PCNA Ubiquitylation: Instructive or Permissive to DNA Damage Tolerance Pathways?
Abstract
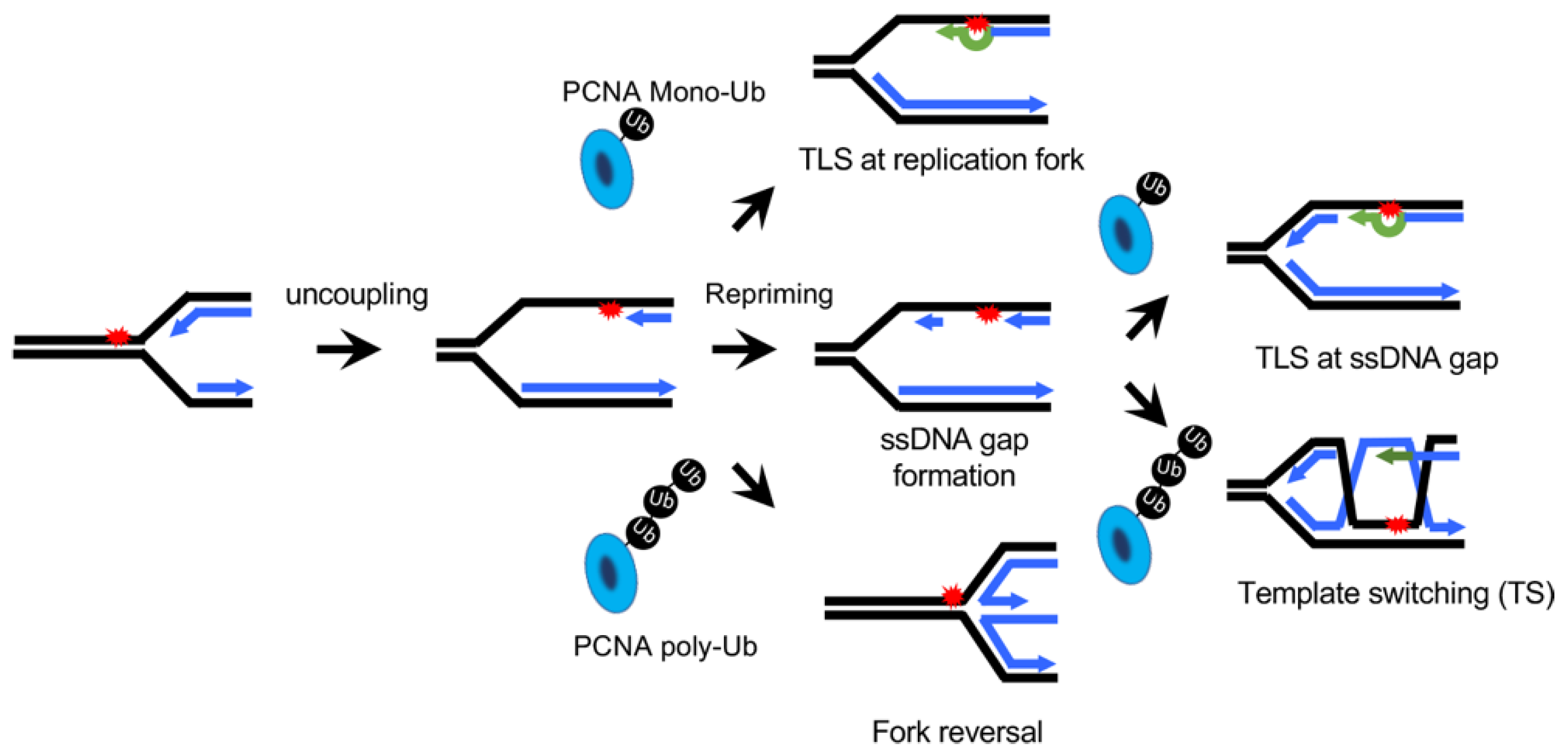
:1. The Type of DNA Lesion Determines the Choice between TLS and TS
2. Hypothesis: Kinetics of TLS or TS Determines the Choice between TLS and TS
3. Steps Affect the Kinetics of TLS Potentially
4. PCNA Mono-Ub Likely Does Not Instruct Which TLS Polymerase to Be Used
5. Stoichiometry and Dynamics of PCNA Ubiquitylation
6. Damage Load Impacts on TLS/TS Choice
7. Other Factors Affecting TLS/TS Choice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C. Suffering in silence: The tolerance of DNA damage. Nat. Rev. Mol. Cell Biol. 2005, 6, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Branzei, D.; Psakhye, I. DNA damage tolerance. Curr. Opin. Cell Biol. 2016, 40, 137–144. [Google Scholar] [CrossRef]
- Masłowska, K.H.; Laureti, L.; Pagès, V. iDamage: A method to integrate modified DNA into the yeast genome. Nucleic Acids Res. 2019, 47, e124. [Google Scholar] [CrossRef] [PubMed]
- Marians, K.J. Lesion Bypass and the Reactivation of Stalled Replication Forks. Annu. Rev. Biochem. 2018, 87, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Hoege, C.; Pfander, B.; Moldovan, G.L.; Pyrowolakis, G.; Jentsch, S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 2002, 419, 135–141. [Google Scholar] [CrossRef]
- Hochstrasser, M. Lingering mysteries of ubiquitin-chain assembly. Cell 2006, 124, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Stelter, P.; Ulrich, H.D. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 2003, 425, 188–191. [Google Scholar] [CrossRef]
- Bienko, M.; Green, C.M.; Crosetto, N.; Rudolf, F.; Zapart, G.; Coull, B.; Kannouche, P.; Wider, G.; Peter, M.; Lehmann, A.R.; et al. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 2005, 310, 1821–1824. [Google Scholar] [CrossRef]
- Franklin, W.A.; Lo, K.M.; Haseltine, W.A. Alkaline lability of fluorescent photoproducts produced in ultraviolet light-irradiated DNA. J. Biol. Chem. 1982, 257, 13535–13543. [Google Scholar] [CrossRef]
- Xiao, W.; Chow, B.L.; Broomfield, S.; Hanna, M. The Saccharomyces cerevisiae RAD6 group is composed of an error-prone and two error-free postreplication repair pathways. Genetics 2000, 155, 1633–1641. [Google Scholar] [CrossRef]
- Broomfield, S.; Chow, B.L.; Xiao, W. MMS2, encoding a ubiquitin-conjugating-enzyme-like protein, is a member of the yeast error-free postreplication repair pathway. Proc. Natl. Acad. Sci. USA 1998, 95, 5678–5683. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.P.; Levine, A.S.; Woodgate, R. The Saccharomyces cerevisiae RAD30 gene, a homologue of Escherichia coli dinB and umuC, is DNA damage inducible and functions in a novel error-free postreplication repair mechanism. Genetics 1997, 147, 1557–1568. [Google Scholar] [CrossRef]
- Chang, D.J.; Lupardus, P.J.; Cimprich, K.A. Monoubiquitination of proliferating cell nuclear antigen induced by stalled replication requires uncoupling of DNA polymerase and mini-chromosome maintenance helicase activities. J. Biol. Chem. 2006, 281, 32081–32088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, A.A.; Huttner, D.; Daigaku, Y.; Chen, S.; Ulrich, H.D. Activation of ubiquitin-dependent DNA damage bypass is mediated by replication protein a. Mol. Cell 2008, 29, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Foiani, M.; Sogo, J.M. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell 2006, 21, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Daigaku, Y.; Davies, A.A.; Ulrich, H.D. Ubiquitin-dependent DNA damage bypass is separable from genome replication. Nature 2010, 465, 951–955. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.P.; Garcia-Rodriguez, N.; Zilio, N.; Hanulova, M.; Ulrich, H.D. Processing of DNA Polymerase-Blocking Lesions during Genome Replication Is Spatially and Temporally Segregated from Replication Forks. Mol. Cell 2020, 77, 3–16.e14. [Google Scholar] [CrossRef]
- Karras, G.I.; Jentsch, S. The RAD6 DNA damage tolerance pathway operates uncoupled from the replication fork and is functional beyond S phase. Cell 2010, 141, 255–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karras, G.I.; Fumasoni, M.; Sienski, G.; Vanoli, F.; Branzei, D.; Jentsch, S. Noncanonical role of the 9-1-1 clamp in the error-free DNA damage tolerance pathway. Mol. Cell 2013, 49, 536–546. [Google Scholar] [CrossRef] [Green Version]
- García-Rodríguez, N.; Wong, R.P.; Ulrich, H.D. The helicase Pif1 functions in the template switching pathway of DNA damage bypass. Nucleic Acids Res. 2018, 46, 8347–8356. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Johnson, R.E.; Prakash, L. Eukaryotic translesion synthesis DNA polymerases: Specificity of structure and function. Annu. Rev. Biochem. 2005, 74, 317–353. [Google Scholar] [CrossRef]
- Daraba, A.; Gali, V.K.; Halmai, M.; Haracska, L.; Unk, I. Def1 promotes the degradation of Pol3 for polymerase exchange to occur during DNA-damage--induced mutagenesis in Saccharomyces cerevisiae. PLoS Biol. 2014, 12, e1001771. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Washington, M.T. Translesion Synthesis: Insights into the Selection and Switching of DNA Polymerases. Genes 2017, 8, 24. [Google Scholar] [CrossRef]
- Waters, L.S.; Walker, G.C. The critical mutagenic translesion DNA polymerase Rev1 is highly expressed during G(2)/M phase rather than S phase. Proc. Natl. Acad. Sci. USA 2006, 103, 8971–8976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plachta, M.; Halas, A.; McIntyre, J.; Sledziewska-Gojska, E. The steady-state level and stability of TLS polymerase eta are cell cycle dependent in the yeast S. cerevisiae. DNA Repair 2015, 29, 147–153. [Google Scholar] [CrossRef]
- Watanabe, K.; Tateishi, S.; Kawasuji, M.; Tsurimoto, T.; Inoue, H.; Yamaizumi, M. Rad18 guides poleta to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J. 2004, 23, 3886–3896. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, S.D.; Kokoska, R.J.; Masutani, C.; Iwai, S.; Hanaoka, F.; Kunkel, T.A. Preferential cis-syn thymine dimer bypass by DNA polymerase eta occurs with biased fidelity. Nature 2004, 428, 97–100. [Google Scholar] [CrossRef]
- Johnson, R.E.; Prakash, S.; Prakash, L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Poleta. Science 1999, 283, 1001–1004. [Google Scholar] [CrossRef]
- Quinet, A.; Vessoni, A.T.; Rocha, C.R.; Gottifredi, V.; Biard, D.; Sarasin, A.; Menck, C.F.; Stary, A. Gap-filling and bypass at the replication fork are both active mechanisms for tolerance of low-dose ultraviolet-induced DNA damage in the human genome. DNA Repair 2014, 14, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Bazan, M.A.; Gallo-Fernandez, M.; Saugar, I.; Jimenez-Martin, A.; Vazquez, M.V.; Tercero, J.A. Rad5 plays a major role in the cellular response to DNA damage during chromosome replication. Cell Rep. 2014, 9, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Vandewiele, D.; Borden, A.; O’Grady, P.I.; Woodgate, R.; Lawrence, C.W. Efficient translesion replication in the absence of Escherichia coli Umu proteins and 3′-5′ exonuclease proofreading function. Proc. Natl. Acad. Sci. USA 1998, 95, 15519–15524. [Google Scholar] [CrossRef] [Green Version]
- Gon, S.; Napolitano, R.; Rocha, W.; Coulon, S.; Fuchs, R.P. Increase in dNTP pool size during the DNA damage response plays a key role in spontaneous and induced-mutagenesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2011, 108, 19311–19316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, J.L.; Bielen, A.B.; Dikic, I.; Ulrich, H.D. Contributions of ubiquitin- and PCNA-binding domains to the activity of Polymerase eta in Saccharomyces cerevisiae. Nucleic Acids Res. 2007, 35, 881–889. [Google Scholar] [CrossRef]
- Ramasubramanyan, S.; Coulon, S.; Fuchs, R.P.; Lehmann, A.R.; Green, C.M. Ubiquitin-PCNA fusion as a mimic for mono-ubiquitinated PCNA in Schizosaccharomyces pombe. DNA Repair 2010, 9, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Lu, M.; Xu, X.; Hanna, M.; Shiomi, N.; Xiao, W. DNA-damage tolerance mediated by PCNA*Ub fusions in human cells is dependent on Rev1 but not Polη. Nucleic Acids Res. 2013, 41, 7356–7369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.S.; Wollscheid, H.P.; Lowther, J.; Ulrich, H.D. Effects of chain length and geometry on the activation of DNA damage bypass by polyubiquitylated PCNA. Nucleic Acids Res. 2020, 48, 3042–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrabaszcz, É.; Laureti, L.; Pagès, V. DNA lesions proximity modulates damage tolerance pathways in Escherichia coli. Nucleic Acids Res. 2018, 46, 4004–4012. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, J.; Hong, X.; Rao, H. PCNA Ubiquitylation: Instructive or Permissive to DNA Damage Tolerance Pathways? Biomolecules 2021, 11, 1543. https://doi.org/10.3390/biom11101543
Che J, Hong X, Rao H. PCNA Ubiquitylation: Instructive or Permissive to DNA Damage Tolerance Pathways? Biomolecules. 2021; 11(10):1543. https://doi.org/10.3390/biom11101543
Chicago/Turabian StyleChe, Jun, Xin Hong, and Hai Rao. 2021. "PCNA Ubiquitylation: Instructive or Permissive to DNA Damage Tolerance Pathways?" Biomolecules 11, no. 10: 1543. https://doi.org/10.3390/biom11101543
APA StyleChe, J., Hong, X., & Rao, H. (2021). PCNA Ubiquitylation: Instructive or Permissive to DNA Damage Tolerance Pathways? Biomolecules, 11(10), 1543. https://doi.org/10.3390/biom11101543





