Glycoside Hydrolases and Glycosyltransferases from Hyperthermophilic Archaea: Insights on Their Characteristics and Applications in Biotechnology
Abstract
:1. Introduction
2. Hyperthermophilic GHs from Archaea
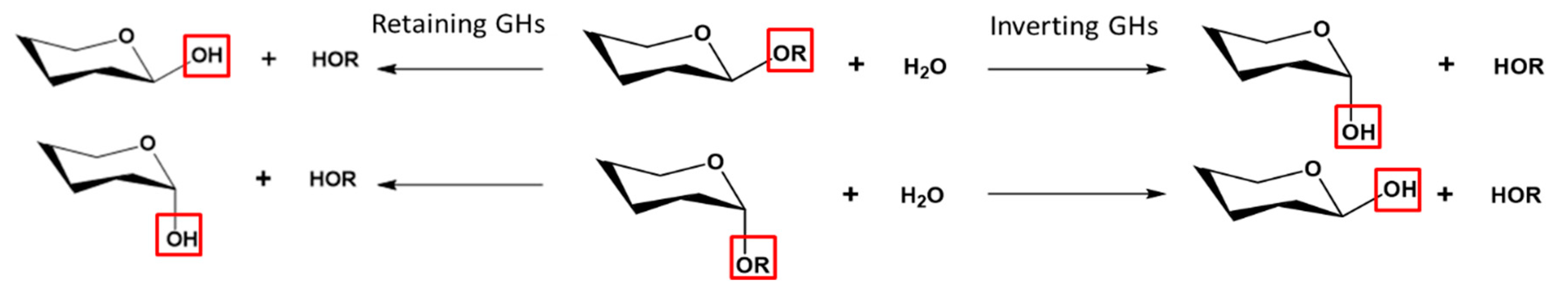
2.1. General Features and Reaction Mechanisms
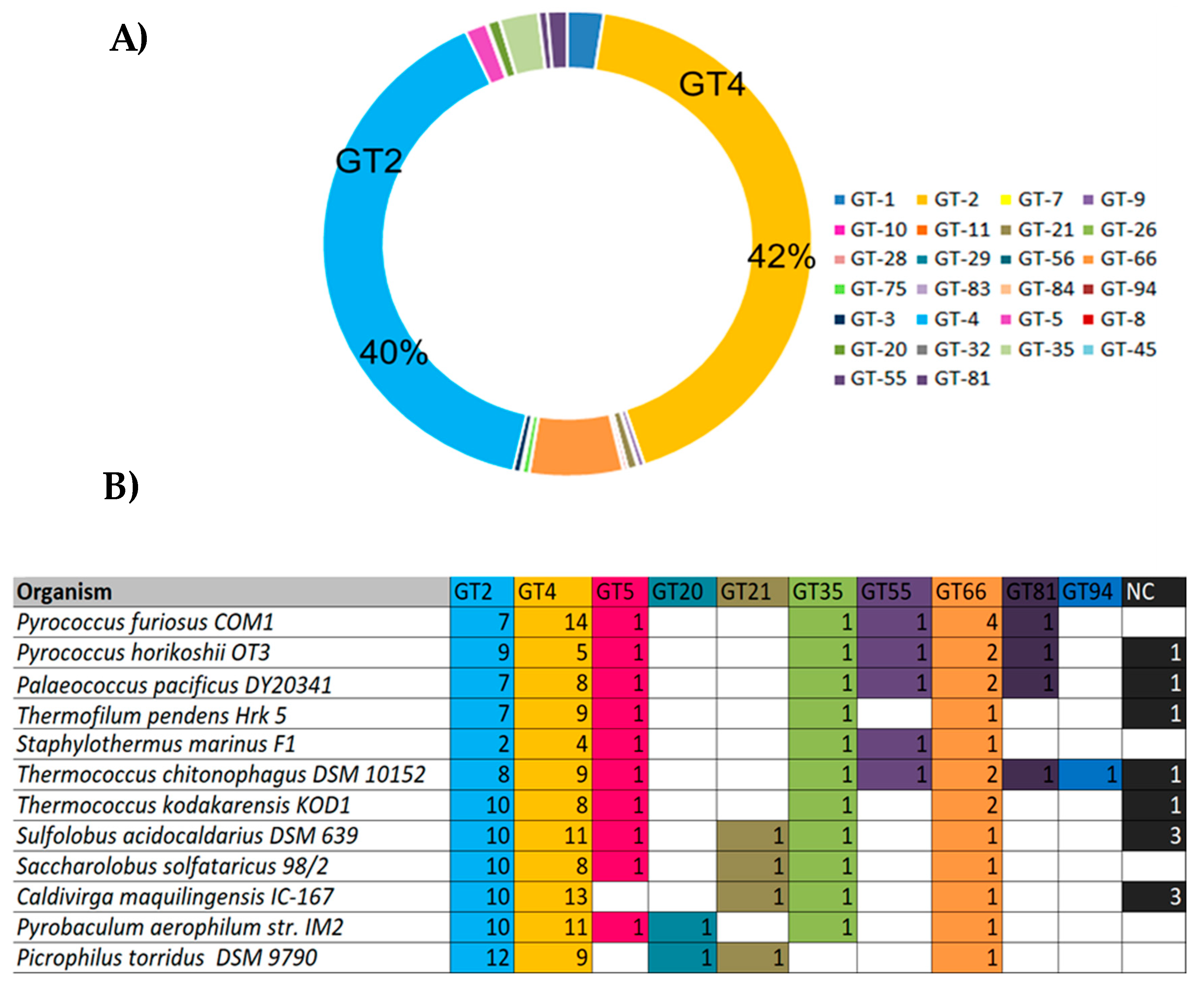
2.2. Glycoside Hydrolases (GHs) in the Genomes of Hyperthermophilic Archaea
2.3. Biochemical Features and Biotechnological Applications
2.3.1. Starch Degrading Enzymes
α-Amylases
β-Amylases and Glucoamylases
Pullulanases
Isoamylases
α-Glucosidases
Cyclodextrin Glucanotransferases (CGTases)
Amylomaltases
2.3.2. Cellulose Hydrolyzing Enzymes

Endoglucanases
β-Glucosidases
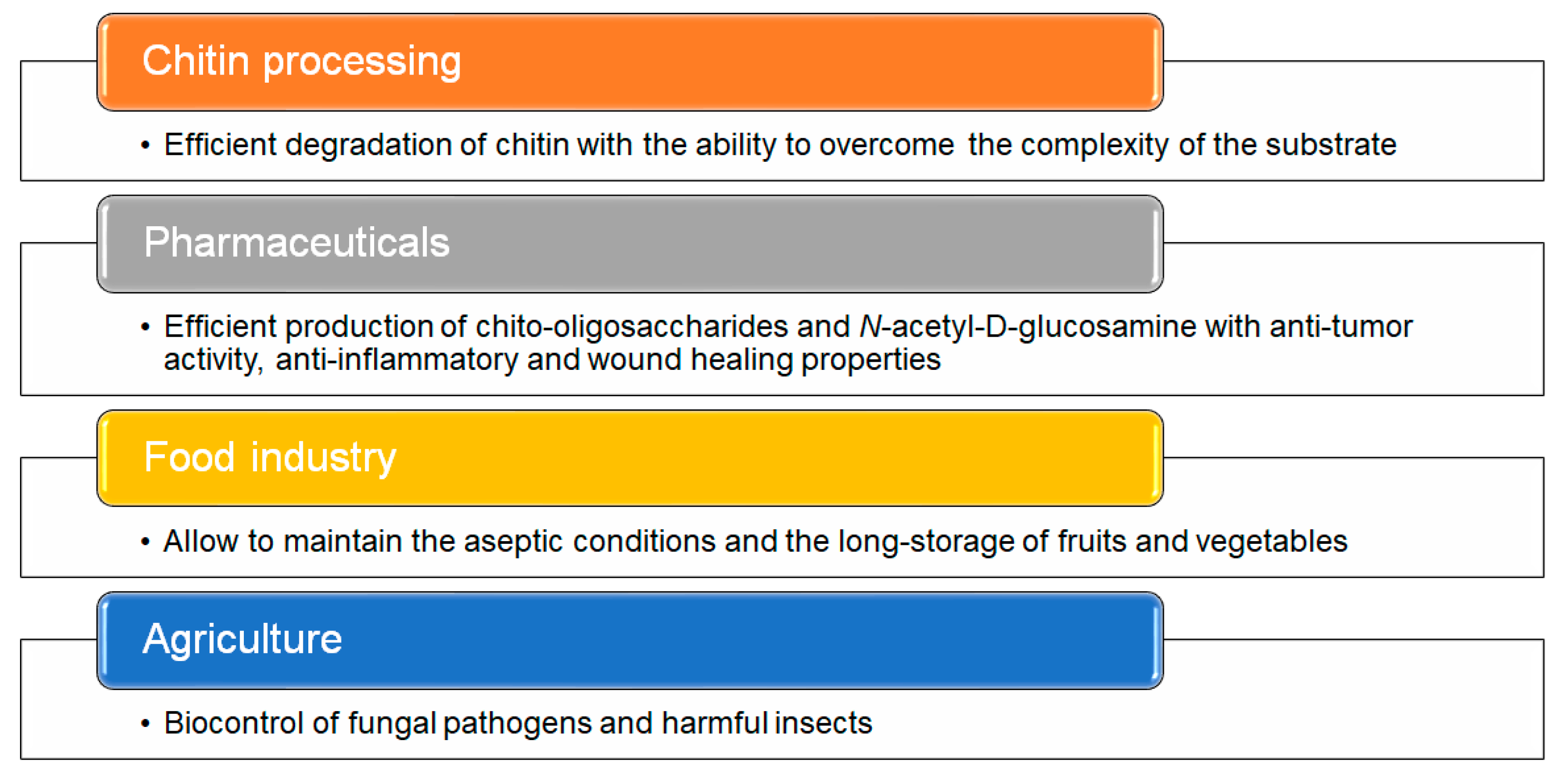
2.3.3. Chitinases
2.4. Metagenomics of Extreme Environments for the Discovery of GHs from Hyperthermophilic Archaea
3. Glycosyltransferases (GTs)
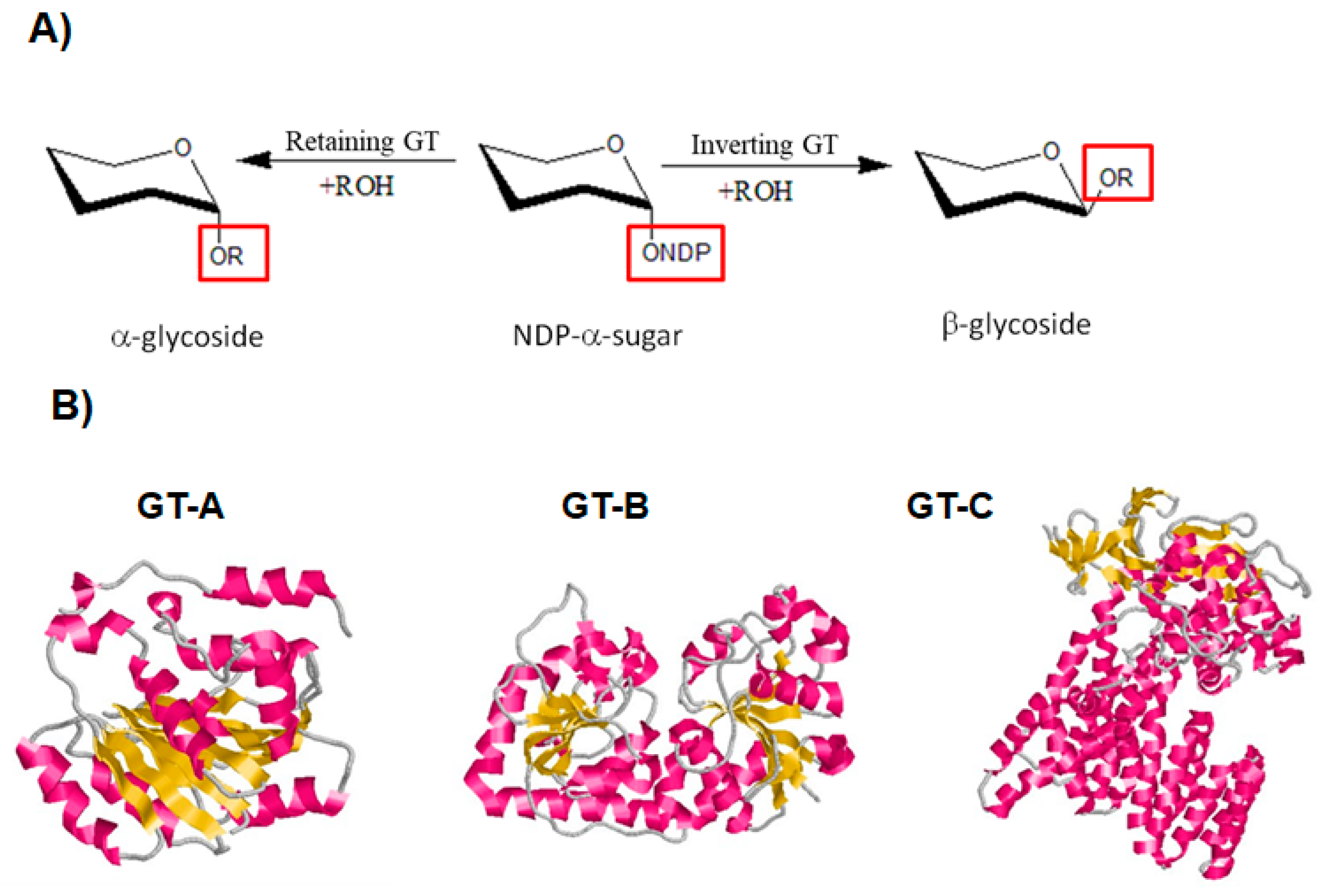
3.1. General Features and Reaction Mechanism
3.2. Hyperthermophilic GTs in Archaea: Biochemical Features and Biotechnological Applications
3.3. Membrane-Associated Archaeal GTs: The Protein N-Glycosylation Pathway
3.4. Soluble GTs
3.4.1. Glycogen Synthase (GS)
3.4.2. α-Glucan Phosphorylase/Maltodextrin Phosphorylases
3.4.3. Trehalose Synthases
Trehalose 6-Phosphate Synthases
3.4.4. Mannosyl-3-Phosphoglycerate (MPG) Synthases
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfeifer, K.; Ergal, İ.; Koller, M.; Basen, M.; Schuster, B.; Rittmann, S.K.R. Archaea Biotechnology. Biotechnol. Adv. 2021, 47, 107668. [Google Scholar] [CrossRef]
- Zeldes, B.M.; Keller, M.W.; Loder, A.J.; Straub, C.T.; Adams, M.W.; Kelly, R.M. Extremely thermophilic microorganisms as metabolic engineering platforms for production of fuels and industrial chemicals. Front. Microbiol. 2015, 6, 1209. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Lee, B.; Park, K. Extremophilic Carbohydrate Active Enzymes (CAZymes). J. Nutr. Health Food Eng. 2017, 7, 230–237. [Google Scholar]
- Dumorné, K.; Córdova, D.C.; Astorga-Eló, M.; Renganathan, P. Extremozymes: A Potential Source for Industrial Applications. J. Microbiol. Biotechnol. 2017, 27, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic. Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic. Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [Green Version]
- Gloster, T.M. Advances in understanding glycosyltransferases from a structural perspective. Curr. Opin. Struct. Biol. 2014, 28, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Cobucci-Ponzano, B.; Rossi, M.; Moracci, M. Extremophiles Handbook; Springer: Tokyo, Japan, 2011; pp. 427–441. [Google Scholar]
- Cabrera, M.; Blamey, J.M. Biotechnological applications of archaeal enzymes from extreme environments. Biol. Res. 2018, 51, 37. [Google Scholar] [CrossRef] [Green Version]
- Ebaid, R.; Wang, H.; Sha, C.; Abomohra, A.E.-F.; Shao, W. Recent trends in hyperthermophilic enzymes production and future perspectives for biofuel industry: A critical review. J. Clean. Prod. 2019, 238, 117925. [Google Scholar] [CrossRef]
- Straub, C.T.; Counts, J.A.; Nguyen, D.M.N.; Wu, C.H.; Zeldes, B.M.; Crosby, J.R.; Conway, J.M.; Otten, J.K.; Lipscomb, G.L.; Schut, G.J.; et al. Biotechnology of extremely thermophilic archaea. FEMS Microbiol. Rev. 2018, 42, 543–578. [Google Scholar] [CrossRef] [Green Version]
- Davies, G.; Henrissat, B. Structures and mechanisms of glycosyl hydrolases. Structure 1995, 3, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Naumoff, D. Hierarchical classification of glycoside hydrolases. Biochemistry (Moscow) 2011, 76, 622–635. [Google Scholar] [CrossRef]
- Ati, J.; Lafite, P.; Daniellou, R. Enzymatic synthesis of glycosides: From natural O- and N-glycosides to rare C- and S-glycosides. Beilstein. J. Org. Chem. 2017, 13, 1857–1865. [Google Scholar] [CrossRef] [Green Version]
- Sjogren, J.; Collin, M. Bacterial glycosidases in pathogenesis and glycoengineering. Future Microbiol. 2014, 9, 1039–1051. [Google Scholar] [CrossRef]
- Vermassen, A.; Leroy, S.; Talon, R.; Provot, C.; Popowska, M.; Desvaux, M. Cell Wall Hydrolases in Bacteria: Insight on the Diversity of Cell Wall Amidases, Glycosidases and Peptidases Toward Peptidoglycan. Front. Microbiol. 2019, 10, 331. [Google Scholar] [CrossRef]
- Henrissat, B.; Romeu, A. Families, superfamilies and subfamilies of glycosyl hydrolases. Biochem. J. 1995, 311, 350–351. [Google Scholar] [CrossRef] [Green Version]
- Vuong, T.V.; Wilson, D.B. Glycoside hydrolases: Catalytic base/nucleophile diversity. Biotechnol. Bioeng. 2010, 107, 195–205. [Google Scholar] [CrossRef]
- McCarter, J.D.; Withers, S.G. Mechanisms of enzymatic glycoside hydrolysis. Curr. Opin. Struct. Biol. 1994, 4, 885–892. [Google Scholar] [CrossRef]
- Carbohydrate Active Enzymes Database. Available online: http://www.cazy.org/ (accessed on 1 July 2021).
- Strazzulli, A.; Cobucci-Ponzano, B.; Iacono, R.; Giglio, R.; Maurelli, L.; Curci, N.; Schiano-di-Cola, C.; Santangelo, A.; Contursi, P.; Lombard, V.; et al. Discovery of hyperstable carbohydrate-active enzymes through metagenomics of extreme environments. FEBS J. 2020, 287, 1116–1137. [Google Scholar] [CrossRef] [PubMed]
- Iacono, R.; Cobucci-Ponzano, B. Spatial Metagenomics of Three Geothermal Sites in Pisciarelli Hot Spring Focusing on the Biochemical Resources of the Microbial Consortia. Molecules 2020, 25, 4023. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kumar, J.; Abedin, M.; Sahoo, D.; Pandey, A.; Rai, A.K.; Singh, S.P. Metagenomics revealing molecular profiling of community structure and metabolic pathways in natural hot springs of the Sikkim Himalaya. BMC Microbiol. 2020, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, A.; Sharma, S.; Satyanarayana, T. Thermostable cellulose saccharifying microbial enzymes: Characteristics, recent advances and biotechnological applications. Int. J. Biol. Macromol. 2021, 188, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, M.; Krüger, A.; Antranikian, G. Biomass-degrading glycoside hydrolases of archaeal origin. Biotechnol. Biofuels 2020, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Koch, R.; Spreinat, A.; Lemke, K.; Antranikian, G. Purification and properties of a hyperthermoactive α-amylase from the archaeobacterium Pyrococcus woesei. Arch. Microbiol. 1991, 155, 572–578. [Google Scholar] [CrossRef]
- Andrade, C.M.M.C.; Pereira, N., Jr.; Antranikian, G. Extremely thermophilic microorganisms and their polymer-hidrolytic enzymes. Rev. Microbiol. 1999, 30, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Schiraldi, C.; Giuliano, M.; De Rosa, M. Perspectives on biotechnological applications of archaea. Archaea 2002, 1, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Burg, B.V.D. Extremophiles as a source for novel enzymes. Curr. Opin. Microbiol. 2003, 6, 213–218. [Google Scholar] [CrossRef]
- Desmet, T.; Soetaert, W. Enzymatic glycosyl transfer: Mechanisms and applications. Biocatal. Biotransformation 2011, 29, 1–18. [Google Scholar] [CrossRef]
- Yennamalli, R.M.; Rader, A.J.; Kenny, A.J.; Wolt, J.D.; Sen, T.Z. Endoglucanases: Insights into thermostability for biofuel applications. Biotechnol. Biofuels 2013, 6, 136. [Google Scholar] [CrossRef] [Green Version]
- Leemhuis, H.; Dijkhuizen, L. Hydrolysis and Transglycosylation Reaction Specificity of Cyclodextrin Glycosyltransferases. J. Appl. Glycosci. 2003, 50, 263–271. [Google Scholar] [CrossRef]
- Torres, D.P.; Gonçalves, M.D.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [Green Version]
- Velázquez, J.B.; Villa, T.G. Industrial Applications of Hyperthermophilic Enzymes: A Review. Protein Pept. Lett. 2006, 13, 645–651. [Google Scholar] [CrossRef]
- Egorova, K.; Antranikian, G. Industrial relevance of thermophilic Archaea. Curr. Opin. Microbiol. 2005, 8, 649–655. [Google Scholar] [CrossRef]
- Henrissat, B.; Bairoch, A. Updating the sequence-based classification of glycosyl hydrolases. Biochem. J. 1996, 316, 695–696. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhao, J.; Fu, J.; Pan, Y.; Li, D. Sequence analysis and biochemical properties of an acidophilic and hyperthermophilic amylopullulanase from Thermofilum pendens. Int. J. Biol. Macromol. 2018, 114, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Park, J.-T.; Kim, Y.-W.; Lee, H.-S.; Nyawira, R.; Shin, H.-S.; Park, C.-S.; Yoo, S.-H.; Kim, Y.-R.; Moon, T.-W.; et al. Properties of a Novel Thermostable Glucoamylase from the Hyperthermophilic Archaeon Sulfolobus solfataricus in Relation to Starch Processing. Appl. Environ. Microbiol. 2004, 70, 3933–3940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.-H.; Seo, D.-H.; Holden, J.F.; Park, C.-S. Maltose-forming α-amylase from the hyperthermophilic archaeon Pyrococcus sp. ST04. Appl. Microbiol. Biotechnol. 2014, 98, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Ghafourian, S.; Vafaei, S.; Mohebi, R.; Farzi, M.; Taherikalani, M.; Sadeghifard, N. Cloning, Expression, and Purification of Hyperthermophile α-Amylase from Pyrococcus woesei. Osong. Public. Health Res. Perspect. 2015, 6, 336–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Lu, Z.; Lu, M.; Qin, S.; Liu, H.; Deng, X.; Lin, Q.; Chen, J. Identification of archaeon-producing hyperthermophilic α-amylase and characterization of the α-amylase. Appl. Microbiol. Biotechnol. 2008, 80, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.; Zhang, C.; Guo, F.; Wang, S.; Bie, X.; Lu, F.; Lu, Z. Secreted Expression of a Hyperthermophilic α-Amylase Gene from Thermococcus sp. HJ21 in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 2012, 22, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.S.; Akiba, T.; Kudo, T. Purification and characterization of α-amylase from hyperthermophilic archaeon Thermococcus profundus, which hydrolyzes both α-1,4 and α-1,6 glucosidic linkages. J. Ferment. Bioeng. 1998, 86, 363–367. [Google Scholar] [CrossRef]
- Chung, Y.C.; Kobayashi, T.; Kanai, H.; Akiba, T.; Kudo, T. Purification and Properties of Extracellular Amylase from the Hyperthermophilic Archaeon Thermococcus profundus DT5432. Appl. Environ. Microbiol. 1995, 61, 1502–1506. [Google Scholar] [CrossRef] [Green Version]
- Haseltine, C.; Rolfsmeier, M.; Blum, P. The glucose effect and regulation of alpha-amylase synthesis in the hyperthermophilic archaeon Sulfolobus solfataricus. J. Bacteriol. 1996, 178, 945–950. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Flowers, L.O.; Whiteley, M.; Peeples, T.L. Biochemical confirmation and characterization of the family-57-like alpha-amylase of Methanococcus jannaschii. Folia Microbiol. (Praha) 2001, 46, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, A.; Vieille, C.; Kang, S.; Zeikus, J.G. Pyrococcus furiosus alpha-amylase is stabilized by calcium and zinc. Biochemistry 2002, 41, 6193–6201. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Li, D.; Park, J.-T.; Yoon, S.-M.; Tran, P.L.; Oh, B.-H.; Janeček, S.; Park, S.G.; Woo, E.-J.; Park, K.-H. Association of Novel Domain in Active Site of Archaic Hyperthermophilic Maltogenic Amylase from Staphylothermus marinus. J. Biol. Chem. 2012, 287, 7979–7989. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, Y.; Park, J.T.; Gu, L.; Li, D. An extremely thermostable maltogenic amylase from Staphylothermus marinus: Bacillus expression of the gene and its application in genistin glycosylation. Int. J. Biol. Macromol. 2018, 107, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Park, J.-T.; Li, X.; Kim, S.; Lee, S.; Shim, J.-H.; Park, S.-H.; Cha, J.; Lee, B.; Kim, J.-W.; et al. Overexpression and characterization of an extremely thermostable maltogenic amylase, with an optimal temperature of 100°C, from the hyperthermophilic archaeon Staphylothermus marinus. New Biotechnol. 2010, 27, 300–307. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, Y.-H.; Lee, H.-S.; Yang, S.-J.; Kim, Y.-W.; Lee, M.-H.; Seo, N.-S.; Park, C.-S.; Park, K.-H. Molecular cloning and biochemical characterization of the first archaeal maltogenic amylase from the hyperthermophilic archaeon Thermoplasma volcanium GSS1. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2007, 1774, 661–669. [Google Scholar] [CrossRef]
- Satyanarayana, T.; Noorwez, S.; Kumar, S.; Rao, J.; Ezhilvannan, M.; Kaur, P. Development of an ideal starch saccharification process using amylolytic enzymes from thermophiles. Biochem. Soc. Trans. 2004, 32, 276–278. [Google Scholar] [CrossRef] [Green Version]
- Comfort, D.A.; Chou, C.J.; Conners, S.B.; VanFossen, A.L.; Kelly, R.M. Functional-genomics-based identification and characterization of open reading frames encoding alpha-glucoside-processing enzymes in the hyperthermophilic archaeon Pyrococcus furiosus. Appl. Environ. Microbiol. 2008, 74, 1281–1283. [Google Scholar] [CrossRef] [Green Version]
- Serour, E.; Antranikian, G. Novel thermoactive glucoamylases from the thermoacidophilic Archaea Thermoplasma acidophilum, Picrophilus torridus and Picrophilus oshimae. Antonie. Van. Leeuwenhoek. 2002, 81, 73–83. [Google Scholar] [CrossRef]
- Dock, C.; Hess, M.; Antranikian, G. A thermoactive glucoamylase with biotechnological relevance from the thermoacidophilic Euryarchaeon Thermoplasma acidophilum. Appl. Microbiol. Biotechnol. 2007, 78, 105–114. [Google Scholar] [CrossRef]
- Schepers, B.; Thiemann, V.; Antranikian, G. Characterization of a Novel Glucoamylase from the Thermoacidophilic ArchaeonPicrophilus torridus Heterologously Expressed inE. coli. Eng. Life Sci. 2006, 6, 311–317. [Google Scholar] [CrossRef]
- Satyanarayana, T.; Nisha, M. Archaeal and bacterial thermostable amylopullulanases: Characteristic features and biotechnological applications. Amylase 2018, 2, 44. [Google Scholar] [CrossRef]
- Hii, S.L.; Tan, J.S.; Ling, T.C.; Bin Ariff, A. Pullulanase: Role in Starch Hydrolysis and Potential Industrial Applications. Enzym. Res. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.C.; Zeikus, J.G. Novel highly thermostable pullulanase from thermophiles. Trends Biotechnol. 1989, 7, 234–239. [Google Scholar] [CrossRef]
- Brown, S.H.; Costantino, H.R.; Kelly, R.M. Characterization of Amylolytic Enzyme Activities Associated with the Hyperthermophilic Archaebacterium Pyrococcus furiosus. Appl. Environ. Microbiol. 1990, 56, 1985–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.H.; Kelly, R.M. Characterization of Amylolytic Enzymes, Having Both alpha-1,4 and alpha-1,6 Hydrolytic Activity, from the Thermophilic Archaea Pyrococcus furiosus and Thermococcus litoralis. Appl. Environ. Microbiol. 1993, 59, 2614–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, S.; Ding, N.; Ren, J.; Gu, Z.; Li, C.; Hong, Y.; Cheng, L.; Holler, T.P.; Li, Z. Maltooligosaccharide-forming amylase: Characteristics, preparation, and application. Biotechnol. Adv. 2017, 35, 619–632. [Google Scholar] [CrossRef]
- Ben Ali, M.; Khemakhem, B.; Robert, X.; Haser, R.; Bejar, S. Thermostability enhancement and change in starch hydrolysis profile of the maltohexaose-forming amylase of Bacillus stearothermophilus US100 strain. Biochem. J. 2006, 394, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.; Zhang, F.; Sun, F.; Chen, H.; Zhang, Y.-H.P. Doubling Power Output of Starch Biobattery Treated by the Most Thermostable Isoamylase from an Archaeon Sulfolobus tokodaii. Sci. Rep. 2015, 5, 13184. [Google Scholar] [CrossRef] [Green Version]
- Van der Maarel, M.J.; van der Veen, B.; Uitdehaag, J.C.; Leemhuis, H.; Dijkhuizen, L. Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol. 2002, 94, 137–155. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, D.; Park, K.-H. An extremely thermostable amylopullulanase from Staphylothermus marinus displays both pullulan- and cyclodextrin-degrading activities. Appl. Microbiol. Biotechnol. 2012, 97, 5359–5369. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Zhang, L.; Ding, Z.; Gu, Z.; Shi, G. Investigation of debranching pattern of a thermostable isoamylase and its application for the production of resistant starch. Carbohydr. Res. 2017, 446–447, 93–100. [Google Scholar] [CrossRef]
- Costantino, H.; Brown, S.; Kelly, R. Purification and characterization of an α-glucosidase from a hyperthermophilic archaebacterium, Pyrococcus furiosus, exhibiting a temperature optimum of 105 to 115°C. J. Bacteriol. 1990, 172, 3654–3660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolfsmeier, M.; Blum, P. Purification and characterization of a maltase from the extremely thermophilic crenarchaeote Sulfolobus solfataricus. J. Bacteriol. 1995, 177, 482–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolfsmeier, M.; Haseltine, C.; Bini, E.; Clark, A.; Blum, P. Molecular Characterization of the α-Glucosidase Gene from the Hyperthermophilic Archaeon Sulfolobus solfataricus. J. Bacteriol. 1998, 180, 1287–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.T.; Parker, K.N.; Bauer, M.W.; Kelly, R.M. Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 260–269. [Google Scholar]
- Lee, M.-H.; Yang, S.-J.; Kim, J.-W.; Lee, H.-S.; Kim, J.-W.; Park, K.-H. Characterization of a thermostable cyclodextrin glucanotransferase from Pyrococcus furiosus DSM3638. Extremophiles 2007, 11, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.; Cornista, J.; Ezaki, S.; Fukui, T.; Atomi, H.; Imanaka, T. Characterization of an archaeal cyclodextrin glucanotransferase with a novel C-terminal domain. J. Bacteriol. 2002, 184, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-S.; Park, J.-T.; Kang, H.-K.; Cha, H.; Kim, D.-S.; Kim, J.-W.; Park, K.-H. TreX fromSulfolobus solfataricusATCC 35092 Displays Isoamylase and 4-α-Glucanotransferase Activities. Biosci. Biotechnol. Biochem. 2007, 71, 1348–1352. [Google Scholar] [CrossRef]
- Xavier, K.B.; Peist, R.; Kossmann, M.; Boos, W.; Santos, H. Maltose metabolism in the hyperthermophilic archaeon Thermococcus litoralis: Purification and characterization of key enzymes. J. Bacteriol. 1999, 181, 3358–3367. [Google Scholar] [CrossRef] [Green Version]
- Kaper, T.; Talik, B.; Ettema, T.J.; Bos, H.; van der Maarel, M.J.E.C.; Dijkhuizen, L. Amylomaltase of Pyrobaculum aerophilum IM2 Produces Thermoreversible Starch Gels. Appl. Environ. Microbiol. 2005, 71, 5098–5106. [Google Scholar] [CrossRef] [Green Version]
- Mehboob, S.; Ahmad, N.; Rashid, N.; Imanaka, T.; Akhtar, M. Pcal_0768, a hyperactive 4-α-glucanotransferase from Pyrobacculum calidifontis. Extremophiles 2016, 20, 559–566. [Google Scholar] [CrossRef]
- Mehboob, S.; Ahmad, N.; Munir, S.; Ali, R.; Younas, H.; Rashid, N. Gene cloning, expression enhancement in Escherichia coli and biochemical characterization of a highly thermostable amylomaltase from Pyrobaculum calidifontis. Int. J. Biol. Macromol. 2020, 165, 645–653. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Sim, S.J.; Pandey, A. Thermostable cellulases: Current status and perspectives. Bioresour. Technol. 2019, 279, 385–392. [Google Scholar] [CrossRef]
- Pisani, F.M.; Rella, R.; Raia, C.A.; Rozzo, C.; Nucci, R.; Gambacorta, A.; de Rosa, M.; Rossi, M. Thermostable beta-galactosidase from the archaebacterium Sulfolobus solfataricus Purification and properties. JBIC J. Biol. Inorg. Chem. 1990, 187, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Vieille, C.; Zeikus, G.J. Hyperthermophilic Enzymes: Sources, Uses, and Molecular Mechanisms for Thermostability. Microbiol. Mol. Biol. Rev. 2001, 65, 1–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigoldi, F.; Donini, S.; Redaelli, A.; Parisini, E.; Gautieri, A. Review: Engineering of thermostable enzymes for industrial applications. Appl. Bioeng. 2018, 2, 011501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Vaid, S.; Bhat, B.; Singh, S.; Bajaj, B.K. Biomass, Biofuels and Biochemicals: Advances in Enzyme Technology; Elsevier: Jammu, India, 2019; Volume 17, pp. 469–495. [Google Scholar]
- Turner, P.; Mamo, G.; Karlsson, E.N. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell Factories 2007, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Singhal, G.; Bhagyawant, S.S.; Srivastava, N. Current Status and Future Scope of Microbial Cellulases; Elsevier: Jammu, India, 2021; Volume 3, pp. 39–57. [Google Scholar]
- Kashima, Y.; Mori, K.; Fukada, H.; Ishikawa, K. Analysis of the function of a hyperthermophilic endoglucanase from Pyrococcus horikoshii that hydrolyzes crystalline cellulose. Extremophiles 2005, 9, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.W.; Driskill, L.E.; Callen, W.; Snead, M.A.; Mathur, E.J.; Kelly, R.M. An Endoglucanase, EglA, from the Hyperthermophilic Archaeon Pyrococcus furiosus Hydrolyzes β-1,4 Bonds in Mixed-Linkage (1→3),(1→4)-β-D-Glucans and Cellulose. J. Bacteriol. 1999, 181, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Neelamegam, A.; Rajeswari, M.; Thangavel, B.; Gupta, V. Microbial Enzymes in Bioconversions of Biomass; Springer: Cham, Switzerland, 2016; pp. 37–45. [Google Scholar]
- Girfoglio, M.; Rossi, M.; Cannio, R. Cellulose Degradation by Sulfolobus solfataricus Requires a Cell-Anchored Endo-β-1-4-Glucanase. J. Bacteriol. 2012, 194, 5091–5100. [Google Scholar] [CrossRef] [Green Version]
- Klose, H.; Röder, J.; Girfoglio, M.; Fischer, R.; Commandeur, U. Hyperthermophilic endoglucanase for in planta lignocellulose conversion. Biotechnol. Biofuels 2012, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-Z.; Zhang, Y.-H.P. Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 131–146. [Google Scholar]
- Béguin, P. Molecular biology of cellulose degradation. Annu. Rev. Microbiol. 1990, 44, 219–248. [Google Scholar] [CrossRef]
- Tipparat, H. Hyperthermostable cellulolytic and hemicellulolytic enzymes and their biotechnological applications. SJST 2002, 24, 481–491. [Google Scholar]
- Olajuyigbe, F.M.; Nlekerem, C.M.; Ogunyewo, O.A. Production and Characterization of Highly Thermostable β-Glucosidase during the Biodegradation of Methyl Cellulose by Fusarium oxysporum. Biochem. Res. Int. 2016, 2016, 3978124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Li, X.; Dang, W.; Tran, P.L.; Park, S.H.; Oh, B.C.; Hong, W.S.; Lee, J.S.; Park, K.H. Characterization and application of an acidophilic and thermostable beta-glucosidase from Thermofilum pendens. J. Biosci. Bioeng. 2013, 115, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Hansson, T.; Kaper, T.; van Der Oost, J.; de Vos, W.M.; Adlercreutz, P. Improved oligosaccharide synthesis by protein engineering of beta-glucosidase CelB from hyperthermophilic Pyrococcus furiosus. Biotechnol. Bioeng. 2001, 73, 203–210. [Google Scholar] [CrossRef]
- Voorhorst, W.G.; Eggen, I.R.; Luesink, E.J.; de Vos, W.M. Characterization of the celB gene coding for beta-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus and its expression and site-directed mutation in Escherichia coli. J. Bacteriol. 1995, 177, 7105–7111. [Google Scholar] [CrossRef] [Green Version]
- Kado, Y.; Inoue, T.; Ishikawa, K. Structure of hyperthermophilic β-glucosidase from Pyrococcus furiosus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Lebbink, J.; Kaper, T.; Kengen, S.; Oost, J.; De Vos, W. β-glucosidase CelB from Pyrococcus furiosus: Production by Escherichia coli, purification, and in vitro evolution. Methods Enzym. 2001, 330, 364–379. [Google Scholar]
- Kim, H.W.; Ishikawa, K. Complete saccharification of cellulose at high temperature using endocellulase and beta-glucosidase from Pyrococcus sp. J. Microbiol. Biotechnol. 2010, 20, 889–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.H.; Park, K.H.; Oh, B.C.; Alli, I.; Lee, B.H. Expression and characterization of an extremely thermostable beta-glycosidase (mannosidase) from the hyperthermophilic archaeon Pyrococcus furiosus DSM3638. N Biotechnol. 2011, 28, 639–648. [Google Scholar] [CrossRef]
- Gumerov, V.; Rakitin, A.; Mardanov, A.; Ravin, N.V. A Novel Highly Thermostable Multifunctional Beta-Glycosidase from CrenarchaeonAcidilobus saccharovorans. Archaea 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, A.; Walsh, G. Expression and characterisation of a thermophilic endo-1,4-β-glucanase from Sulfolobus shibatae of potential industrial application. Mol. Biol. Rep. 2018, 45, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Park, N.-Y.; Cha, J.; Kim, D.-O.; Park, C.-S. Enzymatic characterization and substrate specificity of thermostable beta-glycosidase from hyperthermophilic archaea, Sulfolobus shibatae, expressed in E. coli. J. Microbiol. Biotechnol. 2007, 17, 454–460. [Google Scholar]
- Rathore, A.S.; Gupta, R.D. Chitinases from Bacteria to Human: Properties, Applications, and Future Perspectives. Enzym. Res. 2015, 2015, 791907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyeleye, A.; Normi, Y.M. Chitinase: Diversity, limitations, and trends in engineering for suitable applications. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Chen, L.; Wei, Y.; Shi, M.; Li, Z.; Zhang, S.-H. An Archaeal Chitinase With a Secondary Capacity for Catalyzing Cellulose and Its Biotechnological Applications in Shell and Straw Degradation. Front. Microbiol. 2019, 10, 1253. [Google Scholar] [CrossRef] [Green Version]
- Le, B.; Yang, S.H. Microbial chitinases: Properties, current state and biotechnological applications. World J. Microbiol. Biotechnol. 2019, 35, 144. [Google Scholar] [CrossRef]
- Kuzu, S.B.; Güvenmez, H.K.; Denizci, A.A. Production of a Thermostable and Alkaline Chitinase by Bacillus thuringiensis subsp. kurstaki Strain HBK-51. Biotechnol. Res. Int. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Chilukoti, N.; Pvsrn, S.; Jogi, M.; Pallinti, P.N.; Kaur, M.; Dutta, S.; Podile, A.R. Microorganisms in Environmental Management: Microbes and Environment; Elsevier: Dordrecht, The Netherlands, 2012; pp. 135–150. [Google Scholar]
- Tanaka, T.; Fujiwara, S.; Nishikori, S.; Fukui, T.; Takagi, M.; Imanaka, T. A Unique Chitinase with Dual Active Sites and Triple Substrate Binding Sites from the Hyperthermophilic Archaeon Pyrococcus kodakaraensis KOD1. Appl. Environ. Microbiol. 1999, 65, 5338–5344. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Fukui, T.; Imanaka, T. Different cleavage specificities of the dual catalytic domains in chitinase from the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Biol. Chem. 2001, 276, 35629–35635. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Mine, S.; Hagihara, Y.; Ishikawa, K.; Uegaki, K. Structure of the catalytic domain of the hyperthermophilic chitinase fromPyrococcus furiosus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 63, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Chettri, D.; Verma, A.K.; Verma, A.K. Innovations in CAZyme gene diversity and its modification for biorefinery applications. Biotechnol. Rep. 2020, 28, e00525. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.E.; Clark, M.E.; Nadler, D.C.; Huffer, S.; Chokhawala, H.A.; Rowland, S.E.; Blanch, H.W.; Clark, D.S.; Robb, F.T. Identification and characterization of a multidomain hyperthermophilic cellulase from an archaeal enrichment. Nat. Commun. 2011, 2, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavrilov, S.N.; Stracke, C.; Jensen, K.; Menzel, P.; Kallnik, V.; Slesarev, A.; Sokolova, T.; Zayulina, K.; Bräsen, C.; Bonch-Osmolovskaya, E.A.; et al. Isolation and Characterization of the First Xylanolytic Hyperthermophilic Euryarchaeon Thermococcus sp. Strain 2319x1 and Its Unusual Multidomain Glycosidase. Front. Microbiol. 2016, 7, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antranikian, G.; Suleiman, M.; Schäfers, C.; Adams, M.W.W.; Bartolucci, S.; Blamey, J.M.; Birkeland, N.K.; Bonch-Osmolovskaya, E.; da Costa, M.S.; Cowan, D.; et al. Diversity of bacteria and archaea from two shallow marine hydrothermal vents from Vulcano Island. Extremophiles 2017, 21, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.; Eixenberger, D.; Suleiman, M.; Schäfers, C.; Antranikian, G. Characterization of an extremely thermo-active archaeal β-glucosidase and its activity towards glucan and mannan in concert with an endoglucanase. Appl. Microbiol. Biotechnol. 2019, 103, 9505–9514. [Google Scholar] [CrossRef] [PubMed]
- Lewin, A.; Zhou, J.; Pham, V.T.T.; Haugen, T.; Zeiny, M.E.; Aarstad, O.; Liebl, W.; Wentzel, A.; Liles, M.R. Novel archaeal thermostable cellulases from an oil reservoir metagenome. AMB Express. 2017, 7, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushal, G.; Kumar, J.; Sangwan, R.S.; Singh, S.P. Metagenomic analysis of geothermal water reservoir sites exploring carbohydrate-related thermozymes. Int. J. Biol. Macromol. 2018, 119, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Kapitonov, D.; Yu, R.K. Conserved domains of glycosyltransferases. Glycobiology 1999, 9, 961–978. [Google Scholar] [CrossRef] [Green Version]
- Eschmid, J.; Heider, D.; Wendel, N.J.; Esperl, N.; Sieber, V. Bacterial Glycosyltransferases: Challenges and Opportunities of a Highly Diverse Enzyme Class Toward Tailoring Natural Products. Front. Microbiol. 2016, 7, 182. [Google Scholar] [CrossRef] [Green Version]
- Breton, C.; Šnajdrová, L.; Jeanneau, C.; Koca, J.; Imberty, A. Structures and mechanisms of glycosyltransferases. Glycobiology 2005, 16, 29R–37R. [Google Scholar] [CrossRef]
- Liu, J.; Mushegian, A. Three monophyletic superfamilies account for the majority of the known glycosyltransferases. Protein Sci. 2003, 12, 1418–1431. [Google Scholar] [CrossRef] [Green Version]
- Sinnott, M.L. Catalytic mechanism of enzymic glycosyl transfer. Chem. Rev. 1990, 90, 1171–1202. [Google Scholar] [CrossRef]
- Liang, D.-M.; Liu, J.-H.; Wu, H.; Wang, B.-B.; Zhu, H.-J.; Qiao, J.-J. Glycosyltransferases: Mechanisms and applications in natural product development. Chem. Soc. Rev. 2015, 44, 8350–8374. [Google Scholar] [CrossRef]
- Protein Data Bank PDB. Available online: https://www.rcsb.org/ (accessed on 1 October 2021).
- Coutinho, P.M.; Deleury, E.; Davies, G.; Henrissat, B. An Evolving Hierarchical Family Classification for Glycosyltransferases. J. Mol. Biol. 2003, 328, 307–317. [Google Scholar] [CrossRef]
- Fukuda, M.; Bierhuizen, M.F.A.; Nakayama, J. Expression cloning of glycosyltransferases. Glycobiology 1996, 6, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, S.; Shimada, A.; Nyirenda, J.; Igura, M.; Kawano, Y.; Kohda, D. Crystal structures of an archaeal oligosaccharyltransferase provide insights into the catalytic cycle of N-linked protein glycosylation. Proc. Natl. Acad. Sci. USA 2013, 110, 17868–17873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrimal, S.; Gilmore, R. Oligosaccharyltransferase structures provide novel insight into the mechanism of asparagine-linked glycosylation in prokaryotic and eukaryotic cells. Glycobiology 2018, 29, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, K.F.; Ding, Y.; Meyer, B.H.; Albers, S.-V.; Kaminski, L.; Eichler, J. N-Linked Glycosylation in Archaea: A Structural, Functional, and Genetic Analysis. Microbiol. Mol. Biol. Rev. 2014, 78, 304–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Qarn, M.; Giordano, A.; Battaglia, F.; Trauner, A.; Hitchen, P.G.; Morris, H.R.; Dell, A.; Eichler, J. Identification of AglE, a Second Glycosyltransferase Involved in N Glycosylation of the Haloferax volcanii S-Layer Glycoprotein. J. Bacteriol. 2008, 190, 3140–3146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanDyke, D.J.; Wu, J.; Logan, S.M.; Kelly, J.F.; Mizuno, S.; Aizawa, S.; Jarrell, K.F. Identification of genes involved in the assembly and attachment of a novel flagellin N-linked tetrasaccharide important for motility in the archaeon Methanococcus maripaludis. Mol. Microbiol. 2009, 72, 633–644. [Google Scholar] [CrossRef]
- Meyer, B.H.; Peyfoon, E.; Dietrich, C.; Hitchen, P.; Panico, M.; Morris, H.R.; Dell, A.; Albers, S.-V. Agl16, a Thermophilic Glycosyltransferase Mediating the Last Step of N-Glycan Biosynthesis in the Thermoacidophilic Crenarchaeon Sulfolobus acidocaldarius. J. Bacteriol. 2013, 195, 2177. [Google Scholar] [CrossRef] [Green Version]
- Elharar, Y.; Podilapu, A.R.; Guan, Z.; Kulkarni, S.S.; Eichler, J. Assembling Glycan-Charged Dolichol Phosphates: Chemoenzymatic Synthesis of a Haloferax volcanii N-Glycosylation Pathway Intermediate. Bioconjugate Chem. 2017, 28, 2461–2470. [Google Scholar] [CrossRef]
- Igura, M.; Maita, N.; Kamishikiryo, J.; Yamada, M.; Obita, T.; Maenaka, K.; Kohda, D. Structure-guided identification of a new catalytic motif of oligosaccharyltransferase. EMBO J. 2007, 27, 234–243. [Google Scholar] [CrossRef]
- Kohda, D. Structural Basis of Protein Asn-Glycosylation by Oligosaccharyltransferases. Adv. Exp. Med. Biol 2018, 1104, 171–199. [Google Scholar]
- Magidovich, H.; Eichler, J. Glycosyltransferases and oligosaccharyltransferases in Archaea: Putative components of the N-glycosylation pathway in the third domain of life. Fems. Microbiol. Lett. 2009, 300, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Cohen-Rosenzweig, C.; Guan, Z.; Shaanan, B.; Eichler, J. Substrate Promiscuity: AglB, the Archaeal Oligosaccharyltransferase, Can Process a Variety of Lipid-Linked Glycans. Appl. Environ. Microbiol. 2013, 80, 486–496. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.C.; Laine, R.A. Dolichyl-phosphomannose synthase from the Archae Thermoplasma acidophilum. Glycobiology 1996, 6, 811–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanFossen, A.L.; Lewis, D.L.; Nichols, J.D.; Kelly, R.M. Polysaccharide Degradation and Synthesis by Extremely Thermophilic Anaerobes. Ann. N. Y. Acad. Sci. 2008, 1125, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Urushibata, Y.; Ebisu, S.; Matsui, I. A thermostable dolichol phosphoryl mannose synthase responsible for glycoconjugate synthesis of the hyperthermophilic archaeon Pyrococcus horikoshii. Extremophiles 2008, 12, 665–676. [Google Scholar] [CrossRef]
- Takemasa, R.; Yokooji, Y.; Yamatsu, A.; Atomi, H.; Imanaka, T. Thermococcus kodakarensis as a Host for Gene Expression and Protein Secretion. Appl. Environ. Microbiol. 2011, 77, 2392–2398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.D.; Ciulla, R.A.; Roberts, M.F. Osmoadaptation in Archaea. Appl. Environ. Microbiol. 1999, 65, 1815–1825. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.; Dijkhuizen, L.; Leemhuis, H. Starch and α-glucan acting enzymes, modulating their properties by directed evolution. J. Biotechnol. 2009, 140, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Ubiparip, Z.; Beerens, K.; Franceus, J.; Vercauteren, R.; Desmet, T. Thermostable alpha-glucan phosphorylases: Characteristics and industrial applications. Appl. Microbiol. Biotechnol. 2018, 102, 8187–8202. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, Y.; Liu, Q.; Wang, R.; Liu, X.; Liu, B. Enzymatic and regulatory properties of the trehalose-6-phosphate synthase from the thermoacidophilic archaeon Thermoplasma acidophilum. Biochimie 2014, 101, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Tsai, M.-Y.; Lee, G.-C.; Shaw, J.-F. Construction of a Recombinant Thermostable β-Amylase-Trehalose Synthase Bifunctional Enzyme for Facilitating the Conversion of Starch to Trehalose. J. Agric. Food Chem. 2007, 55, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Empadinhas, N.; Marugg, J.; Borges, N.; Santos, H.; da Costa, M. Pathway for the Synthesis of Mannosylglycerate in the Hyperthermophilic Archaeon Pyrococcus horikoshii. J. Biol. Chem. 2001, 276, 43580–43588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horcajada, C.; Guinovart, J.J.; Fita, I.; Ferrer, J.C. Crystal structure of an archaeal glycogen synthase: Insights into oligomerization and substrate binding of eukaryotic glycogen synthases. J. Biol. Chem. 2006, 281, 2923–2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruyer, S.; Legin, E.; Bliard, C.; Ball, S.; Duchiron, F. The Endopolysaccharide Metabolism of the Hyperthermophilic Archeon Thermococcus hydrothermalis: Polymer Structure and Biosynthesis. Curr. Microbiol. 2002, 44, 206–211. [Google Scholar] [CrossRef]
- Zea, C.J.; MacDonell, S.W.; Pohl, N.L. Discovery of the Archaeal Chemical Link between Glycogen (Starch) Synthase Families Using a New Mass Spectrometry Assay. J. Am. Chem. Soc. 2003, 125, 13666–13667. [Google Scholar] [CrossRef]
- Zea, C.J.; Pohl, N.L. Unusual sugar nucleotide recognition elements of mesophilic vs. thermophilic glycogen synthases. Biopolymers 2005, 79, 106–113. [Google Scholar] [CrossRef]
- Mizanur, R.M.; Griffin, A.K.K.; Pohl, N.L. Recombinant production and biochemical characterization of a hyperthermostable α-glucan/maltodextrin phosphorylase fromPyrococcus furiosus. Archaea 2008, 2, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Rathore, R.S.; Garg, N.; Garg, S.; Kumar, A. Starch phosphorylase: Role in starch metabolism and biotechnological applications. Crit. Rev. Biotechnol. 2009, 29, 214–224. [Google Scholar] [CrossRef]
- Zhu, Z.; Kin Tam, T.; Sun, F.; You, C.; Percival Zhang, Y.H. A high-energy-density sugar biobattery based on a synthetic enzymatic pathway. Nat. Commun. 2014, 5, 3026. [Google Scholar] [CrossRef] [Green Version]
- Labes, A.; Schönheit, P. Unusual Starch Degradation Pathway via Cyclodextrins in the Hyperthermophilic Sulfate-Reducing Archaeon Archaeoglobus fulgidus Strain 7324. J. Bacteriol. 2007, 189, 8901–8913. [Google Scholar] [CrossRef] [Green Version]
- Mueller, M.; Takemasa, R.; Schwarz, A.; Atomi, H.; Nidetzky, B. “Short-chain” α-1,4-glucan phosphorylase having a truncated N-terminal domain: Functional expression and characterization of the enzyme from Sulfolobus solfataricus. Biochim. Biophys. Acta 2009, 1794, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, S.; Wang, Y.J. Trehalose: Current use and future applications. J. Pharm. Sci. 2011, 100, 2020–2053. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-H.; Jung, J.-H.; Seo, D.-H.; Ha, S.-J.; Yoo, S.-H.; Kim, C.-H.; Park, C.-S. Novel enzymatic production of trehalose from sucrose using amylosucrase and maltooligosyltrehalose synthase-trehalohydrolase. World J. Microbiol. Biotechnol. 2011, 27, 2851–2856. [Google Scholar] [CrossRef]
- Ryu, S.-I.; Park, C.-S.; Cha, J.; Woo, E.-J.; Lee, S.-B. A novel trehalose-synthesizing glycosyltransferase from Pyrococcus horikoshii: Molecular cloning and characterization. Biochem. Biophys. Res. Commun. 2005, 329, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Lee, S.J.; Boos, W. TreT, a novel trehalose glycosyltransferring synthase of the hyperthermophilic archaeon Thermococcus litoralis. J. Biol. Chem. 2004, 279, 47890–47897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouril, T.; Zaparty, M.; Marrero, J.; Brinkmann, H.; Siebers, B. A novel trehalose synthesizing pathway in the hyperthermophilic Crenarchaeon Thermoproteus tenax: The unidirectional TreT pathway. Arch. Microbiol. 2008, 190, 355. [Google Scholar] [CrossRef]
- Ponnu, J.; Wahl, V.; Schmid, M. Trehalose-6-phosphate: Connecting plant metabolism and development. Front. Plant Sci. 2011, 2, 70. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, S.; Borges, N.; Santos, H.; Matias, P.M. Crystallization and preliminary X-ray analysis of mannosyl-3-phosphoglycerate synthase from Thermus thermophilus HB27. Acta Cryst. Sect. F 2009, 65, 1014–1017. [Google Scholar] [CrossRef] [Green Version]
- Neves, C.; da Costa, M.S.; Santos, H. Compatible Solutes of the Hyperthermophile Palaeococcus ferrophilus: Osmoadaptation and Thermoadaptation in the Order Thermococcales. Appl. Environ. Microbiol. 2005, 71, 8091–8098. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, K.; Tranchimand, S.; Benvegnu, T.; Abdel-Razzak, Z.; Chamieh, H. Glycoside Hydrolases and Glycosyltransferases from Hyperthermophilic Archaea: Insights on Their Characteristics and Applications in Biotechnology. Biomolecules 2021, 11, 1557. https://doi.org/10.3390/biom11111557
Amin K, Tranchimand S, Benvegnu T, Abdel-Razzak Z, Chamieh H. Glycoside Hydrolases and Glycosyltransferases from Hyperthermophilic Archaea: Insights on Their Characteristics and Applications in Biotechnology. Biomolecules. 2021; 11(11):1557. https://doi.org/10.3390/biom11111557
Chicago/Turabian StyleAmin, Khadija, Sylvain Tranchimand, Thierry Benvegnu, Ziad Abdel-Razzak, and Hala Chamieh. 2021. "Glycoside Hydrolases and Glycosyltransferases from Hyperthermophilic Archaea: Insights on Their Characteristics and Applications in Biotechnology" Biomolecules 11, no. 11: 1557. https://doi.org/10.3390/biom11111557
APA StyleAmin, K., Tranchimand, S., Benvegnu, T., Abdel-Razzak, Z., & Chamieh, H. (2021). Glycoside Hydrolases and Glycosyltransferases from Hyperthermophilic Archaea: Insights on Their Characteristics and Applications in Biotechnology. Biomolecules, 11(11), 1557. https://doi.org/10.3390/biom11111557






