Functional Assembly of Caenorhabditis elegans Cytochrome b-2 (Cecytb-2) into Phospholipid Bilayer Nanodisc with Enhanced Iron Reductase Activity
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Expression and Purification of Cecytb-2
2.3. Reconstitution of Cecytb-2 into Nanodisc
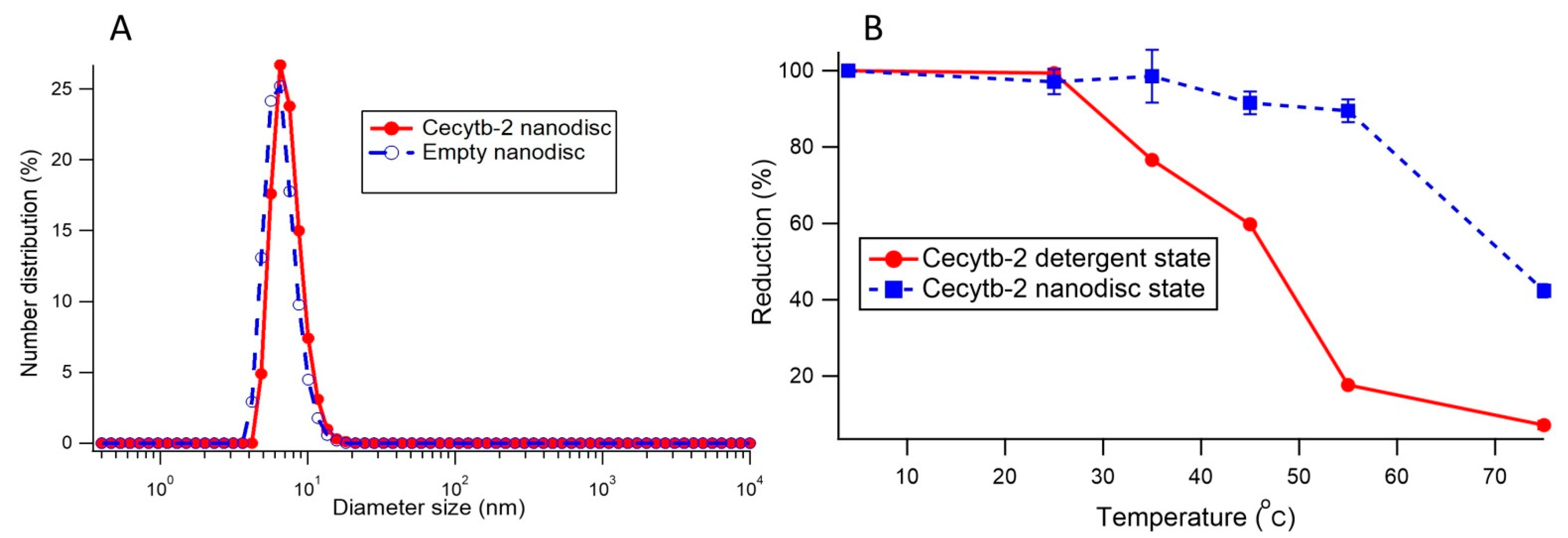
2.4. Homogeneity and Particle Size Distribution of Nanodisc
2.5. Thermal Stability of Cecytb-2 in a Nanodisc and in a Detergent Micelle State
2.6. Measurements of Ferric Reductase Activity of Cecytb-2
2.7. Measurements of Ferric Reductase Activity by Nitroso-PSAP Assay
3. Results
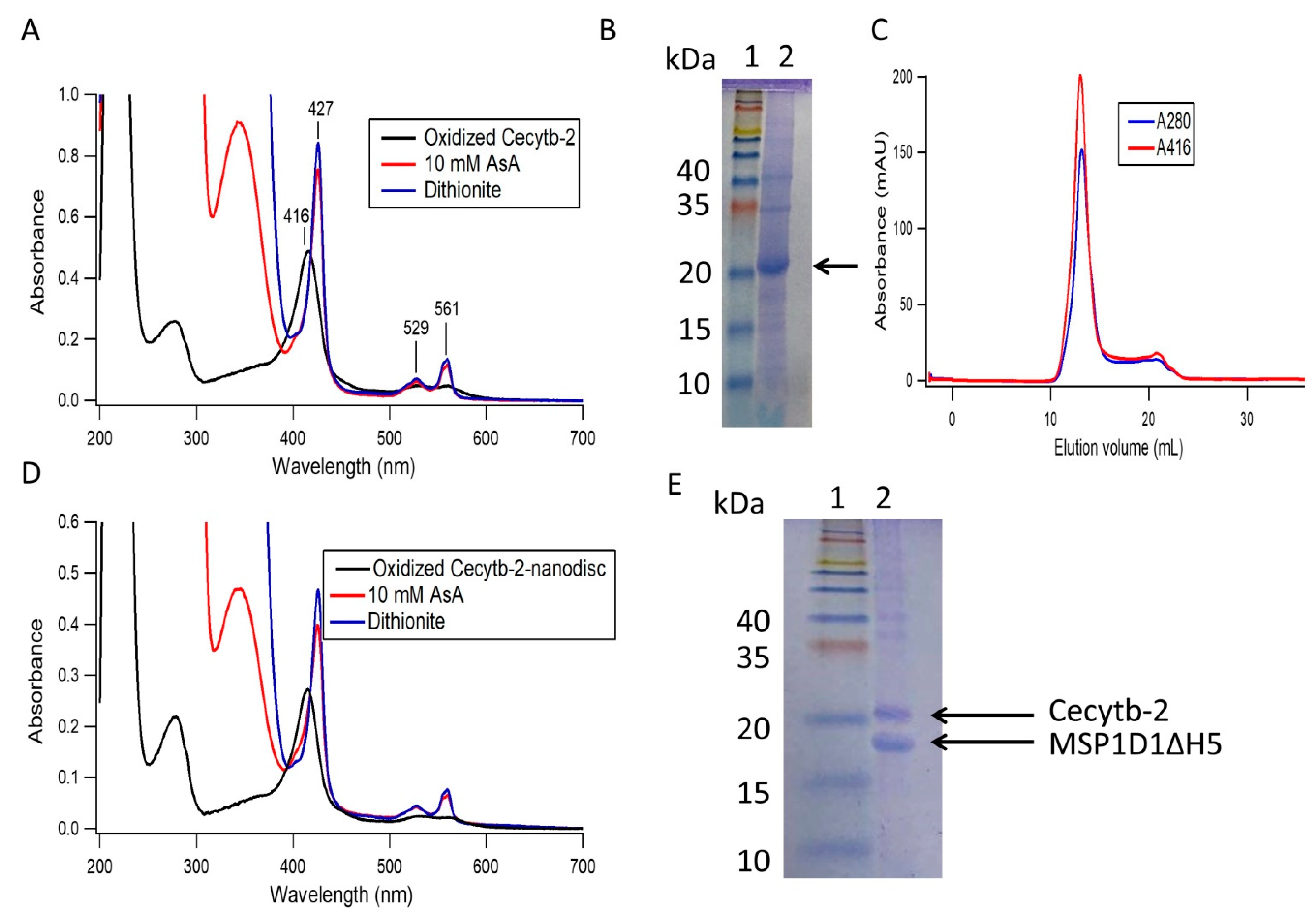
3.1. Cecytb-2 Purification and its Assembly into a Nanodisc
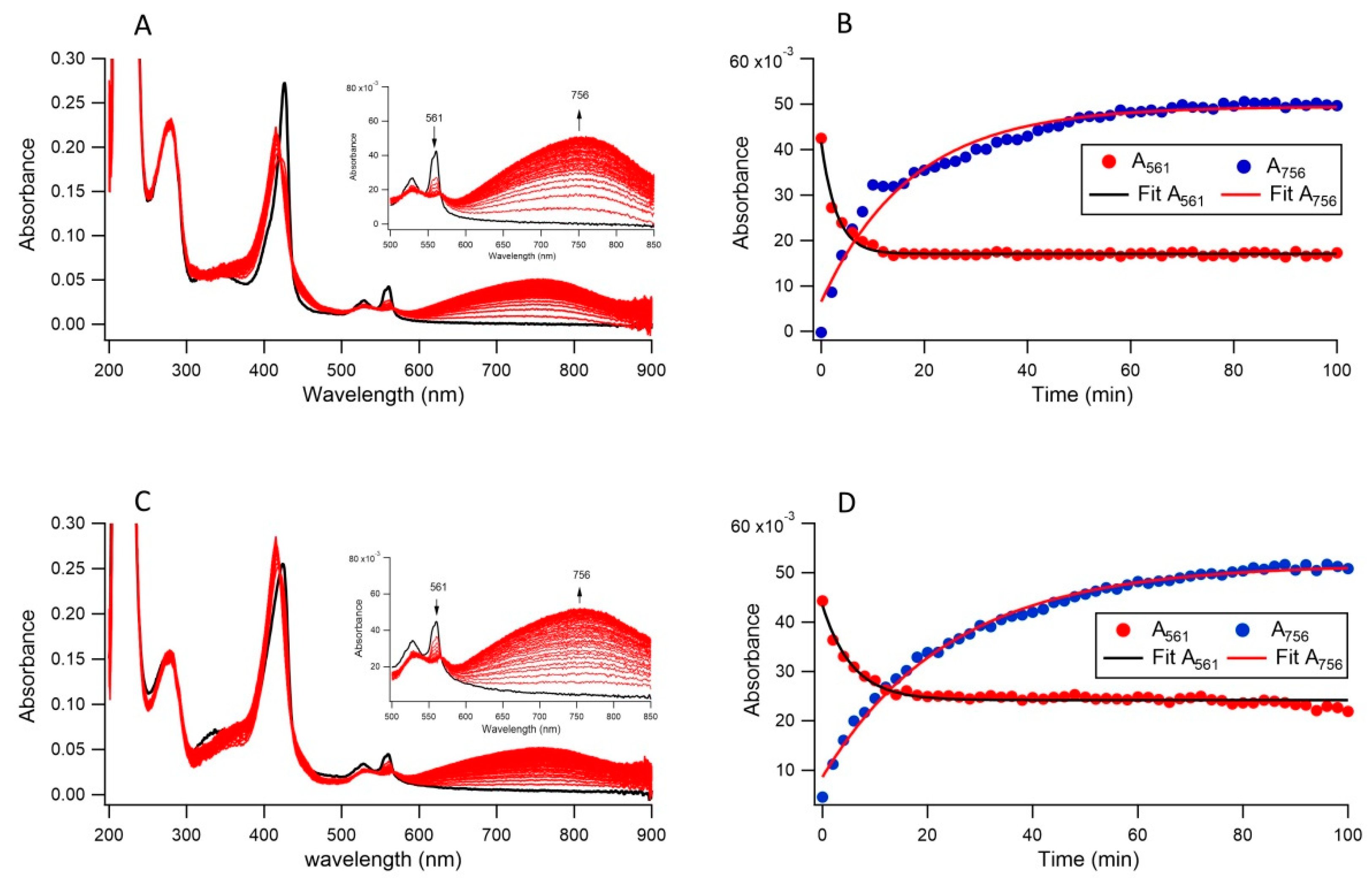
3.2. Ferric Reductase Activity of Cecytb-2 in DDM Detergent State
3.3. Ferric Reductase Activity of Cecytb-2 in the Nanodisc State
3.4. Ferric Reductase Activity of Cecytb-2 in Nanodisc and DDM Detergent State Measured by Nitroso-PSAP Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsubaki, M.; Takeuchi, F.; Nakanishi, N. Cytochrome b561 protein family: Expanding roles and versatile transmembrane electron transfer abilities as predicted by a new classification system and protein sequence motif analyses. Biochim. Biophys. Acta 2005, 1753, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Alajos, B.; Laszlo, Z. The Trans-Membrane Cytochrome b561 Proteins: Structural Information and Biological Function. Curr. Protein Pept. Sci. 2014, 15, 745–760. [Google Scholar] [CrossRef]
- Asard, H.; Barbaro, R.; Trost, P.; Bérczi, A. Cytochromes b561: Ascorbate-mediated trans-membrane electron transport. Antioxid. Redox Signal. 2013, 19, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; da Silva, G.F.; Wu, G.; Palmer, G.; Tsai, A.-L.; Kulmacz, R.J. Functional and structural roles of residues in the third extramembrane segment of adrenal cytochrome b 561. Biochemistry 2011, 50, 3149–3160. [Google Scholar] [CrossRef][Green Version]
- McKie, A.; Latunde-Dada, G.; Miret, S.; McGregor, J.; Anderson, G.; Vulpe, C.; Wrigglesworth, J.; Simpson, R. Molecular Evidence for the Role of a Ferric Reductase in Iron Transport.; Portland Press Ltd.: London, UK, 2002. [Google Scholar]
- Kafina, M.D.; Paw, B.H. Intracellular iron and heme trafficking and metabolism in developing erythroblasts. Metallomics 2017, 9, 1193–1203. [Google Scholar] [CrossRef]
- Glanfield, A.; McManus, D.P.; Smyth, D.J.; Lovas, E.M.; Loukas, A.; Gobert, G.N.; Jones, M.K. A Cytochrome b561 with Ferric Reductase Activity from the Parasitic Blood Fluke, Schistosoma japonicum. PLoS Negl. Trop. Dis. 2010, 4, e884. [Google Scholar] [CrossRef]
- Mackenzie, E.L.; Iwasaki, K.; Tsuji, Y. Intracellular iron transport and storage: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 997–1030. [Google Scholar] [CrossRef]
- Su, D.; Asard, H. Three mammalian cytochromes b561 are ascorbate-dependent ferrireductases. FEBS J. 2006, 273, 3722–3734. [Google Scholar] [CrossRef]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef]
- Bérczi, A.; Su, D.; Asard, H. An Arabidopsis cytochrome b561 with trans-membrane ferrireductase capability. FEBS Lett. 2007, 581, 1505–1508. [Google Scholar] [CrossRef]
- Mizutani, A.; Sanuki, R.; Kakimoto, K.; Kojo, S.; Taketani, S. Involvement of 101F6, a homologue of cytochrome b561, in the reduction of ferric ions. J. Biochem. 2007, 142, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, M.; Round, E.; Gushchin, I.; Polovinkin, V.; Balandin, T.; Kuzmichev, P.; Shevchenko, V.; Borshchevskiy, V.; Kuklin, A.; Round, A. Integral membrane proteins can be crystallized directly from nanodiscs. Cryst. Growth Des. 2017, 17, 945–948. [Google Scholar] [CrossRef]
- Hagn, F.; Nasr, M.L.; Wagner, G. Assembly of phospholipid nanodiscs of controlled size for structural studies of membrane proteins by NMR. Nat. Protoc. 2018, 13, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Marty, M.T.; Wilcox, K.C.; Klein, W.L.; Sligar, S.G. Nanodisc-solubilized membrane protein library reflects the membrane proteome. Anal. Bioanal. Chem. 2013, 405, 4009–4016. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; Ravula, T.; Ramamoorthy, A. Detergent free extraction, reconstitution and characterization of membrane-anchored cytochrome-b5 in native lipids. Chem. Commun. 2020, 56, 6511–6514. [Google Scholar] [CrossRef]
- Barnaba, C.; Ramamoorthy, A. Picturing the Membrane-Assisted Choreography of Cytochrome P450 with Lipid Nanodiscs. ChemPhysChem 2018, 19, 2603–2613. [Google Scholar] [CrossRef]
- Gentry, K.A.; Zhang, M.; Im, S.-C.; Waskell, L.; Ramamoorthy, A. Substrate mediated redox partner selectivity of cytochrome P450. Chem. Commun. 2018, 54, 5780–5783. [Google Scholar] [CrossRef]
- Barnaba, C.; Sahoo, B.R.; Ravula, T.; Medina-Meza, I.G.; Im, S.C.; Anantharamaiah, G.; Waskell, L.; Ramamoorthy, A. Cytochrome-P450-induced ordering of microsomal membranes modulates affinity for drugs. Angew. Chem. Int. Ed. 2018, 57, 3391–3395. [Google Scholar] [CrossRef]
- Ravula, T.; Barnaba, C.; Mahajan, M.; Anantharamaiah, G.M.; Im, S.-C.; Waskell, L.; Ramamoorthy, A. Membrane environment drives cytochrome P450’s spin transition and its interaction with cytochrome b5. Chem. Commun. 2017, 53, 12798–12801. [Google Scholar] [CrossRef]
- Yokogawa, M.; Fukuda, M.; Osawa, M. Nanodiscs for structural biology in a membranous environment. Chem. Pharm. Bull. (Tokyo) 2019, 67, 321–326. [Google Scholar] [CrossRef]
- Ritchie, T.; Grinkova, Y.; Bayburt, T.; Denisov, I.; Zolnerciks, J.; Atkins, W.; Sligar, S. Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 2009, 464, 211–231. [Google Scholar] [PubMed]
- El Behery, M.; Fujimura, M.; Kimura, T.; Tsubaki, M. Direct measurements of ferric reductase activity of human 101F6 and its enhancement upon reconstitution into phospholipid bilayer nanodisc. Biochem. Biophys. Rep. 2020, 21, 100730. [Google Scholar] [CrossRef] [PubMed]
- Recuenco, M.C.; Fujito, M.; Rahman, M.; Sakamoto, Y.; Takeuchi, F.; Tsubaki, M. Functional expression and characterization of human 101F6 protein, a homologue of cytochrome b_{561} and a candidate tumor suppressor gene product. BioFactors 2009, 34, 219–230. [Google Scholar] [CrossRef]
- Recuenco, M.C.; Rahman, M.M.; Takeuchi, F.; Kobayashi, K.; Tsubaki, M. Electron transfer reactions of candidate tumor suppressor 101F6 protein, a cytochrome b 561 homologue, with ascorbate and monodehydroascorbate radical. Biochemistry 2013, 52, 3660–3668. [Google Scholar] [CrossRef] [PubMed]
- Recuenco, M.C.; Watanabe, S.; Takeuchi, F.; Park, S.-Y.; Tsubaki, M. Properties of human tumor suppressor 101F6 protein as a cytochrome b561 and its preliminary crystallization trials. Tumor Suppressor Genes 2012, 295. [Google Scholar] [CrossRef][Green Version]
- Recuenco, M.C.; Rahman, M.M.; Sakamoto, Y.; Takeuchi, F.; Hori, H.; Tsubaki, M. Functional characterization of the recombinant human tumour suppressor 101F6 protein, a cytochrome b561 homologue. J. Biochem. 2012, 153, 233–242. [Google Scholar] [CrossRef]
- Rahman, M.; Nakanishi, N.; Takigami, T.; Hase, T.; Park, S.; Tsubaki, M. Purification and Biochemical Analyses of Zea mays Cytochrome b561 Heterologously Expressed in Pichia pastoris. In Proceedings of the 2007 International Symposium on Micro-NanoMechatronics and Human Science, Nagoya, Japan, 11–14 November 2007; pp. 108–112. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Takeuchi, F.; Kobayashi, K.; Tagawa, S.; Tsubaki, M. Ascorbate inhibits the carbethoxylation of two histidyl and one tyrosyl residues indispensable for the transmembrane electron transfer reaction of cytochrome b 561. Biochemistry 2001, 40, 4067–4076. [Google Scholar] [CrossRef]
- Bayburt, T.H.; Grinkova, Y.V.; Sligar, S.G. Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2002, 2, 853–856. [Google Scholar] [CrossRef]
- Bayburt, T.H.; Sligar, S.G. Self-assembly of single integral membrane proteins into soluble nanoscale phospholipid bilayers. Protein Sci. 2003, 12, 2476–2481. [Google Scholar] [CrossRef]
- Yokota, F.; Abe, S. Solid phase colorimetry of trace metal ions based on a tristimulus chromaticity diagram. Simultaneous determination of iron (II) and iron (III). Anal. Commun. 1997, 34, 111–112. [Google Scholar] [CrossRef]
- Oakhill, J.S.; Marritt, S.J.; Gareta, E.G.; Cammack, R.; McKie, A.T. Functional characterization of human duodenal cytochrome b (Cybrd1): Redox properties in relation to iron and ascorbate metabolism. Biochim. Biophys. Acta BBA Bioenerg. 2008, 1777, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liu, Y.; Culhane, K.J.; DeVree, B.T.; Yang, Y.; Sunahara, R.K.; Yan, E.C. Purification of family BG protein-coupled receptors using nanodiscs: Application to human glucagon-like peptide-1 receptor. PLoS ONE 2017, 12, e0179568. [Google Scholar] [CrossRef] [PubMed]
- Corin, K.; Baaske, P.; Geissler, S.; Wienken, C.J.; Duhr, S.; Braun, D.; Zhang, S. Structure and function analyses of the purified GPCR human vomeronasal type 1 receptor 1. Sci. Rep. 2011, 1, 172. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.; Lerch, M.; Kunji, E.; Slotboom, D.-J.; de Gier, J.-W. Optimization of membrane protein overexpression and purification using GFP fusions. Nat. Methods 2006, 3, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M. The secretory-vesicle ascorbate-regenerating system: A chain of concerted H+/e−-transfer reactions. Biochim. Biophys. Acta 1993, 1144, 235–248. [Google Scholar] [CrossRef]
- Tsubaki, M.; Nakayama, M.; Okuyama, E.; Ichikawa, Y.; Hori, H. Existence of two heme B centers in cytochromeb 561 from bovine adrenal chromaffin vesicles as revealed by a new purification procedure and EPR spectroscopy. J. Biol. Chem. 1997, 272, 23206–23210. [Google Scholar] [CrossRef]
- Pollock, N.L.; Lee, S.C.; Patel, J.H.; Gulamhussein, A.A.; Rothnie, A.J. Structure and function of membrane proteins encapsulated in a polymer-bound lipid bilayer. Biochim. Biophys. Acta 2018, 1860, 809–817. [Google Scholar] [CrossRef]
- Hagn, F.; Etzkorn, M.; Raschle, T.; Wagner, G. Optimized phospholipid bilayer nanodiscs facilitate high-resolution structure determination of membrane proteins. J. Am. Chem. Soc. 2013, 135, 1919–1925. [Google Scholar] [CrossRef]
- Hernández-Rocamora, V.M.; Reija, B.; García, C.; Natale, P.; Alfonso, C.; Minton, A.P.; Zorrilla, S.; Rivas, G.; Vicente, M. Dynamic interaction of the Escherichia coli cell division ZipA and FtsZ proteins evidenced in nanodiscs. J. Biol. Chem. 2012, 287, 30097–30104. [Google Scholar] [CrossRef] [PubMed]
- Dörr, J.M.; Koorengevel, M.C.; Schäfer, M.; Prokofyev, A.V.; Scheidelaar, S.; van der Cruijsen, E.A.; Dafforn, T.R.; Baldus, M.; Killian, J.A. Detergent-free isolation, characterization, and functional reconstitution of a tetrameric K+ channel: The power of native nanodiscs. Proc. Natl. Acad. Sci. USA 2014, 111, 18607–18612. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Jamshad, M.; Knowles, T.J.; Morrison, K.A.; Downing, R.; Cant, N.; Collins, R.; Koenderink, J.B.; Ford, R.C.; Overduin, M. Detergent-free purification of ABC (ATP-binding-cassette) transporters. Biochem. J. 2014, 461, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.D.; Herpers, B.; McKie, A.T.; Gledhill, S.; McDonnell, J.; van den Heuvel, M.; Davies, K.E.; Ponting, C.P. Stromal cell-derived receptor 2 and cytochrome b561 are functional ferric reductases. Biochim. Biophys. Acta 2003, 1651, 116–123. [Google Scholar] [CrossRef]
- Choi, J.; Masaratana, P.; Latunde-Dada, G.O.; Arno, M.; Simpson, R.J.; McKie, A.T. Duodenal reductase activity and spleen iron stores are reduced and erythropoiesis is abnormal in Dcytb knockout mice exposed to hypoxic conditions. J. Nutr. 2012, 142, 1929–1934. [Google Scholar] [CrossRef]
- Bacon, B.R.; Tavill, A.S. Role of the liver in normal iron metabolism. Semin. Liver Dis. 1984, 4, 181–192. [Google Scholar] [CrossRef]
- Sun, I.L.; Navas, P.; Crane, F.L.; Morre, D.; Löw, H. NADH diferric transferrin reductase in liver plasma membrane. J. Biol. Chem. 1987, 262, 15915–15921. [Google Scholar] [CrossRef]

| Sample | Dithionite-Reducible b561 Content (nmoles) | Total Protein (mg) | Specific Content (nmole/mg) | Yield (%) | Fold |
|---|---|---|---|---|---|
| DDM-solubilized fraction | 718.2 | 382.64 | 1.88 | 100 | 1 |
| Ni-NTA Sepharose fraction | 162.2 | 4.148 | 39.1 | 22.58 | 21.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abosharaf, H.A.; Sakamoto, Y.; Radwan, A.M.; Yuzu, K.; Fujimura, M.; Diab, T.; Mohamed, T.M.; Chatani, E.; Kimura, T.; Tsubaki, M. Functional Assembly of Caenorhabditis elegans Cytochrome b-2 (Cecytb-2) into Phospholipid Bilayer Nanodisc with Enhanced Iron Reductase Activity. Biomolecules 2021, 11, 96. https://doi.org/10.3390/biom11010096
Abosharaf HA, Sakamoto Y, Radwan AM, Yuzu K, Fujimura M, Diab T, Mohamed TM, Chatani E, Kimura T, Tsubaki M. Functional Assembly of Caenorhabditis elegans Cytochrome b-2 (Cecytb-2) into Phospholipid Bilayer Nanodisc with Enhanced Iron Reductase Activity. Biomolecules. 2021; 11(1):96. https://doi.org/10.3390/biom11010096
Chicago/Turabian StyleAbosharaf, Hamed A., Yuki Sakamoto, Aliaa M. Radwan, Keisuke Yuzu, Mika Fujimura, Thoria Diab, Tarek M. Mohamed, Eri Chatani, Tetsunari Kimura, and Motonari Tsubaki. 2021. "Functional Assembly of Caenorhabditis elegans Cytochrome b-2 (Cecytb-2) into Phospholipid Bilayer Nanodisc with Enhanced Iron Reductase Activity" Biomolecules 11, no. 1: 96. https://doi.org/10.3390/biom11010096
APA StyleAbosharaf, H. A., Sakamoto, Y., Radwan, A. M., Yuzu, K., Fujimura, M., Diab, T., Mohamed, T. M., Chatani, E., Kimura, T., & Tsubaki, M. (2021). Functional Assembly of Caenorhabditis elegans Cytochrome b-2 (Cecytb-2) into Phospholipid Bilayer Nanodisc with Enhanced Iron Reductase Activity. Biomolecules, 11(1), 96. https://doi.org/10.3390/biom11010096







