The Effect of Mycotoxins and Silymarin on Liver Lipidome of Mice with Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Chemicals
2.2. Animals and In Vivo Study Design
2.3. Laboratory Analyses
2.4. Liver Histology
2.5. Sample Preparation for Lipidomic Analyses
2.6. Lipidomic U-HPLC-HRMS/MS Analysis
2.7. Lipid Identification and Statistical Analyses
3. Results
3.1. The Effect of Mycotoxin Exposure and Silymarin Treatment on Clinical and Laboratory Characteristics of Mice Fed High-Fat MCD Diet
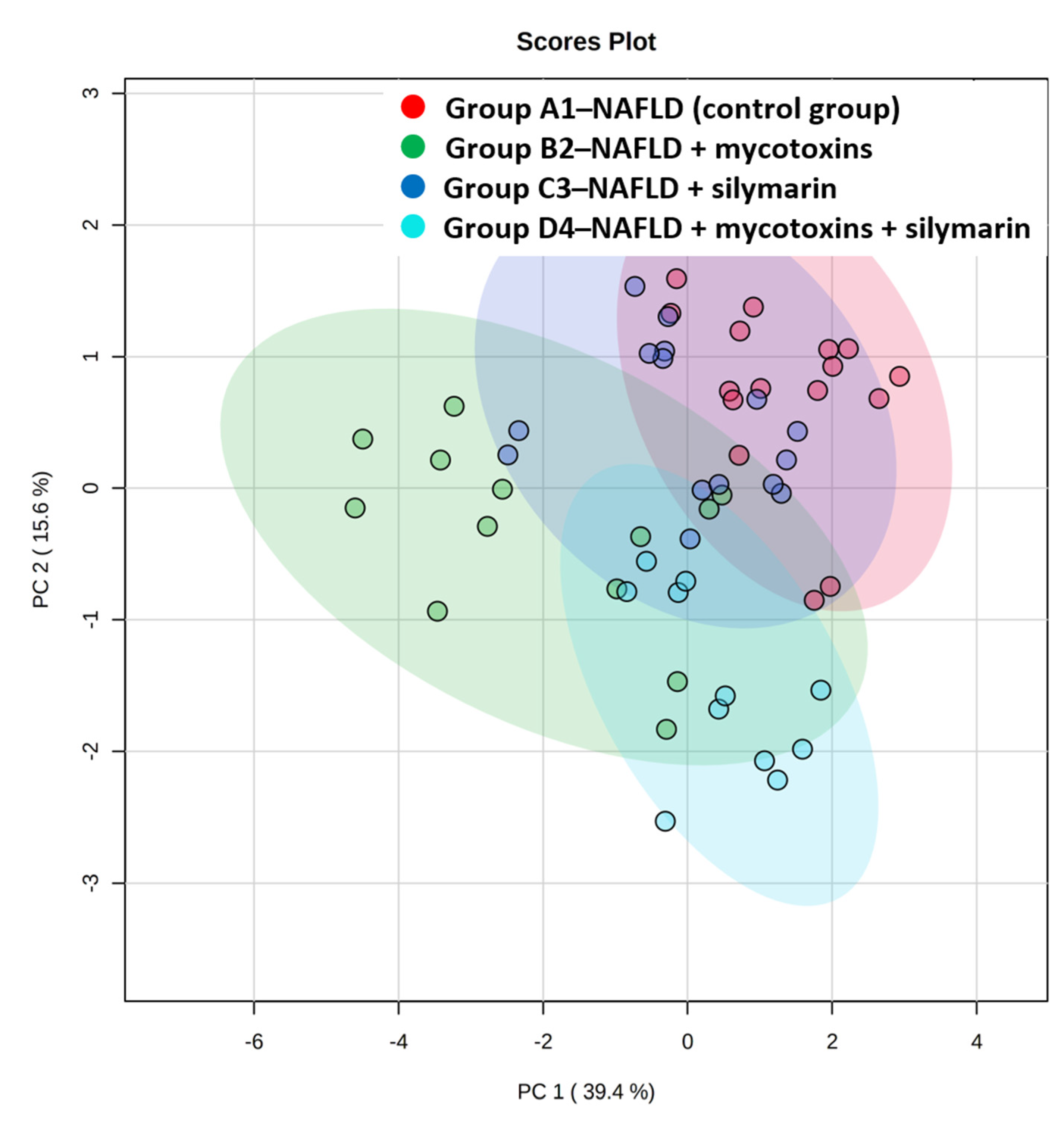
3.2. Liver Lipidome Analysis
4. Discussion
5. Conclusions
- ▪
- Exposure to mycotoxins and silymarin, frequently occurring together in the dietary supplements intended for liver disease treatment, plays a significant role in the lipid metabolic response, which occurs earlier than the clinical manifestation.
- ▪
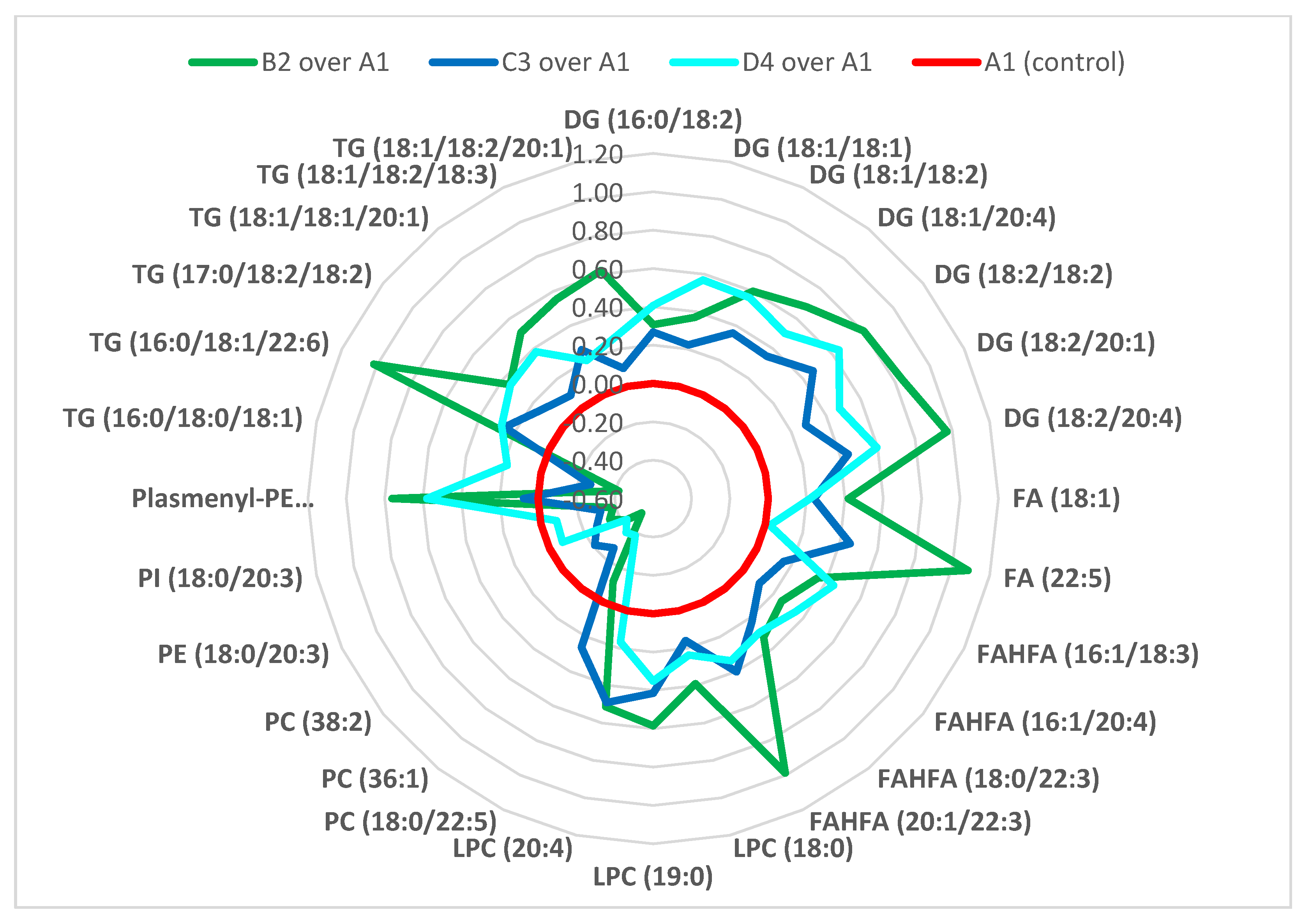
- The majority of fatty acids present in lipid classes differentiating the experimental groups, i.e., groups of mice exposed to mycotoxins, mycotoxins + silymarin, and silymarin, were (poly)unsaturated fatty acids of 18 carbons and more, in particular C18: 1, C18: 2, C18: 3, C20: 4, C22: 3, C22: 5 and C22: 6.
- ▪
- The administration of silymarin and/or mycotoxins led to changed metabolic regulation of TG, DGs, FAs, FAHFAs, LPCs, PCs, PIs, PEs, plasmenyl-PEs and ceramides, where ceramides, formation of which is closely associated with the oxidative stress, was the only lipid group unequivocally downregulated in silymarin administered groups, and upregulated in the groups where mycotoxins were fed.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fenclova, M.; Novakova, A.; Viktorova, J.; Jonatova, P.; Dzuman, Z.; Ruml, T.; Kren, V.; Hajslova, J.; Vitek, L.; Stranska-Zachariasova, M. Poor Chemical and Microbiological Quality of the Commercial Milk Thistle-Based Dietary Supplements May Account for Their Reported Unsatisfactory and Non-Reproducible Clinical Outcomes. Sci. Rep. 2019, 9, 11118. [Google Scholar] [CrossRef]
- Peraica, M.; Radić, B.; Lucić, A.; Pavlović, M. Toxic Effects of Mycotoxins in Humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar] [PubMed]
- Ahmed Adam, M.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of Different Mycotoxins on Humans, Cell Genome and Their Involvement in Cancer (Review). Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prosperini, A.; Berrada, H.; Ruiz, M.J.; Caloni, F.; Coccini, T.; Spicer, L.J.; Perego, M.C.; Lafranconi, A. A Review of the Mycotoxin Enniatin B. Front. Public Health 2017, 5, 304. [Google Scholar] [CrossRef]
- Tralamazza, S.M.; Piacentini, K.C.; Iwase, C.H.T.; Rocha, L. de O. Toxigenic Alternaria Species: Impact in Cereals Worldwide. Curr. Opin. Food Sci. 2018, 23, 57–63. [Google Scholar] [CrossRef]
- Marín, S.; Cano-Sancho, G.; Sanchis, V.; Ramos, A.J. The Role of Mycotoxins in the Human Exposome: Application of Mycotoxin Biomarkers in Exposome-Health Studies. Food Chem. Toxicol. 2018, 121, 504–518. [Google Scholar] [CrossRef] [Green Version]
- Belser-Ehrlich, S.; Harper, A.; Hussey, J.; Hallock, R. Human and Cattle Ergotism since 1900: Symptoms, Outbreaks, and Regulations. Toxicol. Ind. Health 2013, 29, 307–316. [Google Scholar] [CrossRef]
- Krishnamachari, K.A.; Bhat, R.V.; Nagarajan, V.; Tilak, T.B. Hepatitis Due to Aflatoxicosis. An Outbreak in Western India. Lancet 1975, 1, 1061–1063. [Google Scholar] [CrossRef]
- Meggs, W.J. Epidemics of Mold Poisoning Past and Present. Toxicol. Ind. Health 2009, 25, 571–576. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, Toxicology, and Exposure Assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Eshelli, M.; Qader, M.M.; Jambi, E.J.; Hursthouse, A.S.; Rateb, M.E. Current Status and Future Opportunities of Omics Tools in Mycotoxin Research. Toxins 2018, 10, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Yan, T.; Wang, J.; Huang, Z.; Chen, X.; Jia, G.; Wu, C.; Zhao, H.; Xue, B.; Xiao, L.; et al. Biological System Responses to Zearalenone Mycotoxin Exposure by Integrated Metabolomic Studies. J. Agric. Food Chem. 2013, 61, 11212–11221. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhu, P.; Cui, F.; Pi, F.; Zhang, Y.; Li, Y.; Wang, J.; Sun, X. The Antagonistic Effect of Mycotoxins Deoxynivalenol and Zearalenone on Metabolic Profiling in Serum and Liver of Mice. Toxins 2017, 9, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.; Zhu, P.; Blaženović, I.; Cui, F.; Gholami, M.; Sun, J.; Habimana, J.; Zhang, Y.; Sun, X. Explaining Combinatorial Effects of Mycotoxins Deoxynivalenol and Zearalenone in Mice with Urinary Metabolomic Profiling. Sci. Rep. 2018, 8, 3762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Balogh, K.; Bázár, G.; Kocsó, D.; Ali, O.; Kovács, M. Individual and Combined Effects of Fumonisin B1, Deoxynivalenol and Zearalenone on the Hepatic and Renal Membrane Lipid Integrity of Rats. Toxins 2017, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.-C.; Hymery, N.; Troadec, S.; Pawtowski, A.; Coton, E.; Madec, S. Hepatotoxicity of Fusariotoxins, Alone and in Combination, towards the HepaRG Human Hepatocyte Cell Line. Food Chem. Toxicol. 2017, 109, 439–451. [Google Scholar] [CrossRef]
- Grenier, B.; Oswald, I. Mycotoxin Co-Contamination of Food and Feed: Meta-Analysis of Publications Describing Toxicological Interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New Insights into Mycotoxin Mixtures: The Toxicity of Low Doses of Type B Trichothecenes on Intestinal Epithelial Cells Is Synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef]
- Veprikova, Z.; Zachariasova, M.; Dzuman, Z.; Zachariasova, A.; Fenclova, M.; Slavikova, P.; Vaclavikova, M.; Mastovska, K.; Hengst, D.; Hajslova, J. Mycotoxins in Plant-Based Dietary Supplements: Hidden Health Risk for Consumers. J. Agric. Food Chem. 2015, 63, 6633–6643. [Google Scholar] [CrossRef]
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The Silymarin Composition… and Why Does It Matter??? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef]
- Khatoon, A.; Khan, M.Z.; Khan, A.; Saleemi, M.K.; Javed, I. Amelioration of Ochratoxin A-Induced Immunotoxic Effects by Silymarin and Vitamin E in White Leghorn Cockerels. J. Immunotoxicol. 2013, 10, 25–31. [Google Scholar] [CrossRef]
- Sozmen, M.; Devrim, A.K.; Tunca, R.; Bayezit, M.; Dag, S.; Essiz, D. Protective Effects of Silymarin on Fumonisin B1-Induced Hepatotoxicity in Mice. J. Vet. Sci. 2014, 15, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Naseer, O.; Khan, J.A.; Khan, M.S.; Omer, M.O.; Chishti, G.A.; Sohail, M.L.; Saleem, M.U. Comparative Efficacy of Silymarin and Choline Chloride (Liver Tonics) in Preventing the Effects of Aflatoxin B1 in Bovine Calves. Pol. J. Vet. Sci. 2016, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaleghipour, B.; Khosravinia, H.; Toghiyani, M.; Azarfar, A. Efficacy of Silymarin-Nanohydrogle Complex in Attenuation of Aflatoxins Toxicity in Japanese Quails. Ital. J. Anim. Sci. 2020, 19, 351–359. [Google Scholar] [CrossRef] [Green Version]
- El-Sheshtawy, S.M.; El-Zoghby, A.F.; Shawky, N.A.; Samak, D.H. Aflatoxicosis in Pekin Duckling and the Effects of Treatments with Lycopene and Silymarin. Vet. World 2021, 14, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Egresi, A.; Süle, K.; Szentmihályi, K.; Blázovics, A.; Fehér, E.; Hagymási, K.; Fébel, H. Impact of Milk Thistle (Silybum Marianum) on the Mycotoxin Caused Redox-Homeostasis Imbalance of Ducks Liver. Toxicon 2020, 187, 181–187. [Google Scholar] [CrossRef]
- Raj, J.; Vasiljević, M.; Tassis, P.; Farkaš, H.; Männer, K. Efficacy of a Multicomponent Mycotoxin Detoxifying Agent on Concurrent Exposure to Zearalenone and T-2 Mycotoxin in Weaned Pigs. Livest. Sci. 2020, 242, 104295. [Google Scholar] [CrossRef]
- Viktorova, J.; Stranska-Zachariasova, M.; Fenclova, M.; Vitek, L.; Hajslova, J.; Kren, V.; Ruml, T. Complex Evaluation of Antioxidant Capacity of Milk Thistle Dietary Supplements. Antioxidants 2019, 8, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šuk, J.; Jašprová, J.; Biedermann, D.; Petrásková, L.; Valentová, K.; Křen, V.; Muchová, L.; Vítek, L. Isolated Silymarin Flavonoids Increase Systemic and Hepatic Bilirubin Concentrations and Lower Lipoperoxidation in Mice. Oxid. Med. Cell. Longev. 2019, 2019, 6026902. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Koelmel, J.P.; Kroeger, N.M.; Ulmer, C.Z.; Bowden, J.A.; Patterson, R.E.; Cochran, J.A.; Beecher, C.W.W.; Garrett, T.J.; Yost, R.A. LipidMatch: An Automated Workflow for Rule-Based Lipid Identification Using Untargeted High-Resolution Tandem Mass Spectrometry Data. BMC Bioinform. 2017, 18, 331. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Pierantonelli, I.; Torquato, P.; Marinelli, R.; Ferreri, C.; Chatgilialoglu, C.; Bartolini, D.; Galli, F. Lipidomic Biomarkers and Mechanisms of Lipotoxicity in Non-Alcoholic Fatty Liver Disease. Free Radic. Biol. Med. 2019, 144, 293–309. [Google Scholar] [CrossRef]
- Smith, T.; Kawa, K.; Eckl, V.; Morton, C.; Stredney, R. Herbal Supplement Sales in US Increase 7.7% in 2016 Consumer Preferences Shifing toward Ingredients with General Wellness Benefits, Driving Growth of Adaptogens and Digestive Health Products. HerbalGram 2017, 115, 56–65. [Google Scholar]
- Féher, J.; Lengyel, G. Silymarin in the Prevention and Treatment of Liver Diseases and Primary Liver Cancer. Curr. Pharm. Biotechnol. 2012, 13, 210–217. [Google Scholar] [CrossRef]
- Zhong, S.; Fan, Y.; Yan, Q.; Fan, X.; Wu, B.; Han, Y.; Zhang, Y.; Chen, Y.; Zhang, H.; Niu, J. The Therapeutic Effect of Silymarin in the Treatment of Nonalcoholic Fatty Disease: A Meta-Analysis (PRISMA) of Randomized Control Trials. Medicine 2017, 96, e9061. [Google Scholar] [CrossRef]
- Ferenci, P.; Scherzer, T.; Kerschner, H.; Rutter, K.; Beinhardt, S.; Hofer, H.; Schöniger–Hekele, M.; Holzmann, H.; Steindl–Munda, P. Silibinin Is a Potent Antiviral Agent in Patients with Chronic Hepatitis C Not Responding to Pegylated Interferon/Ribavirin Therapy. Gastroenterology 2008, 135, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Seeff, L.B.; Bonkovsky, H.L.; Navarro, V.J.; Wang, G. Herbal Products and the Liver: A Review of Adverse Effects and Mechanisms. Gastroenterology 2015, 148, 517–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Avelar, C.R.; Pereira, E.M.; de Farias Costa, P.R.; de Jesus, R.P.; de Oliveira, L.P.M. Effect of Silymarin on Biochemical Indicators in Patients with Liver Disease: Systematic Review with Meta-Analysis. World J. Gastroenterol. 2017, 23, 5004–5017. [Google Scholar] [CrossRef]
- Fried, M.W.; Navarro, V.J.; Afdhal, N.; Belle, S.H.; Wahed, A.S.; Hawke, R.L.; Doo, E.; Meyers, C.M.; Reddy, K.R. Silymarin in NASH and C Hepatitis (SyNCH) Study Group Effect of Silymarin (Milk Thistle) on Liver Disease in Patients with Chronic Hepatitis C Unsuccessfully Treated with Interferon Therapy: A Randomized Controlled Trial. JAMA 2012, 308, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhuang, L.; Lu, Y.; Xu, Q.; Chen, X. Effects and Tolerance of Silymarin (Milk Thistle) in Chronic Hepatitis C Virus Infection Patients: A Meta-Analysis of Randomized Controlled Trials. Biomed. Res. Int. 2014, 2014, 941085. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lopez, S.; Hayward, D.G.; Park, H.Y.; Wong, J.W.; Kim, S.S.; Wan, J.; Reddy, R.M.; Quinn, D.J.; Steiniger, D. Determination of Multiresidue Pesticides in Botanical Dietary Supplements Using Gas Chromatography–Triple-Quadrupole Mass Spectrometry (GC-MS/MS). J. Agric. Food Chem. 2016, 64, 6125–6132. [Google Scholar] [CrossRef]
- Nardin, T.; Piasentier, E.; Barnaba, C.; Larcher, R. Targeted and Untargeted Profiling of Alkaloids in Herbal Extracts Using Online Solid-Phase Extraction and High-Resolution Mass Spectrometry (Q-Orbitrap). J. Mass Spectrom. 2016, 51, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Tournas, V.H.; Rivera Calo, J.; Sapp, C. Fungal Profiles in Various Milk Thistle Botanicals from US Retail. Int. J. Food Microbiol. 2013, 164, 87–91. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Multiclass Mycotoxin Analysis in Silybum Marianum by Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry Using a Procedure Based on QuEChERS and Dispersive Liquid-Liquid Microextraction. J. Chromatogr. A 2013, 1282, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tournas, V.H.; Sapp, C.; Trucksess, M.W. Occurrence of Aflatoxins in Milk Thistle Herbal Supplements. Food Addit. Contam. Part A 2012, 29, 994–999. [Google Scholar] [CrossRef]
- Gorden, D.L.; Myers, D.S.; Ivanova, P.T.; Fahy, E.; Maurya, M.R.; Gupta, S.; Min, J.; Spann, N.J.; McDonald, J.G.; Kelly, S.L.; et al. Biomarkers of NAFLD Progression: A Lipidomics Approach to an Epidemic1. J. Lipid Res. 2015, 56, 722–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayo, R.; Crespo, J.; Martínez-Arranz, I.; Banales, J.M.; Arias, M.; Mincholé, I.; Aller de la Fuente, R.; Jimenez-Agüero, R.; Alonso, C.; de Luis, D.A.; et al. Metabolomic-based Noninvasive Serum Test to Diagnose Nonalcoholic Steatohepatitis: Results from Discovery and Validation Cohorts. Hepatol. Commun. 2018, 2, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Mato, J.; Alonso, C.; Noureddin, M.; Lu, S. Biomarkers and Subtypes of Deranged Lipid Metabolism in Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2019, 25, 3009–3020. [Google Scholar] [CrossRef]
- Gorden, D.L.; Ivanova, P.T.; Myers, D.S.; McIntyre, J.O.; VanSaun, M.N.; Wright, J.K.; Matrisian, L.M.; Brown, H.A. Increased Diacylglycerols Characterize Hepatic Lipid Changes in Progression of Human Nonalcoholic Fatty Liver Disease; Comparison to a Murine Model. PLoS ONE 2011, 6, e22775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.W.L.; Arendt, B.M.; Hillyer, L.M.; Fung, S.K.; McGilvray, I.; Guindi, M.; Allard, J.P. Plasma Phospholipids and Fatty Acid Composition Differ between Liver Biopsy-Proven Nonalcoholic Fatty Liver Disease and Healthy Subjects. Nutr. Diabetes 2016, 6, e220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjani, K.; Lhomme, M.; Sokolovska, N.; Poitou, C.; Aron-Wisnewsky, J.; Bouillot, J.-L.; Lesnik, P.; Bedossa, P.; Kontush, A.; Clement, K.; et al. Circulating Phospholipid Profiling Identifies Portal Contribution to NASH Signature in Obesity. J. Hepatol. 2015, 62, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Birerdinc, A.; Younossi, Z. Can NASH Lipidome Provide Insight into the Pathogenesis of Obesity-Related Non-Alcoholic Fatty Liver Disease? J. Hepatol. 2015, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montefusco, D.J.; Allegood, J.C.; Spiegel, S.; Cowart, L.A. Non-Alcoholic Fatty Liver Disease: Insights from Sphingolipidomics. Biochem. Biophys. Res. Commun. 2018, 504, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Nikolova-Karakashian, M. Sphingolipids at the Crossroads of NAFLD and Senescence. Adv. Cancer Res. 2018, 140, 155–190. [Google Scholar] [CrossRef] [PubMed]
- Bony, S.; Carcelen, M.; Olivier, L.; Devaux, A. Genotoxicity Assessment of Deoxynivalenol in the Caco-2 Cell Line Model Using the Comet Assay. Toxicol. Lett. 2006, 166, 67–76. [Google Scholar] [CrossRef]
- Da Silva, E.O.; Bracarense, A.P.; Oswald, I.P. Mycotoxins and Oxidative Stress: Where Are We? World Mycotoxin J. 2018, 11, 113–134. [Google Scholar] [CrossRef]
- Wu, Q.-H.; Wang, X.; Yang, W.; Nüssler, A.K.; Xiong, L.-Y.; Kuča, K.; Dohnal, V.; Zhang, X.-J.; Yuan, Z.-H. Oxidative Stress-Mediated Cytotoxicity and Metabolism of T-2 Toxin and Deoxynivalenol in Animals and Humans: An Update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef]
- Ferrer, E.; Juan-García, A.; Font, G.; Ruiz, M.J. Reactive Oxygen Species Induced by Beauvericin, Patulin and Zearalenone in CHO-K1 Cells. Toxicol. Vitro 2009, 23, 1504–1509. [Google Scholar] [CrossRef]
| Administered Compound | mg/kg b.w. and Day | |
|---|---|---|
| Mycotoxins | AOH | 0.16 |
| AME | 0.06 | |
| DON | 0.15 | |
| ZEA | 0.01 | |
| T-2 | 0.14 | |
| HT-2 | 0.07 | |
| DAS | 0.001 | |
| TEN | 0.05 | |
| BEA | 0.09 | |
| ENN-A | 0.02 | |
| ENN-A1 | 0.03 | |
| ENN-B | 0.07 | |
| ENN-B1 | 0.04 | |
| silymarin | 30 | |
| Group A1 (Control) | Group B2 (Mycotoxins) | Group C3 (Silymarin) | Group D4 (Mycotoxins + Silymarin) | p-Value * | |
|---|---|---|---|---|---|
| Body weight (g) | 31.5 ± 3.2 | 29.7 ± 2.4 | 30 ± 4 | 31.9 ± 2.5 | NS |
| Liver weight (g) | 1.58 ± 0.19 | 1.3 ± 0.5 | 1.33 ± 0.2 | 1.48 ± 0.1 | NS |
| ALT (U/L) | 28 (23–41) | 22 (15–35) | 36 (17–46) | 34 (32–43) | NS |
| AST (U/L) | 90 (64–127) | 58 (48–83) | 66 (56–73) | 71 (67–76) | NS |
| ALP (U/L) | 85 ± 7.9 | 76 ± 9.6 | 83 ± 7 | 80 ± 12 | NS |
| LD (U/L) | 249 ± 76 | 220 ± 29 | 282 ± 114 | 251 ± 45 | NS |
| Total cholesterol (mmol/L) | 3 ± 0.5 | 2.5 ± 0.6 | 2.8 ± 0.4 | 3 ± 0.4 | NS |
| LDL cholesterol (mmol/L) | 0.64 ± 0.2 | 0.49 ± 0.2 | 0.56 ± 0.1 | 0.7 ± 0.1 | NS |
| HDL cholesterol (mmol/L) | 2.15 ± 0.3 | 1.89 ± 0.3 | 2.06 ± 0.3 | 2.3 ± 0.1 | NS |
| Triacylglycerols (mmol/L) | 0.62 ± 0.1 | 0.53 ± 0.1 | 0.59 ± 0.1 | 0.57 ± 0.1 | NS |
| Glucose (mmol/L) | 11.3 ± 1.4 | 12.2 ± 1 | 11.5 ± 1.9 | 12.3 ± 0.9 | NS |
| Bilirubin in serum (μmol/L) | 2.3 ± 0.8 | 2.3 ± 0.7 | 2.3 ± 0.1 | 2.2 ± 0.6 | NS |
| Bilirubin in liver (μmol/g) | 1.01 (0.8–1.1) | 0.83 (0.7–2.2) | 0.63 (0.6–0.9) | 1.08 (0.7–1.2) | NS |
| Fold Change (FC) Expressed as log2(FC) | ||||
|---|---|---|---|---|
| Lipid Name | B2 over A1 | C3 over A1 | D4 over A1 | A1 (Control) |
| DG (16: 0/18: 2) | 0.31 * | 0.27 * | 0.41 * | 0 |
| DG (18: 1/18: 1) | 0.37 * | 0.22 | 0.57 * | 0 |
| DG (18: 1/18: 2) | 0.60 * | 0.36 * | 0.56 * | 0 |
| DG (18: 1/20: 4) | 0.68 * | 0.35 | 0.50 * | 0 |
| DG (18: 2/18: 2) | 0.80 * | 0.47 * | 0.64 * | 0 |
| DG (18: 2/20: 1) | 0.84 * | 0.28 | 0.48 * | 0 |
| DG (18: 2/20: 4) | 0.97 * | 0.44 * | 0.60 * | 0 |
| FA (18: 1) | 0.42 * | 0.24 | 0.22 | 0 |
| FA (22: 5) | 1.08 * | 0.45 | 0.02 | 0 |
| FAHFA (16: 1/18: 3) | 0.35 * | 0.16 | 0.44 * | 0 |
| FAHFA (16: 1/20: 4) | 0.26 * | 0.11 | 0.35 * | 0 |
| FAHFA (18: 0/22: 3) | 0.32 * | 0.22 * | 0.29 * | 0 |
| FAHFA (20: 1/22: 3) | 0.99 * | 0.40 | 0.34 | 0 |
| LPC (18: 0) | 0.39 * | 0.16 | 0.24 | 0 |
| LPC (19: 0) | 0.58 * | 0.41 * | 0.35 * | 0 |
| LPC (20: 4) | 0.51 * | 0.49 * | 0.17 | 0 |
| PC (18: 0/22: 5) | −0.12 | 0.26 | −0.39 * | 0 |
| PC (36: 1) | −0.50 * | −0.27 * | −0.37 * | 0 |
| PC (38: 2) | −0.42 * | −0.21 * | −0.43 * | 0 |
| PE (18: 0/20: 3) | −0.35 * | −0.27 * | −0.08 | 0 |
| PI (18: 0/20: 3) | −0.39 * | −0.32 * | −0.08 | 0 |
| plasmenyl-PE (18: 1/18: 1) | 0.76 * | 0.08 | 0.58 * | 0 |
| TG (16: 0/18: 0/18: 1) | −0.42 * | −0.27 * | 0.18 | 0 |
| TG (16: 0/18: 1/22: 6) | 1.02 * | 0.26 | 0.28 | 0 |
| TG (17: 0/18: 2/18: 2) | 0.35 * | 0.14 | 0.35 * | 0 |
| TG (18: 1/18: 1/20: 1) | 0.51 * | 0.09 | 0.38 * | 0 |
| TG (18: 1/18: 2/18: 3) | 0.56 * | 0.26 | 0.20 | 0 |
| TG (18: 1/18: 2/20: 1) | 0.62 * | 0.10 | 0.27 | 0 |
| Lipid Name | Correlation |
|---|---|
| TG (16: 0/18: 2/18: 3) | positive |
| TG (16: 0/18: 3/18: 3) | positive |
| TG (16: 0/18: 3/18: 3) a | positive |
| FAHFA (16: 1/20: 4 ) b | positive |
| plasmenyl-PE (18: 1/18: 1) a,b | positive |
| PC (34: 3) a | positive |
| PE (34: 3) | positive |
| PE (36: 3) | positive |
| PE (38: 5) | positive |
| PC (16: 0/22: 6) | negative |
| PC (18: 2/22: 6) | negative |
| PC (18: 0/22: 5) b | negative |
| PC (40: 8) | negative |
| PE (16: 0/22: 6) | negative |
| PE (18: 1/20: 4) | negative |
| Lipid Name | Correlation |
|---|---|
| Cer (18: 1/16: 0) | positive |
| Cer (18: 1/23: 0) | positive |
| Cer (18: 2/23: 0) | positive |
| Cer (25: 2/15: 0) | positive |
| TG (16: 0/18: 2/22: 6) | positive |
| TG (16: 0/18: 3/18: 3) a | positive |
| TG (18: 1/18: 1/20: 1) b | positive |
| TG (18: 1/18: 2/20: 1) b | positive |
| TG (18: 2/18: 2/18: 3) | positive |
| DG (18: 2/20: 1) b | positive |
| plasmenyl-PE (18: 1/18: 1) a,b | positive |
| plasmenyl-PE (18: 1/20: 1) | positive |
| PC (34: 3) a | positive |
| plasmenyl-PE (16: 0/22: 4) | negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bechynska, K.; Kosek, V.; Fenclova, M.; Muchova, L.; Smid, V.; Suk, J.; Chalupsky, K.; Sticova, E.; Hurkova, K.; Hajslova, J.; et al. The Effect of Mycotoxins and Silymarin on Liver Lipidome of Mice with Non-Alcoholic Fatty Liver Disease. Biomolecules 2021, 11, 1723. https://doi.org/10.3390/biom11111723
Bechynska K, Kosek V, Fenclova M, Muchova L, Smid V, Suk J, Chalupsky K, Sticova E, Hurkova K, Hajslova J, et al. The Effect of Mycotoxins and Silymarin on Liver Lipidome of Mice with Non-Alcoholic Fatty Liver Disease. Biomolecules. 2021; 11(11):1723. https://doi.org/10.3390/biom11111723
Chicago/Turabian StyleBechynska, Kamila, Vit Kosek, Marie Fenclova, Lucie Muchova, Vaclav Smid, Jakub Suk, Karel Chalupsky, Eva Sticova, Kamila Hurkova, Jana Hajslova, and et al. 2021. "The Effect of Mycotoxins and Silymarin on Liver Lipidome of Mice with Non-Alcoholic Fatty Liver Disease" Biomolecules 11, no. 11: 1723. https://doi.org/10.3390/biom11111723
APA StyleBechynska, K., Kosek, V., Fenclova, M., Muchova, L., Smid, V., Suk, J., Chalupsky, K., Sticova, E., Hurkova, K., Hajslova, J., Vitek, L., & Stranska, M. (2021). The Effect of Mycotoxins and Silymarin on Liver Lipidome of Mice with Non-Alcoholic Fatty Liver Disease. Biomolecules, 11(11), 1723. https://doi.org/10.3390/biom11111723







