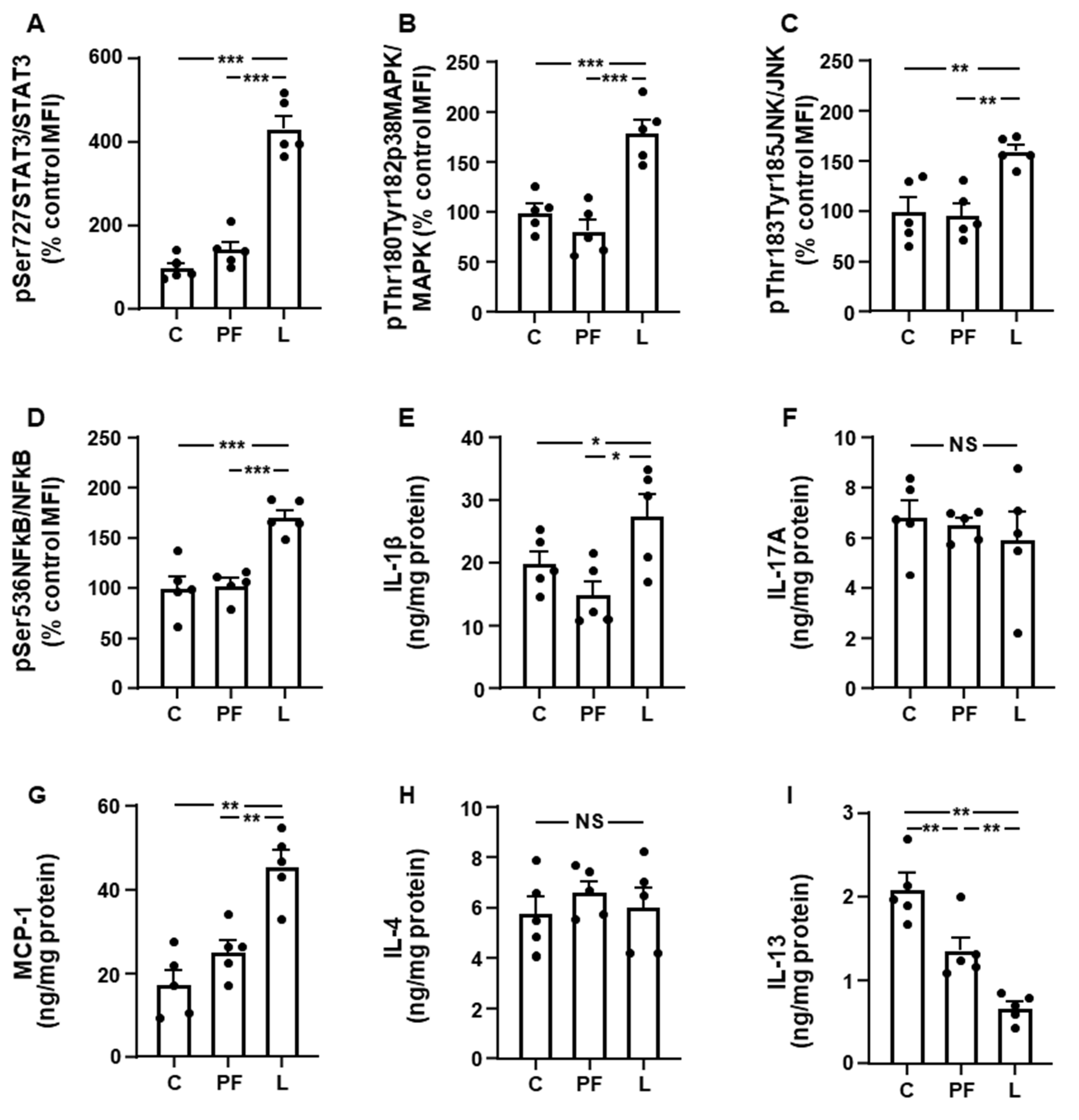
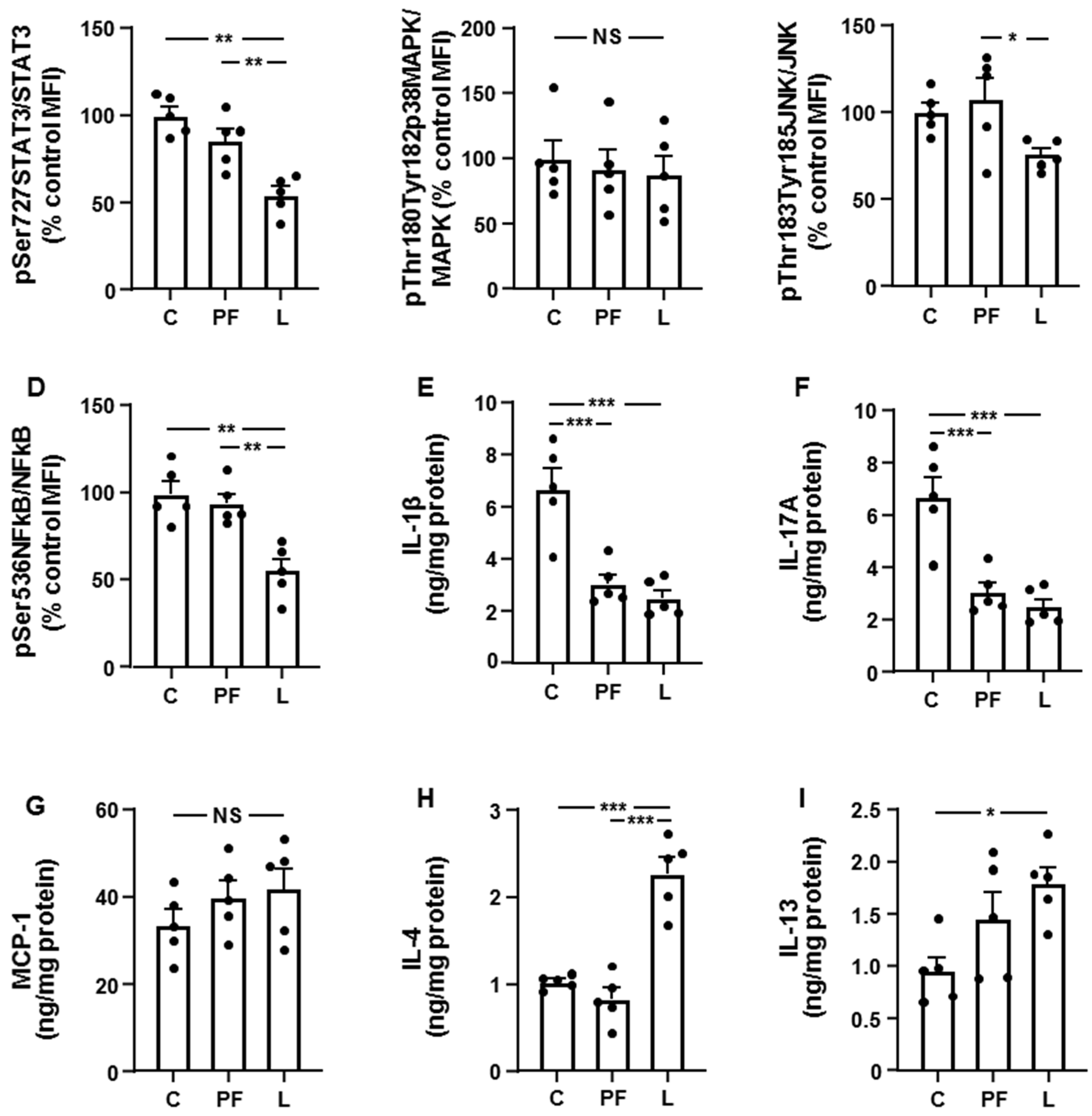
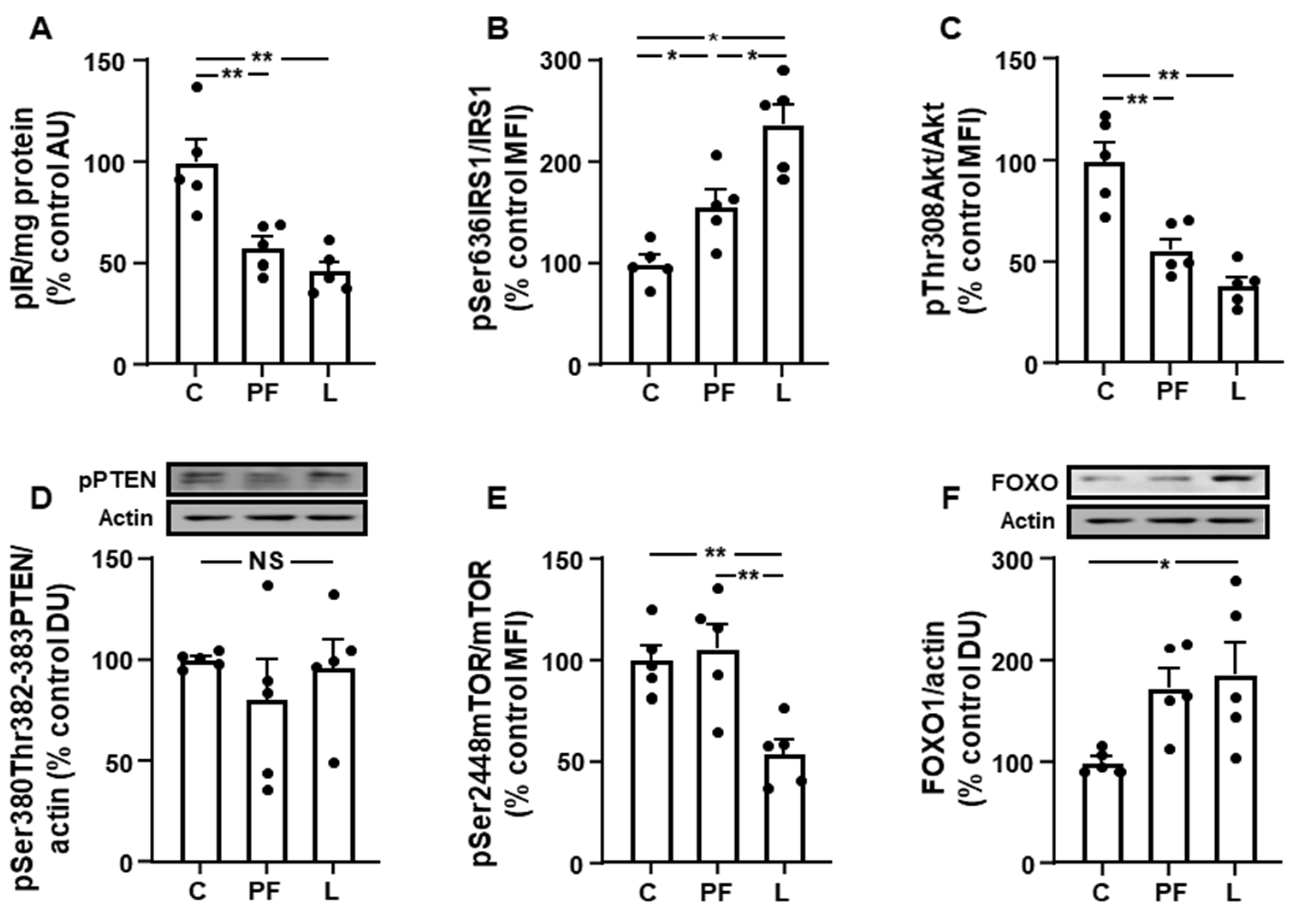
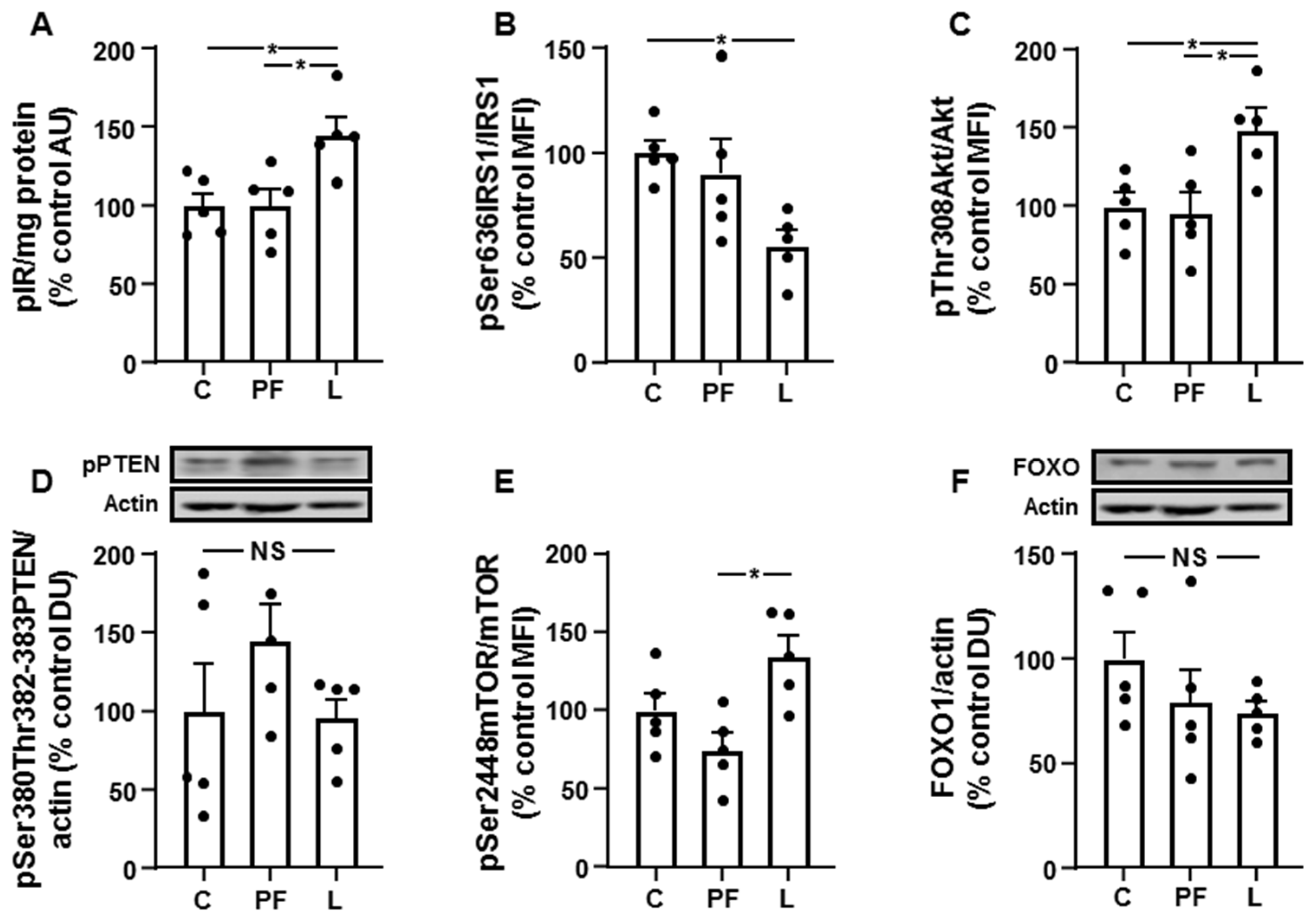
| Parameter | Control | Pair-Fed | Leptin |
|---|---|---|---|
| Inguinal fat weight (%) | 1.02 ± 0.08 | 0.94 ± 0.09 | 0.64 ± 0.08 ** ## |
| Liver weight (%) | 3.62 ± 0.23 | 3.82 ± 0.13 | 3.99 ± 0.10 |
| Serum IL-1β (pg/mL) | 25.56 ± 2.63 | 17.63 ± 2.40 | 39.32 ± 4.77 * # |
| Serum IL-17A (pg/mL) | 8.95 ± 1.55 | 7.51 ± 1.02 | 6.40 ± 0.78 |
| Serum MCP-1 (pg/mL) | 36.96 ± 3.82 | 31.48 ± 2.87 | 36.86 ± 3.67 |
| Serum IL-4 (pg/mL) | 3.25 ± 0.31 | 2.93 ± 0.60 | 11.09 ± 1.00 *** ### |
| Serum IL-13 (pg/mL) | 1.95 ± 0.18 | 3.13 ± 0.30 * | 5.65 ± 0.62 * # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrios, V.; Campillo-Calatayud, A.; Guerra-Cantera, S.; Canelles, S.; Martín-Rivada, Á.; Frago, L.M.; Chowen, J.A.; Argente, J. Opposite Effects of Chronic Central Leptin Infusion on Activation of Insulin Signaling Pathways in Adipose Tissue and Liver Are Related to Changes in the Inflammatory Environment. Biomolecules 2021, 11, 1734. https://doi.org/10.3390/biom11111734
Barrios V, Campillo-Calatayud A, Guerra-Cantera S, Canelles S, Martín-Rivada Á, Frago LM, Chowen JA, Argente J. Opposite Effects of Chronic Central Leptin Infusion on Activation of Insulin Signaling Pathways in Adipose Tissue and Liver Are Related to Changes in the Inflammatory Environment. Biomolecules. 2021; 11(11):1734. https://doi.org/10.3390/biom11111734
Chicago/Turabian StyleBarrios, Vicente, Ana Campillo-Calatayud, Santiago Guerra-Cantera, Sandra Canelles, Álvaro Martín-Rivada, Laura M. Frago, Julie A. Chowen, and Jesús Argente. 2021. "Opposite Effects of Chronic Central Leptin Infusion on Activation of Insulin Signaling Pathways in Adipose Tissue and Liver Are Related to Changes in the Inflammatory Environment" Biomolecules 11, no. 11: 1734. https://doi.org/10.3390/biom11111734
APA StyleBarrios, V., Campillo-Calatayud, A., Guerra-Cantera, S., Canelles, S., Martín-Rivada, Á., Frago, L. M., Chowen, J. A., & Argente, J. (2021). Opposite Effects of Chronic Central Leptin Infusion on Activation of Insulin Signaling Pathways in Adipose Tissue and Liver Are Related to Changes in the Inflammatory Environment. Biomolecules, 11(11), 1734. https://doi.org/10.3390/biom11111734






