Possible Role of Leptin in Atopic Dermatitis: A Literature Review
Abstract
:1. Introduction
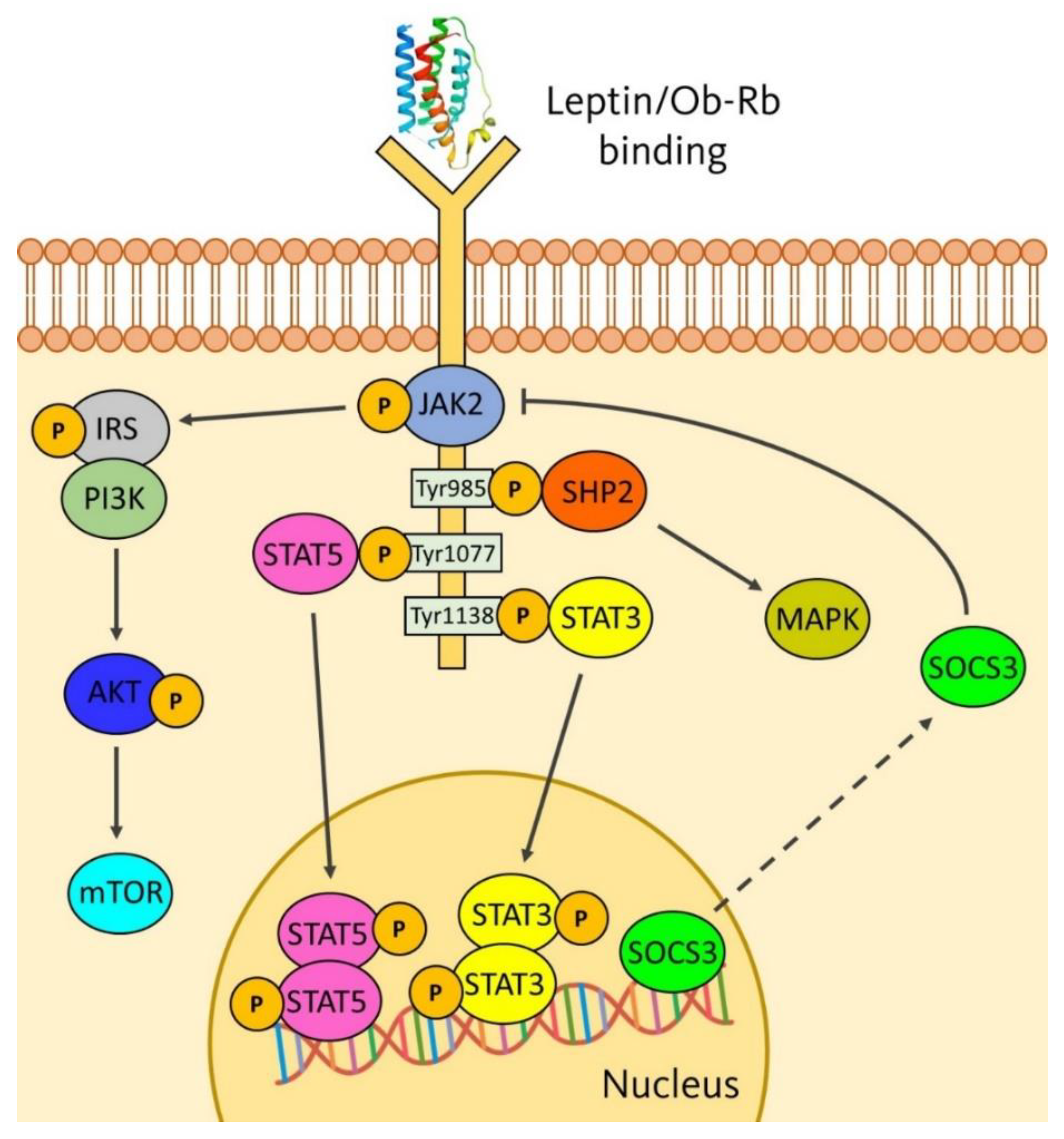
2. Leptin at a Glance
3. Role of Obesity in AD
4. Role of Leptin in AD
5. Overview and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nutten, S. Atopic dermatitis: Global epidemiology and risks factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Karimkhani, C.; Silverberg, J.I.; Dellavalle, R.P. Defining intrinsic vs. extrinsic atopic dermatitis. Dermatol. Online J. 2015, 21, 13030. [Google Scholar]
- Silverberg, J.I.; Vakharia, P.P.; Chopra, R.; Sacotte, R.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.Y. Phenotypical differences of childhood- and adult-onset atopic dermatitis. J. Allergy Clin. Immunol. Pract. 2018, 6, 1306–1312. [Google Scholar] [CrossRef]
- Kaufman, B.P.; Guttman-Yassky, E.; Alexis, A.F. Atopic dermatitis in diverse racial and ethnic groups-Variations in epidemiology, genetics, clinical presentation and treatment. Exp. Dermatol. 2018, 27, 340–357. [Google Scholar] [CrossRef] [Green Version]
- Lifschitz, C. The impact of atopic dermatitis on quality of life. Ann. Nutr. Metab. 2015, 66, 34–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, Z.; Ulrik, C.S.; Agner, T.; Thomsen, S.F. Is atopic dermatitis associated with obesity? A systematic review of observational studies. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Bhurosy, T.; Jeewon, R. Overweight and Obesity Epidemic in Developing Countries: A Problem with Diet, Physical Activity, or Socioeconomic Status? Sci. World J. 2014, 2014, 964236. [Google Scholar] [CrossRef] [Green Version]
- Versini, M.; Jeandel, P.-Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of leptin in inflammation and vice versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar]
- Eichenfield, L.F.; Tom, W.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis. Part 1: Diagnosis and Assessment of Atopic Dermatitis. J. Am. Acad. Dermatol. 2014, 70, 338–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thijs, J.L.; Nierkens, S.; Herath, A.; Bruijnzeel-Koomen, C.A.F.; Knol, E.F.; Giovannone, B.; de Bruin-Weller, M.S.; Hijnen, D. A panel of biomarkers for disease severity in atopic dermatitis. Clin. Exp. Allergy 2015, 45, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Thijs, J.; Krastev, T.; Weidinger, S.; Buckens, C.F.; de Bruin-Weller, M.; Bruijnzeel-Koomen, C.; Flohr, C.; Hijnen, D. Biomarkers for atopic dermatitis: A systematic review and meta-analysis. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 453–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esaki, H.; Brunner, P.M.; Renert-Yuval, Y.; Czarnowicki, T.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; Johnson, D.B.; et al. Early-onset pediatric atopic dermatitis is Th2 but also Th17 polarized in skin. J. Allergy Clin. Immunol. 2016, 138, 1639–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabanillas, B.; Brehler, A.-C.; Novak, N. Atopic dermatitis phenotypes and the need for personalized medicine. Curr. Opin. Allergy. Clin. Immunol. 2017, 17, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Nygaard, U.; Hvid, M.; Johansen, C.; Buchner, M.; Fölster-Holst, R.; Deleuran, M.; Vestergaard, C. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1930–1938. [Google Scholar] [CrossRef]
- Kabashima, K. New concept of the pathogenesis of atopic dermatitis: Interplay among the barrier, allergy, and pruritus as a trinity. J. Dermatol. Sci. 2013, 70, 3–11. [Google Scholar]
- Arbes, S.J., Jr.; Gergen, P.J.; Elliott, L.; Zeldin, D.C. Prevalences of positive skin test responses to 10 common allergens in the US population: Results from the third National Health and Nutrition Examination Survey. J. Allergy Clin. Immunol. 2005, 116, 377–383. [Google Scholar] [CrossRef]
- Schulte-Herbruggen, O.; Folster-Holst, R.; von Elstermann, M.; Augustin, M.; Hellweg, R. Clinical relevance of nerve growth factor serum levels in patients with atopic dermatitis and psoriasis. Int. Arch. Allergy Immunol. 2007, 144, 211–216. [Google Scholar] [CrossRef]
- Dhar, S.; Malakar, R.; Chattopadhyay, S.; Banerjee, R.; Ghosh, A. Correlation of the severity of atopic dermatitis with absolute eosinophil counts in peripheral blood and serum IgE levels. Indian J. Dermatol. Venereol. Leprol. 2005, 71, 246–249. [Google Scholar] [PubMed]
- Gerdes, S.; Kurrat, W.; Mrowietz, U. Serum mast cell tryptase is not a useful marker for disease severity in psoriasis or atopic dermatitis. Br. J. Dermatol. 2009, 160, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Fariñas, M.; Dhingra, N.; Gittler, J.; Shemer, A.; Cardinale, I.; de Guzman-Strong, C.; Krueger, J.G.; Guttman-Yassky, E. Intrinsic atopic dermatitis shows similar Th2 and higher immune activation compared with extrinsic atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dopytalska, K.; Baranowska-Bik, A.; Roszkiewicz, M.; Bik, W.; Walecka, I. The role of leptin in selected skin diseases. Lipids Health Dis. 2020, 19, 215. [Google Scholar] [CrossRef]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose Tissue in Obesity-Related Inflammation and Insulin Resistance: Cells, Cytokines, and Chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef] [Green Version]
- Mahbouli, S.; Der Vartanian, A.; Ortega, S.; Rougé, S.; Vasson, M.-P.; Rossary, A. Leptin induces ROS via NOX5 in healthy and neoplastic mammary epithelial cells. Oncol. Rep. 2017, 38, 3254–3264. [Google Scholar] [PubMed] [Green Version]
- Ingalls, A.M.; Dickie, M.M.; Snell, G.D. Obese, a new mutation in the house mouse. J. Hered. 1950, 41, 317–318. [Google Scholar] [PubMed]
- Hummel, K.P.; Dickie, M.M.; Coleman, D.L. Diabetes, a new mutation in the mouse. Science 1966, 153, 1127–1128. [Google Scholar]
- Zhang, Y.; Proenca, R.; Maffei, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Zhang, F.; Basinski, M.B.; Beals, J.M.; Briggs, S.L.; Churgay, L.M.; Clawson, D.K.; DiMarchi, R.D.; Furman, T.C.; Hale, J.E.; Hsiung, H.M.; et al. Crystal structure of the obese protein leptin-E100. Nature 1997, 387, 206–209. [Google Scholar] [CrossRef]
- Gambino, Y.P.; Pérez-Pérez, A.; Dueñas, J.L.; Calvo, J.C.; Sánchez-Margalet, V.; Varone, C.L. Regulation of leptin expression by 17beta-estradiol in human placental cells involves membrane associated estrogen receptor alpha. Biochim. Biophys. Acta 2012, 1823, 900–910. [Google Scholar] [PubMed] [Green Version]
- Hu, X.; Juneja, S.C.; Maihle, N.J.; Cleary, M.P. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J. Natl. Cancer Inst. 2002, 94, 1704–1711. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, C.; Lindell, K.; Svensson, E.; Bergh, C.; Lind, P.; Billig, H.; Carlsson, L.M.S.; Carlsson, B. Expression of functional leptin receptors in the human ovary. J. Clin. Endocrinol. Metab. 1997, 82, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Popovic, V.; Damjanovic, S.; Dieguez, C.; Casanueva, F.F. Leptin and the pituitary. Pituitary 2001, 4, 7–14. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Maymó, J.; Dueñas, J.L.; Varone, C.; Sánchez-Margalet, V. Role of leptin in female reproduction. Clin. Chem. Lab. Med. 2015, 53, 15–28. [Google Scholar]
- Procaccini, C.; Pucino, V.; Mantzoros, C.S.; Matarese, G. Leptin in autoimmune diseases. Metabolism. 2015, 64, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Denroche, H.C.; Huynh, F.K.; Kieffer, T.J. The role of leptin in glucose homeostasis. J. Diabetes Investig. 2012, 3, 115–129. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Cortegana, C.; García-Galey, A.; Tami, M.; Del Pino, P.; Carmona, I.; López, S.; Alba, G.; Sánchez-Margalet, V. Role of leptin in non-alcoholic fatty liver disease. Biomedicines 2021, 9, 762. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, F.; Pérez-Pérez, A.; De la Cruz-Merino, L.; Sánchez-Margalet, V. Obesity and Breast cancer: Role of leptin. Front. Oncol. 2019, 9, 596. [Google Scholar] [CrossRef]
- Jiménez-Cortegana, C.; López-Saavedra, A.; Sánchez-Jiménez, F.; Pérez-Pérez, A.; Castiñeiras, J.; Virizuela-Echaburu, J.A.; De la Cruz-Merino, L.; Sánchez-Margalet, V. Leptin, both bad and Good actor in cancer. Biomolecules 2021, 11, 913. [Google Scholar]
- Li, S.; Li, X. Leptin in normal physiology and leptin resistance. Sci. Bull. 2016, 61, 1480–1488. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-K.; Ahima, R.S. Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metabolism 2016, 64, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernández, C.; Mera, A.; Gonzalez-Gay, M.A.; Gómez, R.; Gualillo, O. Obesity, Fat Mass and Immune System: Role for Leptin. Front. Physiol. 2018, 9, 640. [Google Scholar] [PubMed] [Green Version]
- Zhou, Y.; Rui, L. Leptin signaling and leptin resistance. Front. Med. 2013, 7, 207–222. [Google Scholar] [CrossRef]
- Yang, R.; Barouch, L.A. Leptin signaling and obesity: Cardiovascular consequences. Circ. Res. 2007, 101, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ishida-Takahashi, R.; Villanueva, E.C.; Fingar, D.C.; Münzberg, H.; Myers Jr, M.G. The long form of leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J. Biol. Chem. 2007, 282, 31019–31027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Hulver, M.; McMillan, R.P.; Cai, L.; Kershaw, E.E.; Yu, L.; Xue, B.; Shi, H. Regulation of insulin and leptin signaling by muscle suppressor of cytokine signaling 3 (SOCS3). PLoS ONE 2012, 7, e47493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR signaling in cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [Green Version]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Gomez-Llorente, M.; Romero, R.; Chueca, N.; Martinez-Cañavate, A.; Gomez-Llorente, C. Obesity and Asthma: A Missing Link. Int. J. Mol. Sci. 2017, 18, 1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shipman, A.R.; Millington, G.W.M. Obesity and the skin. Br. J. Dermatol. 2011, 165, 743–750. [Google Scholar] [PubMed]
- Silverberg, J.I.; Kleiman, E.; Lev-Tov, H.; Silverberg, N.B.; Durkin, H.G.; Joks, R.; Smith-Norowitz, T.A. Association between obesity and atopic dermatitis in childhood: A case-control study. J. Allergy Clin. Immunol. 2011, 127, 1180. [Google Scholar]
- Baumann, S.; Lorentz, A. Obesity—A Promoter of Allergy? Int. Arch. Allergy Immunol. 2013, 162, 205–213. [Google Scholar] [CrossRef]
- Zhang, A.; Silverberg, J.I. Association of atopic dermatitis with being overweight and obese: A systematic review and metaanalysis. J. Am. Acad. Dermatol. 2015, 72, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Guida, B.; Nino, M.; Perrino, N.R.; Laccetti, R.; Trio, R.; Labella, S.; Balato, N. The impact of obesity on skin disease and epidermal permeability barrier status. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 191–195. [Google Scholar] [CrossRef]
- Hirt, P.A.; Castillo, D.E.; Yosipovitch, G.; Keri, J.E. Skin changes in the obese patient. J. Am. Acad. Dermatol. 2019, 81, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Lindgren, T.H.; Koch, C.A.; Brodell, R.T. Obesity as a risk factor for malignant melanoma and non-melanoma skin cancer. Rev. Endocr. Metab. Disord. 2016, 17, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Pothiawala, S.; Qureshi, A.A.; Li, Y.; Han, J. Obesity and the incidence of skin cancer in US Caucasians. Cancer Causes Control 2012, 23, 717–726. [Google Scholar]
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.L.; Margolis, D.J.; De Bruin-Weller, M.; Eckert, L. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 2018, 73, 1284–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—the biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Nahm, D.-H. Associations of Atopic Dermatitis with Obesity and Unmarried Status in Young Adults: Evidence for Atopic Dermatitis as a Life-Style Disorder with High Social Impact. Allergy Asthma Immunol. Res. 2016, 8, 89–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulet, L.P. Obesity and atopy. Clin. Exp. Allergy 2015, 45, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Brandwein, M.; Katz, I.; Katz, A.; Kohen, R. Beyond the gut: Skin microbiome compositional changes are associated with BMI. Hum. Microbiome, J. 2019, 13, 100063. [Google Scholar] [CrossRef]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.-C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddel, S.; Del Chierico, F.; Quagliariello, A.; Giancristoforo, S.; Vernocchi, P.; Russo, A.; Fiocchi, A.; Rossi, P.; Putignani, L.; El Hachem, M. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci. Rep. 2019, 9, 4996. [Google Scholar] [CrossRef] [Green Version]
- Son, M.J.; Yang, G.-J.; Jo, E.-H.; Shim, Y.-H.; Kang, S.-J.; Hong, J.-E.; Kim, Y.-E.; Lee, J.-E.; Chun, J.; Park, S.; et al. Association of atopic dermatitis with obesity via a multi-omics approach. Medicine 2019, 98, e16527. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, A.K.; Szepietowski, J.C.; Szafraniec, K.; Jaworek, M.; Halubiec, P.; Wojas-Pelc, A.; Pokorski, M. Adipokines as biomarkers of atopic dermatitis in adults. J. Clin. Med. 2020, 9, 2858. [Google Scholar]
- Banihani, S.A.; Abu-Alia, K.F.; Khabour, O.F.; Alzoubi, K.H. Association between Resistin Gene Polymorphisms and Atopic Dermatitis. Biomolecules 2018, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farag, A.G.A.; Hammam, M.A.; Khaled, H.N.; Soliman, S.; Tayel, N.R.; El-Shamendy, A.A.; Shehata, W.A. Resistin adipokin in atopic dermatitis patients: A clinical, biochemical, and genetic study. J. Cosmet. Dermatol. 2020, 19, 2929–2935. [Google Scholar] [PubMed]
- Machura, E.; Szczepanska, M.; Ziora, K.; Ziora, D.; Swietochowska, E.; Barc-Czarnecka, M.; Kasperska-Zajac, A. Evaluation of adipokines: Apelin, visfatin, and resistin in children with atopic dermatitis. Mediators Inflamm. 2013, 2013, 760691. [Google Scholar] [PubMed]
- Kimata, H. Elevated serum leptin in AEDS. Allergy 2002, 57, 179. [Google Scholar] [CrossRef] [PubMed]
- Kimata, H. Elevation of breast milk leptin levels by laughter. Horm. Metab. Res. 2004, 36, 254–256. [Google Scholar]
- Bostanci, I.; Atli, O.; Celebi, N.; Tasar, A.; Alpkarakoç, E.; Dallar, Y. Serum leptin level in children with atopic dermatitis-treated topical steorids. Pediatr. Allergy Immunol. 2004, 15, 267–269. [Google Scholar] [CrossRef]
- Kimata, H. Increased incidence of fatty liver in non-obese Japanese children under 1 year of age with or without atopic dermatitis. Public Health 2006, 120, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Koenig, W.; Rapp, K.; Wabitsch, M.; Zoellner, I.; Weiland, S.K. Associations of adipokines with asthma, rhinoconjuctivitis, and eczema in German schoolchildren. Pediatr. Allergy Immunol. 2009, 20, 81–88. [Google Scholar]
- Balato, N.; Nino, M.; Patruno, C.; Matarese, G.; Ayala, F. “Eczemas” and leptin. Dermatitis 2011, 22, 320–323. [Google Scholar]
- Jeong, K.-Y.; Lee, J.; Li, C.; Han, T.; Lee, S.-B.; Lee, H.; Back, S.K.; Na, H.S. Juvenile Obesity aggravates disease severity in a rat model of atopic dermatitis. Allergy Asthma Immunol. Res. 2015, 7, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, S.; Yoon, W.S.; Cho, Y.; Park, S.H.; Choung, J.T.; Yoo, Y. Leptin and atopic dermatitis in Korean Elementary School Children. J. Allergy Asthma Immunol. 2016, 15, 138–144. [Google Scholar]
- Han, B.; Wu, H.B.; Bae, J.M.; Son, S.-J.; Lee, J.-H.; Han, T.Y. Serum leptin and adiponectin in atopic dermatitis (AD) and their relation to disease severity. J. Am. Acad. Dermatol. 2016, 75, 629–631. [Google Scholar]
- Mohamed, S.A.; Alsayad, T.; El-Askary, A.; Alwafa, H.A. Serum Leptin Level among School Children with Atopic Dermatitis. Neonat. Pediatr. Med. 2017, 3, 130. [Google Scholar] [CrossRef]
- Jung, M.J.; Kim, H.R.; Kang, S.Y.; Kim, H.O.; Chung, B.Y.; Park, C.W. Effect of weight reduction on treatment outcomes for patients with atopic dermatitis. Ann. Dermatol. 2020, 32, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Pugliarello, S.; Cozzi, A.; Gisondi, P.; Girolomoni, G. Phenotypes of atopic dermatitis. J. Dtsch. Dermatol. Ges. 2011, 9, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Frazier, W.; Bhardwai, N. Atopic dermatitis: Diagnosis and treatment. Am. Fam. Physician 2020, 101, 590–598. [Google Scholar] [PubMed]
- Ng, P.C.; Lam, C.W.K.; Lee, C.H.; Fok, T.F.; C., K.; Wong, E.; Chan, I.H.S. Changes in serum leptin concentration after corticosteroid treatment in preterm infants. Acta Paediatr. 2002, 91, 684–690. [Google Scholar] [CrossRef]
- Banihani, S.A.; Elmadhoun, R.A.; Khabour, O.F.; Alzoubi, K.H. The rs2167270 polymorphism of leptin gene is associated with atopic dermatitis. Dermato-Endocrinology 2018, 10, e1454191. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Skin barrier abnormalities and immune dysfunction in atopic dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furue, M.; Chiba, T.; Tsuji, G.; Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Kadono, T. Atopic dermatitis: Immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Ilergol. Int. 2017, 66, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.M.; Elbehidy, R.M.; Shokry, D.M.; Elbehidy, E.M. The influence of leptin on Th1/Th2 balance in obese children with asthma. J. Bras. Pneumol. 2013, 39, 562–568. [Google Scholar]
- Mattioli, B.; Giordani, L.; Quaranta, M.G.; Viora, M. Leptin exerts an anti-apoptotic effect on human dendritic cells via the PI3K-Akt signaling pathway. FEBS Lett. 2009, 583, 1102–1106. [Google Scholar] [CrossRef] [Green Version]
- Brunner, P.M.; Suárez-Fariñas, M.; He, H.; Malik, K.; Wen, H.-C.; Gonzalez, J.; Chan, T.C.-C.; Estrada, Y.; Zheng, X.; Khattri, S.; et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci. Rep. 2017, 7, 8707. [Google Scholar] [PubMed]
- De Rosa, V.; Procaccini, C.; Calì, G.; Pirozzi, G.; Fontana, S.; Zappacosta, S.; La Cava, A.; Matarese, G. A key role of leptin in the control of regulatory T cell proliferation. Immunity 2007, 26, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, R.; Wisniewski, J.; Woodfolk, J.A. The role of regulatory T cells in atopic dermatitis. Curr. Probl. Dermatol. 2011, 41, 112–124. [Google Scholar] [PubMed] [Green Version]
- Roesner, L.M.; Floess, S.; Witte, T.; Olek, S.; Huehn, J.; Werfel, T. Foxp3(+) regulatory T cells are expanded in severe atopic dermatitis patients. Allergy 2015, 70, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Procaccini, C.; De Rosa, V.; Horvath, T.L.; La Cava, A. Regulatory T cells in obesity: The leptin connection. Trends Mol. Med. 2010, 16, 247–256. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, A.; Agnihothri, R.; McGirt, L.Y.; Bank, L.G.; Beck, L.A. Atopic dermatitis: A disease caused by innate immune defects? J. Investig. Dermatol. 2009, 129, 14–30. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Zhang, X.; Castillo, E.F.; Luo, Y.; Liu, M.; Yang, X.O. Leptin enhances Th2 and ILC2 responses in allergic airway disease. J. Biol. Chem. 2016, 291, 22043–22052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka, K.-I.; Mizutani, H. The role of cytokines/chemokines in the pathogenesis of atopic dermatitis. Curr. Probl. Dermatol. 2011, 41, 80–92. [Google Scholar] [PubMed]
- Klonowska, J.; Glen, J.; Nowicki, R.J.; Trzeciak, M. New cytokines in the pathogenesis of atopic dermatitis–New therapeutic targets. Int. J. Mol. Sci. 2018, 19, 3086. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.-T.; Goodarzi, H.; Chen, H.-Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310. [Google Scholar] [PubMed]
- Amorim, N.R.T.; Souza-Almeida, G.; Luna-Gomes, T.; Bozza, P.T.; Canetti, C.; Diaz, B.L.; Maya-Monteiro, C.M.; Bandeira-Melo, C. Leptin elicits in vivo eosinophil migration and activation: Key role of mast cell-derived PGD2. Front. Endocrinol. 2020, 11, 572113. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Vilariño-García, T.; Fernández-Riejos, P.; Martín-González, J.; Segura-Egea, J.J.; Sánchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef]
- Grotta, M.B.; Squebola-Cola, D.M.; Toro, A.A.; Ribeiro, M.A.; Mazon, S.B.; Ribeiro, J.D. Antunes, E. Obesity increases eosinophil activity in asthmatic children and adolescents. BMC Pulm. Med. 2013, 13, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzukawa, M.; Nagase, H.; Ogahara, I.; Han, K.; Tashimo, H.; Shibui, A.; Koketsu, R.; Nakae, S.; Yamaguchi, M.; Ohta, K. Leptin enhances survival and induces migration, degranulation, and cytokine synthesis of human basophils. J. Immunol. 2011, 186, 5254–5260. [Google Scholar]
- Żelechowska, P.; Agier, J.; Różalska, S.; Wiktorska, M.; Brzezińska-Błaszczyk, E. Leptin stimulates tissue rat mast cell pro-inflammatory activity and migratory response. Inflamm. Res. 2018, 67, 789–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Zeng, Q.; Luo, X.; Ma, R.; Tang, Y.; Liu, W. Leptin promoted IL-17 production from ILC2s in allergic rhinitis. Mediators Inflamm. 2020, 2020, 9248479. [Google Scholar] [CrossRef] [PubMed]
- Gittler, J.K.; Shemer, A.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.F.; Mitsui, H.; Cardinale, I.; De Guzman-Strong, C.; Krueger, J.G.; et al. Progressive activation of Th2/Th22 cytokines and selective epidermal proteins characterizes acute and chronic dermatitis. J. Allergy. Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Fariñas, M.; Gittler, J.K.; Shemer, A.; Cardinale, I.; Krueger, J.G.; Guttman-Yassky, E. Residual genomic signature of atopic dermatitis despite clinical resolution with narrow-band UVB. J. Allergy Clin. Immunol. 2013, 131, 577–579. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, D.; Wu, X.; Zhang, X.; Zhou, Q.; Luo, Y.; Yang, X.; Chock, C.J.; Liu, M.; Yang, X.O. Leptin promotes allergic airway inflammation through targeting the unfolded protein response pathway. Sci. Rep. 2018, 8, 8905. [Google Scholar] [CrossRef] [PubMed]

| Study (Year) | Country | Type of Study | Life Stage | Subjects * | Correlation Leptin-AD | Correlation Leptin-IgE |
|---|---|---|---|---|---|---|
| Jaworek et al., (2020) [68] | Poland | Humans | Adults | 79 | Positive | No correlation |
| Kimata (2002) [72] | Japan | Humans | Children | 50 | Positive | Positive |
| Kimata (2004) [73] | Japan | Humans | Adults | 60 | Negative | NM |
| Bostanci et al., (2004) [74] | Turkey | Humans | Children | 40 | No correlation | NM |
| Kimata (2006) [75] | Japan | Humans | Children | 1226 ** | NM | NM |
| Nagel et al., (2009) [76] | Germany | Humans | Children | 462 | NM | NM |
| Balato et al., (2011) [77] | Italy | Humans | Teenagers and adults | 138 | No correlation | NM |
| Jeong et al., (2015) [78] | Korea | Rats | Pups | 33 | Positive | NM |
| Seo et al., (2016) [79] | Korea | Humans | Children | 227 | Negative | NM |
| Han et al., (2016) [80] | Korea | Humans | Children, teenagers, and adults | 64 | No correlation | NM |
| Mohammed et al., (2017) [81] | Egypt | Humans | Children | 90 | Positive | Negative |
| Jung et al., (2020) [82] | Korea | Humans | Teenagers and adults | 40 | Positive | No correlation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Cortegana, C.; Ortiz-García, G.; Serrano, A.; Moreno-Ramírez, D.; Sánchez-Margalet, V. Possible Role of Leptin in Atopic Dermatitis: A Literature Review. Biomolecules 2021, 11, 1642. https://doi.org/10.3390/biom11111642
Jiménez-Cortegana C, Ortiz-García G, Serrano A, Moreno-Ramírez D, Sánchez-Margalet V. Possible Role of Leptin in Atopic Dermatitis: A Literature Review. Biomolecules. 2021; 11(11):1642. https://doi.org/10.3390/biom11111642
Chicago/Turabian StyleJiménez-Cortegana, Carlos, Germán Ortiz-García, Amalia Serrano, David Moreno-Ramírez, and Víctor Sánchez-Margalet. 2021. "Possible Role of Leptin in Atopic Dermatitis: A Literature Review" Biomolecules 11, no. 11: 1642. https://doi.org/10.3390/biom11111642
APA StyleJiménez-Cortegana, C., Ortiz-García, G., Serrano, A., Moreno-Ramírez, D., & Sánchez-Margalet, V. (2021). Possible Role of Leptin in Atopic Dermatitis: A Literature Review. Biomolecules, 11(11), 1642. https://doi.org/10.3390/biom11111642






