Correlative Light-Environmental Scanning Electron Microscopy of Plasma Membrane Efflux Carriers of Plant Hormone Auxin
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
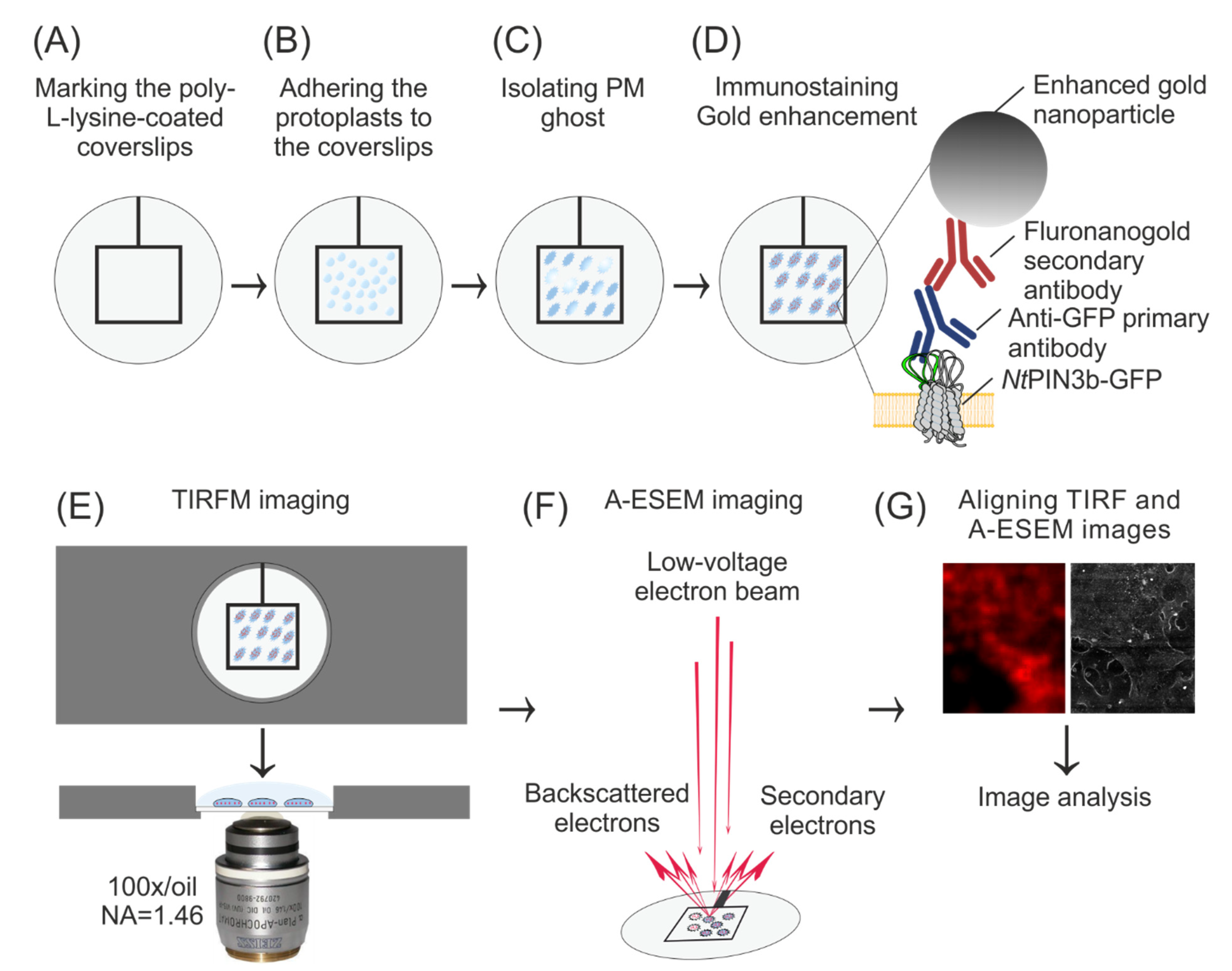
2.2. Preparation of Coverslips, Protoplasts and PM Ghosts
2.3. Immunostaining of PM Ghosts
2.4. TIRF Microscopy
2.5. A-ESEM and CLEM
2.6. Image Analysis and Statistics
3. Results
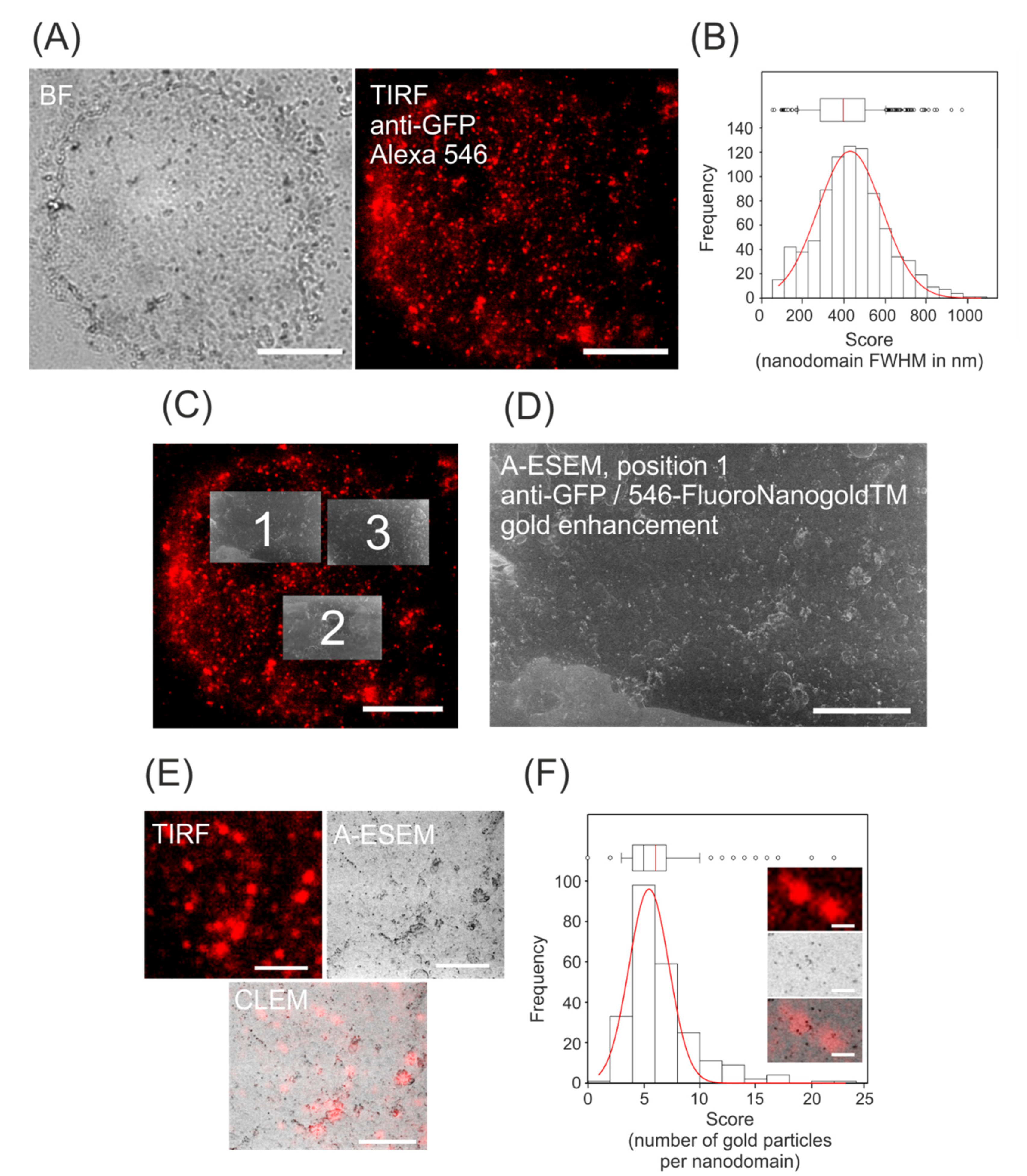
3.1. Immunolocalization of GFP-Tagged Auxin Efflux Carrier NtPIN3
3.2. CLEM of NtPIN3b-GFP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. The Content of an Appendix is Contained within the Sections Subordinate to the Major Heading

References
- Gronnier, J.; Legrand, A.; Loquet, A.; Habenstein, B.; Germain, V.; Mongrand, S. Mechanisms governing subcompartmentalization of biological membranes. Curr. Opin. Plant Biol. 2019, 52, 114–123. [Google Scholar] [CrossRef]
- Malinsky, J.; Opekarová, M.; Grossmann, G.; Tanner, W. Membrane microdomains, rafts, and detergent-resistant membranes in plants and fungi. Annu. Rev. Plant Biol. 2013, 64, 501–529. [Google Scholar] [CrossRef] [PubMed]
- Gronnier, J.; Gerbeau-Pissot, P.; Germain, V.; Mongrand, S.; Simon-Plas, F. Divide and rule: Plant plasma membrane organization. Trends Plant Sci. 2018, 23, 899–917. [Google Scholar] [CrossRef] [PubMed]
- Ott, T. Membrane nanodomains and microdomains in plant–microbe interactions. Curr. Opin. Plant Biol. 2017, 40, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Cui, Y.; Zhang, X.; Li, R.; Lin, J. Organization and dynamics of functional plant membrane microdomains. Cell. Mol. Life Sci. 2020, 77, 275–287. [Google Scholar] [CrossRef]
- Mamode Cassim, A.; Gouguet, P.; Gronnier, J.; Laurent, N.; Germain, V.; Grison, M.; Boutté, Y.; Gerbeau-Pissot, P.; Simon-Plas, F.; Mongrand, S. Plant lipids: Key players of plasma membrane organization and function. Prog. Lipid Res. 2019, 73, 1–27. [Google Scholar] [CrossRef]
- Smokvarska, M.; Francis, C.; Platre, M.P.; Fiche, J.B.; Alcon, C.; Dumont, X.; Nacry, P.; Bayle, V.; Nollmann, M.; Maurel, C.; et al. A plasma membrane nanodomain ensures signal specificity during osmotic signaling in plants. Curr. Biol. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Furlan, A.L.; Laurin, Y.; Botcazon, C.; Rodríguez-Moraga, N.; Rippa, S.; Deleu, M.; Lins, L.; Sarazin, C.; Buchoux, S. Contributions and limitations of biophysical approaches to study of the interactions between amphiphilic molecules and the plant plasma membrane. Plants 2020, 9, 648. [Google Scholar] [CrossRef]
- Adamowski, M.; Friml, J. PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell Online 2015, 27, 20–32. [Google Scholar] [CrossRef]
- Langowski, L.; Wabnik, K.; Li, H.; Vanneste, S.; Naramoto, S.; Tanaka, H.; Friml, J. Cellular mechanisms for cargo delivery and polarity maintenance at different polar domains in plant cells. Cell Discov. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Hille, S.; Akhmanova, M.; Glanc, M.; Johnson, A.; Friml, J. Relative contribution of PIN-containing secretory vesicles and plasma membrane pins to the directed auxin transport: Theoretical estimation. Int. J. Mol. Sci. 2018, 19, 3566. [Google Scholar] [CrossRef] [PubMed]
- Komis, G.; Novák, D.; Ovečka, M.; Šamajová, O.; Šamaj, J. Advances in Imaging plant cell dynamics. Plant Physiol. 2018, 176, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Komis, G.; Šamajová, O.; Ovečka, M.; Šamaj, J. Super-resolution microscopy in plant cell imaging. Trends Plant Sci. 2015, 20, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Tapken, W.; Murphy, A.S. Membrane nanodomains in plants: Capturing form, function, and movement. J. Exp. Bot. 2015, 66, 1573–1586. [Google Scholar] [CrossRef]
- Gouguet, P.; Gronnier, J.; Legrand, A.; Perraki, A.; Jolivet, M.D.; Deroubaix, A.F.; Retana, S.G.; Boudsocq, M.; Habenstein, B.; Mongrand, S.; et al. Connecting the dots: From nanodomains to physiological functions of REMORINs. Plant Physiol. 2021, 185, 632–649. [Google Scholar] [CrossRef]
- Daněk, M.; Angelini, J.; Malínská, K.; Andrejch, J.; Amlerová, Z.; Kocourková, D.; Brouzdová, J.; Valentová, O.; Martinec, J.; Petrášek, J. Cell wall contributes to the stability of plasma membrane nanodomain organization of Arabidopsis thaliana FLOTILLIN2 and HYPERSENSITIVE INDUCED REACTION1 proteins. Plant J. 2020, 101, 619–636. [Google Scholar] [CrossRef]
- McKenna, J.F.; Rolfe, D.J.; Webb, S.E.D.; Tolmie, A.F.; Botchway, S.W.; Martin-Fernandez, M.L.; Hawes, C.; Runions, J. The cell wall regulates dynamics and size of plasma-membrane nanodomains in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 12857–12862. [Google Scholar] [CrossRef]
- Vizcay-Barrena, G.; Webb, S.E.D.; Martin-Fernandez, M.L.; Wilson, Z.A. Subcellular and single-molecule imaging of plant fluorescent proteins using total internal reflection fluorescence microscopy (TIRFM). J. Exp. Bot. 2011, 62, 5419–5428. [Google Scholar] [CrossRef]
- Johnson, A.; Vert, G. Single event resolution of plant plasma membrane protein endocytosis by TIRF microscopy. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Lukeš, T.; Glatzová, D.; Kvíčalová, Z.; Levet, F.; Benda, A.; Letschert, S.; Sauer, M.; Brdička, T.; Lasser, T.; Cebecauer, M. Quantifying protein densities on cell membranes using super-resolution optical fluctuation imaging. Nat. Commun. 2017, 8, 1731. [Google Scholar] [CrossRef]
- Gustafsson, N.; Culley, S.; Ashdown, G.; Owen, D.M.; Pereira, P.M.; Henriques, R. Fast live-cell conventional fluorophore nanoscopy with ImageJ through super-resolution radial fluctuations. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marion, J.; Le Bars, R.; Satiat-Jeunemaitre, B.; Boulogne, C. Optimizing CLEM protocols for plants cells: GMA embedding and cryosections as alternatives for preservation of GFP fluorescence in Arabidopsis roots. J. Struct. Biol. 2017, 198, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, J.; Cui, Y.; Klumpe, S.; Xiang, Y.; Erdmann, P.S.; Jiang, L. Membrane imaging in the plant endomembrane system. Plant Physiol. 2021, 185, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Jahn, K.A.; Barton, D.A.; Kobayashi, K.; Ratinac, K.R.; Overall, R.L.; Braet, F. Correlative microscopy: Providing new understanding in the biomedical and plant sciences. Micron 2012, 43, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Jing, Y.; Wu, H.; Lin, J. Tracking tonoplast protein behaviors in intact vacuoles isolated from arabidopsis leaves. Mol. Plant 2017, 10, 349–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, P.; Kang, B.-H. Correlative light and electron microscopy imaging of the plant trans-golgi network. In Plant Endosomes; Humana: New York, NY, USA, 2020; Volume 2177, pp. 59–67. [Google Scholar]
- Neděla, V.; Tihlaříková, E.; Hřib, J. The low-temperature method for study of coniferous tissues in the environmental scanning electron microscope. Microsc. Res. Tech. 2015, 78, 13–21. [Google Scholar] [CrossRef]
- Tihlaříková, E.; Neděla, V.; Đorđević, B. In-Situ preparation of plant samples in ESEM for energy dispersive x-ray microanalysis and repetitive observation in SEM and ESEM. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Nagata, T.; Nemoto, Y.; Hasezawa, S. Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int. Rev. Cytol. 1992, 132, 1–30. [Google Scholar]
- Müller, K.; Hošek, P.; Laňková, M.; Vosolsobě, S.; Malínská, K.; Čarná, M.; Fílová, M.; Dobrev, P.I.; Helusová, M.; Hoyerová, K.; et al. Transcription of specific auxin efflux and influx carriers drives auxin homeostasis in tobacco cells. Plant J. 2019, 100, 627–640. [Google Scholar] [CrossRef]
- Sonobe, S.; Takahashi, S. Association of microtubules with the plasma membrane of tobacco BY-2 Cells in Vitro. Plant Cell Physiol. 1994, 35, 451–460. [Google Scholar] [CrossRef]
- Krtková, J.; Zimmermann, A.; Schwarzerová, K.; Nick, P. Hsp90 binds microtubules and is involved in the reorganization of the microtubular network in angiosperms. J. Plant Physiol. 2012, 169, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, E.V.; Polishchuk, R.S. Analysis of Golgi Complex Function Using Correlative Light-Electron Microscopy, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume118, ISBN 9780124171640. [Google Scholar]
- Neděla, V.; Tihlaříková, E.; Runštuk, J.; Hudec, J. High-efficiency detector of secondary and backscattered electrons for low-dose imaging in the ESEM. Ultramicroscopy 2018, 184, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; von Wangenheim, D.; Zhang, X.; Tan, S.; Darwish-Miranda, N.; Naramoto, S.; Wabnik, K.; De Rycke, R.; Kaufmann, W.A.; Gütl, D.; et al. Cellular requirements for PIN polar cargo clustering in Arabidopsis thaliana. New Phytol. 2021, 229, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Feraru, E.; Feraru, M.I.; Kleine-Vehn, J.; Martinière, A.; Mouille, G.; Vanneste, S.; Vernhettes, S.; Runions, J.; Friml, J. PIN polarity maintenance by the cell wall in Arabidopsis. Curr. Biol. 2011, 21, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Martiniere, A.; Lavagi, I.; Nageswaran, G.; Rolfe, D.J.; Maneta-Peyret, L.; Luu, D.-T.; Botchway, S.W.; Webb, S.E.D.; Mongrand, S.; Maurel, C.; et al. Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 12805–12810. [Google Scholar] [CrossRef]
- Noack, L.C.; Bayle, V.; Armengot, L.; Rozier, F.; Mamode-Cassim, A.; Stevens, F.D.; Caillaud, M.-C.; Munnik, T.; Mongrand, S.; Pleskot, R.; et al. A nanodomain-anchored scaffolding complex is required for the function and localization of phosphatidylinositol 4-kinase alpha in plants. Plant Cell 2021, 12, 56. [Google Scholar] [CrossRef]
- Platre, M.P.; Bayle, V.; Armengot, L.; Bareille, J.; del Marquès-Bueno, M.; Creff, A.; Maneta-Peyret, L.; Fiche, J.-B.; Nollmann, M.; Miège, C.; et al. Developmental control of plant Rho GTPase nano-organization by the lipid phosphatidylserine. Science 2019, 364, 57–62. [Google Scholar] [CrossRef]
- Gronnier, J.; Crowet, J.-M.; Habenstein, B.; Nasir, M.N.; Bayle, V.; Hosy, E.; Platre, M.P.; Gouguet, P.; Raffaele, S.; Martinez, D.; et al. Structural basis for plant plasma membrane protein dynamics and organization into functional nanodomains. Elife 2017, 6, 1–24. [Google Scholar] [CrossRef]
- Ke, M.; Ma, Z.; Wang, D.; Sun, Y.; Wen, C.; Huang, D.; Chen, Z.; Yang, L.; Tan, S.; Li, R.; et al. Salicylic acid regulates PIN2 auxin transporter hyperclustering and root gravitropic growth via Remorin-dependent lipid nanodomain organisation in Arabidopsis thaliana. New Phytol. 2021, 229, 963–978. [Google Scholar] [CrossRef]
- Pan, X.; Fang, L.; Liu, J.; Senay-Aras, B.; Lin, W.; Zheng, S.; Zhang, T.; Guo, J.; Manor, U.; Van Norman, J.; et al. Auxin-induced signaling protein nanoclustering contributes to cell polarity formation. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, X.; Kong, W.; Yang, X.-L.; Molnár, G.; Vondráková, Z.; Filepová, R.; Petrášek, J.; Friml, J.; Xue, H.-W. The lipid code-dependent phosphoswitch PDK1–D6PK activates PIN-mediated auxin efflux in Arabidopsis. Nat. Plants 2020, 6, 556–569. [Google Scholar] [CrossRef]
- Shaw, R.; Tian, X.; Xu, J. Single-cell transcriptome analysis in plants: Advances and challenges. Mol. Plant 2021, 14, 115–126. [Google Scholar] [CrossRef]
- Van Elsland, D.M.; Bos, E.; Pawlak, J.B.; Overkleeft, H.S.; Koster, A.J.; Van Kasteren, S.I. Correlative light and electron microscopy reveals discrepancy between gold and fluorescence labelling. J. Microsc. 2017, 267, 309–317. [Google Scholar] [CrossRef]
- Prost, S.; Kishen, R.E.B.; Kluth, D.C.; Bellamy, C.O.C. Working with commercially available quantum dots for immunofluorescence on tissue sections. PLoS ONE 2016, 11, e0163856. [Google Scholar] [CrossRef]
- Ellisman, M.H.; Deerinck, T.J.; Shu, X.; Sosinsky, G.E. Picking faces out of a crowd: Genetic labels for identification of proteins in correlated light and electron microscopy imaging. Methods Cell Biol. 2012, 111, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.; Wojcik, M.; Kim, D.; Mahmoudi, M.; Li, W.; Xu, K. Correlative super-resolution microscopy: New dimensions and new opportunities. Chem. Rev. 2017, 117, 7428–7456. [Google Scholar] [CrossRef]
- Neděla, V.; Tihlaříková, E.; Maxa, J.; Imrichová, K.; Bučko, M.; Gemeiner, P. Simulation-based optimisation of thermodynamic conditions in the ESEM for dynamical in-situ study of spherical polyelectrolyte complex particles in their native state. Ultramicroscopy 2020, 211, 112954. [Google Scholar] [CrossRef] [PubMed]
- Lace, B.; Prandi, C. Shaping Small bioactive molecules to untangle their biological function: A focus on fluorescent plant hormones. Mol. Plant 2016, 9, 1099–1118. [Google Scholar] [CrossRef]
- Peckys, D.B.; Korf, U.; de Jonge, N. Local variations of HER2 dimerization in breast cancer cells discovered by correlative fluorescence and liquid electron microscopy. Sci. Adv. 2015, 1, e1500165. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelate, A.; Tihlaříková, E.; Schwarzerová, K.; Neděla, V.; Petrášek, J. Correlative Light-Environmental Scanning Electron Microscopy of Plasma Membrane Efflux Carriers of Plant Hormone Auxin. Biomolecules 2021, 11, 1407. https://doi.org/10.3390/biom11101407
Stelate A, Tihlaříková E, Schwarzerová K, Neděla V, Petrášek J. Correlative Light-Environmental Scanning Electron Microscopy of Plasma Membrane Efflux Carriers of Plant Hormone Auxin. Biomolecules. 2021; 11(10):1407. https://doi.org/10.3390/biom11101407
Chicago/Turabian StyleStelate, Ayoub, Eva Tihlaříková, Kateřina Schwarzerová, Vilém Neděla, and Jan Petrášek. 2021. "Correlative Light-Environmental Scanning Electron Microscopy of Plasma Membrane Efflux Carriers of Plant Hormone Auxin" Biomolecules 11, no. 10: 1407. https://doi.org/10.3390/biom11101407
APA StyleStelate, A., Tihlaříková, E., Schwarzerová, K., Neděla, V., & Petrášek, J. (2021). Correlative Light-Environmental Scanning Electron Microscopy of Plasma Membrane Efflux Carriers of Plant Hormone Auxin. Biomolecules, 11(10), 1407. https://doi.org/10.3390/biom11101407