Protocatechuic Acid, a Simple Plant Secondary Metabolite, Induced Apoptosis by Promoting Oxidative Stress through HO-1 Downregulation and p21 Upregulation in Colon Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Treatments
2.3. MTT Bioassay
2.4. Annexin V Determination
2.5. Lactic Dehydrogenase Release
2.6. Reactive Oxygen Species Assay
2.7. Thiol Group Determination
2.8. HO-1 Protein Expression
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. PCA Cytotoxicity and Cell Death Induction
3.1.1. Effects of PCA on Cell Viability of CaCo-2
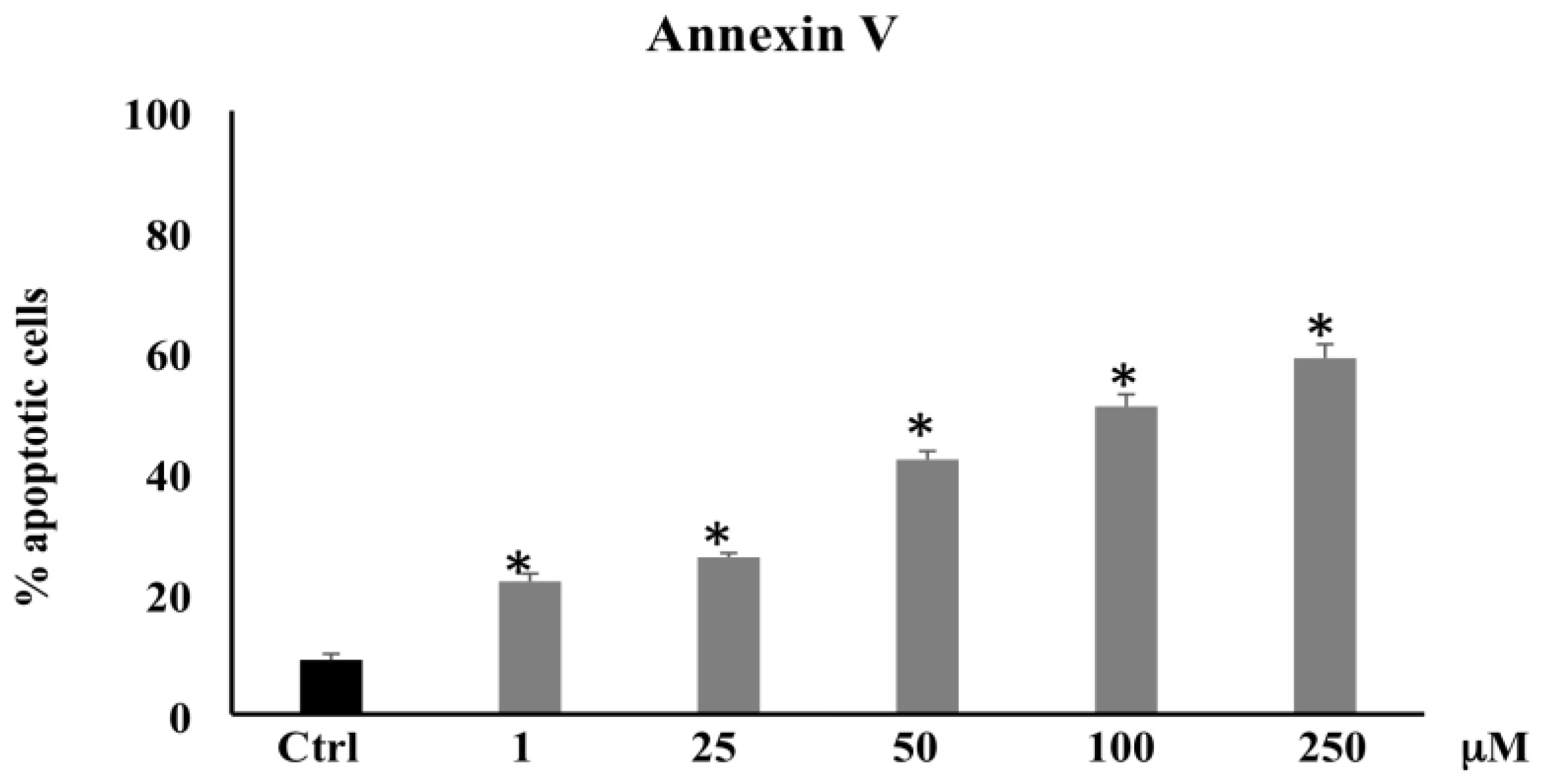
3.1.2. Annexin V Determination
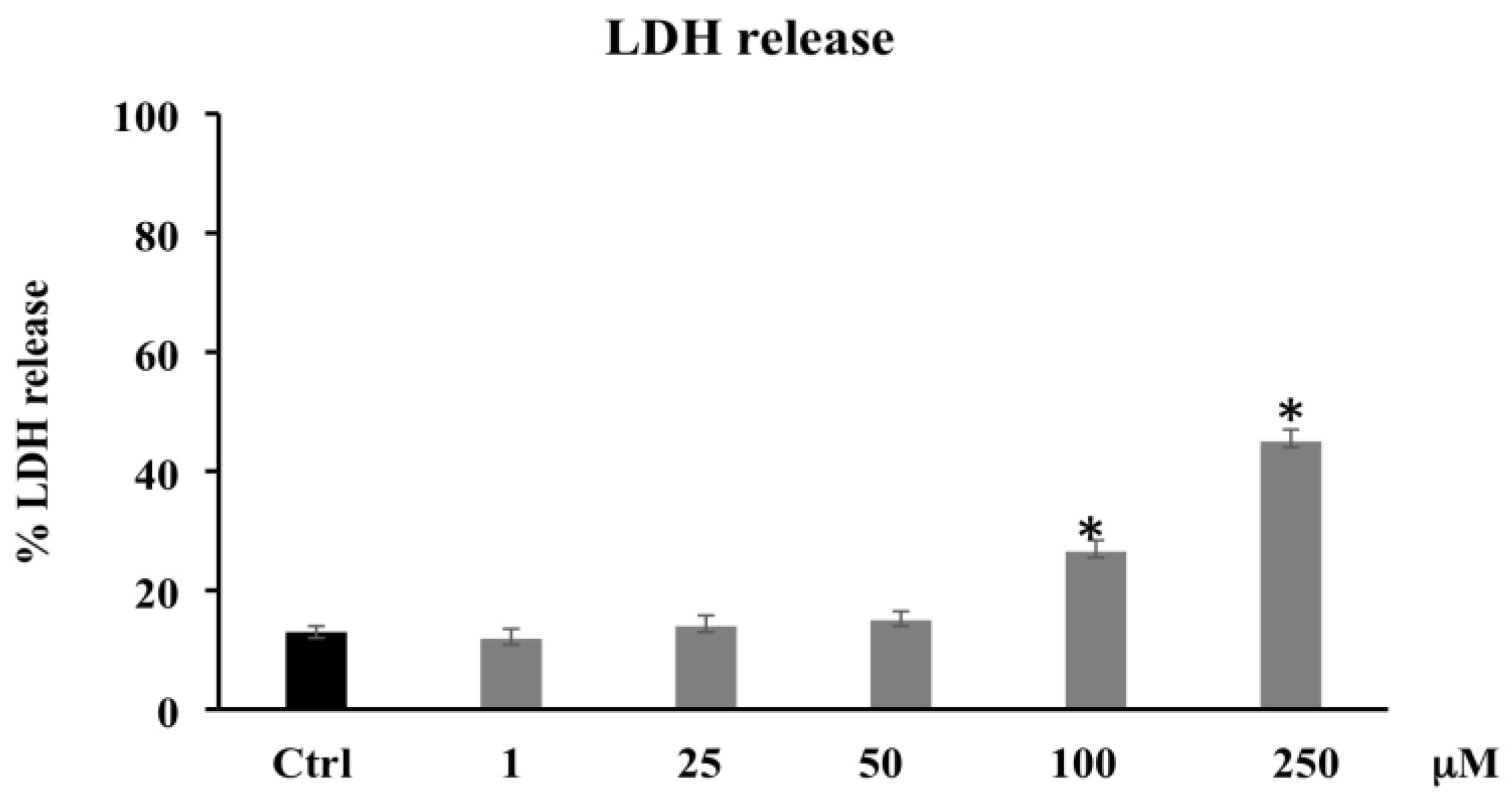
3.1.3. LDH Release
3.2. PCA Oxidative Properties
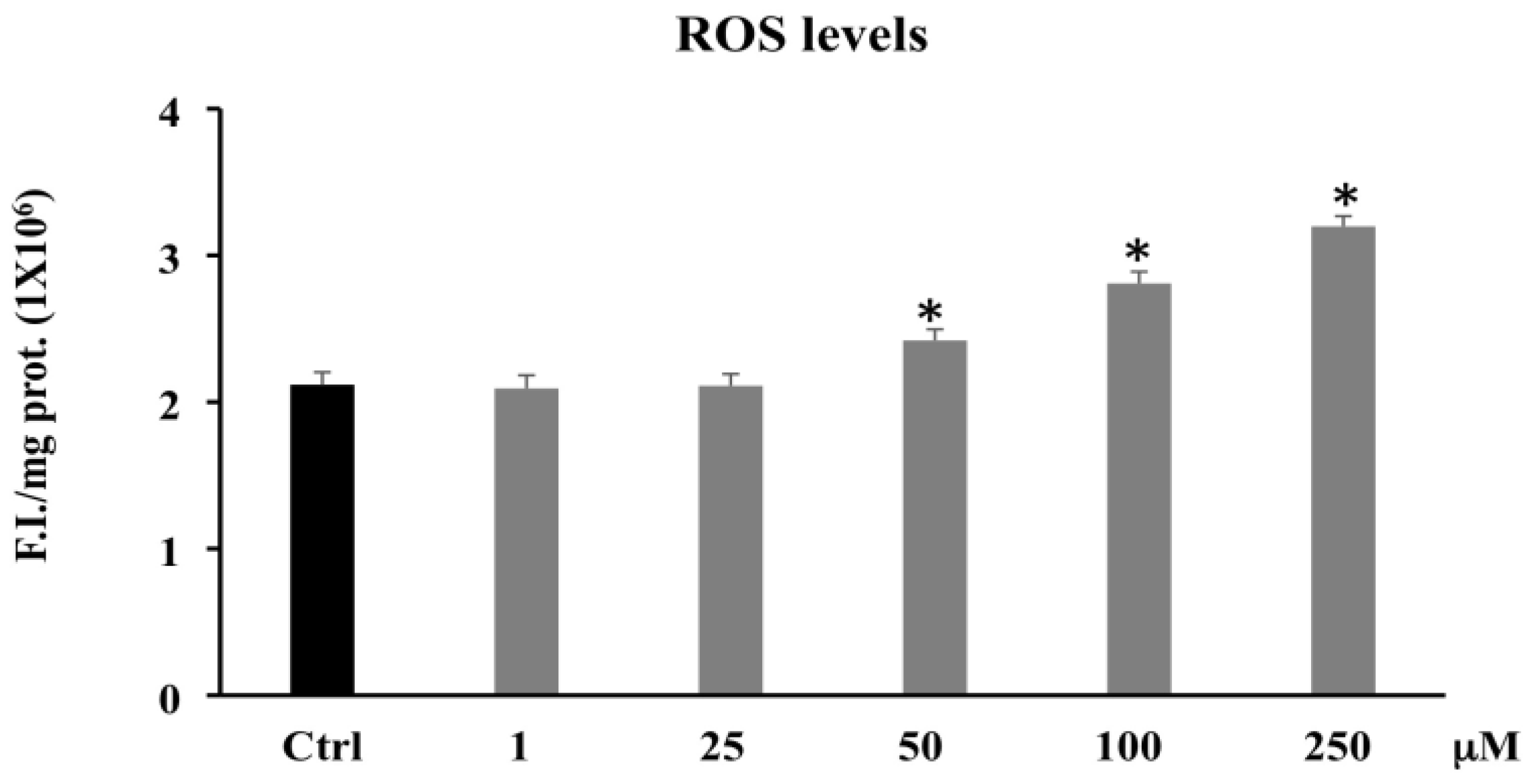
3.2.1. Reactive Oxygen Species (ROS)
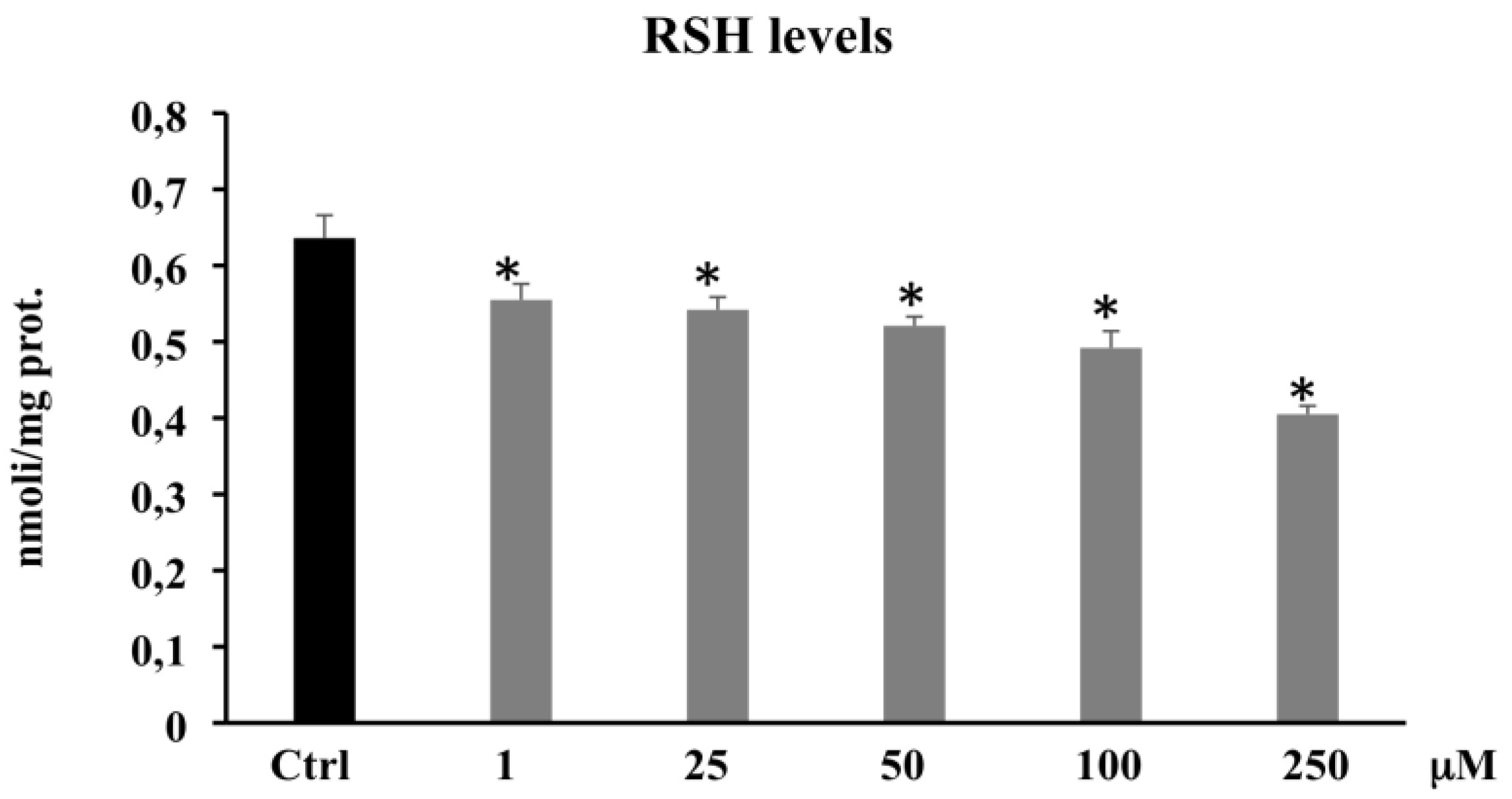
3.2.2. Thiol Group Determination (RSH)
3.3. PCA and Proteins Expression
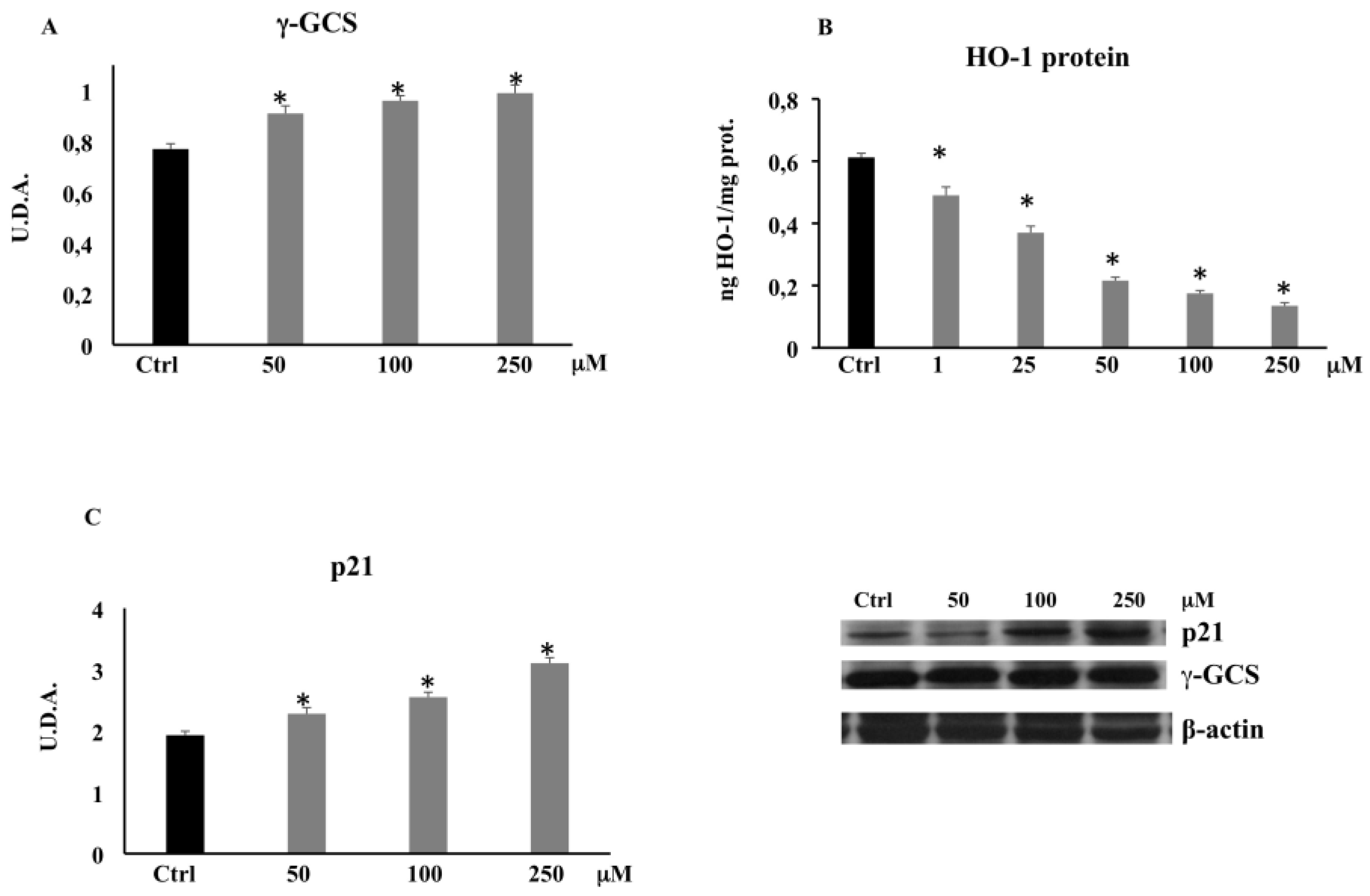
3.3.1. γ-Glutamylcysteine Synthetase (γ-GCS) by Western Blot
3.3.2. HO-1 Expression by ELISA
3.3.3. p21 Expression by Western Blot
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | colorectal cancer |
| DCFH-DA | dichlorofluorescein diacetate |
| CaCo-2 | colon cancer cell line |
| HO-1 | heme oxygenase-1 |
| LDH | lactic dehydrogenase |
| ROS | Reactive oxygen species |
| LOOH | Lipid hydroperoxide |
| RSH | Thiol groups |
| γ-GCS | γ-glutamylcysteine synthetase |
| GSH | Glutathione |
References
- Weisburger, J.H. Causes, relevant mechanisms, and prevention of large bowel cancer. Semin. Oncol. 1991, 18, 316–336. [Google Scholar] [PubMed]
- Ahmed, F.E. Effect of nutrition on the health of the elderly. J. Am. Diet. Assoc. 1992, 92, 1102–1108. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 1981, 66, 1191–1308. [Google Scholar] [CrossRef]
- Ames, B.N. Dietary carcinogens and anticarcinogens: Oxygen radicals and degenerative diseases. Science 1983, 221, 256–1264. [Google Scholar] [CrossRef] [Green Version]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer type. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef] [Green Version]
- Johnson, I. New approaches to the role of diet in the prevention of cancers of the alimentary tract. Mutat. Res. 2004, 551, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce, W.R.; Giacca, A.; Medline, A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1271–1279. [Google Scholar]
- Bultman, S.J. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol. Nutr. Food Res. 2017, 61, 1500902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, A.; Paganini-Hill, A.; Ross, R.K.; Henderson, B.E. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: A prospective study. Br. J. Cancer 1992, 66, 673–679. [Google Scholar] [CrossRef] [Green Version]
- Weisburger, J.H. Effective human cancer prevention as goal for the regulation of environmental chemicals. J. Environ. Sci. Health Part C 1991, 8, 339–351. [Google Scholar] [CrossRef]
- Nakamura, Y.; Torikai, K.; Ohto, Y.; Murakami, A.; Tanaka, T.; Ohigashi, H. A simple phenolic antioxidant protocatechuic acid enhances tumor promotion and oxidative stress in female ICR mouse skin: Dose- and timing-dependent enhancement and involvement of bioactivation by tyrosinase. Carcinogenesis 2000, 21, 1899–1907. [Google Scholar] [CrossRef] [Green Version]
- Acquaviva, R.; Malfa, G.A.; Di Giacomo, C. Plant-Based bioactive molecules in improving health and preventing lifestyle diseases. Int. J. Mol. Sci. 2021, 22, 2991. [Google Scholar] [CrossRef]
- Tomasello, B.; Di Mauro, M.D.; Malfa, G.A.; Acquaviva, R.; Sinatra, F.; Spampinato, G.; Laudani, S.; Villaggio, G.; Bielak-Zmijewska, A.; Grabowska, W.; et al. Rapha Myr®, a blend of sulforaphane and myrosinase, exerts antitumor and anoikis-sensitizing effects on human astrocytoma cells modulating sirtuins and DNA methylation. Int. J. Mol. Sci. 2020, 21, 5328. [Google Scholar] [CrossRef]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2017, 8, 15996–16016. [Google Scholar] [CrossRef] [Green Version]
- Tenhunen, R.; Marver, H.S.; Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Podkalicka, P.; Mucha, O.; Jòzkowicz, A.; Dulak, J.; Loboda, A. Heme oxygenase inhibition in cancers: Possible tools and targets. Contemp. Oncol. 2018, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Luu Hoang, K.N.; Anstee, J.E.; Arnold, J.N. The diverse roles of heme oxygenase-1 in tumor progression. Front. Immunol. 2021, 12, 658315. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Fernàndez-Fierro, A.; Coviàn, C.; Bueno, S.M.; Riedel, C.A.; Mackern-Oberti, J.P.; Kalergis, A.M. Naturally derived heme-oxygenase 1 inducers and their therapeutic application to immune-mediated diseases. Front. Immunol. 2020, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Malfa, G.A.; Tomasello, B.; Acquaviva, R.; Genovese, C.; La Mantia, A.; Cammarata, F.P.; Ragusa, M.; Renis, M.; Di Giacomo, C. Betula etnensis Raf. (Betulaceae) extract induced HO-1 expression and ferroptosis cell death in human colon cancer cells. Int. J. Mol. Sci. 2019, 20, 2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanella, L.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Li Volti, G.; Cardile, V.; Abraham, N.G.; Sorrenti, V. Effects of ellagic Acid on angiogenic factors in prostate cancer cells. Cancers 2013, 5, 726–738. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.L.; Tsai, J.R.; Charles, A.L.; Hwang, J.J.; Chou, S.H.; Ping, Y.H.; Lin, F.Y.; Chen, Y.L.; Hung, C.Y.; Chen, W.C.; et al. Resveratrol inhibits human lung adenocarcinoma cell metastasis by suppressing heme oxygenase 1-mediated nuclear factor-kappaB pathway and subsequently downregulating expression of matrix metalloproteinases. Mol. Nutr. Food Res. 2010, 54, S196–S204. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Osawa, T. Absorption and metabolism of cyanidin 3-O-beta-D-glucoside in rats. FEBS Lett. 1999, 449, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Mohsen, M.A.E.; Marks, J.; Kuhnle, G.; Moore, K.; Debnam, E.; Srai, S.K.; Rice-Evans, C.; Spencer, J. Absorption, tissue distribution and excretion of pelargonidin and its metabolites following oral administration to rats. Br. J. Nutr. 2006, 95, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Gustavsson, K.; Andersson, S.; Nilsson, A.; Duan, R. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J. Agric. Food Chem. 2004, 52, 7264–7271. [Google Scholar] [CrossRef]
- Seeram, N.; Adams, L.; Hardy, M.; Heber, D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef]
- Glei, M.; Matuschek, M.; Steiner, C.; Bohm, V.; Persin, C.; Pool-Zobel, B. Initial in vitro toxicity testing of functional foods rich in catechins and anthocyanins in human cells. Toxicol. In Vitro 2003, 17, 723–729. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Bùsselberg, D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Acquaviva, R.; Sorrenti, V.; Santangelo, R.; Cardile, V.; Tomasello, B.; Malfa, G.; Vanella, L.; Amodeo, A.; Mastrojeni, S.; Pugliese, M.; et al. Effects of extract of Celtis aetnensis (Tornab.) Strobl twigs in human colon cancer cell cultures. Oncol. Rep. 2016, 36, 2298–2304. [Google Scholar] [CrossRef]
- Li Volti, G.; Galvano, F.; Frigiola, A.; Guccione, S.; Di Giacomo, C.; Forte, S.; Tringali, G.; Caruso, M.; Adekoya, O.A.; Gazzolo, D. Potential immunoregulatory role of heme oxygenase-1 in human milk: A combined biochemical and molecular modeling approach. J. Nutr. Biochem. 2010, 21, 865–871. [Google Scholar] [CrossRef]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Santangelo, C.; D’Archivio, M.; Li Volti, G.; Giovannini, C.; Galvano, F. Protocatechuic acid and human disease prevention: Biological activities and molecular mechanisms. Curr. Med. Chem. 2012, 19, 2901–2917. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Lin, C.C.; Wu, H.C.; Tsao, S.M.; Hsu, C.K. Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: Potential mechanisms of action. J. Agric. Food Chem. 2009, 57, 6468–6473. [Google Scholar] [CrossRef] [PubMed]
- Yip, E.C.; Chan, A.S.; Pang, H.; Tam, Y.K.; Wong, Y.H. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol. Toxicol. 2006, 22, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.H.; Chen, J.H.; Huang, C.C.; Wang, C.J. Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK/p38 MAPK signaling activation. Int. J. Cancer 2007, 120, 2306–2316. [Google Scholar] [CrossRef]
- Lin, H.H.; Chen, J.H.; Chou, F.P.; Wang, C.J. Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-Œ∫B pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 2011, 162, 237–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Kawamori, T.; Ohnishi, M.; Okamoto, K.; Mori, H.; Hara, A. Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis by dietary protocatechuic acid during initiation and postinitiation phases. Cancer Res. 1994, 54, 2359–2365. [Google Scholar] [PubMed]
- Tanaka, T.; Kojima, T.; Suzui, M.; Mori, H. Chemoprevention of colon carcinogenesis by the natural product of a simple phenolic compound protocatechuic acid: Suppressing effects on tumor development and biomarkers expression of colon tumorigenesis. Cancer Res. 1993, 53, 3908–3913. [Google Scholar]
- Xie, Z.; Guo, Z.; Wang, Y.; Lei, J.; Yu, J. Protocatechuic acid inhibits the growth of ovarian cancer cells by inducing apoptosis and autophagy. Phytother. Res. 2018, 32, 2256–2263. [Google Scholar] [CrossRef]
- Tsao, S.M.; Hsia, T.C.; Yin, M.C. Protocatechuic acid inhibits lung cancer cells by modulating FAK, MAPK, and NF-Œ∫B pathways. Nutr. Cancer 2014, 66, 1331–1341. [Google Scholar] [CrossRef]
- Ueda, S.; Masutani, H.; Nakamura, H.; Tanaka, T.; Ueno, M.; Yodoi, J. Redox control of cell death. Antioxid. Redox Signal. 2002, 4, 405–414. [Google Scholar] [CrossRef]
- Ueda, J.; Saito, N.; Shimazu, Y.; Ozawa, T. A comparison of scavenging abilities of antioxidants against hydroxyl radicals. Arch. Biochem. Biophys. 1996, 333, 377–384. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Huang, J.J.; Cheung, P.C.K. Mechanistic study of the in vitro and in vivo inhibitory effects of protocatechuic acid and syringic acid on VEGF-induced angiogenesis. J. Agric. Food Chem. 2018, 66, 6742–6751. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evid. Based Complement. Altern. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef] [Green Version]
- Zeraik, M.L.; Petronio, M.S.; Coelho, D.; Regasini, L.O.; Silva, D.H.; da Fonseca, L.M.; Machado, S.A.; Bolzani, V.S.; Ximenes, V.F. Improvement of pro-oxidant capacity of protocatechuic acid by esterification. PLoS ONE 2014, 9, e110277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leòn-Gonzàlez, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Habibu, R.S.; Saidu, K.; Haliru, F.Z.; Ajiboye, H.O.; Aliyu, N.O.; Ibitoye, O.B.; Uwazie, J.N.; Muritala, H.F.; Bello, S.A.; et al. Involvement of oxidative stress in protocatechuic acid-mediated bacterial lethality. Microbiologyopen 2017, 6, e00472. [Google Scholar] [CrossRef]
- Kondo, T.; Higashiyama, Y.; Goto, S.; Iida, T.; Cho, S.; Iwanaga, M.; Mori, K.; Tani, M.; Urata, Y. Regulation of gamma-glutamylcysteine synthetase expression in response to oxidative stress. Free Radic. Res. 1999, 31, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Mierla, A.L.; Minelli, A. Nrf2 and NF-Œ∫B and their concerted modulation in cancer pathogenesis and progression. Cancers 2010, 2, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Chau, L.Y. Heme oxygenase-1: Emerging target of cancer therapy. J. Biomed. Sci. 2015, 22, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitti, M.; Piras, S.; Marinari, U.M.; Moretta, L.; Pronzato, M.A.; Furfaro, A.L. HO-1 Induction in cancer progression: A matter of cell adaptation. Antioxidants 2017, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Fang, J.; Liao, L.; Maeda, H.; Su, Q. Upregulation of heme oxygenase-1 in colorectal cancer patients with increased circulation carbon monoxide levels, potentially affects chemotherapeutic sensitivity. BMC Cancer 2014, 14, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uc, A.; Britigan, B.E. Does heme oxygenase-1 have a role in Caco-2 cell cycle progression? Exp. Biol. Med. 2003, 228, 590–595. [Google Scholar] [CrossRef]
- Busserolles, J.; Megías, J.; Terencio, M.C.; Alcaraz, M.J. Heme oxygenase-1 inhibits apoptosis in Caco-2 cells via activation of Akt pathway. Int. J. Biochem. Cell Biol. 2006, 38, 1510–1517. [Google Scholar] [CrossRef]
- Weng, C.J.; Yen, G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef]
- Kweon, M.H.; Adhami, V.M.; Lee, J.S.; Mukhtar, H. Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J. Biol. Chem. 2006, 281, 33761–33772. [Google Scholar] [CrossRef] [Green Version]
- Andrès, N.C.; Fermento, M.E.; Gandini, N.A.; Romero, A.L.; Ferro, A.; Donna, L.G.; Curino, A.C.; Facchinetti, M.M. Heme oxygenase-1 has antitumoral effects in colorectal cancer: Involvement of p53. Exp. Mol. Pathol. 2014, 97, 321–331. [Google Scholar] [CrossRef]
- Inoue, T.; Kato, K.; Kato, H.; Asanoma, K.; Kuboyama, A.; Ueoka, Y.; Yamaguchi, S.; Ohgami, T.; Wake, N. Level of reactive oxygen species induced by p21Waf1/CIP1 is critical for the determination of cell fate. Cancer Sci. 2009, 100, 1275–1283. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acquaviva, R.; Tomasello, B.; Di Giacomo, C.; Santangelo, R.; La Mantia, A.; Naletova, I.; Sarpietro, M.G.; Castelli, F.; Malfa, G.A. Protocatechuic Acid, a Simple Plant Secondary Metabolite, Induced Apoptosis by Promoting Oxidative Stress through HO-1 Downregulation and p21 Upregulation in Colon Cancer Cells. Biomolecules 2021, 11, 1485. https://doi.org/10.3390/biom11101485
Acquaviva R, Tomasello B, Di Giacomo C, Santangelo R, La Mantia A, Naletova I, Sarpietro MG, Castelli F, Malfa GA. Protocatechuic Acid, a Simple Plant Secondary Metabolite, Induced Apoptosis by Promoting Oxidative Stress through HO-1 Downregulation and p21 Upregulation in Colon Cancer Cells. Biomolecules. 2021; 11(10):1485. https://doi.org/10.3390/biom11101485
Chicago/Turabian StyleAcquaviva, Rosaria, Barbara Tomasello, Claudia Di Giacomo, Rosa Santangelo, Alfonsina La Mantia, Irina Naletova, Maria Grazia Sarpietro, Francesco Castelli, and Giuseppe Antonio Malfa. 2021. "Protocatechuic Acid, a Simple Plant Secondary Metabolite, Induced Apoptosis by Promoting Oxidative Stress through HO-1 Downregulation and p21 Upregulation in Colon Cancer Cells" Biomolecules 11, no. 10: 1485. https://doi.org/10.3390/biom11101485
APA StyleAcquaviva, R., Tomasello, B., Di Giacomo, C., Santangelo, R., La Mantia, A., Naletova, I., Sarpietro, M. G., Castelli, F., & Malfa, G. A. (2021). Protocatechuic Acid, a Simple Plant Secondary Metabolite, Induced Apoptosis by Promoting Oxidative Stress through HO-1 Downregulation and p21 Upregulation in Colon Cancer Cells. Biomolecules, 11(10), 1485. https://doi.org/10.3390/biom11101485







