Immunosuppressive Roles of Galectin-1 in the Tumor Microenvironment
Abstract
:1. Introduction
2. Gal-1 Molecular Structures and Biological Functions in Human Cancers
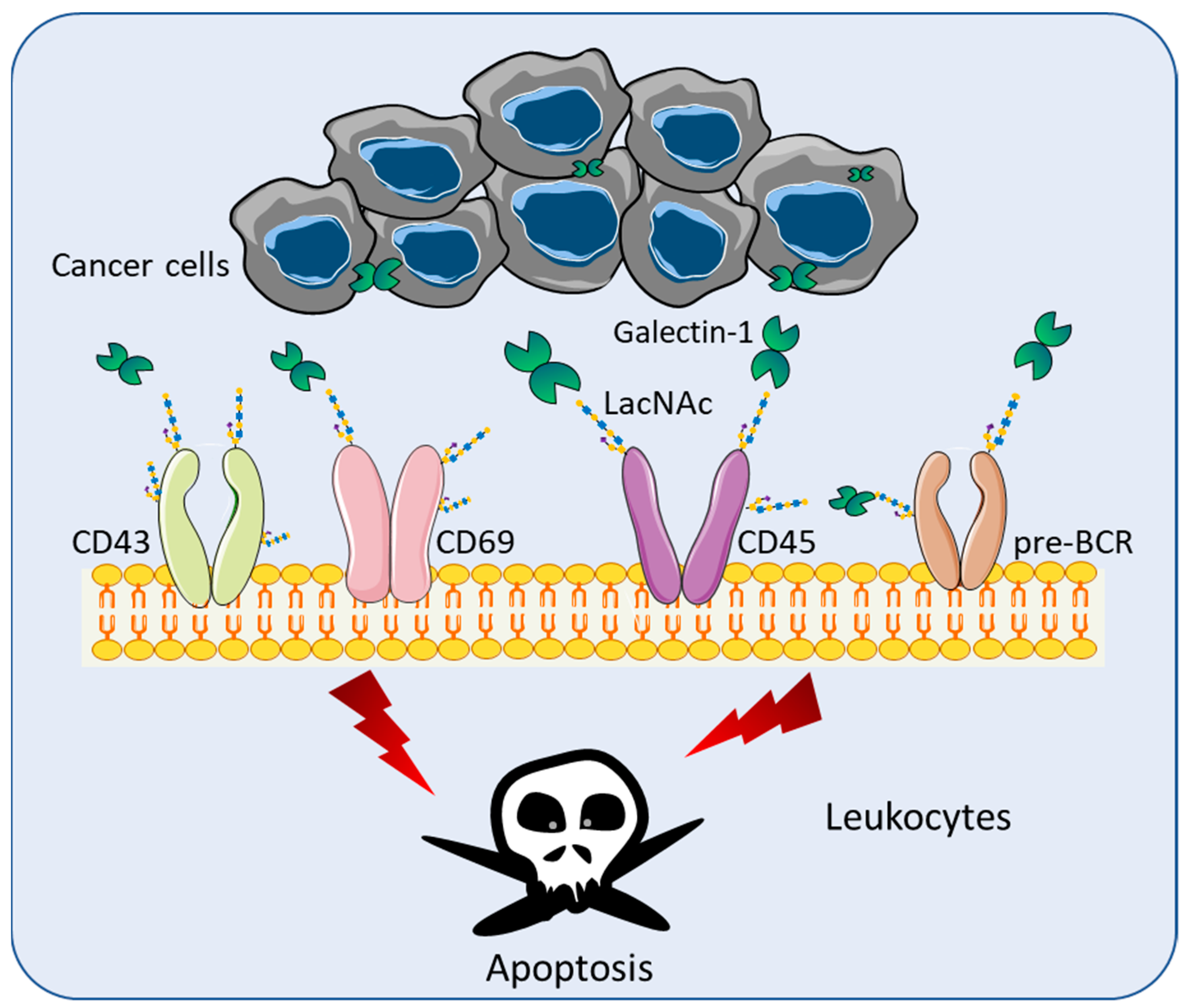
3. Gal-1 in T-Cell Immunodeficiency Diseases
4. Role of Galectins in Cancer Immune Surveillance
4.1. Lymphoma
4.2. Head and Neck Cancer (HNC)
4.3. Glioblastoma (GBM)
4.4. Pancreatic Ductal Adenocarcinoma (PDAC)
4.5. Lung Cancers
4.6. Breast Cancer
4.7. Melanomas
4.8. Neuroblastoma (NB)
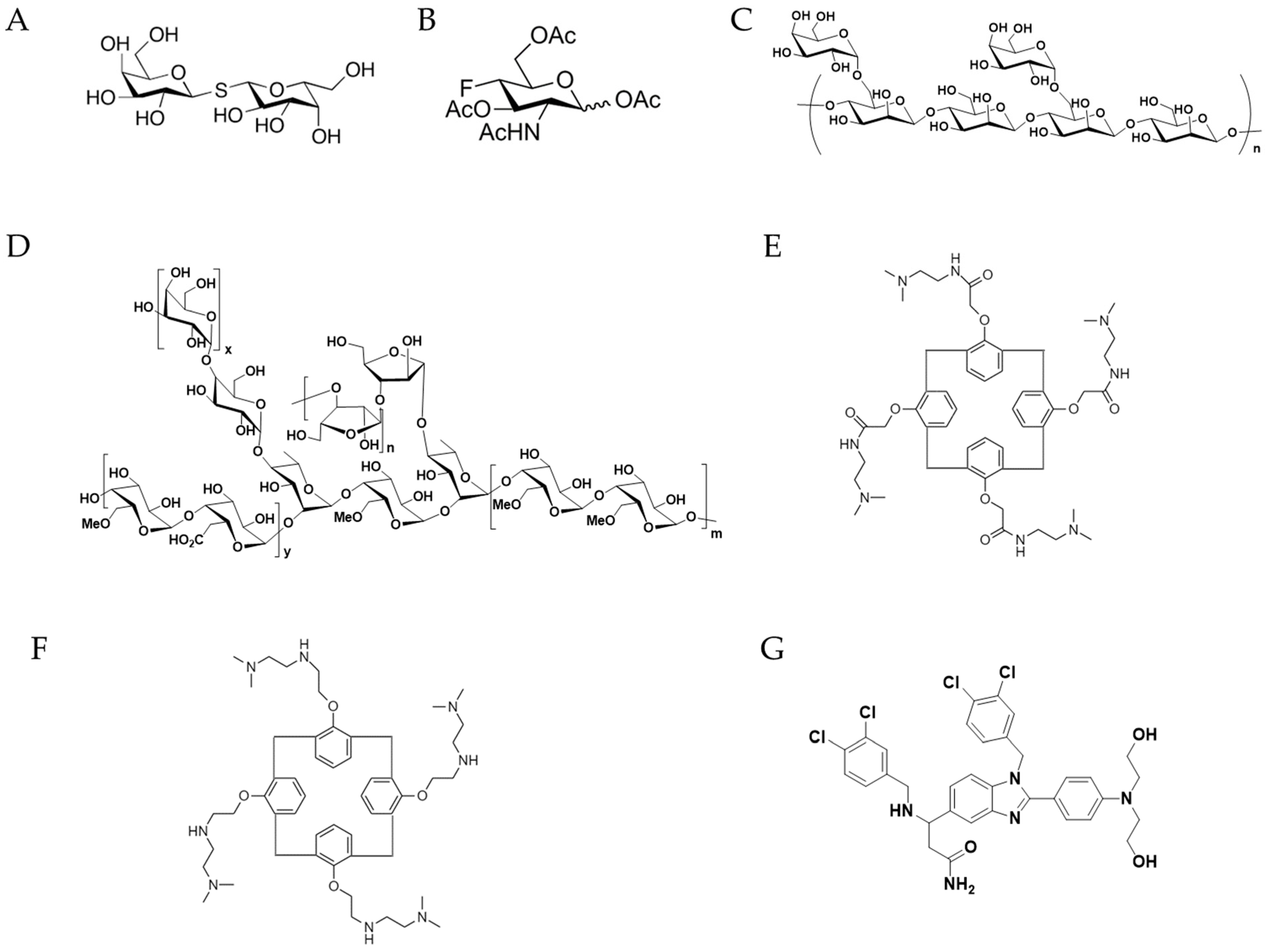
5. Therapeutic Agents against Gal-1 Signaling
| Inhibitors | Materials | Disease | Effect | Study Trials | Ref |
|---|---|---|---|---|---|
| Thiodigalactoside | Disaccharides | Breast cancer | Inhibition of tumor growth. Synergistic effect with immunotherapy. | Rat | [73] |
| Peracetylated 4-fluoro-glucosamine (4-F-GlcNAc) | Glycan | B16 melanomas and EL-4 lymphomas | Inhibition of tumor growth. Elicit anti-melanoma CTLs and lower levels of IL-10. | Mouse | [77] |
| AP-74 M-545 | Aptamer | Lewis lung carcinoma | T cell apoptosis restoration and tumor growth inhibition | Mouse | [78] |
| GM-CT-01 (Davanat) and/or 5-Fluorouracil | Polysaccharide and/or chemotherapeutic chemicals | Metastatic colorectal cancer | Enhancement in longevity of the patients and reduction in serious adverse effects. | Pre-clinical in Phase I and Phase II | [80] ClinicalTrials.gov: NCT00110721 and NCT00054977 |
| GR-MD-02 (Belapectin) | Polysaccharide | NASH cirrhosis patients | Significant treatment effects in NASH cirrhosis patients without esophageal varices. | Phase II clinical trial | [81] ClinicalTrials.gov: NCT02462967 |
| GR-MD-02 (Belapectin) with pembrolizumab | Polysaccharide and antibody respectively | Patients with advanced melanoma, non-small cell lung cancer, and head and neck squamous cell cancer | --- | Phase I clinical trial | ClinicalTrials.gov: NCT02575404 |
| OTX008 | Small molecule | Advanced solid tumors | --- | Phase I of the clinical studies | ClinicalTrials.gov: NCT01724320 |
| PTX013 | Small molecule | Human cancer cell lines and drug resistant cancer cells | Strong inhibitory effect on human cancer cell lines and drug-resistant cancer cells. | Cancer cells and drug- resistant cancer cells | [82] |
| LLS30 | Small molecule | Human metastatic castration-resistant prostate cancer | Increasing the anti-tumor effect of docetaxel and inhibiting the invasion and metastasis of prostate cancer cells in vivo. | Castration-resistant prostate cancer xenograft | [83] |
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.N.; Guevara-Patiño, J.A. Immune recognition of self in immunity against cancer. J. Clin. Investig. 2004, 114, 468–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnaar, R.L. Glycans and glycan-binding proteins in immune regulation: A concise introduction to glycobiology for the allergist. J. Allergy Clin. Immunol. 2015, 135, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Morell, A.G.; Gregoriadis, G.; Scheinberg, I.H.; Hickman, J.; Ashwell, G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J. Biol. Chem. 1971, 246, 1461–1467. [Google Scholar] [CrossRef]
- Teichberg, V.I.; Silman, I.; Beitsch, D.D.; Resheff, G. A beta-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc. Natl. Acad. Sci. USA 1975, 72, 1383–1387. [Google Scholar] [CrossRef] [Green Version]
- de Waard, A.; Hickman, S.; Kornfeld, S. Isolation and properties of beta-galactoside binding lectins of calf heart and lung. J. Biol. Chem. 1976, 251, 7581–7587. [Google Scholar] [CrossRef]
- Nowak, T.P.; Kobiler, D.; Roel, L.E.; Barondes, S.H. Developmentally regulated lectin from embryonic chick pectoral muscle. Purification by affinity chromatography. J. Biol. Chem. 1977, 252, 6026–6030. [Google Scholar] [CrossRef]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef]
- Chou, F.-C.; Chen, H.-Y.; Kuo, C.-C.; Sytwu, H.-K. Role of galectins in tumors and in clinical immunotherapy. Int. J. Mol. Sci. 2018, 19, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.; et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Earl, L.A.; Bi, S.; Baum, L.G. Galectin multimerization and lattice formation are regulated by linker region structure. Glycobiology 2011, 21, 6–12. [Google Scholar] [CrossRef] [Green Version]
- López-Lucendo, M.F.; Solís, D.; André, S.; Hirabayashi, J.; Kasai, K.; Kaltner, H.; Gabius, H.J.; Romero, A. Growth-regulatory human galectin-1: Crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J. Mol. Biol. 2004, 343, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.M.; Trujillo, M.; Estrin, D.A.; Rabinovich, G.A.; Di Lella, S. Impact of human galectin-1 binding to saccharide ligands on dimer dissociation kinetics and structure. Glycobiology 2016, 26, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Tracey, B.M.; Feizi, T.; Abbott, W.M.; Carruthers, R.A.; Green, B.N.; Lawson, A.M. Subunit molecular mass assignment of 14,654 Da to the soluble beta-galactoside-binding lectin from bovine heart muscle and demonstration of intramolecular disulfide bonding associated with oxidative inactivation. J. Biol. Chem. 1992, 267, 10342–10347. [Google Scholar] [CrossRef]
- Paz, A.; Haklai, R.; Elad-Sfadia, G.; Ballan, E.; Kloog, Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 2001, 20, 7486–7493. [Google Scholar] [CrossRef] [Green Version]
- Vyakarnam, A.; Dagher, S.F.; Wang, J.L.; Patterson, R.J. Evidence for a role for galectin-1 in pre-mRNA splicing. Mol. Cell. Biol. 1997, 17, 4730–4737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.W.; Voss, P.G.; Grabski, S.; Wang, J.L.; Patterson, R.J. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001, 29, 3595–3602. [Google Scholar] [CrossRef]
- Ose, R.; Oharaa, O.; Nagase, T. Galectin-1 and galectin-3 mediate protocadherin-24-dependent membrane localization of β-catenin in colon cancer cell line HCT116. Curr. Chem. Genom. 2012, 6, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Bosch, N.; Fernández-Barrena, M.G.; Moreno, M.; Ortiz-Zapater, E.; Munné-Collado, J.; Iglesias, M.; André, S.; Gabius, H.J.; Hwang, R.F.; Poirier, F.; et al. Galectin-1 drives pancreatic carcinogenesis through stroma remodeling and Hedgehog signaling activation. Cancer Res. 2014, 74, 3512–3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlini, M.J.; Roitman, P.; Nuñez, M.; Pallotta, M.G.; Boggio, G.; Smith, D.; Salatino, M.; Joffé, E.D.; Rabinovich, G.A.; Puricelli, L.I. Clinical relevance of galectin-1 expression in non-small cell lung cancer patients. Lung Cancer 2014, 84, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.J.; Moon, H.G.; Cho, B.I.; Jeong, C.Y.; Joo, Y.T.; Lee, Y.J.; Hong, S.C.; Choi, S.K.; Ha, W.S.; Kim, J.W.; et al. Galectin-1 expression in cancer-associated stromal cells correlates tumor invasiveness and tumor progression in breast cancer. Int. J. Cancer 2007, 120, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- White, N.M.; Masui, O.; Newsted, D.; Scorilas, A.; Romaschin, A.D.; Bjarnason, G.A.; Siu, K.W.; Yousef, G.M. Galectin-1 has potential prognostic significance and is implicated in clear cell renal cell carcinoma progression through the HIF/mTOR signaling axis. Br. J. Cancer 2014, 110, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, P.; Shi, B.; Zhou, M.; Jiang, H.; Zhang, H.; Pan, X.; Gao, H.; Sun, H.; Li, Z. Galectin-1 overexpression promotes progression and chemoresistance to cisplatin in epithelial ovarian cancer. Cell Death Dis. 2014, 5, e991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leffler, H.; Carlsson, S.; Hedlund, M.; Qian, Y.; Poirier, F. Introduction to galectins. Glycoconj. J. 2002, 19, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Muller, W.E.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta 2002, 1572, 232–254. [Google Scholar] [CrossRef]
- Perillo, N.L.; Pace, K.E.; Seilhamer, J.J.; Baum, L.G. Apoptosis of T cells mediated by galectin-1. Nature 1995, 378, 736–739. [Google Scholar] [CrossRef]
- Hahn, H.P.; Pang, M.; He, J.; Hernandez, J.D.; Yang, R.Y.; Li, L.Y.; Wang, X.; Liu, F.T.; Baum, L.G. Galectin-1 induces nuclear translocation of endonuclease G in caspase and cytochrome c-independent T cell death. Cell Death Differ. 2004, 11, 1277–1286. [Google Scholar] [CrossRef]
- Ion, G.; Fajka-Boja, R.; Tóth, G.K.; Caron, M.; Monostori, É. Role of p56lck and ZAP70-mediated tyrosine phosphorylation in galectin-1-induced cell death. Cell Death Differ. 2005, 12, 1145–1147. [Google Scholar] [CrossRef]
- Matarrese, P.; Tinari, A.; Mormone, E.; Bianco, G.A.; Toscano, M.A.; Ascione, B.; Rabinovich, G.A.; Malorni, W. Galectin-1 sensitizes resting human T lymphocytes to Fas (CD95)-mediated cell death via mitochondrial hyperpolarization, budding, and fission. J. Biol. Chem. 2005, 280, 6969–6985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinovich, G.A.; Alonso, C.R.; Sotomayor, C.E.; Durand, S.; Bocco, J.L.; Riera, C.M. Molecular mechanisms implicated in galectin-1-induced apoptosis: Activation of the AP-1 transcription factor and downregulation of Bcl-2. Cell Death Differ. 2000, 7, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toscano, M.A.; Bianco, G.A.; Ilarregui, J.M.; Croci, D.O.; Correale, J.; Hernandez, J.D.; Zwirner, N.W.; Poirier, F.; Riley, E.M.; Baum, L.G.; et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007, 8, 825–834. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Daly, G.; Dreja, H.; Tailor, H.; Riera, C.M.; Hirabayashi, J.; Chernajovsky, Y. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J. Exp. Med. 1999, 190, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Santucci, L.; Fiorucci, S.; Rubinstein, N.; Mencarelli, A.; Palazzetti, B.; Federici, B.; Rabinovich, G.A.; Morelli, A. Galectin-1 suppresses experimental colitis in mice. Gastroenterology 2003, 124, 1381–1394. [Google Scholar] [CrossRef]
- Toscano, M.A.; Commodaro, A.G.; Ilarregui, J.M.; Bianco, G.A.; Liberman, A.; Serra, H.M.; Hirabayashi, J.; Rizzo, L.V.; Rabinovich, G.A. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J. Immunol. 2006, 176, 6323–6332. [Google Scholar] [CrossRef] [Green Version]
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological decision-making: How does the immune system decide to mount a helper T-cell response? Immunology 2008, 123, 326–338. [Google Scholar] [CrossRef]
- Steidl, C.; Connors, J.M.; Gascoyne, R.D. Molecular pathogenesis of Hodgkin’s lymphoma: Increasing evidence of the importance of the microenvironment. J. Clin. Oncol. 2011, 29, 1812–1826. [Google Scholar] [CrossRef]
- Anderson, K.C.; Bates, M.P.; Slaughenhoupt, B.L.; Pinkus, G.S.; Schlossman, S.F.; Nadler, L.M. Expression of human B cell-associated antigens on leukemias and lymphomas: A model of human B cell differentiation. Blood 1984, 63, 1424–1433. [Google Scholar] [CrossRef] [Green Version]
- Juszczynski, P.; Ouyang, J.; Monti, S.; Rodig, S.J.; Takeyama, K.; Abramson, J.; Chen, W.; Kutok, J.L.; Rabinovich, G.A.; Shipp, M.A. The AP1-dependent secretion of galectin-1 by reed–sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA 2007, 104, 13134–13139. [Google Scholar] [CrossRef] [Green Version]
- Rodig, S.J.; Ouyang, J.; Juszczynski, P.; Currie, T.; Law, K.; Neuberg, D.S.; Rabinovich, G.A.; Shipp, M.A.; Kutok, J.L. AP1-dependent galectin-1 expression delineates classical Hodgkin and anaplastic large cell lymphomas from other lymphoid malignancies with shared molecular features. Clin. Cancer Res. 2008, 14, 3338–3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykken, J.M.; Horikawa, M.; Minard-Colin, V.; Kamata, M.; Miyagaki, T.; Poe, J.C.; Tedder, T.F. Galectin-1 drives lymphoma CD20 immunotherapy resistance: Validation of a preclinical system to identify resistance mechanisms. Blood 2016, 127, 1886–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillenwater, A.; Xu, X.-C.; El-Naggar, A.K.; Clayman, G.L.; Lotan, R. Expression of galectins in head and neck squamous cell carcinoma. Head Neck 1996, 18, 422–432. [Google Scholar] [CrossRef]
- Valach, J.; Fík, Z.; Strnad, H.; Chovanec, M.; Plzák, J.; Čada, Z.; Szabo, P.; Šáchová, J.; Hroudová, M.; Urbanová, M.; et al. Smooth muscle actin-expressing stromal fibroblasts in head and neck squamous cell carcinoma: Increased expression of galectin-1 and induction of poor prognosis factors. Int. J. Cancer 2012, 131, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Warren, T.A.; Wockner, L.F.; Lambie, D.L.; Brown, I.S.; Martin, T.P.; Khanna, R.; Leggatt, G.R.; Panizza, B.J. Galectin-1 is associated with poor prognosis in patients with cutaneous head and neck cancer with perineural spread. Cancer Immunol. Immunother. 2016, 65, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, D.K.; Aguilera, T.; Cao, H.; Kwok, S.; Kong, C.; Bloomstein, J.; Wang, Z.; Rangan, V.S.; Jiang, D.; von Eyben, R.; et al. Galectin-1-driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance. J. Clin. Investig. 2019, 129, 5553–5567. [Google Scholar] [CrossRef] [Green Version]
- Saussez, S.; Camby, I.; Toubeau, G.; Kiss, R. Galectins as modulators of tumor progression in head and neck squamous cell carcinomas. Head Neck 2007, 29, 874–884. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaoka, K.; Mishima, K.; Nagashima, Y.; Asai, A.; Sanai, Y.; Kirino, T. Expression of galectin-1 mRNA correlates with the malignant potential of human gliomas and expression of antisense galectin-1 inhibits the growth of 9 glioma cells. J. Neurosci. Res. 2000, 59, 722–730. [Google Scholar] [CrossRef]
- Rorive, S.; Belot, N.; Decaestecker, C.; Lefranc, F.; Gordower, L.; Micik, S.; Maurage, C.A.; Kaltner, H.; Ruchoux, M.M.; Danguy, A.; et al. Galectin-1 is highly expressed in human gliomas with relevance for modulation of invasion of tumor astrocytes into the brain parenchyma. Glia 2001, 33, 241–255. [Google Scholar] [CrossRef]
- Camby, I.; Belot, N.; Rorive, S.; Lefranc, F.; Maurage, C.A.; Lahm, H.; Kaltner, H.; Hadari, Y.; Ruchoux, M.M.; Brotchi, J.; et al. Galectins are differentially expressed in supratentorial pilocytic astrocytomas, astrocytomas, anaplastic astrocytomas and glioblastomas, and significantly modulate tumor astrocyte migration. Brain Pathol. 2001, 11, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Verschuere, T.; Toelen, J.; Maes, W.; Poirier, F.; Boon, L.; Tousseyn, T.; Mathivet, T.; Gerhardt, H.; Mathieu, V.; Kiss, R.; et al. Glioma-derived galectin-1 regulates innate and adaptive antitumor immunity. Int. J. Cancer 2014, 134, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Han, B.; Meng, X.; Duan, C.; Yang, C.; Wu, Z.; Magafurov, D.; Zhao, S.; Safin, S.; Jiang, C.; et al. Immunogenomic analysis reveals LGALS1 contributes to the immune heterogeneity and immunosuppression in glioma. Int. J. Cancer 2019, 145, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Park, S.; Phillips, P.A.; Santucci, N.; Goldstein, D.; Kumar, R.K.; Ramm, G.A.; Buchler, M.; Friess, H.; McCarroll, J.A.; et al. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas 2004, 29, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef]
- Qian, D.; Lu, Z.; Xu, Q.; Wu, P.; Tian, L.; Zhao, L.; Cai, B.; Yin, J.; Wu, Y.; Staveley-O’Carroll, K.F.; et al. Galectin-1-driven upregulation of SDF-1 in pancreatic stellate cells promotes pancreatic cancer metastasis. Cancer Lett. 2017, 397, 43–51. [Google Scholar] [CrossRef]
- Orozco, C.A.; Martinez-Bosch, N.; Guerrero, P.E.; Vinaixa, J.; Dalotto-Moreno, T.; Iglesias, M.; Moreno, M.; Djurec, M.; Poirier, F.; Gabius, H.-J.; et al. Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor-stroma crosstalk. Proc. Natl. Acad. Sci. USA 2018, 115, E3769–E3778. [Google Scholar] [CrossRef] [Green Version]
- Szoke, T.; Kayser, K.; Baumhakel, J.D.; Trojan, I.; Furak, J.; Tiszlavicz, L.; Horvath, A.; Szluha, K.; Gabius, H.J.; Andre, S. Prognostic significance of endogenous adhesion/growth-regulatory lectins in lung cancer. Oncology 2005, 69, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, L.Y.; Tang, S.J.; Sun, G.H.; Chou, T.Y.; Yeh, T.S.; Yu, S.L.; Sun, K.H. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin. Cancer Res. 2012, 18, 4037–4047. [Google Scholar] [CrossRef] [Green Version]
- Kuo, P.L.; Hung, J.Y.; Huang, S.K.; Chou, S.H.; Cheng, D.E.; Jong, Y.J.; Hung, C.H.; Yang, C.J.; Tsai, Y.M.; Hsu, Y.L.; et al. Lung cancer-derived galectin-1 mediates dendritic cell anergy through inhibitor of DNA binding 3/IL-10 signaling pathway. J. Immunol. 2011, 186, 1521–1530. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.-L.; Hung, J.-Y.; Chiang, S.-Y.; Jian, S.-F.; Wu, C.-Y.; Lin, Y.-S.; Tsai, Y.-M.; Chou, S.-H.; Tsai, M.-J.; Kuo, P.-L. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget 2016, 7, 27584–27598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalotto-Moreno, T.; Croci, D.O.; Cerliani, J.P.; Martinez-Allo, V.C.; Dergan-Dylon, S.; Méndez-Huergo, S.P.; Stupirski, J.C.; Mazal, D.; Osinaga, E.; Toscano, M.A.; et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013, 73, 1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.-E.; Hung, J.-Y.; Huang, M.-S.; Hsu, Y.-L.; Lu, C.-Y.; Tsai, E.-M.; Hou, M.-F.; Kuo, P.-L. Myosin IIa activation is crucial in breast cancer derived galectin-1 mediated tolerogenic dendritic cell differentiation. Biochim. Biophys. Acta (BBA) 2014, 1840, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Hersey, P. Apoptosis and melanoma: How new insights are effecting the development of new therapies for melanoma. Curr. Opin. Oncol. 2006, 18, 189–196. [Google Scholar] [CrossRef]
- Ivanov, V.N.; Bhoumik, A.; Ronai, Z.E. Death receptors and melanoma resistance to apoptosis. Oncogene 2003, 22, 3152–3161. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, N.; Alvarez, M.; Zwirner, N.W.; Toscano, M.A.; Ilarregui, J.M.; Bravo, A.; Mordoh, J.; Fainboim, L.; Podhajcer, O.L.; Rabinovich, G.A. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell 2004, 5, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Yazawa, E.M.; Geddes-Sweeney, J.E.; Cedeno-Laurent, F.; Walley, K.C.; Barthel, S.R.; Opperman, M.J.; Liang, J.; Lin, J.Y.; Schatton, T.; Laga, A.C.; et al. Melanoma cell galectin-1 ligands functionally correlate with malignant potential. J. Investig. Dermatol. 2015, 135, 1849–1862. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, V.; Le Mercier, M.; De Neve, N.; Sauvage, S.; Gras, T.; Roland, I.; Lefranc, F.; Kiss, R. Galectin-1 knockdown increases sensitivity to temozolomide in a B16F10 mouse metastatic melanoma model. J. Investig. Dermatol. 2007, 127, 2399–2410. [Google Scholar] [CrossRef]
- Sitek, B.; Apostolov, O.; Stühler, K.; Pfeiffer, K.; Meyer, H.E.; Eggert, A.; Schramm, A. Identification of dynamic proteome changes upon ligand activation of Trk-receptors using two-dimensional fluorescence difference gel electrophoresis and mass spectrometry. Mol. Cell. Proteom. 2005, 4, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Soldati, R.; Berger, E.; Zenclussen, A.C.; Jorch, G.; Lode, H.N.; Salatino, M.; Rabinovich, G.A.; Fest, S. Neuroblastoma triggers an immunoevasive program involving galectin-1-dependent modulation of T cell and dendritic cell compartments. Int. J. Cancer 2012, 131, 1131–1141. [Google Scholar] [CrossRef]
- Weiss, W.A.; Aldape, K.; Mohapatra, G.; Feuerstein, B.G.; Bishop, J.M. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997, 16, 2985–2995. [Google Scholar] [CrossRef] [PubMed]
- Büchel, G.; Schulte, J.H.; Harrison, L.; Batzke, K.; Schüller, U.; Hansen, W.; Schramm, A. Immune response modulation by Galectin-1 in a transgenic model of neuroblastoma. Oncoimmunology 2016, 5, e1131378. [Google Scholar] [CrossRef] [Green Version]
- Stannard, K.A.; Collins, P.M.; Ito, K.; Sullivan, E.M.; Scott, S.A.; Gabutero, E.; Darren Grice, I.; Low, P.; Nilsson, U.J.; Leffler, H.; et al. Galectin inhibitory disaccharides promote tumour immunity in a breast cancer model. Cancer Lett. 2010, 299, 95–110. [Google Scholar] [CrossRef]
- Blanchard, H.; Bum-Erdene, K.; Bohari, M.H.; Yu, X. Galectin-1 inhibitors and their potential therapeutic applications: A patent review. Expert Opin. Ther. Pat. 2016, 26, 537–554. [Google Scholar] [CrossRef]
- Sethi, A.; Sanam, S.; Alvala, R.; Alvala, M. An updated patent review of galectin-1 and galectin-3 inhibitors and their potential therapeutic applications (2016-present). Expert Opin. Pat. 2021, 31, 709–721. [Google Scholar] [CrossRef]
- Laaf, D.; Bojarová, P.; Elling, L.; Křen, V. Galectin-carbohydrate interactions in biomedicine and biotechnology. Trends Biotechnol. 2019, 37, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Cedeno-Laurent, F.; Opperman, M.J.; Barthel, S.R.; Hays, D.; Schatton, T.; Zhan, Q.; He, X.; Matta, K.L.; Supko, J.G.; Frank, M.H.; et al. Metabolic inhibition of galectin-1-binding carbohydrates accentuates antitumor immunity. J. Investig. Derm. 2012, 132, 410–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.T.; Liang, C.H.; Yu, J.H.; Huang, K.C.; Tung, C.H.; Wu, J.E.; Wu, Y.Y.; Chang, C.H.; Hong, T.M.; Chen, Y.L. A DNA aptamer targeting galectin-1 as a novel immunotherapeutic strategy for lung cancer. Mol. Ther. Nucleic Acids 2019, 18, 991–998. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.C.; Lin, H.Y.; Tu, Z.; Kuo, Y.H.; Hsu, S.D.; Lin, C.H. Dissecting the structure-activity relationship of galectin-ligand interactions. Int. J. Mol. Sci. 2018, 19, 392. [Google Scholar] [CrossRef] [Green Version]
- Klyosov, A.; Zomer, E.; Platt, D. studies. In Glycobiology and Drug Design; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; Volume 1102, pp. 89–130. [Google Scholar]
- Chalasani, N.; Abdelmalek, M.F.; Garcia-Tsao, G.; Vuppalanchi, R.; Alkhouri, N.; Rinella, M.; Noureddin, M.; Pyko, M.; Shiffman, M.; Sanyal, A.; et al. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology 2020, 158, 1334–e1335. [Google Scholar] [CrossRef] [Green Version]
- Dings, R.P.; Levine, J.I.; Brown, S.G.; Astorgues-Xerri, L.; MacDonald, J.R.; Hoye, T.R.; Raymond, E.; Mayo, K.H. Polycationic calixarene PTX013, a potent cytotoxic agent against tumors and drug resistant cancer. Investig. New Drugs 2013, 31, 1142–1150. [Google Scholar] [CrossRef] [Green Version]
- Shih, T.C.; Liu, R.; Wu, C.T.; Li, X.; Xiao, W.; Deng, X.; Kiss, S.; Wang, T.; Chen, X.J.; Carney, R.; et al. Targeting galectin-1 impairs castration-resistant prostate cancer progression and invasion. Clin. Cancer Res. 2018, 24, 4319–4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas, A.; Hamid, O.; Daud, A.; Hodi, F.S.; Wolchok, J.D.; Kefford, R.; Joshua, A.M.; Patnaik, A.; Hwu, W.J.; Weber, J.S.; et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016, 315, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Jenkins, R.W.; Sullivan, R.J. Mechanisms of resistance to immune checkpoint blockade. Am. J. Clin. Derm. 2019, 20, 41–54. [Google Scholar] [CrossRef]
- Fares, C.M.; Allen, E.M.V.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of resistance to immune checkpoint blockade: Why does checkpoint inhibitor immunotherapy not work for all patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 147–164. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wang, H.-C.; Zhao, J.; Wu, M.-H.; Shih, T.-C. Immunosuppressive Roles of Galectin-1 in the Tumor Microenvironment. Biomolecules 2021, 11, 1398. https://doi.org/10.3390/biom11101398
Huang Y, Wang H-C, Zhao J, Wu M-H, Shih T-C. Immunosuppressive Roles of Galectin-1 in the Tumor Microenvironment. Biomolecules. 2021; 11(10):1398. https://doi.org/10.3390/biom11101398
Chicago/Turabian StyleHuang, Yanyu, Hsiao-Chi Wang, Junwei Zhao, Ming-Heng Wu, and Tsung-Chieh Shih. 2021. "Immunosuppressive Roles of Galectin-1 in the Tumor Microenvironment" Biomolecules 11, no. 10: 1398. https://doi.org/10.3390/biom11101398
APA StyleHuang, Y., Wang, H.-C., Zhao, J., Wu, M.-H., & Shih, T.-C. (2021). Immunosuppressive Roles of Galectin-1 in the Tumor Microenvironment. Biomolecules, 11(10), 1398. https://doi.org/10.3390/biom11101398







