LncRNAs: Novel Biomarkers for Pancreatic Cancer
Abstract
:1. Introduction
2. Oncogenic LncRNAs in Pancreatic Cancer
3. Tumor Suppressor LncRNAs in Pancreatic Cancer
4. Diagnostic Role of LncRNAs in Pancreatic Cancer
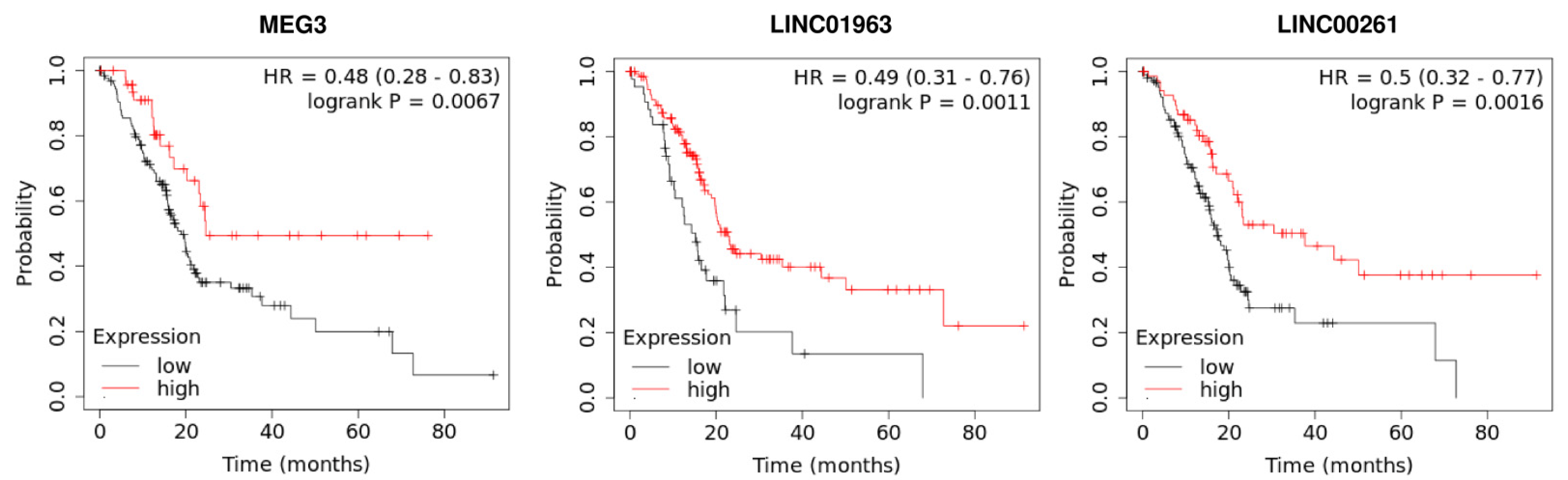
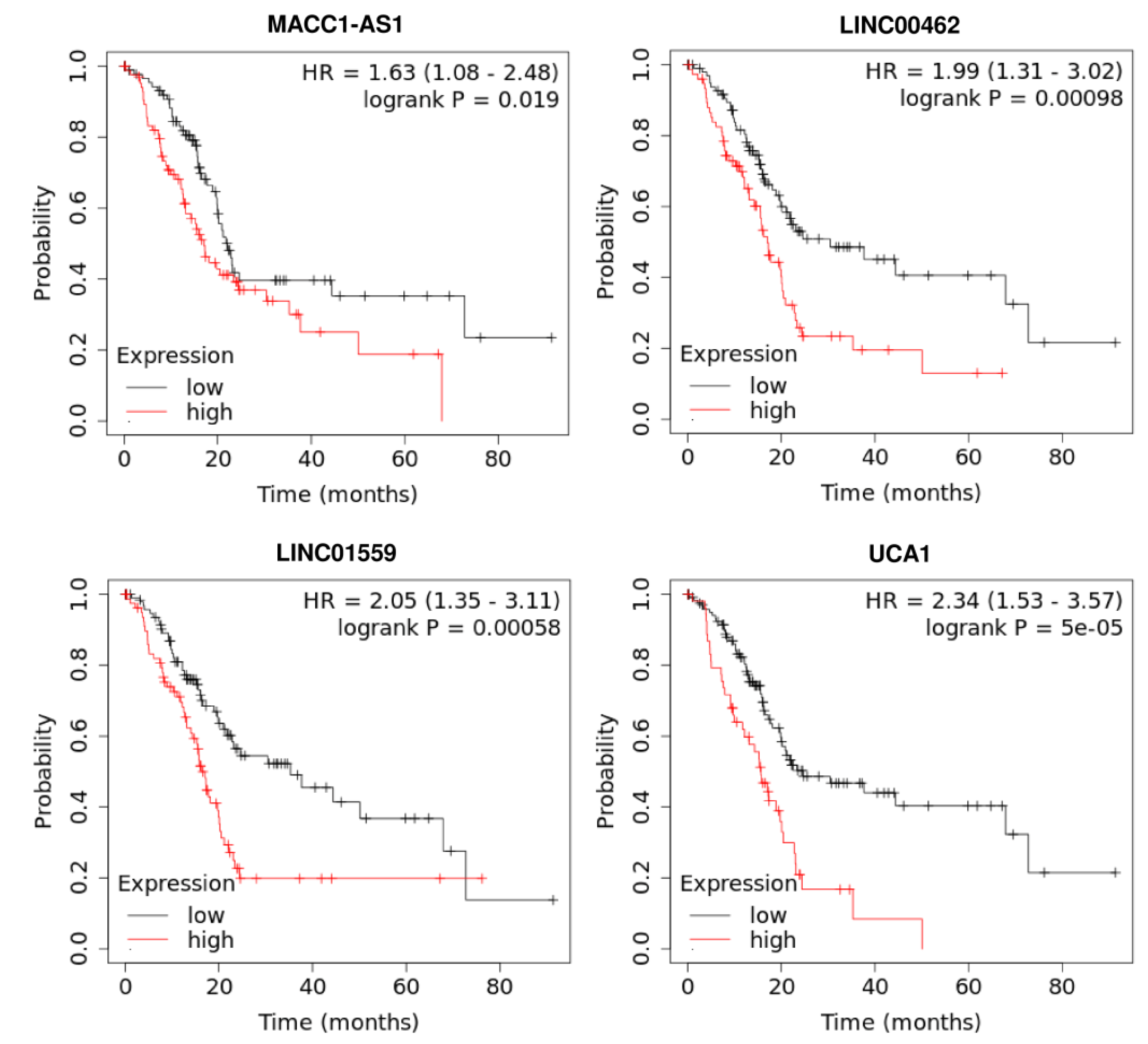
5. Prognostic Role of LncRNAs in Pancreatic Cancer
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020, 159, 335–349.e315. [Google Scholar] [CrossRef]
- Grant, T.J.; Hua, K.; Singh, A. Molecular Pathogenesis of Pancreatic Cancer. Prog. Mol. Biol. Transl. Sci. 2016, 144, 241–275. [Google Scholar]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef] [Green Version]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Qi, C.; Xiaofeng, C.; Dongen, L.; Liang, Y.; Liping, X.; Yue, H.; Jianshuai, J. Long non-coding RNA MACC1-AS1 promoted pancreatic carcinoma progression through activation of PAX8/NOTCH1 signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 1–12. [Google Scholar] [CrossRef]
- Lei, S.; He, Z.; Chen, T.; Guo, X.; Zeng, Z.; Shen, Y.; Jiang, J. Long noncoding RNA 00976 promotes pancreatic cancer progression through OTUD7B by sponging miR-137 involving EGFR/MAPK pathway. J. Exp. Clin. Cancer Res. 2019, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Guo, W.; Sun, C.; Zhang, B.; Zheng, F. Linc00462 promotes pancreatic cancer invasiveness through the miR-665/TGFBR1-TGFBR2/SMAD2/3 pathway. Cell Death Dis. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Agiannitopoulos, K.; Samara, P.; Papadopoulou, M.; Efthymiadou, A.; Papadopoulou, E.; Tsaousis, G.N.; Mertzanos, G.; Babalis, D.; Lamnissou, K. miRNA polymorphisms and risk of premature coronary artery disease. Hell. J. Cardiol. 2020, 62, 278–284. [Google Scholar] [CrossRef]
- Wang, G.; Pan, J.; Zhang, L.; Wei, Y.; Wang, C. Long non-coding RNA CRNDE sponges miR-384 to promote proliferation and metastasis of pancreatic cancer cells through upregulating IRS 1. Cell Prolif. 2017, 50, e12389. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, H.; Matsuda, Y.; Yamamoto, M.; Michishita, M.; Takahashi, K.; Sasaki, N.; Ishikawa, N.; Aida, J.; Takubo, K.; Arai, T.; et al. Reduced expression of the H19 long non-coding RNA inhibits pancreatic cancer metastasis. Lab. Investig. 2018, 98, 814–824. [Google Scholar] [CrossRef]
- Deng, S.; Wang, J.; Zhang, L.; Li, J.; Jin, Y. LncRNA HOTAIR Promotes Cancer Stem-Like Cells Properties by Sponging miR-34a to Activate the JAK2/STAT3 Pathway in Pancreatic Ductal Adenocarcinoma. OncoTargets Ther. 2021, 14, 1883. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Jutooru, I.; Chadalapaka, G.; Corton, J.C.; Safe, S. The long non-coding RNA HOTTIP enhances pancreatic cancer cell proliferation, survival and migration. Oncotarget 2015, 6, 10840–10852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Wei, J.-H.; Feng, Z.-H.; Chen, Z.-H.; Wang, Y.-Q.; Huang, Y.; Fang, Y.; Liang, Y.-P.; Cen, J.-J.; Pan, Y.-H. miR-106b-5p promotes renal cell carcinoma aggressiveness and stem-cell-like phenotype by activating Wnt/β-catenin signalling. Oncotarget 2017, 8, 21461. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wang, J.; Xie, F.; Mou, T.; Zhong, P.; Hua, H.; Liu, P.; Yang, Q. Long noncoding RNA LINC01559 promotes pancreatic cancer progression by acting as a competing endogenous RNA of miR-1343-3p to upregulate RAF1 expression. Aging 2020, 12, 14452. [Google Scholar] [CrossRef]
- Yuan, Z.-J.; Yu, C.; Hu, X.-F.; He, Y.; Chen, P.; Ouyang, S.-X. LINC00152 promotes pancreatic cancer cell proliferation, migration and invasion via targeting miR-150. Am. J. Transl. Res. 2020, 12, 2241. [Google Scholar] [PubMed]
- Chen, S.; Chen, J.-Z.; Zhang, J.-Q.; Chen, H.-X.; Qiu, F.-N.; Yan, M.-L.; Tian, Y.-F.; Peng, C.-H.; Shen, B.-Y.; Chen, Y.-L. Silencing of long noncoding RNA LINC00958 prevents tumor initiation of pancreatic cancer by acting as a sponge of microRNA-330-5p to down-regulate PAX8. Cancer Lett. 2019, 446, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.; Bhattacharyya, A.; Pattnaik, M. Theta autoregressive neural network model for COVID-19 outbreak predictions. Medrxiv 2020. [Google Scholar] [CrossRef]
- Zhu, X.; Niu, X.; Ge, C. Inhibition of LINC00994 represses malignant behaviors of pancreatic cancer cells: Interacting with miR-765-3p/RUNX2 axis. Cancer Biol. Ther. 2019, 20, 799–811. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.-O.; Zhou, W.-Y.; Chang, X.-Y.; Zhang, M.-M.; Zhang, Y.; Yang, X.-H. Long non-coding RNA LINC01207 silencing suppresses AGR2 expression to facilitate autophagy and apoptosis of pancreatic cancer cells by sponging miR-143-5p. Mol. Cell. Endocrinol. 2019, 493, 110424. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Guo, C.; Shao, Y. LncRNA OIP5-AS1 promotes the malignancy of pancreatic ductal adenocarcinoma via regulating miR-429/FOXD1/ERK pathway. Cancer Cell Int. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, P.; Yin, T.; Zhang, F.; Wang, W. Upregulation of LncRNA PVT1 facilitates pancreatic ductal adenocarcinoma cell progression and glycolysis by regulating MiR-519d-3p and HIF-1A. J. Cancer 2020, 11, 2572. [Google Scholar] [CrossRef]
- Huang, R.; Nie, W.; Yao, K.; Chou, J. Depletion of the lncRNA RP11-567G11. 1 inhibits pancreatic cancer progression. Biomed. Pharmacother. 2019, 112, 108685. [Google Scholar] [CrossRef]
- Yin, Z.; Zhou, Y.; Ma, T.; Chen, S.; Shi, N.; Zou, Y.; Hou, B.; Zhang, C. Down-regulated lncRNA SBF2-AS1 in M2 macrophage-derived exosomes elevates miR-122-5p to restrict XIAP, thereby limiting pancreatic cancer development. J. Cell. Mol. Med. 2020, 24, 5028–5038. [Google Scholar] [CrossRef] [Green Version]
- Jian, Y.; Fan, Q. Long non-coding RNA SNHG7 facilitates pancreatic cancer progression by regulating the miR-146b-5p/Robo1 axis. Exp. Ther. Med. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Cao, W.; Zhou, G. LncRNA SNHG12 contributes proliferation, invasion and epithelial–mesenchymal transition of pancreatic cancer cells by absorbing miRNA-320b. Biosci. Rep. 2020, 40, BSR20200805. [Google Scholar] [CrossRef]
- Deng, P.c.; Chen, W.b.; Cai, H.h.; An, Y.; Wu, X.q.; Chen, X.m.; Sun, D.l.; Yang, Y.; Shi, L.q.; Yang, Y. LncRNA SNHG14 potentiates pancreatic cancer progression via modulation of annexin A2 expression by acting as a competing endogenous RNA for miR-613. J. Cell. Mol. Med. 2019, 23, 7222–7232. [Google Scholar] [CrossRef] [Green Version]
- Al-Kafaji, G.; Al-Mahroos, G.; Abdulla Al-Muhtaresh, H.; Sabry, M.A.; Abdul Razzak, R.; Salem, A.H. Circulating endothelium-enriched microRNA-126 as a potential biomarker for coronary artery disease in type 2 diabetes mellitus patients. Biomark 2017, 22, 268–278. [Google Scholar] [CrossRef]
- Guo, W.; Zhong, K.; Wei, H.; Nie, C.; Yuan, Z. Long non-coding RNA SPRY4-IT1 promotes cell proliferation and invasion by regulation of Cdc20 in pancreatic cancer cells. PLoS ONE 2018, 13, e0193483. [Google Scholar] [CrossRef]
- Cui, X.-P.; Wang, C.-X.; Wang, Z.-Y.; Li, J.; Tan, Y.-W.; Gu, S.-T.; Qin, C.-K. LncRNA TP73-AS1 sponges miR-141-3p to promote the migration and invasion of pancreatic cancer cells through the up-regulation of BDH2. Biosci. Rep. 2019, 39, BSR20181937. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Wan, D.; Zheng, D.; Zheng, Q.; Wu, F.; Zhi, Q. Long non-coding RNA UCA1 promotes the tumorigenesis in pancreatic cancer. Biomed. Pharmacother. 2016, 83, 1220–1226. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Ding, W.; Hua, Z.; Wang, L.; Zhu, Y.; Qian, H.; Dai, T. LncRNA UCA1 impacts cell proliferation, invasion, and migration of pancreatic cancer through regulating miR-96/FOXO3. Iubmb Life 2018, 70, 276–290. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhang, Y. LncRNA XIST enhanced TGF-β2 expression by targeting miR-141-3p to promote pancreatic cancer cells invasion. Biosci. Rep. 2019, 39, BSR20190332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Liu, Y.; Lu, Y.; Yang, B.; Tang, L. LncRNA XIST promotes pancreatic cancer proliferation through miR-133a/EGFR. J. Cell. Biochem. 2017, 118, 3349–3358. [Google Scholar] [CrossRef]
- Gao, H.; Gong, N.; Ma, Z.; Miao, X.; Chen, J.; Cao, Y.; Zhang, G. LncRNA ZEB2-AS1 promotes pancreatic cancer cell growth and invasion through regulating the miR-204/HMGB1 axis. Int. J. Biol. Macromol. 2018, 116, 545–551. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Watabe, T. Roles of TGF-β signals in endothelial-mesenchymal transition during cardiac fibrosis. Int. J. Inflamm. 2011, 2011, 724080. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef]
- Pan, S.; Shen, M.; Zhou, M.; Shi, X.; He, R.; Yin, T.; Wang, M.; Guo, X.; Qin, R. Long noncoding RNA LINC01111 suppresses pancreatic cancer aggressiveness by regulating DUSP1 expression via microRNA-3924. Cell Death Dis. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Han, H.; Gu, W.; Cao, C.; Zheng, P. Long non-coding RNA LINC01963 inhibits progression of pancreatic carcinoma by targeting miR-641/TMEFF2. Biomed. Pharmacother. 2020, 129, 110346. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, J.; Shi, M.; Zhan, Q.; Shen, B.; Peng, C. lncRNA MEG3 had anti-cancer effects to suppress pancreatic cancer activity. Biomed. Pharmacother. 2017, 89, 1269–1276. [Google Scholar] [CrossRef]
- Gao, Z.-Q.; Wang, J.-f.; Chen, D.-H.; Ma, X.-S.; Wu, Y.; Tang, Z.; Dang, X.-W. Long non-coding RNA GAS5 suppresses pancreatic cancer metastasis through modulating miR-32-5p/PTEN axis. Cell Biosci. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhou, S.; Fan, T.; Feng, X. lncRNA DGCR 5/miR-27a-3p/BNIP3 promotes cell apoptosis in pancreatic cancer by regulating the p38 MAPK pathway. Int. J. Mol. Med. 2020, 46, 729–739. [Google Scholar] [CrossRef]
- Sun, Y.W.; Chen, Y.F.; Li, J.; Huo, Y.M.; Liu, D.J.; Hua, R.; Zhang, J.F.; Liu, W.; Yang, J.Y.; Fu, X.L.; et al. A novel long non-coding RNA ENST00000480739 suppresses tumour cell invasion by regulating OS-9 and HIF-1α in pancreatic ductal adenocarcinoma. Br. J. Cancer 2014, 111, 2131–2141. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gao, X.; Tian, J.; Zhang, R.; Qiao, Y.; Hua, X.; Shi, G. LINC00261 inhibits progression of pancreatic cancer by down-regulating miR-23a-3p. Arch. Biochem. Biophys. 2020, 689, 108469. [Google Scholar] [CrossRef]
- Li, D.-D.; Fu, Z.-Q.; Lin, Q.; Zhou, Y.; Zhou, Q.-B.; Li, Z.-H.; Tan, L.-P.; Chen, R.-F.; Liu, Y.-M. Linc00675 is a novel marker of short survival and recurrence in patients with pancreatic ductal adenocarcinoma. World J. Gastroenterol. 2015, 21, 9348. [Google Scholar] [CrossRef]
- Ge, J.-N.; Di Yan, C.-L.G.; Wei, M.-J. LncRNA C9orf139 can regulate the growth of pancreatic cancer by mediating the miR-663a/Sox12 axis. World J. Gastrointest. Oncol. 2020, 12, 1272. [Google Scholar] [CrossRef]
- Liu, P.; Sun, Q.-Q.; Liu, T.-X.; Lu, K.; Zhang, N.; Zhu, Y.; Chen, M. Serum lncRNA-UFC1 as a potential biomarker for diagnosis and prognosis of pancreatic cancer. Int. J. Clin. Exp. Pathol. 2019, 12, 4125. [Google Scholar]
- Huang, X.; Ta, N.; Zhang, Y.; Gao, Y.; Hu, R.; Deng, L.; Zhang, B.; Jiang, H.; Zheng, J. Microarray analysis of the expression profile of long non-coding RNAs indicates lncRNA RP11-263F15. 1 as a biomarker for diagnosis and prognostic prediction of pancreatic ductal adenocarcinoma. J. Cancer 2017, 8, 2740. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Feng, W.; Liu, W.; Kong, X.; Li, L.; He, J.; Wang, D.; Zhang, M.; Zhou, G.; Xu, W. Circulating lncRNA ABHD11-AS1 serves as a biomarker for early pancreatic cancer diagnosis. J. Cancer 2019, 10, 3746. [Google Scholar] [CrossRef] [Green Version]
- Ou, Z.-L.; Luo, Z.; Lu, Y.-B. Long non-coding RNA HULC as a diagnostic and prognostic marker of pancreatic cancer. World J. Gastroenterol. 2019, 25, 6728. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, X.; Li, J.; Guo, Y.; Li, H.; Pan, X.; Jiang, J.; Liu, H.; Wu, B. Salivary HOTAIR and PVT1 as novel biomarkers for early pancreatic cancer. Oncotarget 2016, 7, 25408–25419. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhang, J.; Yin, L.; Wang, X.; Zheng, Y.; Zhang, Y.; Gu, J.; Yang, L.; Yang, J.; Zheng, P. The lncRNA RUNX1-IT1 regulates C-FOS transcription by interacting with RUNX1 in the process of pancreatic cancer proliferation, migration and invasion. Cell Death Dis. 2020, 11, 1–17. [Google Scholar]
- Chen, B.; Zhang, Q.; Wang, X.; Wang, Y.; Cui, J.; Zhuang, H.; Tang, J. The lncRNA ENSG00000254041. 1 promotes cell invasiveness and associates with poor prognosis of pancreatic ductal adenocarcinoma. Aging 2020, 12, 3647. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Chen, G.; Dang, Y.-W.; Li, C.-J.; Luo, D.-Z. Expression and prognostic significance of lncRNA MALAT1 in pancreatic cancer tissues. Asian Pac. J. Cancer Prev. 2014, 15, 2971–2977. [Google Scholar] [CrossRef]
- Ding, Y.-C.; Yu, W.; Ma, C.; Wang, Q.; Huang, C.-S.; Huang, T. Expression of long non-coding RNA LOC285194 and its prognostic significance in human pancreatic ductal adenocarcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 8065. [Google Scholar]
- Li, J.; Liu, D.; Hua, R.; Zhang, J.; Liu, W.; Huo, Y.; Cheng, Y.; Hong, J.; Sun, Y. Long non-coding RNAs expressed in pancreatic ductal adenocarcinoma and lncRNA BC008363 an independent prognostic factor in PDAC. Pancreatol. 2014, 14, 385–390. [Google Scholar] [CrossRef]
- Ma, L.; Wang, F.; Du, C.; Zhang, Z.; Guo, H.; Xie, X.; Gao, H.; Zhuang, Y.; Kornmann, M.; Gao, H. Long non-coding RNA MEG3 functions as a tumour suppressor and has prognostic predictive value in human pancreatic cancer. Oncol. Rep. 2018, 39, 1132–1140. [Google Scholar] [CrossRef]
- Peng, W.; Gao, W.; Feng, J. Long noncoding RNA HULC is a novel biomarker of poor prognosis in patients with pancreatic cancer. Med Oncol. 2014, 31, 346. [Google Scholar] [CrossRef]
- López-Jiménez, E. and E. Andrés-León, The Implications of ncRNAs in the Development of Human Diseases. Non-Coding RNA 2021, 7, 17. [Google Scholar] [CrossRef]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 2013, 10, 925–933. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yue, J.; JB Hanley, S.; Kobayashi, N.; Todo, Y.; Watari, H. Exploring lncRNA-Mediated Regulatory Networks in Endometrial Cancer Cells and the Tumor Microenvironment: Advances and Challenges. Cancers 2019, 11, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019, 112, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.G.; Okada, Y.; Von Hoff, D.; Goel, A. Non-coding RNA biomarkers in pancreatic ductal adenocarcinoma. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Xu, T.; Lin, C.M.; Cheng, S.Q.; Min, J.; Li, L.; Meng, X.M.; Huang, C.; Zhang, L.; Deng, Z.Y.; Li, J. Pathological bases and clinical impact of long noncoding RNAs in prostate cancer: A new budding star. Mol. Cancer 2018, 17, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschovis, D.; Gazouli, M.; Tzouvala, M.; Vezakis, A.; Karamanolis, G. Long non-coding RNA in pancreatic adenocarcinoma and pancreatic neuroendocrine tumors. Ann. Gastroenterol. 2017, 30, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Arun, G.; Diermeier, S.D.; Spector, D.L. Therapeutic Targeting of Long Non-Coding RNAs in Cancer. Trends. Mol. Med. 2018, 24, 257–277. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, J.; Ding, J.; Shen, X.; Zhou, J.; Xu, Z. LncRNA Blnc1 expression and its effect on renal fibrosis in diabetic nephropathy. Am. J. Transl. Res. 2019, 11, 5664. [Google Scholar]

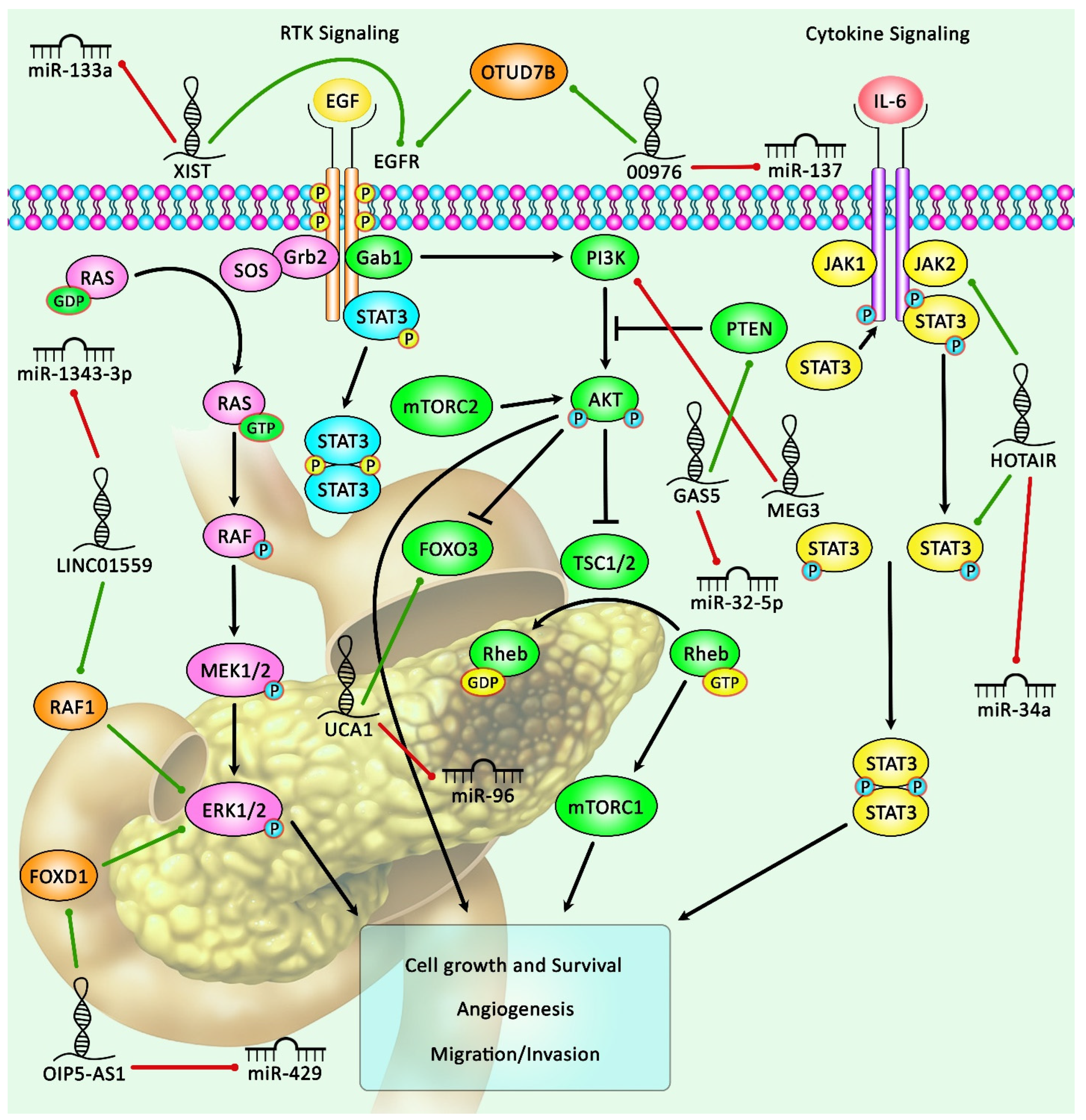
| LncRNA | Sample | Cell Line | Interaction | Signaling Pathway | Clinical Properties | Method | Function | Ref. |
|---|---|---|---|---|---|---|---|---|
| C9orf139 | 54 pairs of tumor and ANTs | AsPC-1, BxPC3, PANC1, PaCa-2, SW-1990, HPDE6-C7 | miR-663a/Sox12 | _ | Tumor stage, lymph nodemetastasis | qRT-PCR, Western blotting, RNA immunoprecipitation, RNA pull-down, luciferase reporter assay | High expression of LncRNA C9orf139 is associated with the poor clinicopathological feature of PC patients | [9] |
| CRNDE | 58 pairs of tumor and ANTs | SW-1990, PANC-1,CAPAN-1, JF305 BxPC-3,HPDE6-C7 | miR-384 | _ | Tumordifferentiation, tumor size, TNM stage, and lymph nodal metastasis | qRT-PCR, luciferase reporter assays, Western blotting, immunohistochemistry (IHC) analysis | LncRNA CRNDE plays an oncogenic role in PC tissue and cell lines via directly targeting miR-384 | [10] |
| H19 | 139 invasive ductal carcinoma samples | PANC-1 | - | - | In situ hybridization, DNA microarray analysis, qRT-PCR | H19 affects cell motility but not cell growth rate | [11] | |
| HOTAIR | _ | HPDE6-C7, SU.86.86, CFPPAC-1, SW-1990, PL45 | miR-34a | JAK2/STAT3 Pathway | _ | qRT-PCR, Western blotting, RNA pull-down | LncRNA HOTAIR can activate the JAK2/STAT3 pathway by targeting miR-34 and then enhancing the proliferation and invasion of PC cells | [12] |
| HOTTIP | Panc-1, L3.6pL, and MiaPaCa2 | HOXA10, HOXB2, HOXA11, HOXA9, and HOXA1 | - | - | Illumina Human V.3 HT12 Beadchip array | HOTTIP regulates the proliferation, apoptosis, and migration of PC cells | [13] | |
| HOXA-AS2 | 16 pairs of tumor and ANTs, 12 pairs of tumor and ANTs | AsPC-1, BxPC-3, PANC-1 | enhancer of zeste homolog 2 (EZH2), lysine-specific demethylase 1 (LSD1) | _ | _ | qRT-PCR | lncRNA HOXA-AS2 plays an oncogenic role in pancreatic cancer tissue | [14] |
| LINC00976 | _ | CFPAC-1, MIA-PaCa-2, PANC-1, BxPC-3,CFPAC-1, ASPC-1, Panc03.27, Capan-2 | miR-137/ OTUD7B | EGFR/MAPK signaling pathway | Tumor size, lymph node metastasis, perineural invasion, vascularinvasion, distant metastasis ability | In situ hybridization (ISH), qRT-PCR | LINC00976 plays an oncogenic role in pancreatic cancer tissue and promotes invasion, migration, and proliferation via up-regulating OTUD7B and then targeting miR-137 | [7] |
| LINC00462 | _ | SW-1990, BxPC3,PANC-1, AsPC-1, CFPAC-1, HPDE6-C7 | miR-665, TGFBR1, TGFBR2 | SMAD2/3 signaling pathway | Large tumor size, poor tumor differentiation, TNM stage, distant metastasis | qRT-PCR, CCK-8 assay, Western blotting, flow cytometry analyses, immunofluorescence | Over-expression of LINC00462 significantly promotes EMT and cell proliferation and suppresses cell apoptosis via up-regulating TGFBR1 and TGFBR2 | [8] |
| LINC01559 | 55 pairs of tumor and ANTs | AsPC-1, BxPC-3, PANC-1, MIA- PaCa-2, SW-1990, HPDE | miR-1343-3p/RAF1 | ERK signaling pathway | Large tumors, lymph node metastasis, | RT-qPCR, RIP assay, CCK-8 assay, Western blotting, immunohistochemistry (IHC) | High expression of LINC01559 enhances proliferation of pancreatic cancer cells and metastasis by up-regulating Raf1 and activating the ERK pathway | [15] |
| LINC00152 | 28 pairs of tumor and ANTs | BxPC3, Panc1, AsPC1, SW-1990, HPDE6-C7 | miR-150 | _ | _ | qRT-PCR, CCK-8 assay, EDU assay, luciferase reporter assay | LINC00152 can suppress miR-150 and then promote pancreatic cancer cells progressions | [16] |
| LINC00958 | _ | PANC-1, Capan-2, SW-1990, BxPC-3,HPDE | miR-330-5p | _ | _ | qRT-PCR, Western blotting, fluorescent in situ hybridization (FISH), RNA immunoprecipitation (RIP) | LINC00958 enhances the EMT process and metastatic ability of PC cells | [17] |
| LUCAT1 | 60 pairs of tumor and ANTs | BxPC-3, Capan2, AsPC-1, PANC-1, HPDE6c7 | miR-539 | _ | tumor size, lymphatic invasion. | qRT-PCR, in situ hybridization, Western blotting | LUCAT1 can enhance the invasion ability of cells by targeting miR-539 | [18] |
| LINC00994 | 10 pairs of tumor and ANTs | PANC-1, AsPC-1, SW-1990 | miR-765-3p/RUNX2 | _ | _ | Microarrays, qRT-PCR, flow cytometry, luciferase assay, Western blotting | LINC00994 acts as an oncogene and its inhibition can suppress RUNX2 by targeting miR-765-3p | [19] |
| LINC01207 | 36 pairs of tumor and ANTs | PANC-1, BxPC-3, Mpanc-96, PaTu-8988 | miR-143-5p | _ | _ | qRT-PCR, RNA pull-down, RNA immunoprecipitation (RIP), flow cytometry, immunofluorescence staining, Western blotting | Its inhibition can induce apoptosis and autophagy activity of PC cells via targeting miR-143-5p | [20] |
| MACC1-AS1 | 98 pairs of tumor and ANTs, 124 pairs of tumor and ANTs | BxPC-3, PANC-1, MIA-PaCa-2, KP-2, AsPC-1, Capan-1 | PAX8 | NOTCH1 signaling pathway | _ | lncRNA microarray, qRT-PCR, luciferase analyses, RNAimmunoprecipitation | High expression of LncRNA MACC1-AS1 can induce pancreatic cancer cells proliferation and promote metastasis through regulating the PAX8/NOTCH1 signaling pathway | [6] |
| OIP5-AS1 | 110 pairs of tumor and ANTs | PANC-1, BxPC-3, AsPC-1, CFPAC-1, HPDE6-C7 | miR-429, FOXD1, ERK pathway | ERK pathway | Tumor size, distant metastasis, TNM stage | qRT-PCR, RNA immunoprecipitation, RNA pull-down, luciferase reporter assay, Western blotting | High expression of LncRNA OIP5-AS1 can increase EMT process, invasion, and PC cell proliferation via activating the ERK pathway | [21] |
| PVT1 | 30 pairs of tumor and ANTs | HPAC, DANG, BxPC-3, PANC1, ASPC-1, H6C7 | miR-519d-3p | glycolysispathway | lymph node metastasis | qRT-PCR, Western blotting, RNA immunoprecipitation (RIP) assay, RNA pull-down assay, immunohistochemistry (IHC) | PTV1 induces downregulation of miR-519d-3p and then promotes the progression of pancreatic cancer | [22] |
| RP11-567G11.1 | 78 tumor tissues and 7 non-tumor tissues | SW-1990, BxPC-3, PANC-1 | _ | NOTCH signaling pathway | _ | In situ hybridization, CCK8 and flow cytometry, Western blotting, qPCR | Inhibition of LncRNA RP11-567G11.1 can induce apoptosis and suppress cancer cell proliferation | [23] |
| SBF2-AS1 | _ | PANC-1, BxPC-3, SW-1990, Capan2, THP-1 | miR-122-5p | SMAD signaling pathway | _ | Flow cytometry, RNA-fluorescence in situ hybridization(FISH), qRT-PCR, Western blotting, RNA immunoprecipitation, RNA pull-down assays | The expression level of SBF2-AS1 is increased in M2 macrophage exosomes and plays an oncogenic role in pancreatic cancer tissue | [24] |
| SNHG7 | 50 pairs of tumor and ANTs | PANC-1, SW-1990, BxPC-3 AsPC-1, HPDE | miR-146b-5, roundabout homolog 1(Robo1) | _ | Tumor size, distant metastasis, lymph node metastasis, | qRT-PCR, Flow cytometry analysis, luciferase reporter assay, RNA immunoprecipitation (RIP) assay, RNA pull-down assay, Western blotting | High expression of LncRNA SNHG7 can promote the progression of PC by positively affecting Robo1 | [25] |
| SNHG12 | 15 pairs of tumor and ANTs | HPDE6, BxPC-3, CAPAN1, PANC1, SW-1990 | miR-320b | _ | _ | qRT-PCR, flow cytometry, luciferase assay | LncRNA SNHG12 can increase the invasion, EMT, and proliferation of cancer cells by negatively affecting miR-320b | [26] |
| SNHG14 | 45 pairs of tumor and ANTs | CFPAC-1, BxPC-3, L3.6pl Panc-1, HPDE6C7 | miR-613 | _ | Poor tumor differentiation, advanced TNM stage, nodal metastasis | qRT-PCR, fluorescent in situ hybridization, flow cytometry, Western blotting | Increased expression of SNHG14 can promote the progression of pancreatic cancer by inhibiting caspase-3 activity and down-regulation of miR-613 | [27] |
| SNHG15 | 48 pairs of tumor and ANTs | AsPC-1, BxPC-3, HPDE6 | zeste homolog 2 | _ | tumor size, TNM stage,lymph node, metastasis | qRT-PCR, Flow cytometry, Western blotting, RNA immunoprecipitation, chromatin immunoprecipitation (ChIP) | SNHG15 plays an oncogenic role in pancreatic cancer tissue by inversely regulating target genes | [28] |
| SPRY4-IT1 | _ | BxPC-3, PANC-1 | Cdc20 | _ | _ | qRT-PCR, Western blotting, wound healing assay, Transwell assay | SPRY4-IT1 acts as an oncogene in PC tissue, and its inhibition induces depletion of PC progression | [29] |
| TP73-AS1 | 77 pairs of tumor and ANTs | HPDE6-C7, SW-1990, CAPAN-1, JF305, PANC-1, BxPC-3 | miR-141 | _ | TNM stage, lymph node metastasis | qRT-PCR, luciferase reporter assays, Western blotting | High expression of lncRNA TP73-AS1 induces migration, invasion, and PC cell proliferation | [30] |
| UCA1 | 120 pairs of tumor and ANTs | PANC-1, BxPC-3, Capan-1, SW-1990, HPDE6C-7 | _ | _ | Tumor size, depth of invasion, CA19-9 level, tumor stage | qRT-PCR, flow cytometry, Western blotting, | Low expression of LncRNA UCA1 can reduce the proliferation of PC cells and induce cell cycle arrest | [31] |
| UCA1 | 36 pairs of tumor and ANTs | HPC-Y5, PANC-1, SW-1990, AsPC-1 | miR-96/FOXO3 | _ | _ | qRT-PCR, Western blotting, immunohistochemistry, flow cytometry, luciferase assay, RNA in situ hybridization | LncRNA UCA1 acts as an oncogene in PC tissue and cell lines via negative regulating miR-96 | [32] |
| XIST | 30 pairs of tumor and ANTs | PANC-1, HEK293T | miR-141-3p, TGF-β2 | TGF-β signaling pathway | _ | qRT-PCR, luciferase reporter assay, Western blotting | LncRNA XIST plays an oncogenic role in PC tissue through targeting miR-141-3p and the TGF-β signaling pathway | [33] |
| XIST | 64 pairs of tumor and ANTs | H6c7, Patu8988,SW-1990, BxPC-3, AsPC-1, CFPAC-1, PANC-1 | miR-133a/EGFR | EGFR/Akt signaling | Larger tumor size, perineuralinvasion, lymph node metastasis, shorter overall survival | qRT-PCR, BrdU cell proliferation assay, luciferase reporter assay | LncRNA XIST can induce PC cell proliferation through negatively regulating miR133a and positively regulating EGFR | [34] |
| ZEB2-AS1 | 39 pairs of tumor and ANTs | AsPC-1, HPAC, Cfpac-1, PANC-1, HPDE | miR-204/ HMGB1 | _ | _ | q-RT-PCR, Western blotting immunofluorescence assay, luciferase reporter assay, RNA-binding protein immunoprecipitation (RIP) assay, LncRNA array | Overexpression of LncRNA ZEB2-AS1 induces cell proliferation and invasion by negatively affecting miR-204 | [35] |
| LncRNA | Sample | Cell Line | Interaction | Signaling Pathway | Clinical Properties | Method | Function | Ref. |
|---|---|---|---|---|---|---|---|---|
| ENST00000480739 | 35 patients with pancreatic cancer | ASPC-1, BXPC-3, CFPAC-1, PANC-1 and SW1990 | OS-9 | - | Tumor node metastasis stage and lymph node metastasis | Transwell invasion assay, ELISA, Western blot | ENST00000480739 participates in tumor metastasis and progression | [43] |
| LINC01111 | _ | HPDE, PANC-1, MIA-PaCa-2,SW-1990, Capan-2, Panc 03.27, BxPC-3, CFPAC-1 | miR-3924 | SAPK/JNK signaling pathway | TNM stage (negatively), survival (positively) | qRT-PCR, EdU incorporation assay, scratch wound healing assays, Western blotting, RNA microarrays, in situ hybridization | LINC01111 plays a tumor-suppressive role in PC tissue and cell lines via inhibition of the SAPK/JNK signaling pathway | [38] |
| LINC01963 | 67 pairs of tumor and ANTs | PANC-1, CFPAC-1, BxPC-3, SW-1990, AsPC1, HPDE6-C7 | miR-641/TMEFF2 | _ | Distantmetastasis, TNM stag | qRT-PCR, flow cytometry assay, luciferase assay, RNA immunoprecipitation, Western blotting | High expression of LncRNA LINC01963 can induce inhibition of pancreatic cancer progression via negatively regulating miR-641 | [39] |
| DGCR5 | 20 pairs of tumor and ANTs | SW-1990, PANC-1, HPDE6-C7 | miR-27a-3p/BNIP3 | p38 MAPK pathway | _ | qRT-PCR, Western blotting, RNA immunoprecipitation (RIP), RNA pull-down assay, luciferase reporter assay, flow cytometric (FCM) analysis | Down-regulation of lncRNA DGCR 5 affects apoptosis through regulating BNIP3 and the p38 MAPK pathway | [42] |
| MEG3 | 30 pairs of tumor and ANTs | PANC-1 | PI3K protein | PI3K/AKT/Bcl-2/Bax/cyclin D1/P53 and PI3K/AKT/MMP-2/MMP-9 signaling pathways | Tumor size, metastasis, and vascular invasion | Immunohistochemistry (IHC) assay, qRT-PCR, Western blotting | LncRNA MEG 3 acts as a tumor-suppressor in PC tissue and cell lines | [40] |
| GAS5 | 22 pairs of tumor and ANTs | PANC-1, BxPC-3, HPDE6-C7 | miR-32-5p | PTEN signaling pathway | _ | qRT-PCR, Western blotting, flow cytometry analysis, RNA immunoprecipitation (RIP) assay, RNA pull-down assay | GAS5 exhibits tumor suppressor activity in PDAC tissue samples | [41] |
| LINC00261 | _ | CFPAC-1, BxPC-3, PANC-1, AsPC-1, HPDE6-C7 | miR-23a-3p | _ | _ | qRT-PCR, flow cytometry, Western blot | A low expression level of LINC00261 can promote PC progression by targeting miR-23a-3p | [44] |
| LncRNA | Expression Pattern | Detection Method for LncRNAs | Sample | Area Under the Curve (AUC) | References |
|---|---|---|---|---|---|
| LncRNA-UFC1 | Up-regulation | qRT-PCR | 48 serum samples of patients | 0.810 | [47] |
| RP11-263F15.1 | Up-regulation | Microarray, qRT-PCR | 71 pairs of tumor and ANTs | 0.843 | [48] |
| ABHD11-AS1 | Up-regulation | qRT-PCR | 15 serum samples of patients and 30 healthy individuals | 0.887 | [49] |
| LINC00675 | Up-regulation | Microarray, qRT-PCR | 45 pairs of tumor and ANTs | 0.928 | [45] |
| HULC | Up-regulation | qRT-PCR | 60 serum samples of patients and 60 healthy individuals | 0.856 | [50] |
| C9orf139 | Up-regulation | qRT-PCR | 54 pairs of tumor and ANTs | 0.923 | [46] |
| PVT1 | Up-regulation | qRT-PCR | Salivary samples from 55 patients with resectable pancreatic cancer, 20 patients with benign pancreatic lesions, and 55 normal controls | 0.84 (cancer vs. benign lesion), 0.90 (cancer vs. healthy state) | [51] |
| HOTAIR | Up-regulation | qRT-PCR | 0.86 (cancer vs. benign lesion), 0.88 (cancer vs. healthy state) |
| LncRNA | Expression Pattern | Sample | Kaplan–Meier Analysis | Multivariate Analysis | References |
|---|---|---|---|---|---|
| RUNX1-IT1 | Up-regulated | 83 tumor tissues and 38 ANTs and 15 normal pancreatic tissues | Overexpression of lncRNA RUNX1-IT1 was associated with poor overall survival | Expression of lncRNA RUNX1-IT1 was identified as an independent prognostic factor for pancreatic cancer patients | [52] |
| ENSG00000254041.1 | Up-regulated | 70 pairs of tumor and ANTs | Its high expression was associated with poor overall survival | Expression of lncRNA ENSG00000254041.1 can be an independent predictor of pancreatic cancer survival | [53] |
| MALAT1 | Up-regulated | 45 pairs of tumor and ANTs | Its high expression was associated with poor disease-free survival | Expression of lncRNA MALAT1 can be an independent prognostic factor for disease-specific survival in patients | [54] |
| LOC285194 | Down-regulated | 85 pairs of tumor and ANTs | Low expression of lncRNA LOC285194 was associated with poor overall survival | Low expression of lncRNA LOC285194 can be an independent poorprognostic factor in pancreatic cancer patients | [55] |
| LncRNA-UFC1 | Up-regulated | 48 serum samples of patients | Overexpression of lncRNA-UFC1 was associated with shorter progression-free survival and overall survival | Expression levels of lncRNA-UFC1 were identified as independent prognostic factors in patients | [47] |
| RP11-263F15.1 | Up-regulated | 71 pairs of tumor and ANTs | Increased lncRNA RP11-263F15.1 expression level was associated with poor overall survival | The expression level of lncRNA RP11-263F15.1 was not independent of prognostic factors in patients | [48] |
| BC008363 | Down-regulated | 30 pairs of tumor and ANTs | Overexpression of lncRNA BC008363 indicated better overall survival | _ | [56] |
| MEG3 | Down-regulated | 25 pairs of tumor and ANTs | Increased LncRNA MEG3 expression was associated with longer overall survival | _ | [57] |
| HULC | Up-regulated | 25 pairs of tumor and ANTs | A high expression level of LncRNA HULC was associated with shorter overall survival | The expression level of LncRNA HULC identified as an independentpredictor for overall survival | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghafouri-Fard, S.; Fathi, M.; Zhai, T.; Taheri, M.; Dong, P. LncRNAs: Novel Biomarkers for Pancreatic Cancer. Biomolecules 2021, 11, 1665. https://doi.org/10.3390/biom11111665
Ghafouri-Fard S, Fathi M, Zhai T, Taheri M, Dong P. LncRNAs: Novel Biomarkers for Pancreatic Cancer. Biomolecules. 2021; 11(11):1665. https://doi.org/10.3390/biom11111665
Chicago/Turabian StyleGhafouri-Fard, Soudeh, Mohadeseh Fathi, Tianyue Zhai, Mohammad Taheri, and Peixin Dong. 2021. "LncRNAs: Novel Biomarkers for Pancreatic Cancer" Biomolecules 11, no. 11: 1665. https://doi.org/10.3390/biom11111665








