Complement Factor H Loss in RPE Cells Causes Retinal Degeneration in a Human RPE-Porcine Retinal Explant Co-Culture Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Porcine Retinal Explants
2.3. Human Retinal Explants
2.4. Immunohistochemistry
2.5. Image Analysis
2.6. TUNEL Assay
2.7. Hematoxylin and Eosin Staining
2.8. Raman Microspectroscopy
2.9. Raman Data Analysis
2.10. RNA Isolation, cDNA Synthesis and qRTPCR
2.11. C3b ELISA
2.12. Statistical Analysis
3. Results
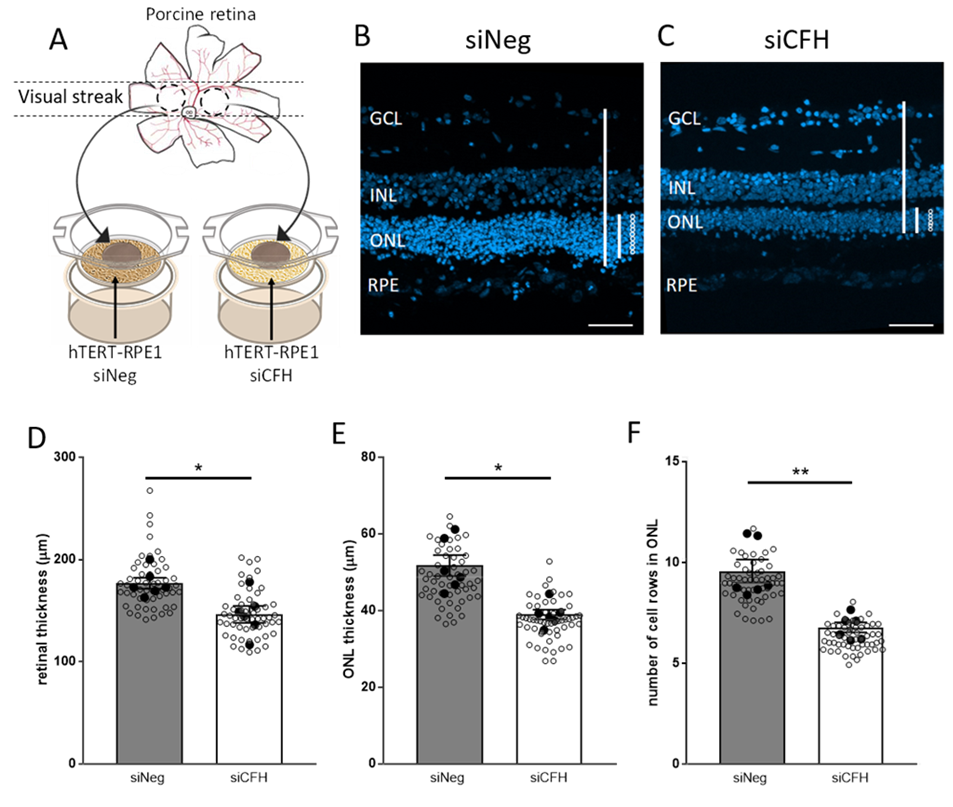
3.1. Porcine Retinal Explants Exposed to FH-Deprived RPE Cells Shows Signs of Degeneration
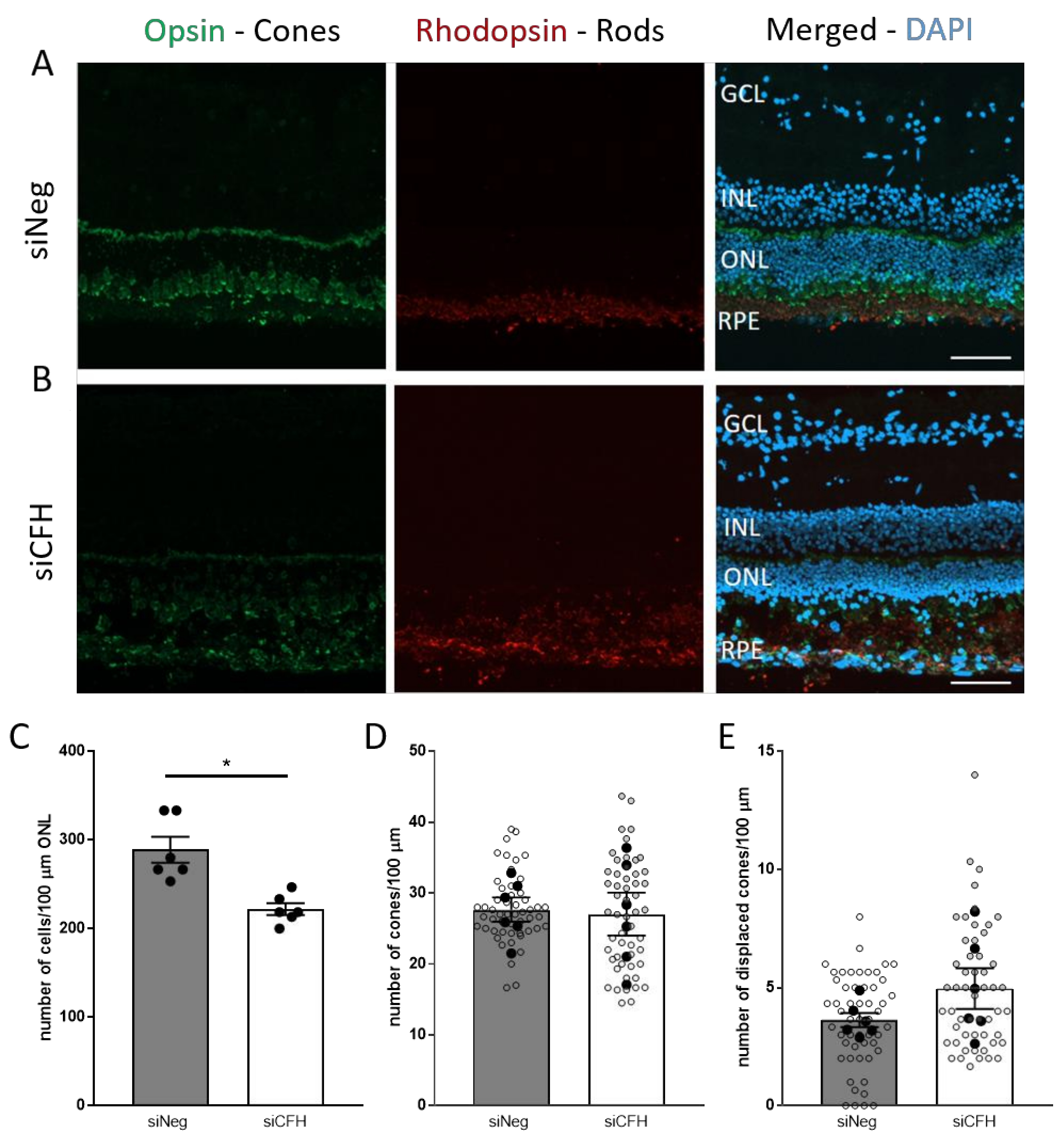
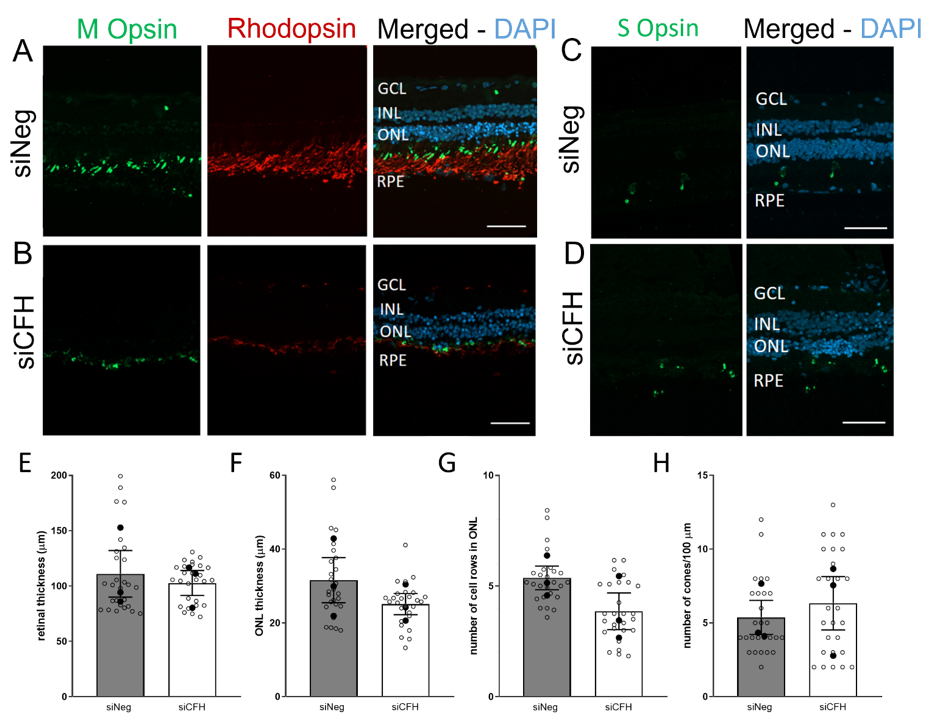
3.2. FH Loss in RPE Cells Mediates Rod Cell Degeneration in the Co-Cultured Porcine Retina
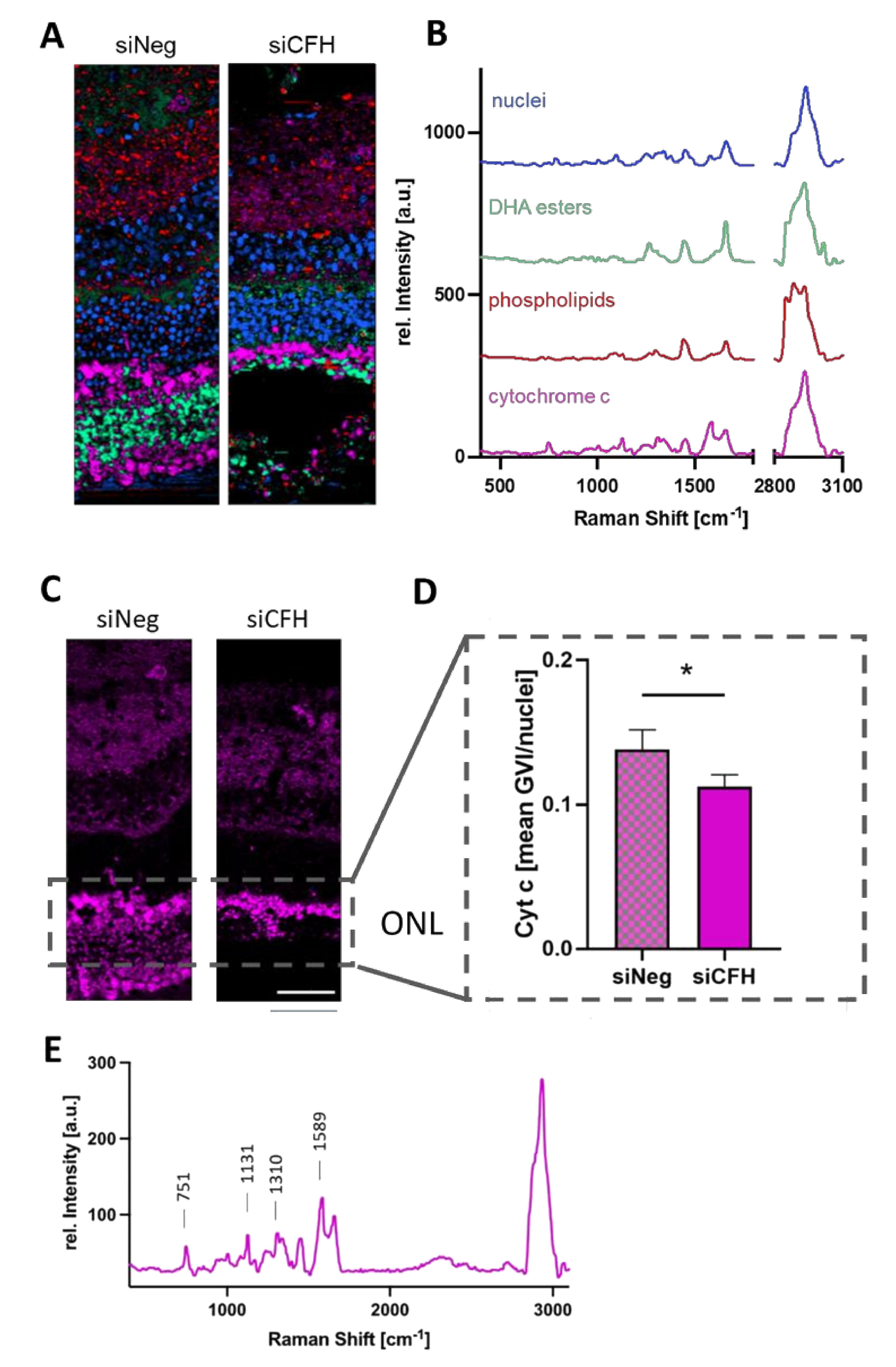
3.3. RPE Cells Deprived of FH Have a Negative Impact on the Mitochondria of Cells in the ONL
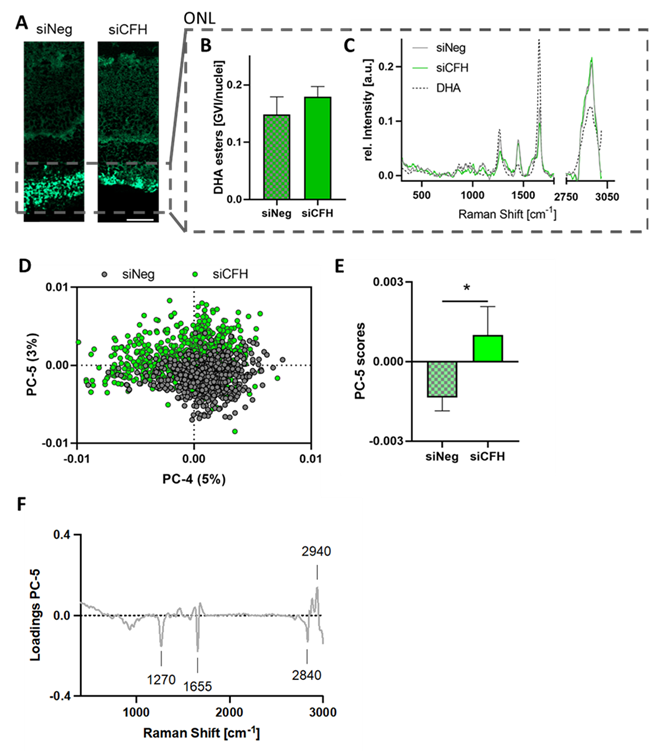
3.4. Retinae Cultured with siCFH RPE Cells Showed Signs of Lipid Oxidation in the ONL
3.5. FH Loss in RPE Cells Leads to Degeneration in Human Retinal Explants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Campagne, M.V.L.; LeCouter, J.; Yaspan, B.; Ye, W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J. Pathol. 2014, 232, 151–164. [Google Scholar] [CrossRef]
- Gehrs, K.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration—emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef]
- Rasoulinejad, S.A.; Zarghami, A.; Hosseini, S.R.; Rajaee, N.; Rasoulinejad, S.E.; Mikaniki, E. Prevalence of age-related macular degeneration among the elderly. Casp. J. Intern. Med. 2015, 6, 141–147. [Google Scholar]
- Armento, A.; Ueffing, M.; Clark, S.J. The complement system in age-related macular degeneration. Cell. Mol. Life Sci. 2021, 78, 4487–4505. [Google Scholar] [CrossRef] [PubMed]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; Hollander, A.I.D. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Ramlogan-Steel, C.A.; Andrzejewski, S.; Steel, J.C.; Layton, C.J. Retinal explant culture: A platform to investigate human neuro-retina. Clin. Exp. Ophthalmol. 2019, 47, 274–285. [Google Scholar] [CrossRef]
- Schnichels, S.; Paquet-Durand, F.; Löscher, M.; Tsai, T.; Hurst, J.; Joachim, S.C.; Klettner, A. Retina in a dish: Cell cultures, retinal explants and animal models for common diseases of the retina. Prog. Retin. Eye Res. 2021, 81, 100880. [Google Scholar] [CrossRef]
- A Curcio, C.; E Medeiros, N.; Millican, C.L. Photoreceptor loss in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1236–1249. [Google Scholar]
- Fritsche, L.G.; Fariss, R.N.; Stambolian, D.; Abecasis, G.R.; Curcio, C.A.; Swaroop, A. Age-Related Macular Degeneration: Genetics and Biology Coming Together. Annu. Rev. Genom. Hum. Genet. 2014, 15, 151–171. [Google Scholar] [CrossRef]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.-Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T.; et al. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Sciences 2005, 308, 385–389. [Google Scholar] [CrossRef]
- Day, A.J.; Willis, A.C.; Ripoche, J.; Sim, R.B. Sequence polymorphism of human complement factor H. Immunogenetics 1988, 27, 211–214. [Google Scholar] [CrossRef]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Borras, C.; Canonica, J.; Jorieux, S.; Abache, T.; El Sanharawi, M.; Klein, C.; Delaunay, K.; Jonet, L.; Salvodelli, M.; Naud, M.-C.; et al. CFH exerts anti-oxidant effects on retinal pigment epithelial cells independently from protecting against membrane attack complex. Sci. Rep. 2019, 9, 13873. [Google Scholar] [CrossRef]
- Shaw, P.X.; Zhang, L.; Zhang, M.; Du, H.; Zhao, L.; Lee, C.; Grob, S.; Lim, S.L.; Hughes, G.; Lee, J.; et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc. Natl. Acad. Sci. USA 2012, 109, 13757–13762. [Google Scholar] [CrossRef]
- Molins, B.; Fuentes-Prior, P.; Adán, A.; Antón, R.; Arostegui, J.I.; Yagüe, J.; Dick, A.D. Complement factor H binding of monomeric C-reactive protein downregulates proinflammatory activity and is impaired with at risk polymorphic CFH variants. Sci. Rep. 2016, 6, 22889. [Google Scholar] [CrossRef]
- Swinkels, M.; Zhang, J.H.; Tilakaratna, V.; Black, G.; Perveen, R.; McHarg, S.; Inforzato, A.; Day, A.J.; Clark, S.J. C-reactive protein and pentraxin-3 binding of factor H-like protein 1 differs from complement factor H: Implications for retinal inflammation. Sci. Rep. 2018, 8, 1643. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.R.; Ferrington, D. Perspective on AMD Pathobiology: A Bioenergetic Crisis in the RPE. Investig. Opthalmol. Vis. Sci. 2018, 59, AMD41–AMD47. [Google Scholar] [CrossRef] [PubMed]
- Armento, A.; Honisch, S.; Panagiotakopoulou, V.; Sonntag, I.; Jacob, A.; Bolz, S.; Kilger, E.; Deleidi, M.; Clark, S.; Ueffing, M. Loss of Complement Factor H impairs antioxidant capacity and energy metabolism of human RPE cells. Sci. Rep. 2020, 10, 10320. [Google Scholar] [CrossRef]
- Daugan, M.V.; Revel, M.; Thouenon, R.; Dragon-Durey, M.-A.; Robe-Rybkine, T.; Torset, C.; Merle, N.S.; Noé, R.; Verkarre, V.; Oudard, S.M.; et al. Intracellular Factor H Drives Tumor Progression Independently of the Complement Cascade. Cancer Immunol. Res. 2021, 9, 909–925. [Google Scholar] [CrossRef]
- Hallam, D.; Collin, J.; Bojic, S.; Chichagova, V.; Buskin, A.; Xu, Y.; Lafage, L.; Otten, E.; Anyfantis, G.; Mellough, C.; et al. An Induced Pluripotent Stem Cell Patient Specific Model of Complement Factor H (Y402H) Polymorphism Displays Characteristic Features of Age-Related Macular Degeneration and Indicates a Beneficial Role for UV Light Exposure. Stem Cells 2017, 35, 2305–2320. [Google Scholar] [CrossRef]
- Ebeling, M.C.; Geng, Z.; Kapphahn, R.J.; Roehrich, H.; Montezuma, S.R.; Dutton, J.R.; Ferrington, D.A. Impaired Mitochondrial Function in iPSC-Retinal Pigment Epithelium with the Complement Factor H Polymorphism for Age-Related Macular Degeneration. Cells 2021, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Armento, A.; Schmidt, T.L.; Sonntag, I.; Merle, D.; Jarboui, M.A.; Kilger, E.; Clark, S.J.; Ueffing, M. CFH Loss in Human RPE Cells Leads to Inflammation and Complement System Dysregulation via the NF-κB Pathway. Int. J. Mol. Sci. 2021, 22, 8727. [Google Scholar] [CrossRef]
- Mohlin, C.; Sandholm, K.; Kvanta, A.; Ekdahl, K.N.; Johansson, K. A model to study complement involvement in experimental retinal degeneration. Upsala J. Med Sci. 2018, 123, 28–42. [Google Scholar] [CrossRef]
- Kostic, C.; Arsenijevic, Y. Animal modelling for inherited central vision loss. J. Pathol. 2016, 238, 300–310. [Google Scholar] [CrossRef]
- Hurst, J.; Kuehn, S.; Jashari, A.; Tsai, T.; Bartz-Schmidt, K.U.; Schnichels, S.; Joachim, S.C. A novel porcine ex vivo retina culture model for oxidative stress induced by H2O2. Altern. Lab Anim. 2017, 45, 11–25. [Google Scholar] [CrossRef]
- Hurst, J.; Mueller-Buehl, A.M.; Hofmann, L.; Kuehn, S.; Herms, F.; Schnichels, S.; Joachim, S.C. iNOS-inhibitor driven neuroprotection in a porcine retina organ culture model. J. Cell Mol. Med. 2020, 24, 4312–4323. [Google Scholar] [CrossRef]
- Wagner, N.; Reinehr, S.; Gammel, M.R.; Greulich, A.; Hurst, J.; Dick, H.B.; Schnichels, S.; Joachim, S.C. Novel Porcine Retina Cultivation Techniques Provide Improved Photoreceptor Preservation. Front. Neurosci. 2020, 14, 556700. [Google Scholar] [CrossRef]
- Marzi, J.; Brauchle, E.M.; Schenke-Layland, K.; Rolle, M.W. Non-invasive functional molecular phenotyping of human smooth muscle cells utilized in cardiovascular tissue engineering. Acta Biomater. 2019, 89, 193–205. [Google Scholar] [CrossRef]
- Spiers, R.M.; Marzi, J.; Brauchle, E.M.; Cross, S.E.; Vaughan, R.H.; Bateman, P.A.; Hughes, S.J.; Schenke-Layland, K.; Johnson, P.R. Donor age significantly influences the Raman spectroscopic biomolecular fingerprint of human pancreatic extracellular matrix proteins following collagenase-based digestion. Acta Biomater. 2019, 99, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef]
- Okada, M.; Smith, N.; Palonpon, A.; Endo, H.; Kawata, S.; Sodeoka, M.; Fujita, K. Label-free Raman observation of cytochrome c dynamics during apoptosis. Proc. Natl. Acad. Sci. USA 2012, 109, 28–32. [Google Scholar] [CrossRef]
- Brazhe, N.A.; Treiman, M.; Brazhe, A.R.; Find, N.L.; Maksimov, G.; Sosnovtseva, O.V. Mapping of Redox State of Mitochondrial Cytochromes in Live Cardiomyocytes Using Raman Microspectroscopy. PLoS ONE 2012, 7, e41990. [Google Scholar] [CrossRef]
- Shindou, H.; Koso, H.; Sasaki, J.; Nakanishi, H.; Sagara, H.; Nakagawa, K.M.; Takahashi, Y.; Hishikawa, D.; Iizuka-Hishikawa, Y.; Tokumasu, F.; et al. Docosahexaenoic acid preserves visual function by maintaining correct disc morphology in retinal photoreceptor cells. J. Biol. Chem. 2017, 292, 12054–12064. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Li, Y.; Driver, M.; Decker, E.; He, L. Lipid and lipid oxidation analysis using surface enhanced Raman spectroscopy (SERS) coupled with silver dendrites. Food Res. Int. 2014, 58, 1–6. [Google Scholar] [CrossRef]
- Machado, N.F.L.; De Carvalho, L.A.E.B.; Otero, J.C.; Marques, M.P.M. The autooxidation process in linoleic acid screened by Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 1991–2000. [Google Scholar] [CrossRef]
- Ding, X.; Patel, M.; Chan, C.-C. Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 2009, 28, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Schnichels, S.; Kiebler, T.; Hurst, J.; Maliha, A.M.; Löscher, M.; Dick, H.B.; Bartz-Schmidt, K.-U.; Joachim, S.C. Retinal Organ Cultures as Alternative Research Models. Altern. Lab. Anim. 2019, 47, 19–29. [Google Scholar] [CrossRef]
- Williams, J.A.E.; Greenwood, J.; Moss, S.E. Retinal Changes Precede Visual Dysfunction in the Complement Factor H Knockout Mouse. PLoS ONE 2013, 8, e68616. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.; Ash, J.D. Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors. Redox Biol. 2019, 24, 101201. [Google Scholar] [CrossRef]
- Owsley, C.; McGwin, G.; Clark, M.E.; Jackson, G.R.; Callahan, M.A.; Kline, L.B.; Witherspoon, C.; Curcio, C.A. Delayed Rod-Mediated Dark Adaptation Is a Functional Biomarker for Incident Early Age-Related Macular Degeneration. Ophthalmology 2016, 123, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Maeda, A.; Chen, Y.; Chauhan, V.; Tang, J.; Palczewska, G.; Sakai, T.; Tsuneoka, H.; Palczewski, K.; Maeda, T. Retinal cone and rod photoreceptor cells exhibit differential susceptibility to light-induced damage. J. Neurochem. 2012, 121, 146–156. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Mir, T.A. The mechanism of cone cell death in Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 62, 24–37. [Google Scholar] [CrossRef]
- Provis, J.M.; Penfold, P.L.; Cornish, E.E.; Sandercoe, T.M.; Madigan, M.C. Anatomy and development of the macula: Specialisation and the vulnerability to macular degeneration. Clin. Exp. Optom. 2005, 88, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Millican, C.L.; Allen, K.A.; Kalina, R.E. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3278–3296. [Google Scholar]
- Shelley, E.J. Cone Degeneration in Aging and Age-Related Macular Degeneration. Arch. Ophthalmol. 2009, 127, 483–492. [Google Scholar] [CrossRef]
- Telander, D.G. Inflammation and age-related macular degeneration (AMD). Semin Ophthalmol. 2011, 26, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Harvey, H.; Durant, S. The role of glial cells and the complement system in retinal diseases and Alzheimer’s disease: Common neural degeneration mechanisms. Exp. Brain Res. 2014, 232, 3363–3377. [Google Scholar] [CrossRef]
- Todd, L.; Palazzo, I.; Suarez, L.; Liu, X.; Volkov, L.; Hoang, T.V.; Campbell, W.A.; Blackshaw, S.; Quan, N.; Fischer, A.J. Reactive microglia and IL1β/IL-1R1-signaling mediate neuroprotection in excitotoxin-damaged mouse retina. J. Neuroinflammation 2019, 16, 118. [Google Scholar] [CrossRef]
- Pfeiffer, R.L.; Marc, R.E.; Kondo, M.; Terasaki, H.; Jones, B.W. Müller cell metabolic chaos during retinal degeneration. Exp. Eye Res. 2016, 150, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Catala, A. Lipid peroxidation of membrane phospholipids in the vertebrate retina. Front. Biosci. 2011, 3, 52–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.-M.; Beversdorf, D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Phoon, C.K.; Schlame, M. Metabolism and function of mitochondrial cardiolipin. Prog. Lipid Res. 2014, 55, 1–16. [Google Scholar] [CrossRef]
- Beltrán-Heredia, E.; Tsai, F.-C.; Salinas-Almaguer, S.; Cao, F.J.; Bassereau, P.; Monroy, F. Membrane curvature induces cardiolipin sorting. Commun. Biol. 2019, 2, 225. [Google Scholar] [CrossRef] [PubMed]
- Spiro, T.G.; Strekas, T.C. Resonance Raman Spectra of Hemoglobin and Cytochrome c: Inverse Polarization and Vibronic Scattering. Proc. Natl. Acad. Sci. USA 1972, 69, 2622–2626. [Google Scholar] [CrossRef] [PubMed]
- Benevides, J.M.; Thomas, G.J., Jr. Characterization of DNA structures by Raman spectroscopy: High-salt and low-salt forms of double helical poly(dG-dC) in H2O and D2O solutions and application to B, Z and A-DNA. Nucleic Acids Res. 1983, 11, 5747–5761. [Google Scholar] [CrossRef] [PubMed]
- Prescott, B.; Steinmetz, W.; Thomas, G.J. Characterization of DNA structures by laser Raman spectroscopy. Biopolymer 1984, 23, 235–256. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armento, A.; Murali, A.; Marzi, J.; Almansa-Garcia, A.C.; Arango-Gonzalez, B.; Kilger, E.; Clark, S.J.; Schenke-Layland, K.; Ramlogan-Steel, C.A.; Steel, J.C.; et al. Complement Factor H Loss in RPE Cells Causes Retinal Degeneration in a Human RPE-Porcine Retinal Explant Co-Culture Model. Biomolecules 2021, 11, 1621. https://doi.org/10.3390/biom11111621
Armento A, Murali A, Marzi J, Almansa-Garcia AC, Arango-Gonzalez B, Kilger E, Clark SJ, Schenke-Layland K, Ramlogan-Steel CA, Steel JC, et al. Complement Factor H Loss in RPE Cells Causes Retinal Degeneration in a Human RPE-Porcine Retinal Explant Co-Culture Model. Biomolecules. 2021; 11(11):1621. https://doi.org/10.3390/biom11111621
Chicago/Turabian StyleArmento, Angela, Aparna Murali, Julia Marzi, Ana C Almansa-Garcia, Blanca Arango-Gonzalez, Ellen Kilger, Simon J Clark, Katja Schenke-Layland, Charmaine A Ramlogan-Steel, Jason C Steel, and et al. 2021. "Complement Factor H Loss in RPE Cells Causes Retinal Degeneration in a Human RPE-Porcine Retinal Explant Co-Culture Model" Biomolecules 11, no. 11: 1621. https://doi.org/10.3390/biom11111621
APA StyleArmento, A., Murali, A., Marzi, J., Almansa-Garcia, A. C., Arango-Gonzalez, B., Kilger, E., Clark, S. J., Schenke-Layland, K., Ramlogan-Steel, C. A., Steel, J. C., & Ueffing, M. (2021). Complement Factor H Loss in RPE Cells Causes Retinal Degeneration in a Human RPE-Porcine Retinal Explant Co-Culture Model. Biomolecules, 11(11), 1621. https://doi.org/10.3390/biom11111621






