Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism
Abstract
:1. Introduction
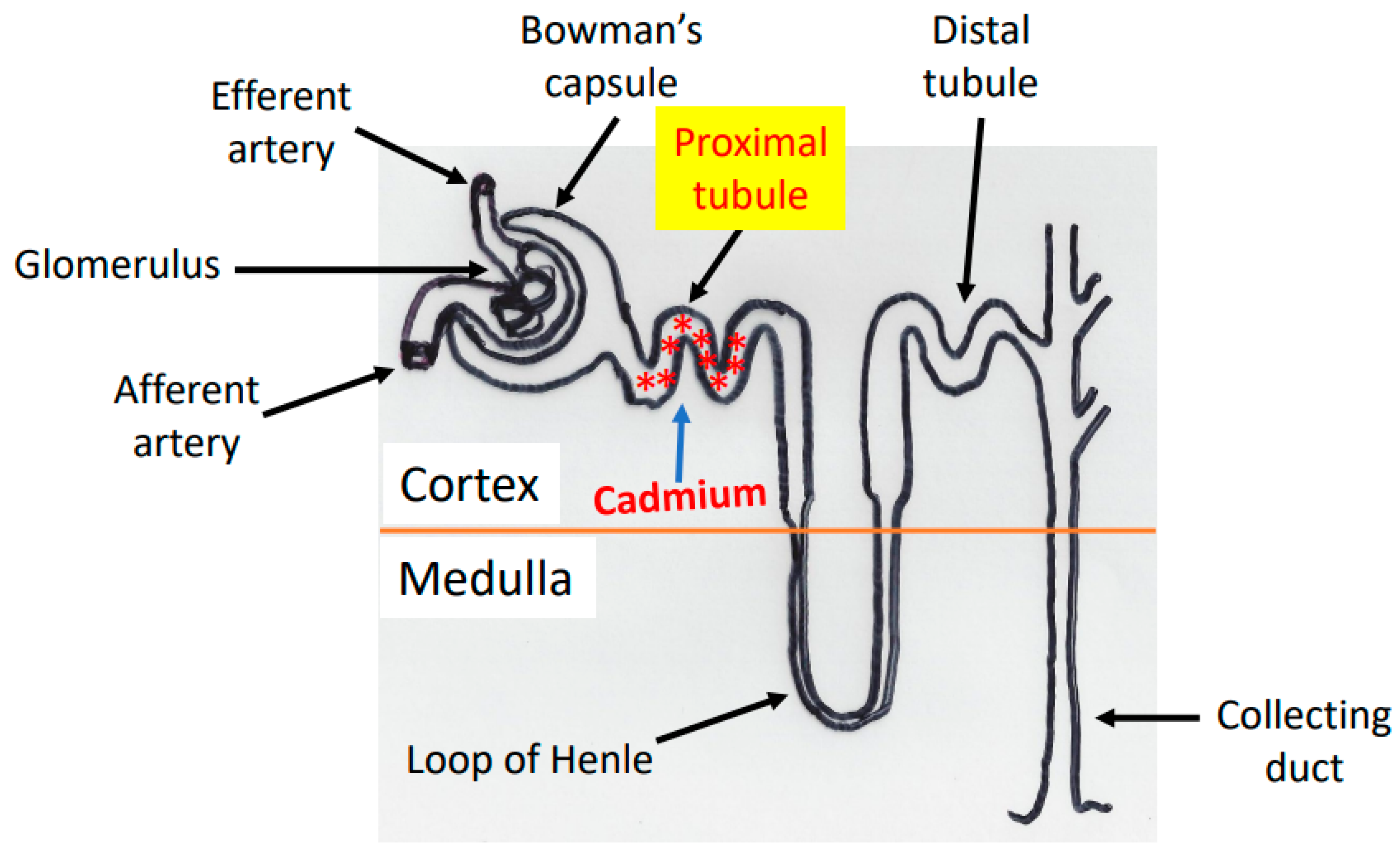
2. Cadmium Absorption, Transportation, and Accumulation in the Kidney
3. Cadmium-Induced Animal Models of Kidney Injury
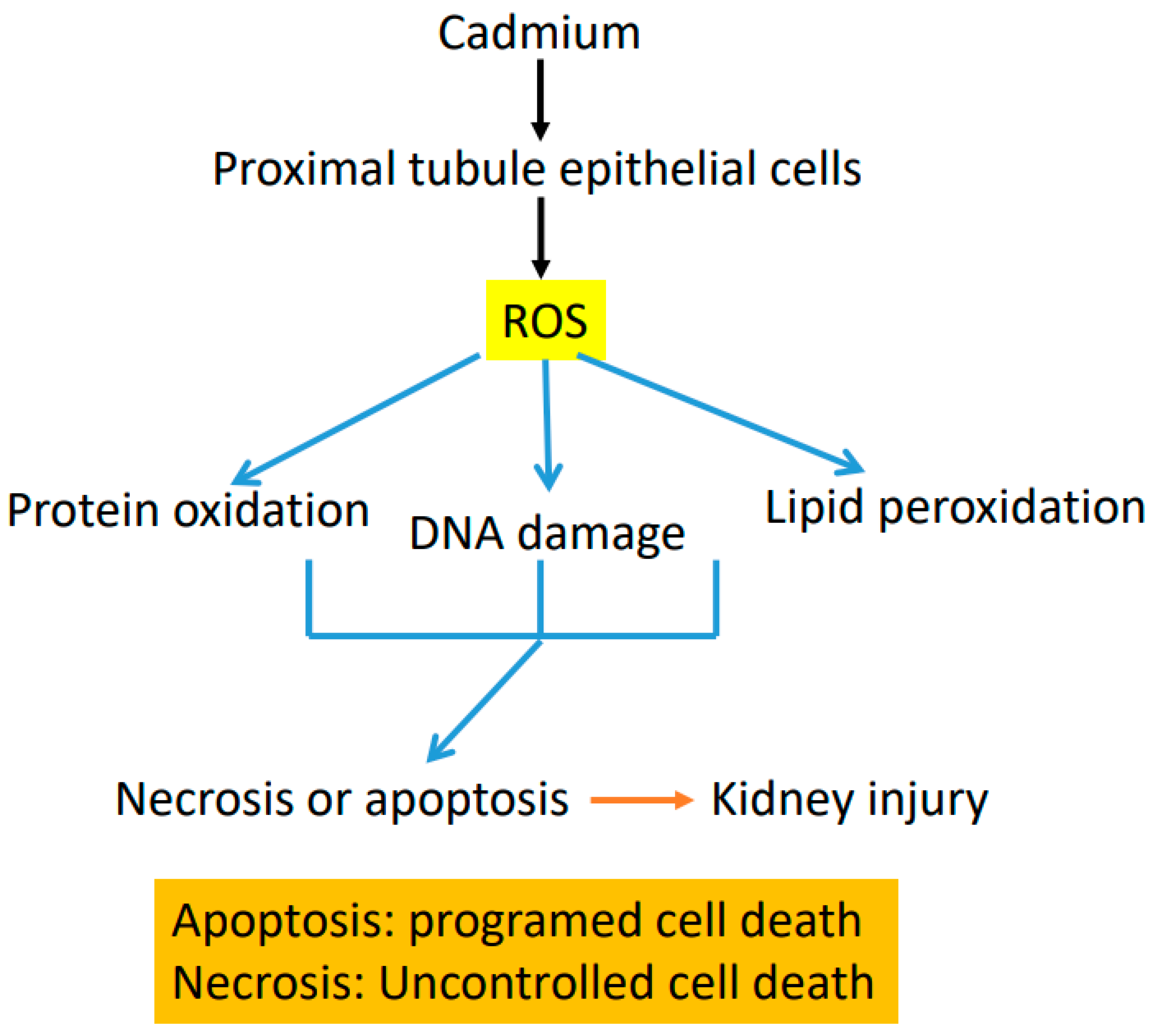
4. Mechanisms of Cadmium-Induced Renal Toxicity
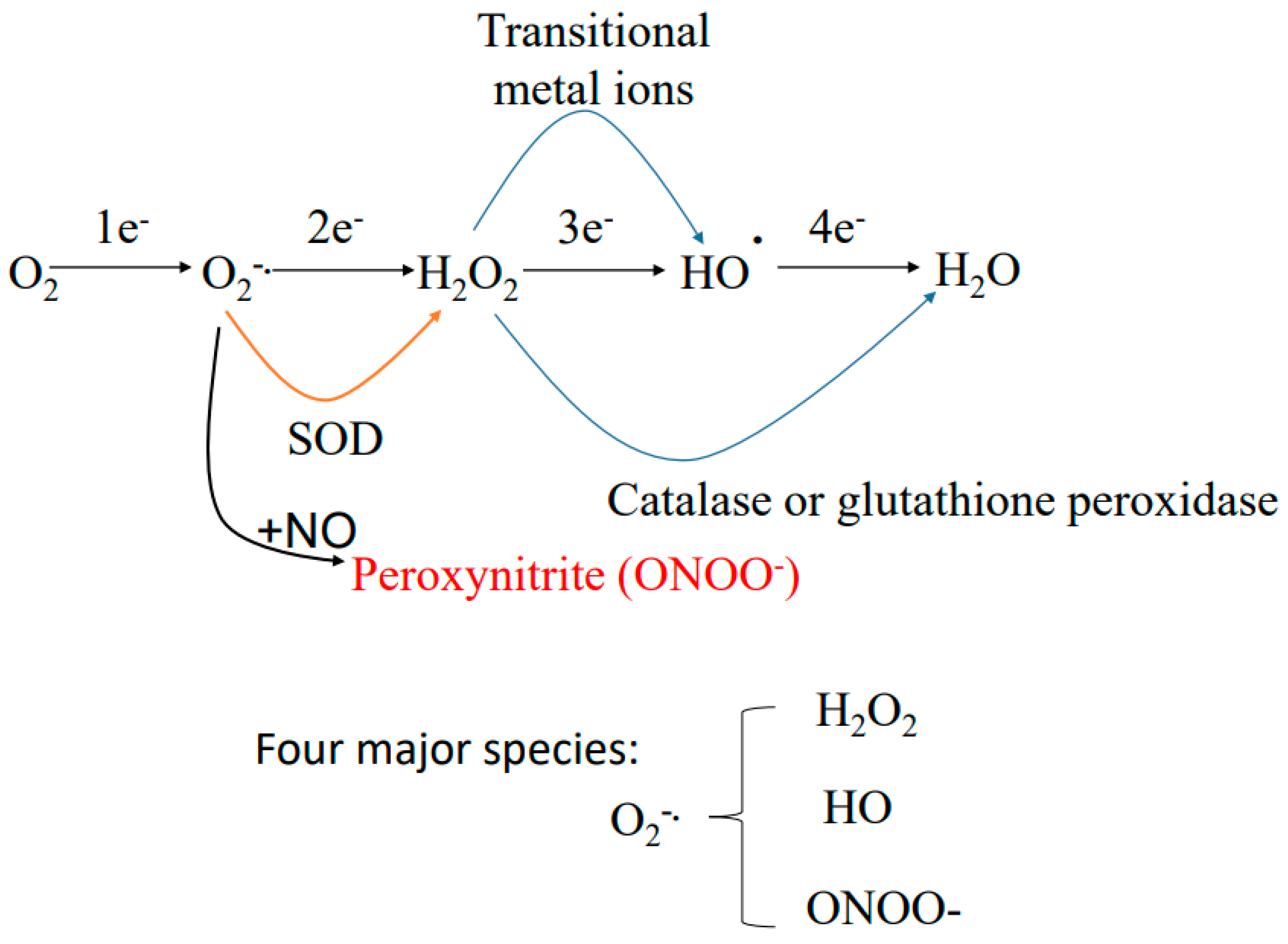
5. Sources of Reactive Oxygen Species
5.1. Mitochondria
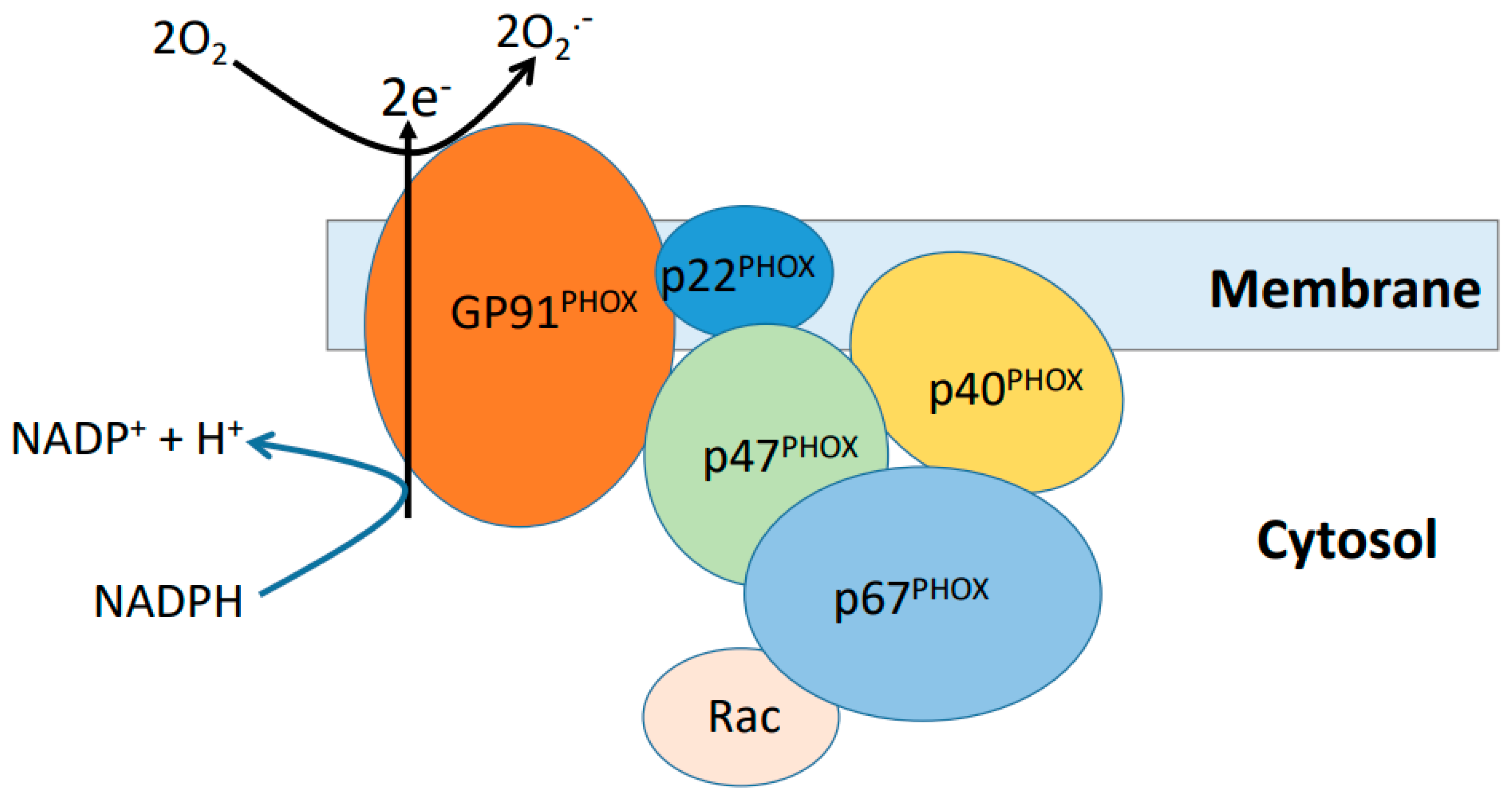
5.2. NADPH Oxidase
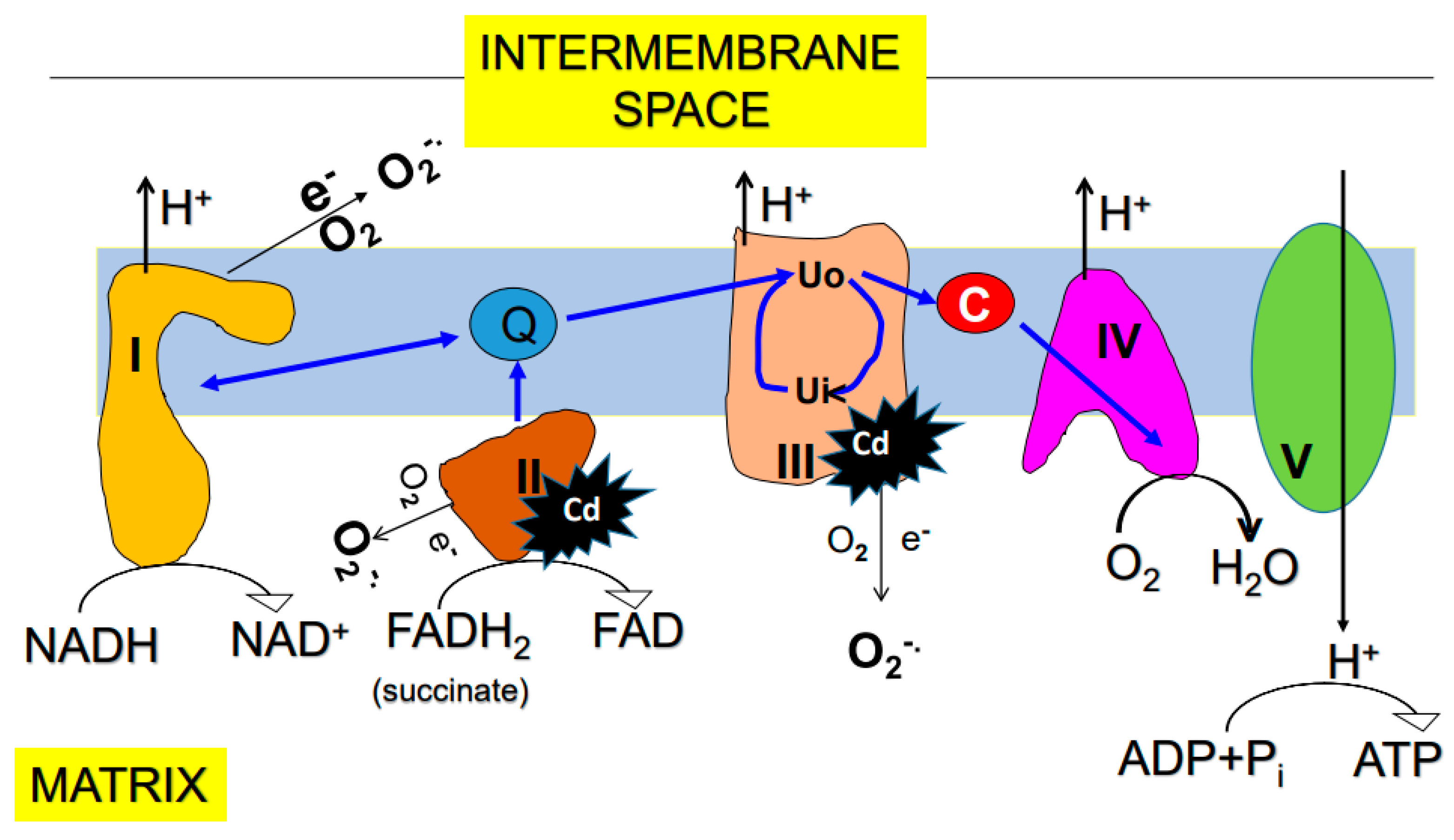
6. Effects of Cadmium on Mitochondrial Function
7. Counteracting Effects of Natural Products, Chemicals and Pharmacological Agents on Cadmium-Induced Kidney Injury
8. Other Potential Interventional Approaches
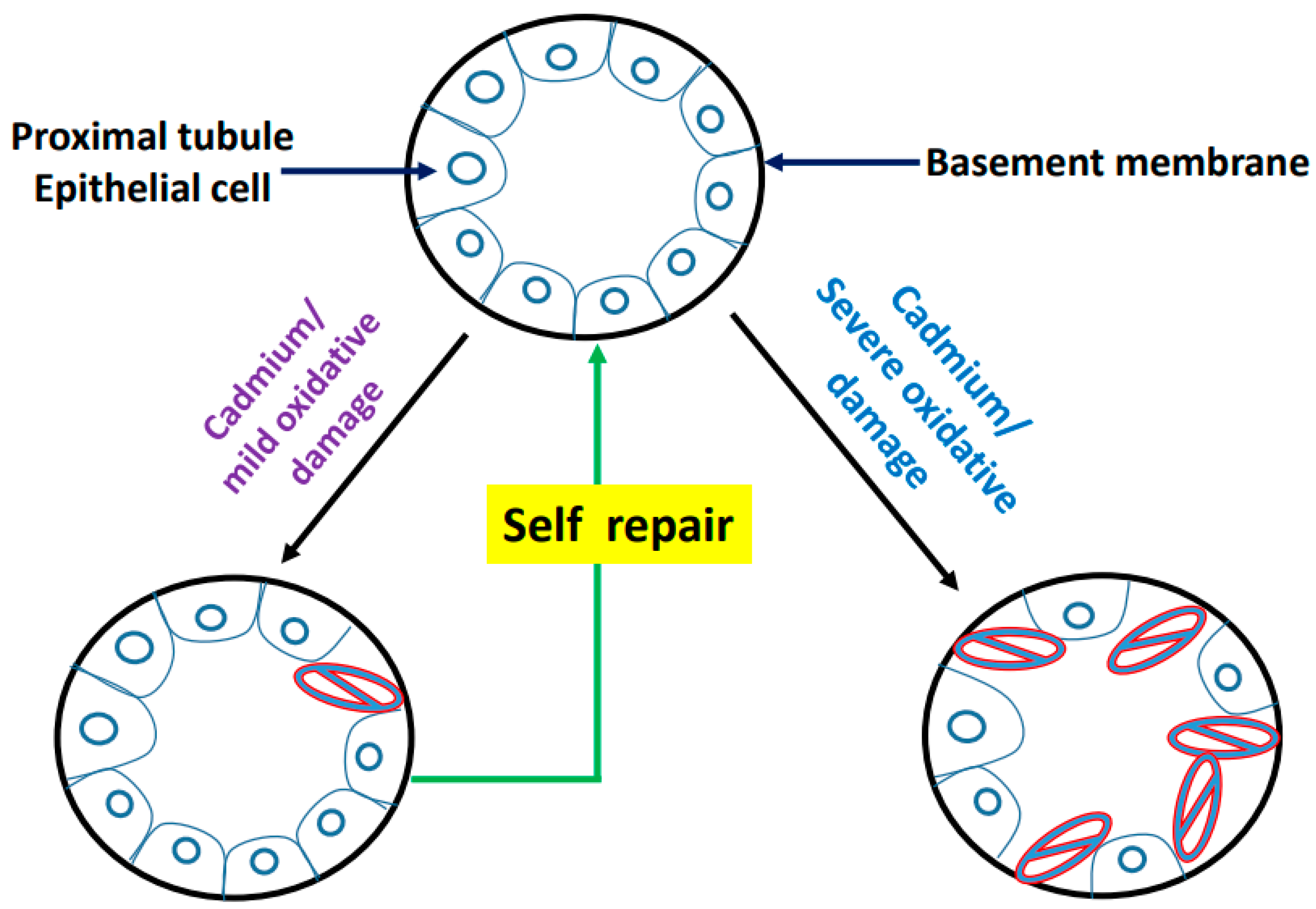
9. Postulated Model of Cadmium-Induced Proximal Tubule Lesion
10. Diagnosis of Cadmium-Induced Kidney Injury
11. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Koeppen, B.M.; Stanton, B.A. Renal Physiology, 5th ed.; Elsevier: Philadelphia, PA, USA, 2013. [Google Scholar]
- Rennke, H.G.; Denker, B.M. Renal Pathology: The Essentials, 5th ed.; Wolters Kluwer: New York, NY, USA, 2020. [Google Scholar]
- Dipiro, J.T.; Talbet, R.L.; Yee, G.C.; Matzke, G.R.; Wells, B.G.; Posey, L.M. Pharmacotherapy: A Pathophysiological Approach, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Sumida, K.D.; Garrett, J.H.; McJilton, W.T.; Hevener, A.L.; Donovan, C.M. Effect of endurance training and fasting on renal gluconeogenic enzymes in the rat. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 323–332. [Google Scholar] [CrossRef]
- Kaneko, K.; Soty, M.; Zitoun, C.; Duchampt, A.; Silva, M.; Philippe, E.; Gautier-Stein, A.; Rajas, F.; Mithieux, G. The role of kidney in the inter-organ coordination of endogenous glucose production during fasting. Mol. Metab. 2018, 16, 203–212. [Google Scholar] [CrossRef]
- Sharma, R.; Tiwari, S. Renal gluconeogenesis in insulin resistance: A culprit for hyperglycemia in diabetes. World J. Diabetes 2021, 12, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.; Marks, A.D. Marks’ Basic Medical Biochemistry: A Clinical Approach, 4th ed.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Molitoris, B.A.; Marrs, J. The role of cell adhesion molecules in ischemic acute renal failure. Am. J. Med. 1999, 106, 583–592. [Google Scholar] [CrossRef]
- Scholz, H.; Boivin, F.J.; Schmidt-Ott, K.M.; Bachmann, S.; Eckardt, K.U.; Scholl, U.I.; Persson, P.B. Kidney physiology and susceptibility to acute kidney injury: Implications for renoprotection. Nat. Rev. Nephrol. 2021, 17, 335–349. [Google Scholar] [CrossRef]
- Zhuang, S.; Lu, B.; Daubert, R.A.; Chavin, K.D.; Wang, L.; Schnellmann, R.G. Suramin promotes recovery from renal ischemia/reperfusion injury in mice. Kidney Int. 2009, 75, 304–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, M.; Tam, D.; Bardia, A.; Bhasin, M.; Rowe, G.C.; Kher, A.; Zsengeller, Z.K.; Akhavan-Sharif, M.R.; Khankin, E.V.; Saintgeniez, M.; et al. Pgc-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Investig. 2011, 121, 4003–4014. [Google Scholar] [CrossRef] [Green Version]
- Dare, A.J.; Bolton, E.A.; Pettigrew, G.J.; Bradley, J.A.; Saeb-Parsy, K.; Murphy, M.P. Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant mitoq. Redox Biol. 2015, 5, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Perazella, M.A. Renal vulnerability to drug toxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 1275–1283. [Google Scholar] [CrossRef]
- Cohen, A.; Ioannidis, K.; Ehrlich, A.; Regenbaum, S.; Cohen, M.; Ayyash, M.; Tikva, S.S.; Nahmias, Y. Mechanism and reversal of drug-induced nephrotoxicity on a chip. Sci. Transl. Med. 2021, 13, eabd6299. [Google Scholar] [CrossRef]
- Hosohata, K. Role of oxidative stress in drug-induced kidney injury. Int. J. Mol. Sci. 2016, 17, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, A.T.; Sharkawi, S.M.Z.; Hassan, M.I.A.; Abo-Youssef, A.M.; Hemeida, R.A.M. Empagli fl ozin and neohesperidin protect against methotrexate-induced renal toxicity via suppression of oxidative stress and inflammation in male rats. Food Chem. Toxicol. 2021, 155, 112406. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.; Sharif, S.; Hasan, A.; McFarlane, I.M. Low-dose methotrexate toxicity in the setting of vancomycin-induced acute kidney injury. Am. J. Med. Case Rep. 2020, 8, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.M.; Trepiccione, F.; Unwin, R.J. Drug toxicity in the proximal tubule: New models, methods and mechanisms. Pediatr. Nephrol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zeng, M.; Shu, Y.; Guo, D.; Sun, Y.; Guo, Z.; Wang, Y.; Liu, Z.; Zhou, H.; Zhang, W. Aging increases the susceptibility of cisplatin-induced nephrotoxicity. Age 2015, 37, 112. [Google Scholar] [CrossRef] [Green Version]
- Lash, L.H. Diverse roles of mitochondria in renal injury from environmental toxicants and therapeutic drugs. Int. J. Mol. Sci. 2021, 22, 4172. [Google Scholar] [CrossRef]
- Bharavi, K.; Reddy, A.G.; Rao, G.S.; Reddy, A.R.; Rao, S.V. Reversal of cadmium-induced oxidative stress in chicken by herbal adaptogens withania somnifera and ocimum sanctum. Toxicol. Int. 2010, 17, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Kataria, A.; Trasande, L.; Trachtman, H. The effects of environmental chemicals on renal function. Nat. Rev. Nephrol. 2015, 11, 610–625. [Google Scholar] [CrossRef]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. Biometals 2010, 23, 783–792. [Google Scholar] [CrossRef]
- Vervaet, B.A.; D’Haese, P.C.; Verhulst, A. Environmental toxin-induced acute kidney injury. Clin. Kidney J. 2017, 10, 747–758. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, S.; Naqshbandi, A.; Farooqui, Z.; Khan, A.A.; Khan, F. Protective effect of dietary flaxseed oil on arsenic-induced nephrotoxicity and oxidative damage in rat kidney. Food Chem. Toxicol. 2014, 68, 99–107. [Google Scholar] [CrossRef]
- Kimura, A.; Ishida, Y.; Hayashi, T.; Wada, T.; Yokoyama, H.; Sugaya, T.; Mukaida, N.; Kondo, T. Interferon-gamma plays protective roles in sodium arsenite-induced renal injury by up-regulating intrarenal multidrug resistance-associated protein 1 expression. Am. J. Pathol. 2006, 169, 1118–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barregard, L.; Bergstrom, G.; Fagerberg, B. Cadmium, type 2 diabetes, and kidney damage in a cohort of middle-aged women. Environ. Res. 2014, 135, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Gangadhariah, M.H.; Luther, J.M.; Garcia, V.; Paueksakon, P.; Zhang, M.Z.; Hayward, S.W.; Love, H.D.; Falck, J.R.; Manthati, V.L.; Imig, J.D.; et al. Hypertension is a major contributor to 20-hydroxyeicosatetraenoic acid-mediated kidney injury in diabetic nephropathy. J. Am. Soc. Nephrol. 2015, 26, 597–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; do Carmo, J.M.; da Silva, A.A.; Fu, Y.; Hall, J.E. Mechanisms of synergistic interactions of diabetes and hypertension in chronic kidney disease: Role of mitochondrial dysfunction and er stress. Curr. Hypertens Rep. 2020, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Ware, K.; Yildiz, V.; Xiao, M.; Medipally, A.; Hemminger, J.; Scarl, R.; Satoskar, A.A.; Hebert, L.; Ivanov, I.; Biederman, L.; et al. Hypertension and the kidney: Reduced kidney mass is bad for both normotensive and hypertensive rats. Am. J. Hypertens 2021. [Google Scholar] [CrossRef] [PubMed]
- Narsipur, S.S.; Peterson, O.W.; Smith, R.; Bigby, T.D.; Parthasarathy, S.; Gabbai, F.B.; Wilson, C.B.; Blantz, R.C. Mechanisms of glomerular immune injury: Effects of antioxidant treatment. J. Am. Soc. Nephrol. 2003, 14, 1748–1755. [Google Scholar] [CrossRef] [Green Version]
- Duann, P.; Lianos, E.A. Mechanisms of ho-1 mediated attenuation of renal immune injury: A gene profiling study. Transl. Res. 2011, 158, 249–261. [Google Scholar] [CrossRef]
- Persson, F.; Rossing, P. Diagnosis of diabetic kidney disease: State of the art and future perspective. Kidney Int. Suppl. 2018, 8, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Hallan, S.; Sharma, K. The role of mitochondria in diabetic kidney disease. Curr. Diabetes Rep. 2016, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.Z.; Szeto, C.C. Mitochondrial dysfunction in diabetic kidney disease. Clin. Chim. Acta 2019, 496, 108–116. [Google Scholar] [CrossRef]
- Yan, L.J. Nadh/nad(+) redox imbalance and diabetic kidney disease. Biomolecules 2021, 11, 730. [Google Scholar] [CrossRef]
- Orr, S.E.; Bridges, C.C. Chronic kidney disease and exposure to nephrotoxic metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.R.; Prozialeck, W.C. Cadmium, diabetes and chronic kidney disease. Toxicol. Appl. Pharmacol. 2009, 238, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Almeer, R.S.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel Moneim, A.E. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 2019, 9, 5825. [Google Scholar] [CrossRef] [Green Version]
- Almeer, R.S.; Kassab, R.B.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel Moneim, A.E. Royal jelly mitigates cadmium-induced neuronal damage in mouse cortex. Mol. Biol. Rep. 2019, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Aurisano, N.; Huang, L.; Mila, I.C.L.; Jolliet, O.; Fantke, P. Chemicals of concern in plastic toys. Environ. Int. 2021, 146, 106194. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Han, X.; Wang, G.; Liu, D.; Bao, L.; Jiao, C.; Luan, J.; Hou, Y.; Xu, Y.; Wang, H.; et al. Nrf2 deficiency aggravates the kidney injury induced by subacute cadmium exposure in mice. Arch. Toxicol. 2021, 95, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Nishijo, M.; Lasker, J.M.; Edwards, R.J.; Moore, M.R. Kidney dysfunction and hypertension: Role for cadmium, p450 and heme oxygenases? Tohoku J. Exp. Med. 2006, 208, 179–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, R.; Song, X.; Sun-Waterhouse, D.; Tan, X.; Li, F.; Li, D. Mir-34a/sirt1/p53 signaling pathway contributes to cadmium-induced nephrotoxicity: A preclinical study in mice. Environ. Pollut 2021, 282, 117029. [Google Scholar] [CrossRef]
- Kim, K.S.; Lim, H.J.; Lim, J.S.; Son, J.Y.; Lee, J.; Lee, B.M.; Chang, S.C.; Kim, H.S. Curcumin ameliorates cadmium-induced nephrotoxicity in sprague-dawley rats. Food Chem. Toxicol. 2018, 114, 34–40. [Google Scholar] [CrossRef]
- Tripathi, S.; Srivastav, A.K. Cytoarchitectural alterations in kidney of wistar rat after oral exposure to cadmium chloride. Tissue Cell 2011, 43, 131–136. [Google Scholar] [CrossRef]
- Rikans, L.E.; Yamano, T. Mechanisms of cadmium-mediated acute hepatotoxicity. J. Biochem. Mol. Toxicol. 2000, 14, 110–117. [Google Scholar] [CrossRef]
- Kim, S.C.; Cho, M.K.; Kim, S.G. Cadmium-induced non-apoptotic cell death mediated by oxidative stress under the condition of sulfhydryl deficiency. Toxicol. Lett. 2003, 144, 325–336. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prozialeck, W.C.; Edwards, J.R. Mechanisms of cadmium-induced proximal tubule injury: New insights with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012, 343, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prozialeck, W.C.; Vaidya, V.S.; Liu, J.; Waalkes, M.P.; Edwards, J.R.; Lamar, P.C.; Bernard, A.M.; Dumont, X.; Bonventre, J.V. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007, 72, 985–993. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, Z.A.; Lucis, O.J. Cadmium and zinc binding in mammalian liver and kidneys. Arch. Environ. Health 1972, 24, 419–425. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Lucis, O.J. Biological differences in cadmium and zinc turnover. Arch. Environ. Health 1972, 24, 410–418. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Habeebu, S.S.; Klaassen, C.D. Susceptibility of mt-null mice to chronic cdcl2-induced nephrotoxicity indicates that renal injury is not mediated by the cdmt complex. Toxicol. Sci. 1998, 46, 197–203. [Google Scholar]
- Klaassen, C.D.; Liu, J. Role of metallothionein in cadmium-induced hepatotoxicity and nephrotoxicity. Drug Metab. Rev. 1997, 29, 79–102. [Google Scholar] [CrossRef]
- Nomiyama, K.; Nomiyama, H. Tissue metallothioneins in rabbits chronically exposed to cadmium, with special reference to the critical concentration of cadmium in the renal cortex. Dev. Toxicol. Environ. Sci. 1982, 9, 47–67. [Google Scholar] [PubMed]
- Nomiyama, K.; Nomiyama, H. Critical concentration of ‘unbound’ cadmium in the rabbit renal cortex. Experientia 1986, 42, 149. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Edwards, J.R.; Lamar, P.C.; Liu, J.; Vaidya, V.S.; Bonventre, J.V. Expression of kidney injury molecule-1 (kim-1) in relation to necrosis and apoptosis during the early stages of cd-induced proximal tubule injury. Toxicol. Appl. Pharmacol. 2009, 238, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fels, J.; Scharner, B.; Zarbock, R.; Zavala Guevara, I.P.; Lee, W.K.; Barbier, O.C.; Thevenod, F. Cadmium complexed with beta2-microglubulin, albumin and lipocalin-2 rather than metallothionein cause megalin:Cubilin dependent toxicity of the renal proximal tubule. Int. J. Mol. Sci. 2019, 20, 2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.R.; Lee, W.K.; Smeets, K.; Swennen, Q.; Sanchez, A.; Thevenod, F.; Cuypers, A. Glutathione and mitochondria determine acute defense responses and adaptive processes in cadmium-induced oxidative stress and toxicity of the kidney. Arch. Toxicol. 2015, 89, 2273–2289. [Google Scholar] [CrossRef]
- Bagchi, D.; Bagchi, M.; Hassoun, E.A.; Stohs, S.J. Cadmium-induced excretion of urinary lipid metabolites, DNA damage, glutathione depletion, and hepatic lipid peroxidation in sprague-dawley rats. Biol. Trace Elem. Res. 1996, 52, 143–154. [Google Scholar] [CrossRef]
- Hassoun, E.A.; Stohs, S.J. Cadmium-induced production of superoxide anion and nitric oxide, DNA single strand breaks and lactate dehydrogenase leakage in j774a.1 cell cultures. Toxicology 1996, 112, 219–226. [Google Scholar] [CrossRef]
- Sotomayor, C.G.; Groothof, D.; Vodegel, J.J.; Eisenga, M.F.; Knobbe, T.J.; IJmker, J.; Lammerts, R.G.M.; de Borst, M.H.; Berger, S.P.; Nolte, I.M.; et al. Plasma cadmium is associated with increased risk of long-term kidney graft failure. Kidney Int. 2021, 99, 1213–1224. [Google Scholar] [CrossRef]
- Fransson, M.N.; Barregard, L.; Sallsten, G.; Akerstrom, M.; Johanson, G. Physiologically-based toxicokinetic model for cadmium using markov-chain monte carlo analysis of concentrations in blood, urine, and kidney cortex from living kidney donors. Toxicol. Sci. 2014, 141, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Jarup, L. Cadmium overload and toxicity. Nephrol. Dial. Transplant. 2002, 17, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Jarup, L.; Persson, B.; Elinder, C.G. Decreased glomerular filtration rate in solderers exposed to cadmium. Occup. Environ. Med. 1995, 52, 818–822. [Google Scholar] [CrossRef] [Green Version]
- Kazantzis, G. Renal tubular dysfunction and abnormalities of calcium metabolism in cadmium workers. Environ. Health Perspect. 1979, 28, 155–159. [Google Scholar] [CrossRef]
- Chen, S.; Liu, G.; Long, M.; Zou, H.; Cui, H. Alpha lipoic acid attenuates cadmium-induced nephrotoxicity via the mitochondrial apoptotic pathways in rat. J. Inorg. Biochem. 2018, 184, 19–26. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R.; Vaidya, V.S.; Bonventre, J.V. Preclinical evaluation of novel urinary biomarkers of cadmium nephrotoxicity. Toxicol. Appl. Pharmacol. 2009, 238, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Sarma, S.N.; Saleem, A.; Lee, J.Y.; Tokumoto, M.; Hwang, G.W.; Man Chan, H.; Satoh, M. Effects of long-term cadmium exposure on urinary metabolite profiles in mice. J. Toxicol. Sci. 2018, 43, 89–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, G.A. Risk factors for toxic nephropathies. Toxicol. Lett. 1989, 46, 269–279. [Google Scholar] [CrossRef]
- Nath, R.; Prasad, R.; Palinal, V.K.; Chopra, R.K. Molecular basis of cadmium toxicity. Prog. Food Nutr. Sci. 1984, 8, 109–163. [Google Scholar]
- Gong, P.; Wang, M.; Yang, W.; Chang, X.; Wang, L.; Chen, F. Integrated metabolomics coupled with pattern recognition and pathway analysis to reveal molecular mechanism of cadmium-induced diabetic nephropathy. Toxicol. Res. 2021, 10, 777–791. [Google Scholar] [CrossRef]
- Gong, P.; Chang, X.; Chen, X.; Bai, X.; Wen, H.; Pi, S.; Yang, W.; Wang, L.; Chen, F. Metabolomics study of cadmium-induced diabetic nephropathy and protective effect of caffeic acid phenethyl ester using uplc-q-tof-ms combined with pattern recognition. Environ. Toxicol. Pharmacol. 2017, 54, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Gentry, A.; Xu, Q.; Young, J.L.; Yan, X.; Pagidas, K.; Yang, Y.; Watson, W.H.; Kong, M.; Cai, L.; et al. Effects of cadmium and high-fat diet on essential metal concentration in the mouse testis. Toxicol. Rep. 2021, 8, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Hagedoorn, I.J.M.; Gant, C.M.; Huizen, S.V.; Maatman, R.; Navis, G.; Bakker, S.J.L.; Laverman, G.D. Lifestyle-related exposure to cadmium and lead is associated with diabetic kidney disease. J. Clin. Med. 2020, 9, 2432. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Iqbal, M.; Karam, J.; Salifu, M.; McFarlane, S.I. Oxidative stress, glucose metabolism, and the prevention of type 2 diabetes: Pathophysiological insights. Antioxid. Redox Signal. 2007, 9, 911–929. [Google Scholar] [CrossRef] [PubMed]
- Sanjeev, S.; Bidanchi, R.M.; Murthy, M.K.; Gurusubramanian, G.; Roy, V.K. Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ. Sci Pollut Res. Int. 2019, 26, 20631–20653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Cannino, G.; Ferruggia, E.; Luparello, C.; Rinaldi, A.M. Cadmium and mitochondria. Mitochondrion 2009, 9, 377–384. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rosca, M.G.; Vazquez, E.J.; Chen, Q.; Kerner, J.; Kern, T.S.; Hoppel, C.L. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes 2012, 61, 2074–2083. [Google Scholar] [CrossRef] [Green Version]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Trewin, A.J.; Bahr, L.L.; Almast, A.; Berry, B.J.; Wei, A.Y.; Foster, T.H.; Wojtovich, A.P. Mitochondrial reactive oxygen species generated at the complex-ii matrix or intermembrane space microdomain have distinct effects on redox signaling and stress sensitivity in caenorhabditis elegans. Antioxid. Redox Signal. 2019, 31, 594–607. [Google Scholar] [CrossRef]
- Ralph, S.J.; Moreno-Sanchez, R.; Neuzil, J.; Rodriguez-Enriquez, S. Inhibitors of succinate: Quinone reductase/complex ii regulate production of mitochondrial reactive oxygen species and protect normal cells from ischemic damage but induce specific cancer cell death. Pharm. Res. 2011, 28, 2695–2730. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. Riding the tiger—Physiological and pathological effects of superoxide and hydrogen peroxide generated in the mitochondrial matrix. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 592–661. [Google Scholar] [CrossRef]
- Brand, M.D.; Goncalves, R.L.; Orr, A.L.; Vargas, L.; Gerencser, A.A.; Borch Jensen, M.; Wang, Y.T.; Melov, S.; Turk, C.N.; Matzen, J.T.; et al. Suppressors of superoxide-h2o2 production at site iq of mitochondrial complex i protect against stem cell hyperplasia and ischemia-reperfusion injury. Cell Metab. 2016, 24, 582–592. [Google Scholar] [CrossRef] [Green Version]
- Ames, B.N.; Shigenaga, M.K. Oxidants are a major contributor to aging. Ann. N. Y. Acad. Sci. 1992, 663, 85–96. [Google Scholar] [CrossRef]
- Yan, L.J. Pathogenesis of chronic hyperglycemia: From reductive stress to oxidative stress. J. Diabetes Res. 2014, 2014, 137919. [Google Scholar] [CrossRef] [Green Version]
- Salvemini, D.; Doyle, T.M.; Cuzzocrea, S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem. Soc. Trans. 2006, 34, 965–970. [Google Scholar] [CrossRef]
- Kassab, A.; Piwowar, A. Cell oxidant stress delivery and cell dysfunction onset in type 2 diabetes. Biochimie 2012, 94, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Klivenyi, P.; Starkov, A.A.; Calingasan, N.Y.; Gardian, G.; Browne, S.E.; Yang, L.; Bubber, P.; Gibson, G.E.; Patel, M.S.; Beal, M.F. Mice deficient in dihydrolipoamide dehydrogenase show increased vulnerability to mptp, malonate and 3-nitropropionic acid neurotoxicity. J. Neurochem. 2004, 88, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Vaubel, R.A.; Rustin, P.; Isaya, G. Mutations in the dimer interface of dihydrolipoamide dehydrogenase promote site-specific oxidative damages in yeast and human cells. J. Biol. Chem. 2011, 286, 40232–40245. [Google Scholar] [CrossRef] [Green Version]
- Ambrus, A.; Torocsik, B.; Tretter, L.; Ozohanics, O.; Adam-Vizi, V. Stimulation of reactive oxygen species generation by disease-causing mutations of lipoamide dehydrogenase. Hum. Mol. Genet. 2011, 20, 2984–2995. [Google Scholar] [CrossRef] [Green Version]
- Bunik, V.I.; Brand, M.D. Generation of superoxide and hydrogen peroxide by side reactions of mitochondrial 2-oxoacid dehydrogenase complexes in isolation and in cells. Biol. Chem. 2018, 399, 407–420. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Leonard, S.S.; Rao, K.M. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 1434–1443. [Google Scholar] [CrossRef]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hebert, R.L. Nadph oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef]
- Suh, Y.A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase mox1. Nature 1999, 401, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Cao, Z.; Xu, X.; van Meir, E.G.; Lambeth, J.D. Homologs of gp91phox: Cloning and tissue expression of nox3, nox4, and nox5. Gene 2001, 269, 131–140. [Google Scholar] [CrossRef]
- Gardiner, G.J.; Deffit, S.N.; McLetchie, S.; Perez, L.; Walline, C.C.; Blum, J.S. A role for nadph oxidase in antigen presentation. Front. Immunol 2013, 4, 295. [Google Scholar] [CrossRef] [Green Version]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: Nadph oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.; Yu, S.L.; Kang, J.; Jeong, B.Y.; Lee, H.Y.; Park, C.G.; Yu, Y.B.; Jin, D.C.; Hwang, W.M.; Yun, S.R.; et al. Nadph oxidase 4 mediates tgf-beta1/smad signaling pathway induced acute kidney injury in hypoxia. PLoS ONE 2019, 14, e0219483. [Google Scholar]
- Liu, Q.; Liang, X.; Liang, M.; Qin, R.; Qin, F.; Wang, X. Ellagic acid ameliorates renal ischemic-reperfusion injury through nox4/jak/stat signaling pathway. Inflammation 2020, 43, 298–309. [Google Scholar] [CrossRef]
- Lima, N.K.S.; Farias, W.R.A.; Cirilo, M.A.S.; Oliveira, A.G.; Farias, J.S.; Aires, R.S.; Muzi-Filho, H.; Paixao, A.D.O.; Vieira, L.D. Renal ischemia-reperfusion leads to hypertension and changes in proximal tubule na(+) transport and renin-angiotensin-aldosterone system: Role of nadph oxidase. Life Sci. 2021, 266, 118879. [Google Scholar] [CrossRef]
- Pinheiro Junior, J.E.G.; Moraes, P.Z.; Rodriguez, M.D.; Simoes, M.R.; Cibin, F.; Pinton, S.; Barbosa Junior, F.; Pecanha, F.M.; Vassallo, D.V.; Miguel, M.; et al. Cadmium exposure activates nadph oxidase, renin-angiotensin system and cyclooxygenase 2 pathways in arteries, inducing hypertension and vascular damage. Toxicol. Lett. 2020, 333, 80–89. [Google Scholar] [CrossRef]
- Asagba, S.O. Alteration in the activity of oxidative enzymes in the tissues of male wistar albino rats exposed to cadmium. Int. J. Occup. Med. Environ. Health 2010, 23, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, E.L.; Paller, M.S. Xanthine oxidase produces o2-. In posthypoxic injury of renal epithelial cells. Am. J. Physiol. 1992, 263, F251–F255. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Noris, M.; Remuzzi, G. Physiology and pathophysiology of nitric oxide in chronic renal disease. Proc. Assoc. Am. Physicians 1999, 111, 602–610. [Google Scholar] [CrossRef]
- Soyupek, S.; Oksay, T.; Sutcu, R.; Armagan, A.; Gokalp, O.; Perk, H.; Delibas, N. The effect of cadmium toxicity on renal nitric oxide synthase isoenzymes. Toxicol. Ind. Health 2012, 28, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Pavon, N.; Buelna-Chontal, M.; Macias-Lopez, A.; Correa, F.; Uribe-Alvarez, C.; Hernandez-Esquivel, L.; Chavez, E. On the oxidative damage by cadmium to kidney mitochondrial functions. Biochem. Cell Biol. 2019, 97, 187–192. [Google Scholar] [CrossRef]
- Thevenod, F.; Lee, W.K.; Garrick, M.D. Iron and cadmium entry into renal mitochondria: Physiological and toxicological implications. Front. Cell Dev. Biol 2020, 8, 848. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, R.; Wang, X.; Shen, X.; Wang, P.; Sun, N.; Li, X.; Li, X.; Hai, C. Effects of sub-chronic, low-dose cadmium exposure on kidney damage and potential mechanisms. Ann. Transl. Med. 2019, 7, 177. [Google Scholar] [CrossRef]
- Ghasemi, H.; Postampour, F.; Ranjbar, A. The role of oxidative stress in metals toxicity/mitochondrial dysfunction as a key player. GMJ 2014, 3, 2–13. [Google Scholar]
- Barnett, L.M.A.; Cummings, B.S. Nephrotoxicity and renal pathophysiology: A contemporary perspective. Toxicol. Sci. 2018, 164, 379–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobe, G.; Crane, D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol. Lett. 2010, 198, 49–55. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, C.; Sun, Y.C.; Zhang, Q.; Lv, M.W.; Guo, K.; Li, J.L. Cadmium exposure triggers mitochondrial dysfunction and oxidative stress in chicken (Gallus gallus) kidney via mitochondrial upr inhibition and nrf2-mediated antioxidant defense activation. Sci. Total Environ. 2019, 689, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Sandbichler, A.M.; Hockner, M. Cadmium protection strategies—A hidden trade-off? Int. J. Mol. Sci 2016, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, H.; Sun, F. Cdse/zns quantum dots exhibited nephrotoxicity through mediating oxidative damage and inflammatory response. Aging 2020, 13, 12194–12206. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, Z.Y.; Zhu, Y.S.; Zhu, S.M.; Fan, R.F.; Wang, L. Alleviation of cadmium-induced oxidative stress by trehalose via inhibiting the nrf2-keap1 signaling pathway in primary rat proximal tubular cells. J. Biochem. Mol. Toxicol. 2018, 32, e22011. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Farkhondeh, T.; Samarghandian, S. Back to nucleus: Combating with cadmium toxicity using nrf2 signaling pathway as a promising therapeutic target. Biol. Trace Elem. Res. 2020, 197, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, C.; Mazumder, R.; Kumar, R.; Dhar, B.; Sengupta, M. Cadmium induced oxystress alters nrf2-keap1 signaling and triggers apoptosis in piscine head kidney macrophages. Aquat. Toxicol. 2021, 231, 105739. [Google Scholar] [CrossRef] [PubMed]
- Dastan, D.; Karimi, S.; Larki-Harchegani, A.; Nili-Ahmadabadi, A. Protective effects of allium hirtifolium boiss extract on cadmium-induced renal failure in rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 18886–18892. [Google Scholar] [CrossRef] [PubMed]
- Handan, B.A.; De Moura, C.F.G.; Cardoso, C.M.; Santamarina, A.B.; Pisani, L.P.; Ribeiro, D.A. Protective effect of grape and apple juices against cadmium intoxication in the kidney of rats. Drug Res. 2020, 70, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Suliman Al-Gebaly, A. Ameliorative effect of arctium lappa against cadmium genotoxicity and histopathology in kidney of wistar rat. Pak. J. Biol. Sci 2017, 20, 314–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Dewanjee, S.; Dua, T.K.; Joardar, S.; Chakraborty, P.; Bhowmick, S.; Saha, A.; Bhattacharjee, S.; De Feo, V. Carnosic acid attenuates cadmium induced nephrotoxicity by inhibiting oxidative stress, promoting nrf2/ho-1 signalling and impairing tgf-beta1/smad/collagen iv signalling. Molecules 2019, 24, 4176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wongmekiat, O.; Peerapanyasut, W.; Kobroob, A. Catechin supplementation prevents kidney damage in rats repeatedly exposed to cadmium through mitochondrial protection. Naunyn. Schmiedebergs Arch. Pharmacol. 2018, 391, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Chen, F.; Liu, X.; Gong, X.; Wang, J.; Ma, Y. Protective effect of caffeic acid phenethyl ester against cadmium-induced renal damage in mice. J. Toxicol. Sci. 2012, 37, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobroob, A.; Chattipakorn, N.; Wongmekiat, O. Caffeic acid phenethyl ester ameliorates cadmium-induced kidney mitochondrial injury. Chem. Biol. Interact. 2012, 200, 21–27. [Google Scholar] [CrossRef]
- Koriem, K.M.; Arbid, M.S.; Asaad, G.F. Chelidonium majus leaves methanol extract and its chelidonine alkaloid ingredient reduce cadmium-induced nephrotoxicity in rats. J. Nat. Med. 2013, 67, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, T.; Sangeetha, N.; Ashokkumar, N. Antihyperglycemic, antihyperlipidemic, and renoprotective effects of chlorella pyrenoidosa in diabetic rats exposed to cadmium. Toxicol. Mech. Methods 2012, 22, 617–624. [Google Scholar] [CrossRef]
- Poontawee, W.; Natakankitkul, S.; Wongmekiat, O. Protective effect of Cleistocalyx nervosum var. Paniala fruit extract against oxidative renal damage caused by cadmium. Molecules 2016, 21, 133. [Google Scholar] [CrossRef] [Green Version]
- Nishio, R.; Tamano, H.; Morioka, H.; Takeuchi, A.; Takeda, A. Intake of heated leaf extract of coriandrum sativum contributes to resistance to oxidative stress via decreases in heavy metal concentrations in the kidney. Plant. Foods Hum. Nutr 2019, 74, 204–209. [Google Scholar] [CrossRef]
- Tubsakul, A.; Sangartit, W.; Pakdeechote, P.; Kukongviriyapan, V.; Apaijit, K.; Kukongviriyapan, U. Curcumin mitigates hypertension, endothelial dysfunction and oxidative stress in rats with chronic exposure to lead and cadmium. Tohoku J. Exp. Med. 2021, 253, 69–76. [Google Scholar] [CrossRef]
- Fan, S.R.; Ren, T.T.; Yun, M.Y.; Lan, R.; Qin, X.Y. Edaravone attenuates cadmium-induced toxicity by inhibiting oxidative stress and inflammation in icr mice. Neurotoxicology 2021, 86, 1–9. [Google Scholar] [CrossRef]
- Kopec, A.; Sikora, E.; Piatkowska, E.; Borczak, B.; Czech, T. Possible protective role of elderberry fruit lyophilizate against selected effects of cadmium and lead intoxication in wistar rats. Environ. Sci. Pollut Res. Int. 2016, 23, 8837–8848. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Du, L.; Li, J.; Song, H. Epigallocatechin-3-gallate attenuates cadmium-induced chronic renal injury and fibrosis. Food Chem. Toxicol. 2016, 96, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Han, L.; Wang, J.; He, W.; Shang, H.; Gao, X.; Wang, T. Eucommia ulmoides bark protects against renal injury in cadmium-challenged rats. J. Med. Food 2012, 15, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Elkhadragy, M.F.; Al-Olayan, E.M.; Al-Amiery, A.A.; Abdel Moneim, A.E. Protective effects of fragaria ananassa extract against cadmium chloride-induced acute renal toxicity in rats. Biol. Trace Elem. Res. 2018, 181, 378–387. [Google Scholar] [CrossRef]
- Onwuka, F.C.; Erhabor, O.; Eteng, M.U.; Umoh, I.B. Protective effects of ginger toward cadmium-induced testes and kidney lipid peroxidation and hematological impairment in albino rats. J. Med. Food 2011, 14, 817–821. [Google Scholar] [CrossRef]
- Veljkovic, A.R.; Nikolic, R.S.; Kocic, G.M.; Pavlovic, D.D.; Cvetkovic, T.P.; Sokolovic, D.T.; Jevtovic, T.M.; Basic, J.T.; Laketic, D.M.; Marinkovic, M.R.; et al. Protective effects of glutathione and lipoic acid against cadmium-induced oxidative stress in rat’s kidney. Ren Fail. 2012, 34, 1281–1287. [Google Scholar] [CrossRef]
- Mohamed, N.E. Effect of aqueous extract of glycyrrhiza glabra on the biochemical changes induced by cadmium chloride in rats. Biol. Trace Elem. Res. 2019, 190, 87–94. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; Li, W.M.; Niu, Y.J.; Guo, H.C.; Liu, X.H.; Hou, Y.C.; Zhao, L.J. The protective effect of grape seed procyanidin extract against cadmium-induced renal oxidative damage in mice. Environ. Toxicol. Pharmacol. 2013, 36, 759–768. [Google Scholar] [CrossRef]
- Claudio, S.R.; Pidone Ribeiro, F.A.; De Lima, E.C.; Santamarina, A.B.; Pisani, L.P.; Pereira, C.S.D.; Fujiyama Oshima, C.T.; Ribeiro, D.A. The protective effect of grape skin or purple carrot extracts against cadmium intoxication in kidney of rats. Pathophysiology 2019, 26, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A. The potential protective effect of green, black, red and white tea infusions against adverse effect of cadmium and lead during chronic exposure—A rat model study. Regul. Toxicol. Pharmacol. 2015, 73, 521–529. [Google Scholar] [CrossRef]
- Ranieri, M.; Di Mise, A.; Difonzo, G.; Centrone, M.; Venneri, M.; Pellegrino, T.; Russo, A.; Mastrodonato, M.; Caponio, F.; Valenti, G.; et al. Green olive leaf extract (ole) provides cytoprotection in renal cells exposed to low doses of cadmium. PLoS ONE 2019, 14, e0214159. [Google Scholar] [CrossRef] [PubMed]
- Dua, T.K.; Dewanjee, S.; Khanra, R.; Bhattacharya, N.; Bhaskar, B.; Zia-Ul-Haq, M.; De Feo, V. The effects of two common edible herbs, ipomoea aquatica and enhydra fluctuans, on cadmium-induced pathophysiology: A focus on oxidative defence and anti-apoptotic mechanism. J. Transl. Med. 2015, 13, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojo, O.A.; Ajiboye, B.O.; Oyinloye, B.E.; Ojo, A.B.; Olarewaju, O.I. Protective effect of irvingia gabonensis stem bark extract on cadmium-induced nephrotoxicity in rats. Interdiscip. Toxicol. 2014, 7, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Aksoy, N.; Dogan, Y.; Iriadam, M.; Bitiren, M.; Uzer, E.; Ozgonul, A.; Aksoy, S. Protective and therapeutic effects of licorice in rats with acute tubular necrosis. J. Ren. Nutr. 2012, 22, 336–343. [Google Scholar] [CrossRef]
- Lan, Z.; Bi, K.S.; Chen, X.H. Ligustrazine attenuates elevated levels of indoxyl sulfate, kidney injury molecule-1 and clusterin in rats exposed to cadmium. Food Chem Toxicol. 2014, 63, 62–68. [Google Scholar] [CrossRef]
- Luo, T.; Liu, G.; Long, M.; Yang, J.; Song, R.; Wang, Y.; Yuan, Y.; Bian, J.; Liu, X.; Gu, J.; et al. Treatment of cadmium-induced renal oxidative damage in rats by administration of alpha-lipoic acid. Environ. Sci. Pollut. Res. Int. 2017, 24, 1832–1844. [Google Scholar] [CrossRef]
- Suru, S.M. Onion and garlic extracts lessen cadmium-induced nephrotoxicity in rats. Biometals 2008, 21, 623–633. [Google Scholar] [CrossRef]
- Shati, A.A. Effects of origanum majorana l. On cadmium induced hepatotoxicity and nephrotoxicity in albino rats. Saudi Med. J. 2011, 32, 797–805. [Google Scholar]
- Osukoya, O.A.; Oyinloye, B.E.; Ajiboye, B.O.; Olokode, K.A.; Adeola, H.A. Nephroprotective and anti-inflammatory potential of aqueous extract from persea americana seeds against cadmium-induced nephrotoxicity in wistar rats. Biometals 2021, 34, 1141–1153. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Al-Quraishy, S.; Diab, M.M.; Othman, M.S.; Aref, A.M.; Abdel Moneim, A.E. The potential protective role of physalis peruviana l. Fruit in cadmium-induced hepatotoxicity and nephrotoxicity. Food Chem Toxicol. 2014, 74, 98–106. [Google Scholar] [CrossRef]
- Yadav, N.; Khandelwal, S. Effect of picroliv on cadmium-induced hepatic and renal damage in the rat. Hum. Exp. Toxicol. 2006, 25, 581–591. [Google Scholar] [CrossRef]
- Jung, H.Y.; Seo, D.W.; Hong, C.O.; Kim, J.Y.; Yang, S.Y.; Lee, K.W. Nephroprotection of plantamajoside in rats treated with cadmium. Environ. Toxicol. Pharmacol. 2015, 39, 125–136. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Diab, M.S.M.; Lokman, M.S.; El-Sayed, H.; Bauomy, A.A.; Al-Shaebi, E.M.; Al-Quraishy, S. Nephroprotective effect of pleurotus ostreatus extract against cadmium chloride toxicity in rats. An. Acad. Bras. Cienc. 2020, 92, e20191121. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Liu, D.; Hou, C.; Liu, D.; Zhao, L.; Cheng, J.; Wang, D.; Bai, D. Protective effect of potentilla anserina polysaccharide on cadmium-induced nephrotoxicity in vitro and in vivo. Food Funct. 2017, 8, 3636–3646. [Google Scholar] [CrossRef]
- Song, X.B.; Liu, G.; Wang, Z.Y.; Wang, L. Puerarin protects against cadmium-induced proximal tubular cell apoptosis by restoring mitochondrial function. Chem. Biol. Interact. 2016, 260, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, G.M.; Al-Qahtani, W.H.; AlFaris, N.A.; Albekairi, N.A.; Alqahtani, S.; Eid, R.; Yagoub, A.E.A.; Al-Harbi, L.N.; Yahya, M.A. Quercetin alleviates cadmium chloride-induced renal damage in rats by suppressing endoplasmic reticulum stress through sirt1-dependent deacetylation of xbp-1s and eif2alpha. Biomed. Pharmacother 2021, 141, 111862. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Ge, J.; Lv, M.W.; Talukder, M.; Guo, K.; Li, Y.H.; Li, J.L. Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and pink1/parkin-mediated mitophagy. Food Funct. 2020, 11, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.N.; Aloliet, R.I.; Ganaie, M.A.; Khan, T.H.; Najeeb Ur, R.; Imam, F.; Hamad, A.M. Roflumilast, a phosphodiesterase 4 inhibitor, attenuates cadmium-induced renal toxicity via modulation of nf-kappab activation and induction of nqo1 in rats. Hum. Exp. Toxicol. 2019, 38, 588–597. [Google Scholar] [CrossRef]
- Joardar, S.; Dewanjee, S.; Bhowmick, S.; Dua, T.K.; Das, S.; Saha, A.; De Feo, V. Rosmarinic acid attenuates cadmium-induced nephrotoxicity via inhibition of oxidative stress, apoptosis, inflammation and fibrosis. Int. J. Mol. Sci. 2019, 20, 2027. [Google Scholar] [CrossRef] [Green Version]
- Abarikwu, S.O.; Njoku, R.C.; Lawrence, C.J.; Charles, I.A.; Ikewuchi, J.C. Rutin ameliorates oxidative stress and preserves hepatic and renal functions following exposure to cadmium and ethanol. Pharm. Biol. 2017, 55, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Rashwan, H.M.; Mohammed, H.E.; El-Nekeety, A.A.; Hamza, Z.K.; Abdel-Aziem, S.H.; Hassan, N.S.; Abdel-Wahhab, M.A. Bioactive phytochemicals from salvia officinalis attenuate cadmium-induced oxidative damage and genotoxicity in rats. Environ. Sci. Pollut Res. Int. 2021. [Google Scholar] [CrossRef]
- He, L.; Zhang, Q.Q.; Lu, X.Y.; Li, X.X. [effects of water extract of salvia miltiorrhiza aganst renal injury on rats exposed to cadmium]. Zhonghua Yi Xue Za Zhi 2017, 97, 57–61. [Google Scholar] [PubMed]
- Albasher, G.; Albrahim, T.; Aljarba, N.; Alharbi, R.I.; Alsultan, N.; Alsaiari, J.; Rizwana, H. Involvement of redox status and the nuclear-related factor 2 in protecting against cadmium-induced renal injury with sana makki (cassia senna l.) pre-treatment in male rats. An. Acad. Bras. Cienc. 2020, 92, e20191237. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, P.; Shen, Z.; Wang, J.; Diao, L. Protective effects of selenium yeast against cadmium-induced necroptosis through mir-26a-5p/pten/pi3k/akt signaling pathway in chicken kidney. Ecotoxicol. Environ. Saf. 2021, 220, 112387. [Google Scholar] [CrossRef]
- Bas, H.; Apaydin, F.G.; Kalender, S.; Kalender, Y. Lead nitrate and cadmium chloride induced hepatotoxicity and nephrotoxicity: Protective effects of sesamol on biochemical indices and pathological changes. J. Food Biochem. 2021, 45, e13769. [Google Scholar] [CrossRef]
- Ansari, M.A.; Raish, M.; Ahmad, A.; Alkharfy, K.M.; Ahmad, S.F.; Attia, S.M.; Alsaad, A.M.S.; Bakheet, S.A. Sinapic acid ameliorate cadmium-induced nephrotoxicity: In vivo possible involvement of oxidative stress, apoptosis, and inflammation via nf-kappab downregulation. Environ. Toxicol. Pharmacol. 2017, 51, 100–107. [Google Scholar] [CrossRef]
- Ramamurthy, C.H.; Subastri, A.; Suyavaran, A.; Subbaiah, K.C.; Valluru, L.; Thirunavukkarasu, C. Solanum torvum swartz. Fruit attenuates cadmium-induced liver and kidney damage through modulation of oxidative stress and glycosylation. Environ. Sci. Pollut. Res. Int. 2016, 23, 7919–7929. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Souid, G.; Timoumi, R.; Le Cerf, D.; Majdoub, H. Partial characterization of the edible spinacia oleracea polysaccharides: Cytoprotective and antioxidant potentials against cd induced toxicity in hct116 and hek293 cells. Int. J. Biol. Macromol. 2019, 136, 332–340. [Google Scholar] [CrossRef]
- Fouad, A.A.; Jresat, I. Protective effect of telmisartan against cadmium-induced nephrotoxicity in mice. Life Sci. 2011, 89, 29–35. [Google Scholar] [CrossRef]
- Mostafa, D.G.; Ahmed, S.F.; Hussein, O.A. Protective effect of tetrahydrobiopterin on hepatic and renal damage after acute cadmium exposure in male rats. Ultrastruct. Pathol. 2018, 42, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Junsi, M.; Takahashi Yupanqui, C.; Usawakesmanee, W.; Slusarenko, A.; Siripongvutikorn, S. Thunbergia laurifolia leaf extract increased levels of antioxidant enzymes and protected human cell-lines in vitro against cadmium. Antioxidants 2020, 9, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M.N.; Rehman, N.U.; Karim, A.; Imam, F.; Hamad, A.M. Protective effect of thymus serrulatus essential oil on cadmium-induced nephrotoxicity in rats, through suppression of oxidative stress and downregulation of nf-kappab, inos, and smad2 mrna expression. Molecules 2021, 26, 1252. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Alwadaani, H.A.; Jresat, I. Protective effect of thymoquinone against nephrotoxicity induced by cadmium in rats. Intenrational Sch. Sci. Res. Innov. 2016, 10, 73–76. [Google Scholar]
- Padma, V.V.; Baskaran, R.; Divya, S.; Priya, L.B.; Saranya, S. Modulatory effect of tinospora cordifolia extract on cd-induced oxidative stress in wistar rats. Integr. Med. Res. 2016, 5, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Fan, R.F.; Li, Z.F.; Zhang, D.; Wang, Z.Y. Involvement of nrf2 and mitochondrial apoptotic signaling in trehalose protection against cadmium-induced kidney injury. Metallomics 2020, 12, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, G.D.; Kumar, P.R.; Bharavi, K.; Annapurna, P.; Rajendar, B.; Patel, P.T.; Kumar, C.S.; Rao, G.S. Protective effect of tribulus terrestris linn on liver and kidney in cadmium intoxicated rats. Indian J. Exp. Biol 2012, 50, 141–146. [Google Scholar]
- Ali, S.; Hussain, S.; Khan, R.; Mumtaz, S.; Ashraf, N.; Andleeb, S.; Shakir, H.A.; Tahir, H.M.; Khan, M.K.A.; Ulhaq, M. Renal toxicity of heavy metals (cadmium and mercury) and their amelioration with ascorbic acid in rabbits. Environ. Sci. Pollut. Res. Int. 2019, 26, 3909–3920. [Google Scholar] [CrossRef]
- Fang, J.; Xie, S.; Chen, Z.; Wang, F.; Chen, K.; Zuo, Z.; Cui, H.; Guo, H.; Ouyang, P.; Chen, Z.; et al. Protective effect of vitamin e on cadmium-induced renal oxidative damage and apoptosis in rats. Biol. Trace Elem. Res. 2021, 199, 4675–4687. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Guo, J.; Tao, W.; Chen, Y.; Fan, X.; Shen, J.; Duan, J.A. Health risk of licorice-yuanhua combination through induction of colonic h2s metabolism. J. Ethnopharmacol. 2019, 236, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, C.; Vandendriessche, C.; Libert, C.; Vandenbroucke, R.E. Caloric restriction: Beneficial effects on brain aging and alzheimer’s disease. Mamm. Genome 2016, 27, 300–319. [Google Scholar] [CrossRef]
- Estrela, G.R.; Wasinski, F.; Batista, R.O.; Hiyane, M.I.; Felizardo, R.J.; Cunha, F.; de Almeida, D.C.; Malheiros, D.M.; Camara, N.O.; Barros, C.C.; et al. Caloric restriction is more efficient than physical exercise to protect from cisplatin nephrotoxicity via ppar-alpha activation. Front. Physiol. 2017, 8, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohal, R.S.; Forster, M.J. Caloric restriction and the aging process: A critique. Free Radic. Biol. Med. 2014, 73, 366–382. [Google Scholar] [CrossRef] [Green Version]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, Z.A.; Jordan, S.A.; Tang, W. Protection against chronic cadmium toxicity by caloric restriction. Toxicology 1999, 133, 93–103. [Google Scholar] [CrossRef]
- Felley-Bosco, E.; Diezi, J. Dietary calcium restriction enhances cadmium-induced metallothionein synthesis in rats. Toxicol. Lett. 1992, 60, 139–144. [Google Scholar] [CrossRef]
- Jin, Z.; Wu, J.; Yan, L.J. Chemical conditioning as an approach to ischemic stroke tolerance: Mitochondria as the target. Int. J. Mol. Sci. 2016, 17, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Li, R.; Li, W.; Ren, M.; Thangthaeng, N.; Sumien, N.; Liu, R.; Yang, S.; Simpkins, J.W.; Forster, M.J.; et al. Administration of 5-methoxyindole-2-carboxylic acid that potentially targets mitochondrial dihydrolipoamide dehydrogenase confers cerebral preconditioning against ischemic stroke injury. Free Radic. Biol. Med. 2017, 113, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Jin, Z.; Yang, X.; Yan, L.J. Post-ischemic administration of 5-methoxyindole-2-carboxylic acid at the onset of reperfusion affords neuroprotection against stroke injury by preserving mitochondrial function and attenuating oxidative stress. Biochem. Biophys. Res. Commun. 2018, 497, 444–450. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [PubMed]
- Akiyama, M.; Unoki, T.; Shinkai, Y.; Ishii, I.; Ida, T.; Akaike, T.; Yamamoto, M.; Kumagai, Y. Environmental electrophile-mediated toxicity in mice lacking nrf2, cse, or both. Environ. Health Perspect. 2019, 127, 67002. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, Y.; Masuda, A.; Akiyama, M.; Xian, M.; Kumagai, Y. Cadmium-mediated activation of the hsp90/hsf1 pathway regulated by reactive persulfides/polysulfides. Toxicol. Sci. 2017, 156, 412–421. [Google Scholar] [PubMed] [Green Version]
- Akiyama, M.; Shinkai, Y.; Unoki, T.; Shim, I.; Ishii, I.; Kumagai, Y. The capture of cadmium by reactive polysulfides attenuates cadmium-induced adaptive responses and hepatotoxicity. Chem. Res. Toxicol. 2017, 30, 2209–2217. [Google Scholar] [CrossRef]
- Bonventre, J.V. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J. Am. Soc. Nephrol. 2003, 14, S55–S61. [Google Scholar] [CrossRef] [Green Version]
- Padanilam, B.J. Cell death induced by acute renal injury: A perspective on the contributions of apoptosis and necrosis. Am. J. Physiol. Renal Physiol. 2003, 284, F608–F627. [Google Scholar] [CrossRef] [Green Version]
- Chang-Panesso, M.; Humphreys, B.D. Cellular plasticity in kidney injury and repair. Nat. Rev. Nephrol. 2017, 13, 39–46. [Google Scholar] [CrossRef]
- Chang-Panesso, M.; Kadyrov, F.F.; Lalli, M.; Wu, H.; Ikeda, S.; Kefaloyianni, E.; Abdelmageed, M.M.; Herrlich, A.; Kobayashi, A.; Humphreys, B.D. Foxm1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J. Clin. Investig. 2019, 129, 5501–5517. [Google Scholar] [CrossRef]
- Moulis, J.M. Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals 2010, 23, 877–896. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Lee, J.Y.; Tokumoto, M.; Satoh, M. Cadmium renal toxicity via apoptotic pathways. Biol. Pharm. Bull. 2012, 35, 1892–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Plant/Extract/Chemical | Rodent Model | Mechanism | Reference |
|---|---|---|---|
| Allium hirtifolium boiss | Rats | Anti-oxidative stress | [125] |
| Apple juice | Rats | Anti-oxidative stress | [126] |
| Arctium lappa | Rats | Anti-oxidative stress | [127] |
| Carnosic acid | Mice and cells | Anti-oxidative damage | [128] |
| Catechin | Rats | Anti-oxidative damage | [129] |
| Caffeic acid phenethyl ester | Rats | Anti-oxidative stress | [130,131] |
| Chelidonium majus leaves | Rats | Antidiuretic | [132] |
| Chorella pyrenoidosa | Rats | Antihyperglycemic | [133] |
| Cleistocalyx nervosum var. paniala | Rats | Increasing antioxidation power | [134] |
| Coriandrum sativum leaf | Mice | Anti-oxidative stress | [135] |
| Curcumin | Rats | Anti-oxidative stress | [136] |
| Edaravone | Mice/Cells | Inhibiting oxidative stress | [137] |
| Elderberry | Rats | Increasing antioxidant enzymes | [138] |
| Epigallocatechin-3-gallate | Rats | Increasing antioxidant defense | [139] |
| Eucommia ulmoides bark | Rats | Anti-oxidative damage | [140] |
| Ferulic acid | Rats | Anti-oxidative stress | [78] |
| Fragaria ananassa | Rats | Anti-oxidative stress | [141] |
| Ginger | Rats | Decrease lipid peroxidation | [142] |
| Glutathione | Rats | Anti-oxidative stress | [143] |
| Glycyrrhiza glabra | Rats | Anti-oxidative stress | [144] |
| Grape seed procyanidin | Mice | Antioxidants | [145] |
| Grape skin/purple carrot | Rats | Anti-oxidative damage | [146] |
| Green/black/red/white tea | Rats | Anti-oxidative damage | [147] |
| Green olive leaf | Renal cells (MCD4) | Anti-oxidative stress | [148] |
| Herbal adaptogens | Chicken | Anti-oxidative damage | [21] |
| Ipomoea aquatic/Enhydra fluctuans | Mice | Anti-oxidation/anti-apoptosis | [149] |
| Irvingia gabonesis stem bark | Rats | Increasing antioxidant defense | [150] |
| Licorice | Rats | Anti-oxidative damage | [151] ** |
| Ligustrazine | Rats | Restoring renal function | [152] |
| Lipoic acid | Rats | Anti-apoptosis | [68,153] |
| Onion/garlic | Rats | Anti-oxidative stress | [154] |
| Origanum majorana L. | Rats | Anti-oxidative damage | [155] |
| Persea americana seeds | Rats | Mitigating oxidative stress | [156] |
| Physalis peruviana L | Rats | Anti-oxidation/Anti-apoptosis | [157] |
| Picroliv | Rats | Anti-oxidative stress | [158] |
| Plantamajoside | Rats | Decrease oxidative damage | [159] |
| Pleurotus ostreatus | Rats (female) | Mitigating oxidative damage | [160] |
| Potentilla anserine | Mice and cells | Anti-oxidative stress | [161] |
| Puerarin | Rat proximal tubule cells | Restoring mitochondrial function | [162] |
| Quercetin | Rats | Suppressing ER stress | [163] |
| Resveratrol | Chickens | Anti-oxidative stress | [164] |
| Roflumilast | Rats | Increasing antioxidant defense | [165] |
| Rosmarinic acid | Mice | Anti-oxidative damage | [166] |
| Royal jelly | Mice (male) | Antioxidation/Nrf2 activation | [39] |
| Rutin | Rats | Inhibiting oxidative stress | [167] |
| Salvia officinalis | Rats | Anti-oxidative damage | [168] |
| Salvia miltiorrhiza | Rats | Anti-oxidative injury | [169] |
| Sana Makki | Rats | Anti-oxidative stress/Nrf2 | [170] |
| Selenium yeast | Chicken | Mitigating necroptosis | [171] |
| Sesamol | Rats | Inhibiting oxidative stress | [172] |
| SInapic acid | Rats | Inhibiting oxidative stress | [173] |
| Solanum torvum Swartz | Rats | Anti-oxidative stress | [174] |
| Spinacia oleracea polysaccharides | HEK293 cells | Anti-oxidative stress | [175] |
| Telmisartan | Mice | Suppressing oxidative stress | [176] |
| Tetrahydrobiopterin | Rats | Maintaining mitochondria integrity | [177] |
| Thunbergia laurifolia leaf | Kidney cells | Increasing antioxidant enzymes | [178] |
| Thymus serrulatus essential oil | Rats | Anti-oxidative stress | [179] |
| Thymoquinone | Rats | Increasing glutathione | [180] |
| Tinospora cordifolia | Rats | Anti-oxidative stress | [181] |
| Trehalose | Rats | Inhibiting oxidative stress | [182] |
| Tribulus terrestris linn | Rats | Anti-oxidation | [183] |
| Vitamin C | Rabbits | Anti-oxidative stress | [184] |
| Vitamin E | Rats | Enhancing antioxidant defense | [185] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.-J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. https://doi.org/10.3390/biom11111575
Yan L-J, Allen DC. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules. 2021; 11(11):1575. https://doi.org/10.3390/biom11111575
Chicago/Turabian StyleYan, Liang-Jun, and Daniel C. Allen. 2021. "Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism" Biomolecules 11, no. 11: 1575. https://doi.org/10.3390/biom11111575
APA StyleYan, L.-J., & Allen, D. C. (2021). Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules, 11(11), 1575. https://doi.org/10.3390/biom11111575







