The Small Molecule Alpha-Synuclein Aggregator, FN075, Enhances Alpha-Synuclein Pathology in Subclinical AAV Rat Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals & Ethical Statement
2.2. Experimental Design
2.3. AAV Virus Production
2.4. Surgery
2.5. Behavioral Testing
2.6. Euthanasia and Tissue Processing
2.7. Immunohistochemistry
2.8. Image Analysis
2.9. Statistical Analysis
3. Results
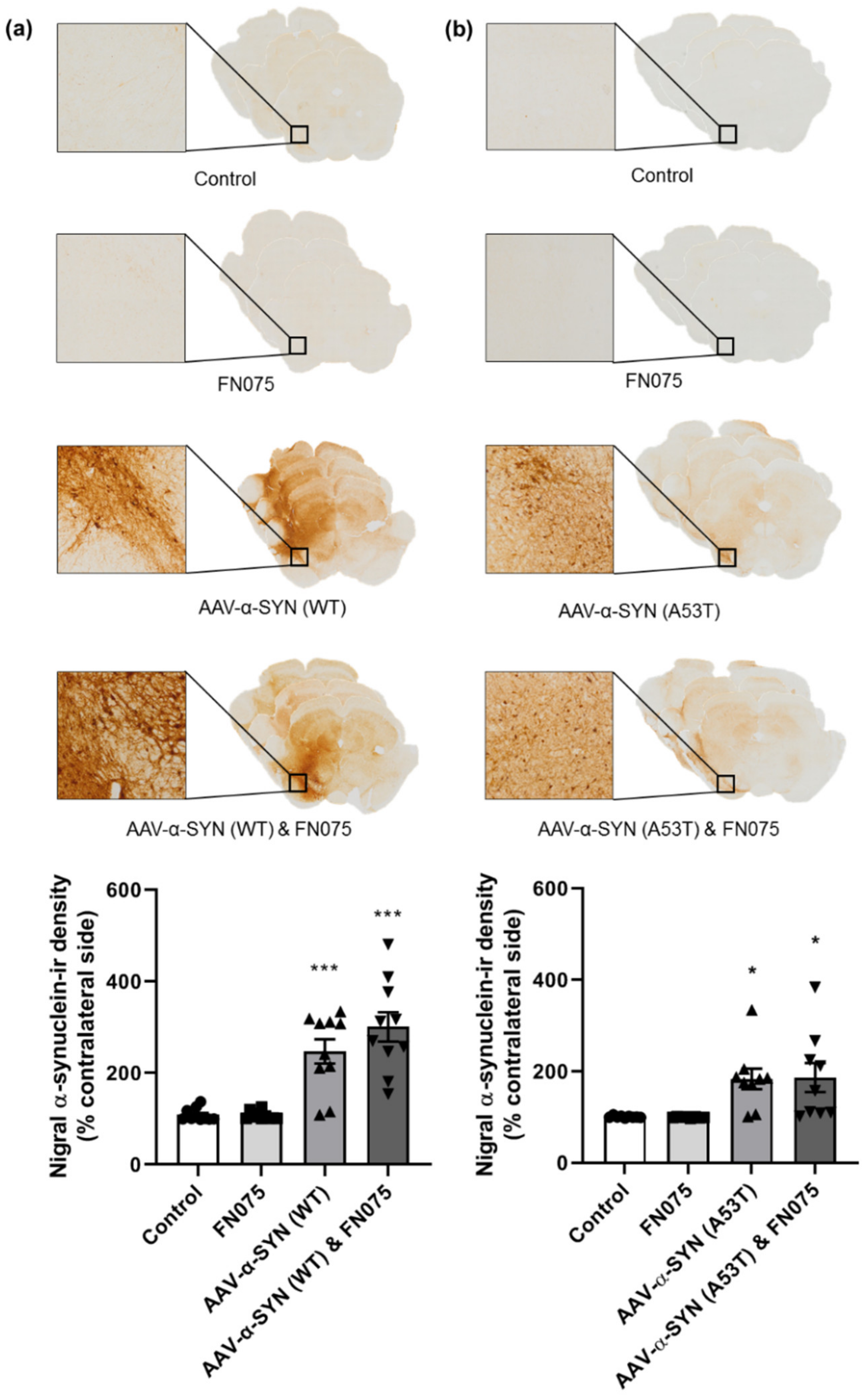
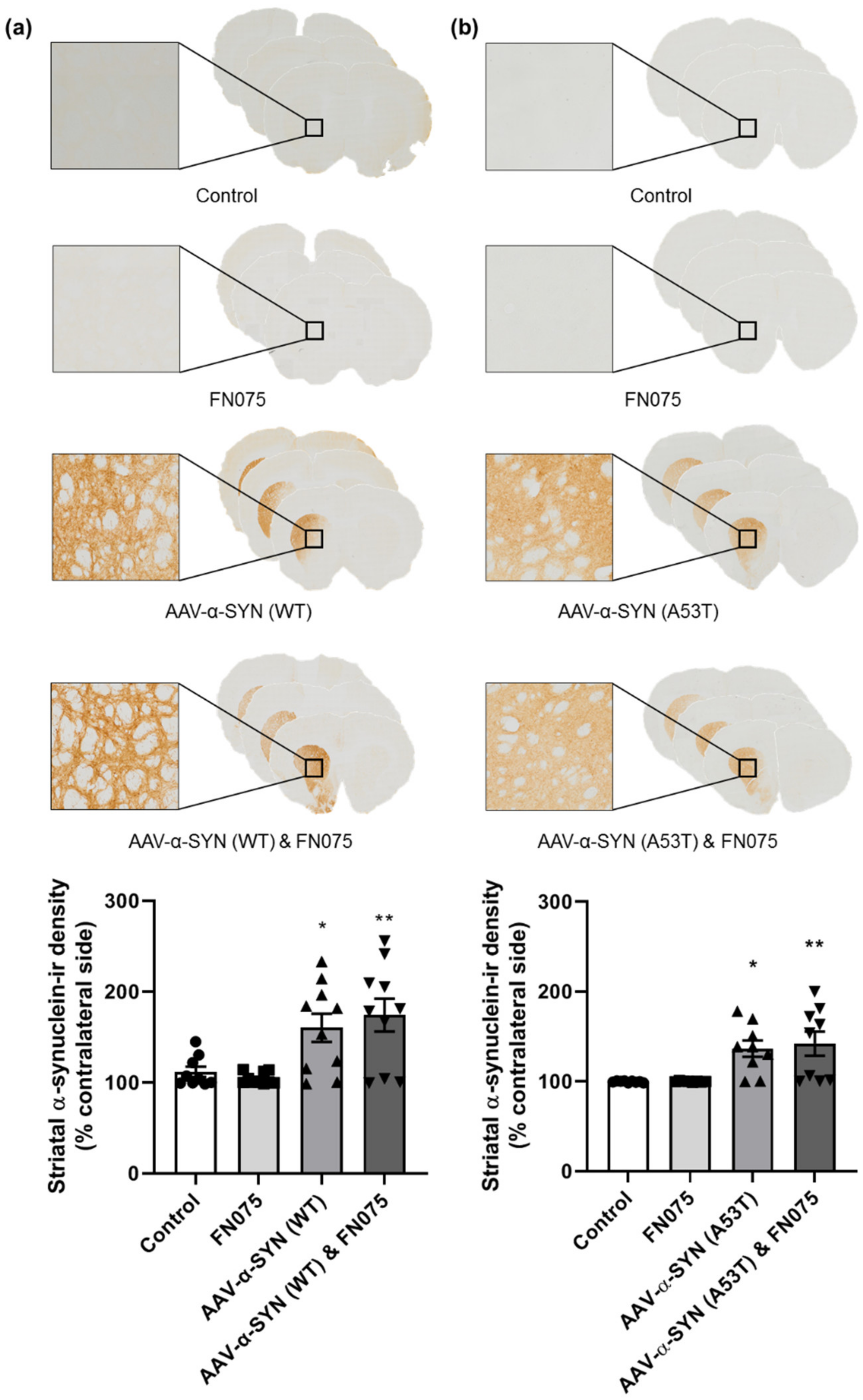
3.1. AAV-α-Synuclein Administration Induced Significant Alpha-Synuclein Expression
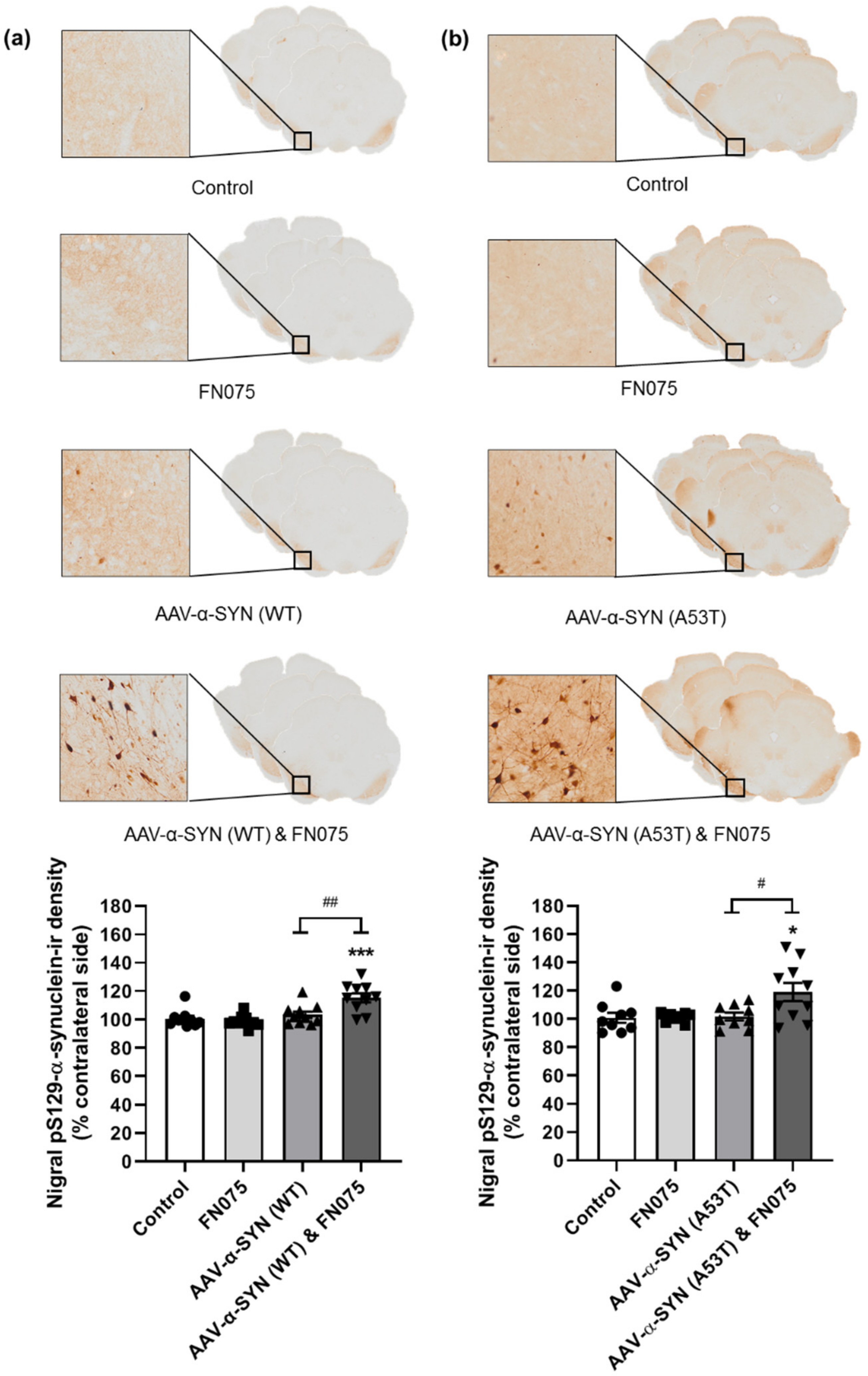
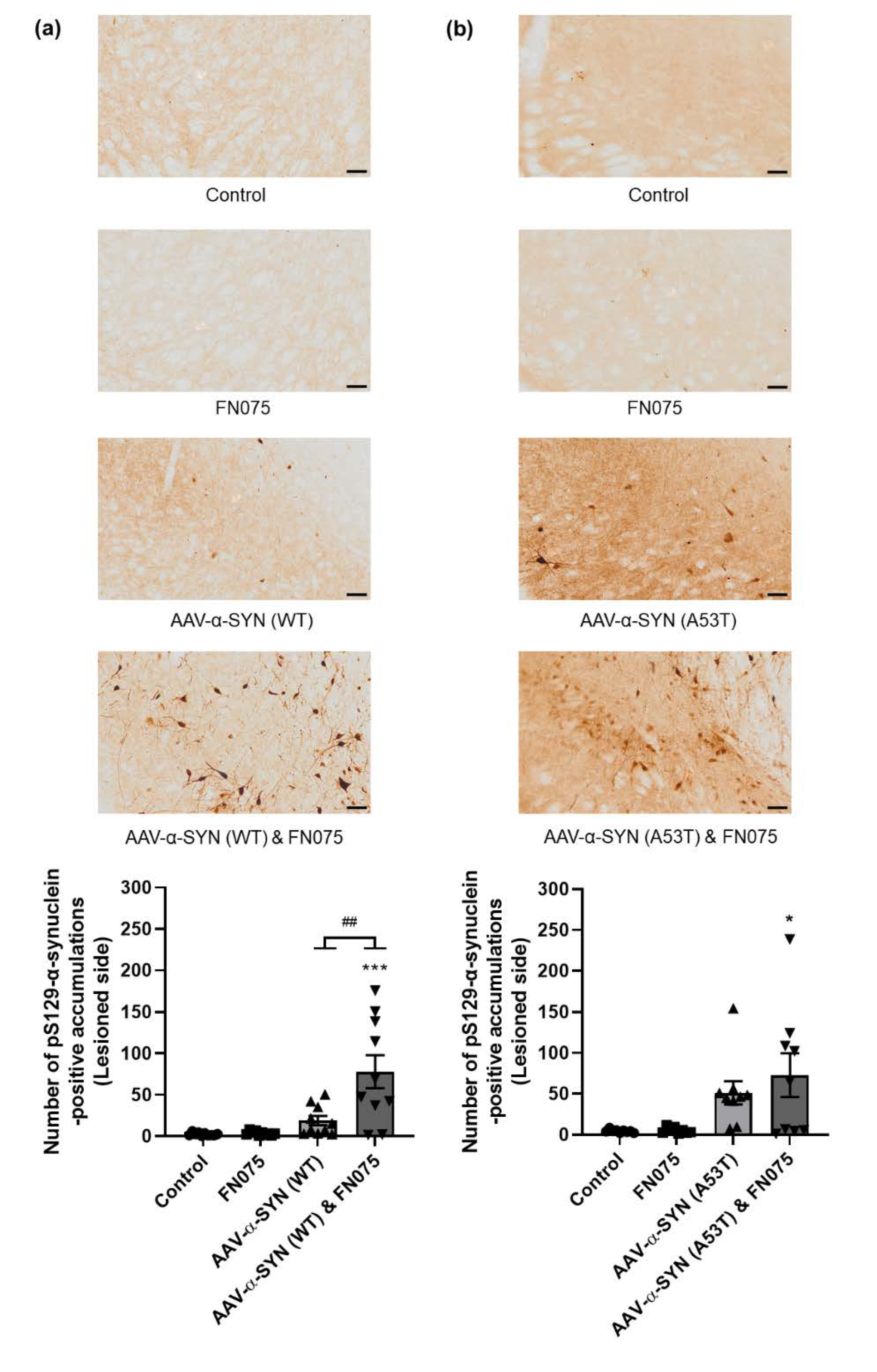
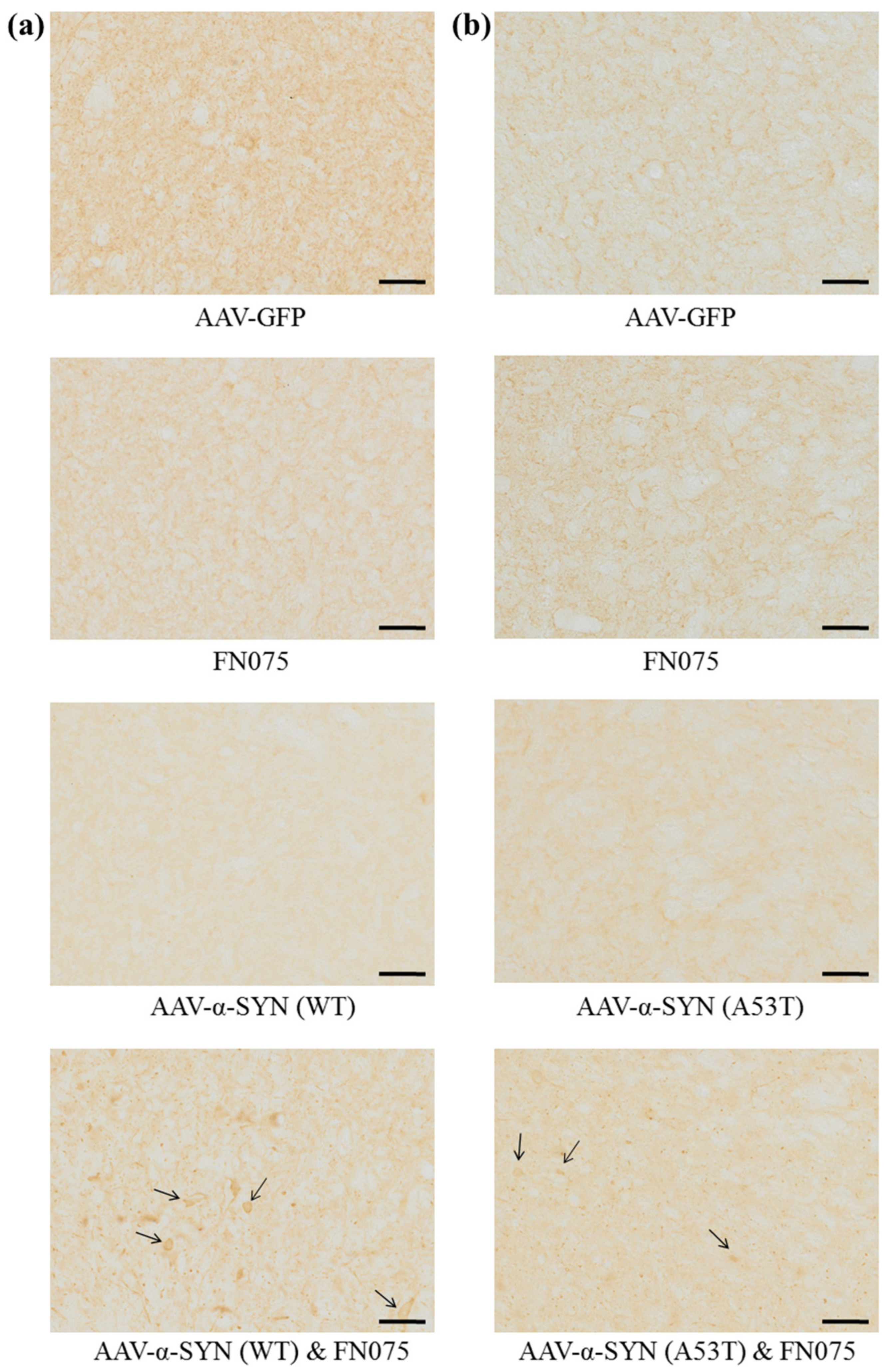
3.2. FN075 Significantly Increased Phosphorylation of Alpha-Synuclein at Serine 129
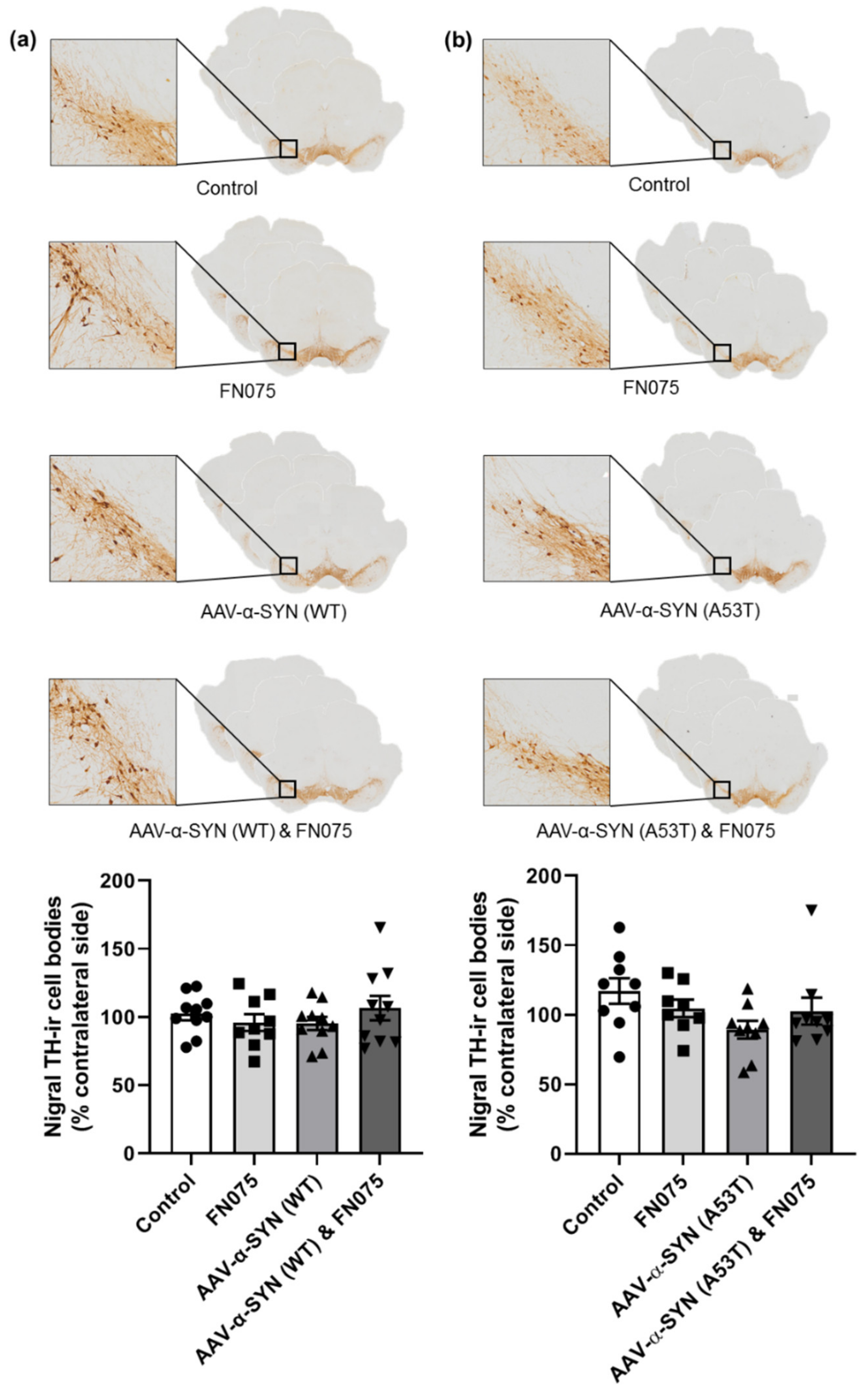
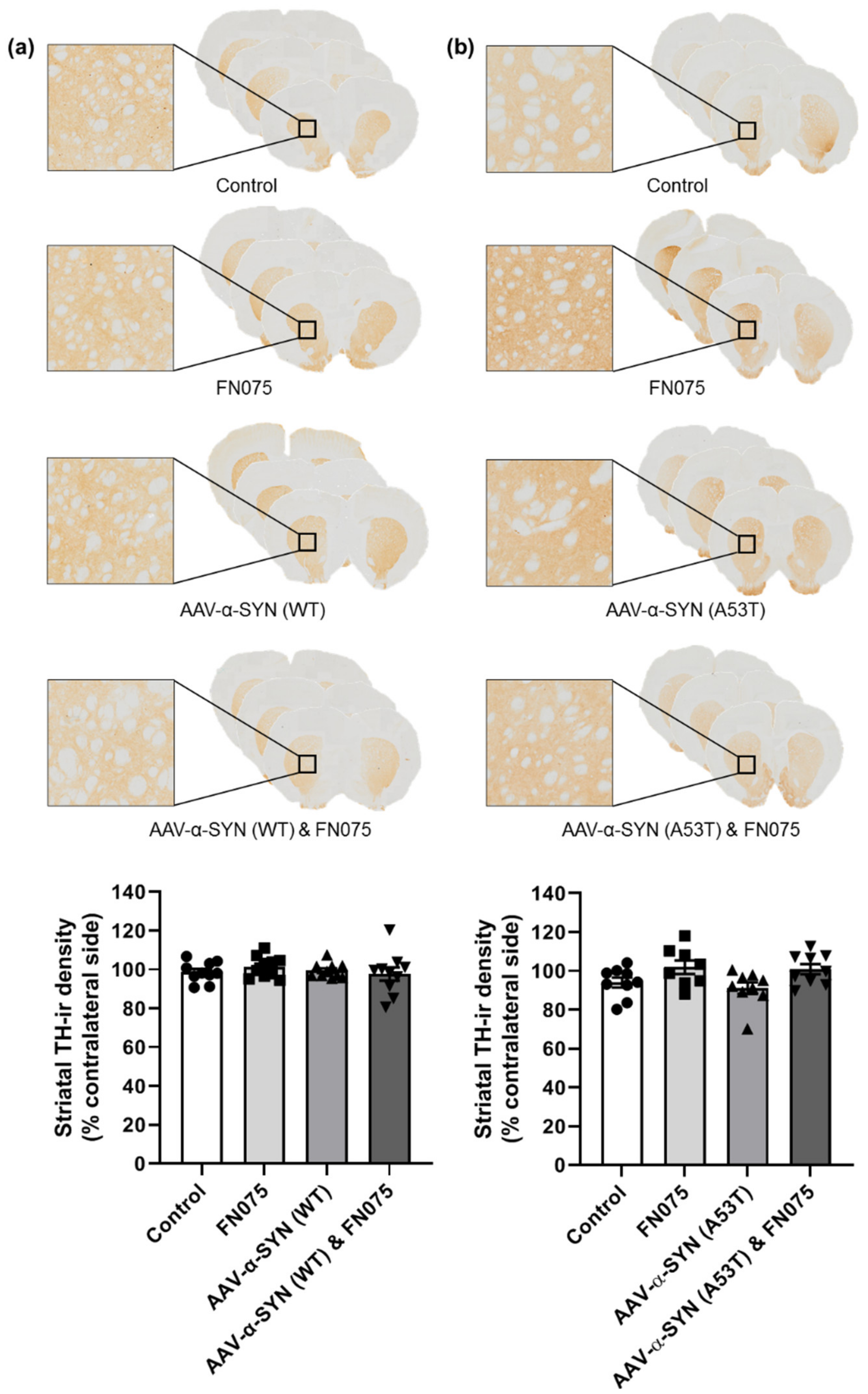
3.3. FN075 Did Not Precipitate Neurodegeneration in the Nigrostriatal Pathway in Preclinical AAV Models
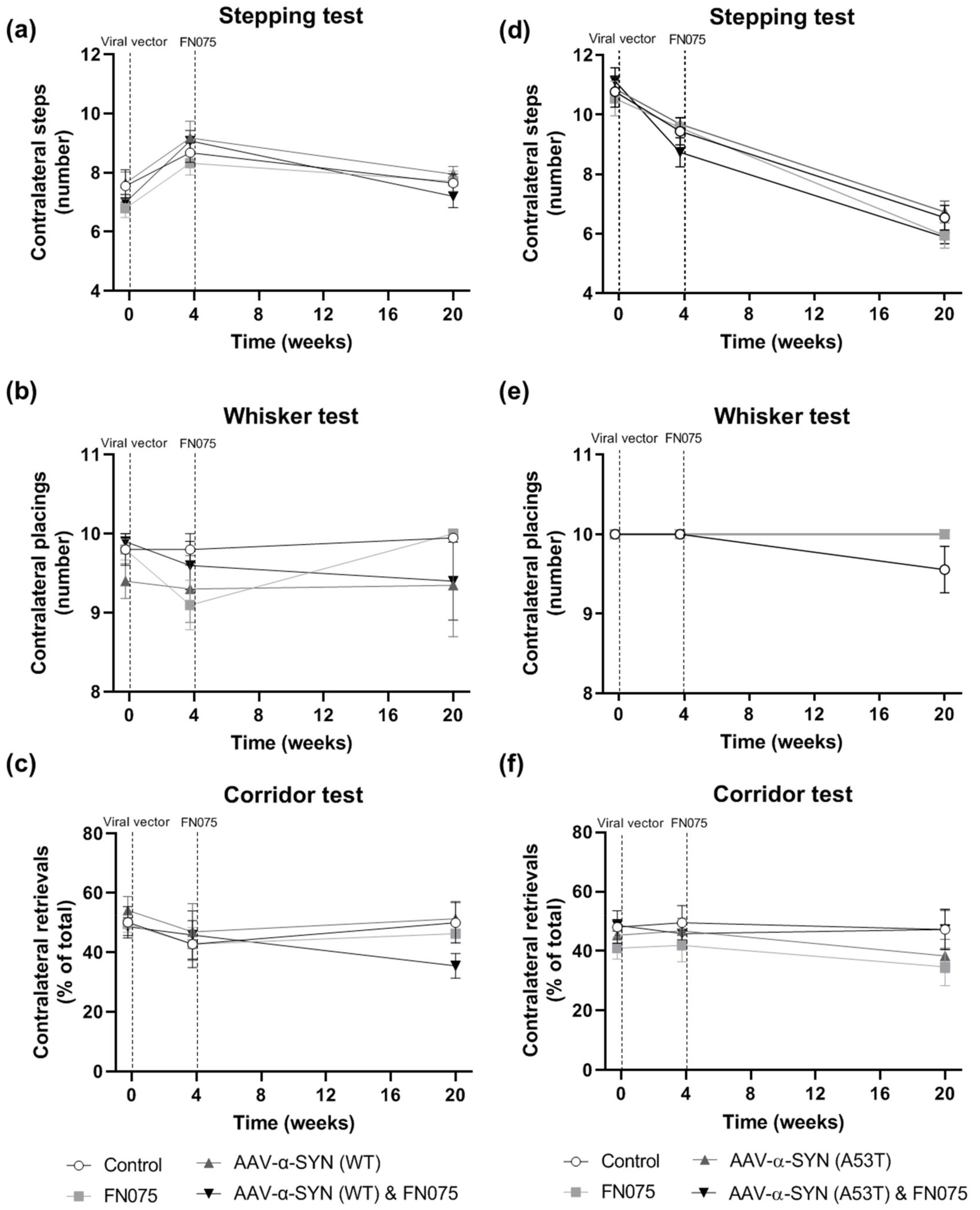
3.4. FN075 Did Not Precipitate Motor Impairment in the Nigrostriatal Pathway in Preclinical AAV Models
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.Y.Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Kirik, D.; Rosenblad, C.; Burger, C.; Lundberg, C.; Johansen, T.E.; Muzyczka, N.; Mandel, R.J.; Björklund, A. Parkinson-Like Neurodegeneration Induced by Targeted Overexpression of α-Synuclein in the Nigrostriatal System. J. Neurosci. 2002, 22, 2780–2791. [Google Scholar] [CrossRef] [PubMed]
- St Martin, J.L.; Klucken, J.; Outeiro, T.F.; Nguyen, P.; Keller-McGandy, C.; Cantuti-Castelvetri, I.; Grammatopoulos, T.N.; Standaert, D.G.; Hyman, B.T.; McLean, P.J. Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J. Neurochem. 2007, 100, 1449–1457. [Google Scholar] [CrossRef]
- Ip, C.W.; Klaus, L.C.; Karikari, A.A.; Visanji, N.P.; Brotchie, J.M.; Lang, A.E.; Volkmann, J.; Koprich, J.B. AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: A new mouse model for Parkinson’s disease. Acta Neuropathol. Commun. 2017, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Kirik, D.; Annett, L.E.; Burger, C.; Muzyczka, N.; Mandel, R.J.; Björklund, A. Nigrostriatal α-synucleinopathy induced by viral vector-mediated overexpression of human α-synuclein: A new primate model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 2884–2889. [Google Scholar] [CrossRef] [Green Version]
- Eslamboli, A.; Romero-Ramos, M.; Burger, C.; Bjorklund, T.; Muzyczka, N.; Mandel, R.J.; Baker, H.; Ridley, R.M.; Kirik, D. Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain. Brain 2007, 130, 799–815. [Google Scholar] [CrossRef]
- Lo Bianco, C.; Ridet, J.L.; Schneider, B.L.; Déglon, N.; Aebischer, P. α-synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2002, 99, 10813–10818. [Google Scholar] [CrossRef] [Green Version]
- Lauwers, E.; Debyser, Z.; Van Dorpe, J.; De Strooper, B.; Nuttin, B.; Baekelandt, V. Neuropathology and neurodegeneration in rodent brain induced by lentiviral vector-mediated overexpression of alpha-synuclein. Brain Pathol. 2003, 13, 364–372. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.; Kirik, D.; Stoyka, L.; Standaert, D.; Harms, A. How can rAAV-α-synuclein and the fibril α-synuclein models advance our understanding of Parkinson’s disease? J. Neurochem. 2016, 139, 131–155. [Google Scholar] [CrossRef]
- Decressac, M.; Mattsson, B.; Lundblad, M.; Weikop, P.; Björklund, A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of α-synuclein in midbrain dopamine neurons. Neurobiol. Dis. 2012, 45, 939–953. [Google Scholar] [CrossRef]
- Cegelski, L.; Pinkner, J.S.; Hammer, N.D.; Cusumano, C.K.; Hung, C.S.; Chorell, E.; Åberg, V.; Walker, J.N.; Seed, P.C.; Almqvist, F.; et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 2009, 5, 913–919. [Google Scholar] [CrossRef] [Green Version]
- Horvath, I.; Weise, C.F.; Andersson, E.K.; Chorell, E.; Sellstedt, M.; Bengtsson, C.; Olofsson, A.; Hultgren, S.J.; Chapman, M.; Wolf-Watz, M.; et al. Mechanisms of protein oligomerization: Inhibitor of functional amyloids templates α-synuclein fibrillation. J. Am. Chem. Soc. 2012, 134, 3439–3444. [Google Scholar] [CrossRef]
- Chermenina, M.; Chorell, E.; Pokrzywa, M.; Antti, H.; Almqvist, F.; Strömberg, I.; Wittung-Stafshede, P. Single injection of small-molecule amyloid accelerator results in cell death of nigral dopamine neurons in mice. NPJ Parkinson’s Dis. 2015, 1, 15024. [Google Scholar] [CrossRef] [Green Version]
- Olsen, L.K.; Cairns, A.G.; Ådén, J.; Moriarty, N.; Cabre, S.; Alamilla, V.R.; Almqvist, F.; Dowd, E.; McKernan, D.P. Viral mimetic priming enhances α-synuclein-induced degeneration: Implications for Parkinson’s disease. Brain. Behav. Immun. 2019, 80, 525–535. [Google Scholar] [CrossRef]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [Green Version]
- Berger, A.; Lorain, S.; Joséphine, C.; Desrosiers, M.; Peccate, C.; Voit, T.; Garcia, L.; Sahel, J.; Bemelmans, A. Repair of rhodopsin mRNA by spliceosome-mediated RNA trans-splicing: A new approach for autosomal dominant retinitis pigmentosa. Mol. Ther. 2015, 23, 918–930. [Google Scholar] [CrossRef] [Green Version]
- Cresto, N.; Gardier, C.; Gaillard, M.-C.; Gubinelli, F.; Roost, P.; Molina, D.; Josephine, C.; Dufour, N.; Auregan, G.; Guillermier, M.; et al. The C-Terminal Domain of LRRK2 with the G2019S Substitution Increases Mutant A53T α-Synuclein Toxicity in Dopaminergic Neurons In Vivo. Int. J. Mol. Sci. 2021, 22, 6760. [Google Scholar] [CrossRef]
- Olsson, M.; Nikkhah, G.; Bentlage, C.; Bjorklund, A. Forelimb akinesia in the rat Parkinson model: Differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J. Neurosci. 1995, 15, 3863–3875. [Google Scholar] [CrossRef]
- Schallert, T.; Fleming, S.M.; Leasure, J.L.; Tillerson, J.L.; Bland, S.T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 2000, 39, 777–787. [Google Scholar] [CrossRef]
- Dowd, E.; Monville, C.; Torres, E.M.; Dunnett, S.B. The Corridor Task: A simple test of lateralised response selection sensitive to unilateral dopamine deafferentation and graft-derived dopamine replacement in the striatum. Brain Res. Bull. 2005, 68, 24–30. [Google Scholar] [CrossRef]
- Fitzsimmons, D.F.; Moloney, T.C.; Dowd, E. Further validation of the corridor task for assessing deficit and recovery in the hemi-Parkinsonian rat: Restoration of bilateral food retrieval by dopamine receptor agonism. Behav. Brain Res. 2006, 169, 352–355. [Google Scholar] [CrossRef]
- Walsh, S.; Finn, D.P.; Dowd, E. Time-course of nigrostriatal neurodegeneration and neuroinflammation in the 6-hydroxydopamine-induced axonal and terminal lesion models of Parkinson’s disease in the rat. Neuroscience 2011, 175, 251–261. [Google Scholar] [CrossRef]
- Hoban, D.B.; Connaughton, E.; Connaughton, C.; Hogan, G.; Thornton, C.; Mulcahy, P.; Moloney, T.C.; Dowd, E. Further characterisation of the LPS model of Parkinson’s disease: A comparison of intra-nigral and intra-striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain. Behav. Immun. 2013, 27, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.T.; Jagannath, S.; Francois, C.; Vanderstichele, H.; Stoops, E.; Lashuel, H.A. How specific are the conformation-specific α-synuclein antibodies? Characterization and validation of 16 α-synuclein conformation-specific antibodies using well-characterized preparations of α-synuclein monomers, fibrils and oligomers with distinct struct. Neurobiol. Dis. 2020, 146, 105086. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Deng, Y.; Qing, H. The phosphorylation of α-synuclein: Development and implication for the mechanism and therapy of the Parkinson’s disease. J. Neurochem. 2015, 135, 4–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oueslati, A. Implication of Alpha-Synuclein Phosphorylation at S129 in Synucleinopathies: What Have We Learned in the Last Decade? J. Parkinson’s Dis. 2016, 6, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehra, S.; Sahay, S.; Maji, S.K. α-Synuclein misfolding and aggregation: Implications in Parkinson’s disease pathogenesis. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 890–908. [Google Scholar] [CrossRef]
- Thakur, P.; Breger, L.S.; Lundblad, M.; Wan, O.W.; Mattsson, B.; Luk, K.C.; Lee, V.M.Y.; Trojanowski, J.Q.; Björklund, A. Modeling Parkinson’s disease pathology by combination of fibril seeds and α-synuclein overexpression in the rat brain. Proc. Natl. Acad. Sci. USA 2017, 114, E8284–E8293. [Google Scholar] [CrossRef] [Green Version]
- Mulcahy, P.; O’Doherty, A.; Paucard, A.; O’Brien, T.; Kirik, D.; Dowd, E. Development and characterisation of a novel rat model of Parkinson’s disease induced by sequential intranigral administration of AAV-α-synuclein and the pesticide, rotenone. Neuroscience 2012, 203, 170–179. [Google Scholar] [CrossRef]
- Naughton, C.; O’Toole, D.; Kirik, D.; Dowd, E. Interaction between subclinical doses of the Parkinson’s disease associated gene, α-synuclein, and the pesticide, rotenone, precipitates motor dysfunction and nigrostriatal neurodegeneration in rats. Behav. Brain Res. 2017, 316, 160–168. [Google Scholar] [CrossRef]
- Nors Perdersen, M.; Foderà, V.; Horvath, I.; van Maarschalkerweerd, A.; Nørgaard Toft, K.; Weise, C.; Almqvist, F.; Wolf-Watz, M.; Wittung-Stafshede, P.; Vestergaard, B. Direct Correlation Between Ligand-Induced α-Synuclein Oligomers and Amyloid-like Fibril Growth. Sci. Rep. 2015, 5, 10422. [Google Scholar] [CrossRef]
- Fujiwara, H.; Hasegawa, M.; Dohmae, N.; Kawashima, A.; Masliah, E.; Goldberg, M.S.; Shen, J.; Takio, K.; Iwatsubo, T. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002, 4, 160–164. [Google Scholar] [CrossRef]
- Anderson, J.P.; Walker, D.E.; Goldstein, J.M.; De Laat, R.; Banducci, K.; Caccavello, R.J.; Barbour, R.; Huang, J.; Kling, K.; Lee, M.; et al. Phosphorylation of Ser-129 Is the Dominant Pathological Modification of α-Synuclein in Familial and Sporadic Lewy Body Disease. J. Biol. Chem. 2006, 281, 29739–29752. [Google Scholar] [CrossRef] [Green Version]
- Stewart, T.; Sossi, V.; Aasly, J.O.; Wszolek, Z.K.; Uitti, R.J.; Hasegawa, K.; Yokoyama, T.; Zabetian, C.P.; Leverenz, J.B.; Stoessl, A.J.; et al. Phosphorylated α-synuclein in Parkinson’s disease: Correlation depends on disease severity. Acta Neuropathol. Commun. 2015, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-H.; Liu, H.-C.; Yang, S.-Y.; Yang, K.-C.; Wu, C.-C.; Chiu, M.-J. Plasma pS129-α-Synuclein Is a Surrogate Biofluid Marker of Motor Severity and Progression in Parkinson’s Disease. J. Clin. Med. 2019, 8, 1601. [Google Scholar] [CrossRef] [Green Version]
- Cariulo, C.; Martufi, P.; Verani, M.; Azzollini, L.; Bruni, G.; Weiss, A.; Deguire, S.M.; Lashuel, H.A.; Scaricamazza, E.; Sancesario, G.M.; et al. Phospho-S129 Alpha-Synuclein Is Present in Human Plasma but Not in Cerebrospinal Fluid as Determined by an Ultrasensitive Immunoassay. Front. Neurosci. 2019, 13, 889. [Google Scholar] [CrossRef] [Green Version]
| Study 1: Wild-Type Experiment | |||
| Group | Virus injection | FN075 injection | n |
| Control | AAV-GFP | Vehicle | 10 |
| FN075 | AAV-GFP | FN075 | 10 |
| AAV-α-SYN (WT) | AAV-α-synuclein (wild-type) | Vehicle | 10 |
| AAV-α-SYN (WT) & FN075 | AAV-α-synuclein (wild-type) | FN075 | 10 |
| Study 2: A53T Mutant Experiment | |||
| Group | Virus injection | FN075 injection | n |
| Control | AAV-GFP | Vehicle | 9 |
| FN075 | AAV-GFP | FN075 | 8 |
| AAV-α-SYN (A53T) | AAV-α-synuclein (A53T mutant) | Vehicle | 9 |
| AAV-α-SYN (A53T) & FN075 | AAV-α-synuclein (A53T mutant) | FN075 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, R.; Cairns, A.G.; Ådén, J.; Almqvist, F.; Bemelmans, A.-P.; Brouillet, E.; Patton, T.; McKernan, D.P.; Dowd, E. The Small Molecule Alpha-Synuclein Aggregator, FN075, Enhances Alpha-Synuclein Pathology in Subclinical AAV Rat Models. Biomolecules 2021, 11, 1685. https://doi.org/10.3390/biom11111685
Kelly R, Cairns AG, Ådén J, Almqvist F, Bemelmans A-P, Brouillet E, Patton T, McKernan DP, Dowd E. The Small Molecule Alpha-Synuclein Aggregator, FN075, Enhances Alpha-Synuclein Pathology in Subclinical AAV Rat Models. Biomolecules. 2021; 11(11):1685. https://doi.org/10.3390/biom11111685
Chicago/Turabian StyleKelly, Rachel, Andrew G. Cairns, Jörgen Ådén, Fredrik Almqvist, Alexis-Pierre Bemelmans, Emmanuel Brouillet, Tommy Patton, Declan P. McKernan, and Eilís Dowd. 2021. "The Small Molecule Alpha-Synuclein Aggregator, FN075, Enhances Alpha-Synuclein Pathology in Subclinical AAV Rat Models" Biomolecules 11, no. 11: 1685. https://doi.org/10.3390/biom11111685
APA StyleKelly, R., Cairns, A. G., Ådén, J., Almqvist, F., Bemelmans, A.-P., Brouillet, E., Patton, T., McKernan, D. P., & Dowd, E. (2021). The Small Molecule Alpha-Synuclein Aggregator, FN075, Enhances Alpha-Synuclein Pathology in Subclinical AAV Rat Models. Biomolecules, 11(11), 1685. https://doi.org/10.3390/biom11111685







