Insulin/IGF Axis in Breast Cancer: Clinical Evidence and Translational Insights
Abstract
1. Introduction
2. Host Metabolism and Breast Cancer Risk
3. Host Metabolism and Breast Cancer Prognosis
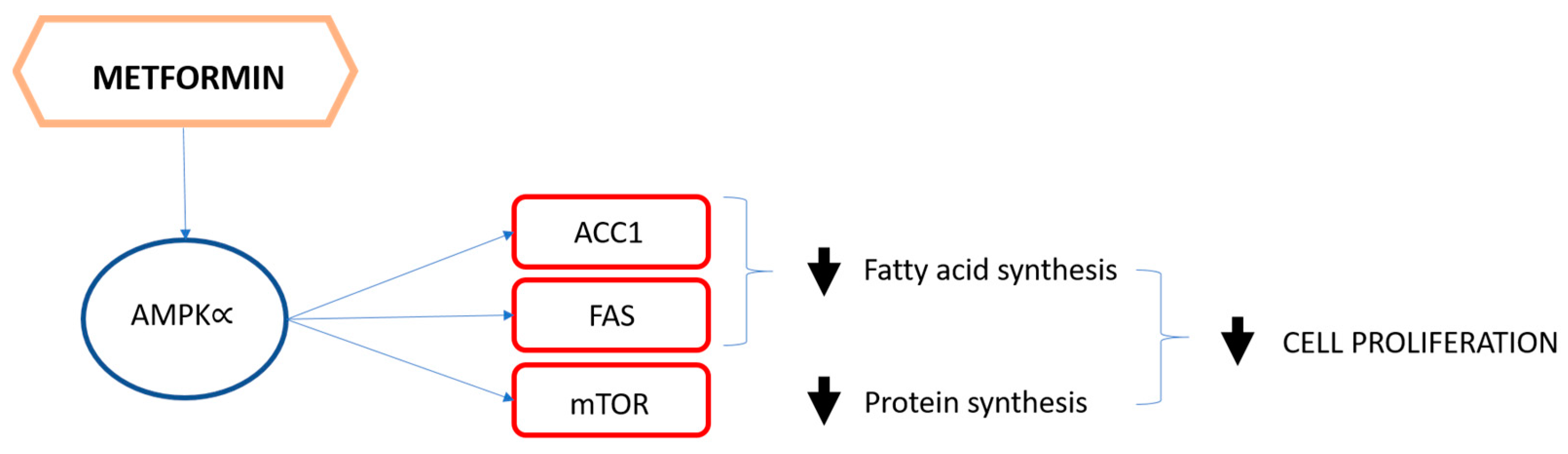
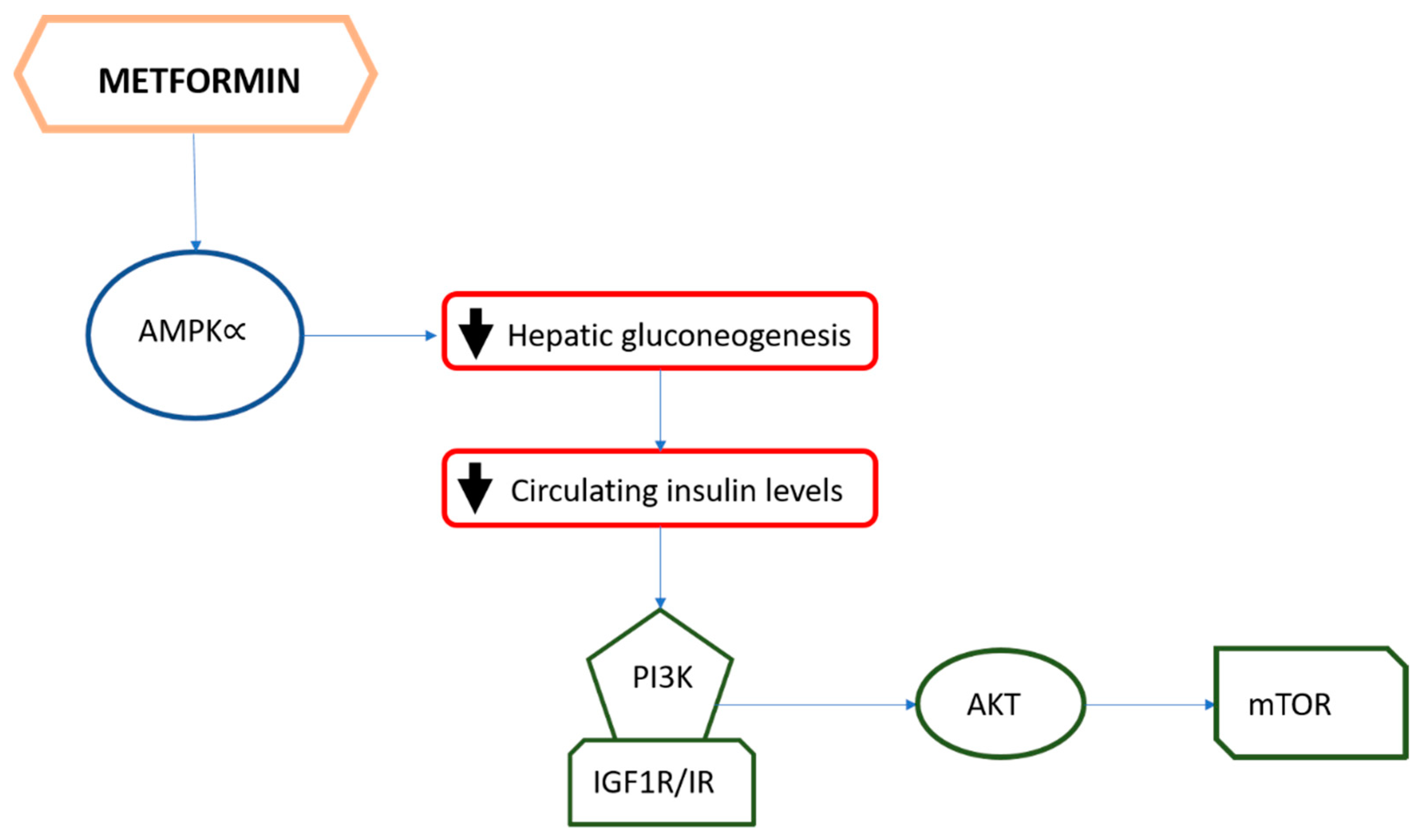
4. The Role of Metformin
Metformin and Breast Cancer: Clinical Evidence from Prospective Randomized Trials
5. Translational Insights
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef]
- Belfiore, A.; Frasca, F.; Pandini, G.; Sciacca, L.; Vigneri, R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009, 30, 586–623. [Google Scholar] [CrossRef]
- Ward, C.W.; Lawrence, M.C. Ligand-induced activation of the insulin receptor: A multi-step process involving structural changes in both the ligand and the receptor. BioEssays 2009, 31, 422–434. [Google Scholar] [CrossRef]
- WHO. World Health Organization, Global Cancer Observatory Online Data. Available online: https://gco.iarc.fr/ (accessed on 19 January 2021).
- Gaudet, M.M.; Press, M.F.; Haile, R.W.; Lynch, C.F.; Glaser, S.L.; Schildkraut, J.; Gammon, M.D.; Thompson, W.D.; Bernstein, J.L. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res. Treat. 2011, 130, 587–597. [Google Scholar] [CrossRef]
- Larsson, S.C.; Mantzoros, C.S.; Wolk, A. Diabetes mellitus and risk of breast cancer: A meta-analysis. Int. J. Cancer 2007, 121, 856–862. [Google Scholar] [CrossRef]
- Papa, V.; Pezzino, V.; Costantino, A.; Belfiore, A.; Giuffrida, D.; Frittitta, L.; Vannelli, G.B.; Brand, R.; Goldfine, I.D.; Vigneri, R. Elevated insulin receptor content in human breast cancer. J. Clin. Investig. 1990, 86, 1503–1510. [Google Scholar] [CrossRef]
- Yakar, S.; Pennisi, P.; Zhao, H.; Zhang, Y.; LeLeroith, D. Circulating IGF-1 and Its Role in Cancer: Lessons from the IGF-1 Gene Deletion (LID) Mouse. Novartis Found. Symp. 2004, 262, 3–18. [Google Scholar] [CrossRef]
- Yakar, S.; Leroith, D.; Brodt, P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: Lessons from animal models. Cytokine Growth Factor Rev. 2005, 16, 407–420. [Google Scholar] [CrossRef]
- Nagle, A.M.; Levine, K.M.; Tasdemir, N.; Scott, J.A.; Burlbaugh, K.; Kehm, J.W.; Katz, T.A.; Boone, D.N.; Jacobsen, B.M.; Atkinson, J.M.; et al. Loss of E-cadherin Enhances IGF1–IGF1R pathway activation and sensitizes breast cancers to anti-IGF1R/InsR inhibitors. Clin. Cancer Res. 2018, 24, 5165–5177. [Google Scholar] [CrossRef]
- Teo, K.; Gómez-Cuadrado, L.; Tenhagen, M.; Byron, A.; Rätze, M.; van Amersfoort, M.; Renes, J.; Strengman, E.; Mandoli, A.; Singh, A.A.; et al. E-cadherin loss induces targetable autocrine activation of growth factor signalling in lobular breast cancer. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Buck, E.; Eyzaguirre, A.; Rosenfeld-Franklin, M.; Thomson, S.; Mulvihill, M.; Barr, S.; Brown, E.; O’Connor, M.; Yao, Y.; Pachter, J.; et al. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008, 68, 8322–8332. [Google Scholar] [CrossRef]
- Luey, B.C.; May, F.E. Insulin-like growth factors are essential to prevent anoikis in oestrogen-responsive breast cancer cells: Importance of the type I IGF receptor and PI3-kinase/Akt pathway. Mol. Cancer 2016, 15, 1–15. [Google Scholar] [CrossRef]
- Menyhart, O.; Santarpia, L.; Gyorffy, B. A Comprehensive outline of trastuzumab resistance biomarkers in HER2 Overexpressing breast cancer. Curr. Cancer Drug Targ. 2015, 15, 665–683. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, R.; Rao, Y.; Du, Y.-K.; Kalembo, F.W. Risk factors for breast cancer and expression of insulin-like growth factor-2 (IGF-2) in women with breast cancer in Wuhan City, China. PLoS ONE 2012, 7, e36497. [Google Scholar] [CrossRef]
- Endogenous Endogenous Hormones and Breast Cancer Collaborative Group; Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.W. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010, 11, 530–542. [Google Scholar] [CrossRef]
- The Premenopausal Breast Cancer Collaborative Group; Schoemaker, M.J.; Nichols, H.B.; Wright, L.B.; Brook, M.N.; Jones, M.E.; O’Brien, K.M.; Adami, H.-O.; Baglietto, L.; Bernstein, L.; et al. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018, 4, e181771. [Google Scholar] [CrossRef]
- Suzuki, R.; Saji, S.; Toi, M. Impact of body mass index on breast cancer in accordance with the life-stage of women. Front. Oncol. 2012, 2, 123. [Google Scholar] [CrossRef]
- Biglia, N.; Peano, E.; Sgandurra, P.; Moggio, G.; Pecchio, S.; Maggiorotto, F.; Sismondi, P. Body mass index (BMI) and breast cancer: Impact on tumor histopatologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol. Endocrinol. 2012, 29, 263–267. [Google Scholar] [CrossRef]
- Kabat, G.C.; Kim, M.Y.; Lee, J.S.; Ho, G.Y.; Going, S.B.; Beebe-Dimmer, J.; Manson, J.E.; Chlebowski, R.T.; Rohan, T.E. Metabolic obesity phenotypes and risk of breast cancer in postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1730–1735. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Arthur, R.; Manson, J.E.; Chlebowski, R.T.; Kroenke, C.H.; Peterson, L.; Cheng, T.-Y.D.; Feliciano, E.C.; Lane, D.; Luo, J.; et al. Association of body fat and risk of breast cancer in postmenopausal women with normal body mass index: A Secondary analysis of a randomized clinical trial and observational study. JAMA Oncol. 2019, 5, 155–163. [Google Scholar] [CrossRef]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Rohan, T.E.; Manson, J.E.; Li, J.; Ho, G.Y.F.; Xue, X.; Anderson, G.L.; et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2008, 101, 48–60. [Google Scholar] [CrossRef]
- Colditz, G.A.; Manson, J.E.; Hankinson, S.E. The nurses’ health study: 20-year contribution to the understanding of health among women. J. Women’s Health 1997, 6, 49–62. [Google Scholar] [CrossRef]
- Schapira, D.V.; Kumar, N.B.; Lyman, G.H.; Cox, C.E. Abdominal obesity and breast cancer risk. Ann. Intern. Med. 1990, 112, 182–186. [Google Scholar] [CrossRef]
- Kang, S.M.; Yoon, J.W.; Ahn, H.Y.; Kim, S.Y.; Lee, K.H.; Shin, H.; Choi, S.H.; Park, K.S.; Jang, H.C.; Lim, S. Android fat depot is more closely associated with metabolic syndrome than abdominal visceral fat in elderly people. PLoS ONE 2011, 6, e27694. [Google Scholar] [CrossRef]
- Iqbal, J.; Thike, A.A.; Cheok, P.Y.; Tse, G.M.-K.; Tan, P.H. Insulin growth factor receptor-1 expression and loss of PTEN protein predict early recurrence in triple-negative breast cancer. Histopathology 2012, 61, 652–659. [Google Scholar] [CrossRef]
- Millikan, R.C.; Newman, B.; Tse, C.-K.; Moorman, P.G.; Conway, K.; Smith, L.V.; Labbok, M.H.; Geradts, J.; Bensen, J.T.; Jackson, S.; et al. Epidemiology of basal-like breast cancer. Breast Cancer Res. Treat. 2007, 109, 123–139. [Google Scholar] [CrossRef]
- Berstad, P.; Coates, R.J.; Bernstein, L.; Folger, S.G.; Malone, K.E.; Marchbanks, P.A.; Weiss, L.K.; Liff, J.M.; McDonald, J.A.; Strom, B.L.; et al. A case-control study of body mass index and breast cancer risk in white and african-american women. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1532–1544. [Google Scholar] [CrossRef]
- Yang, X.R.; Chang-Claude, J.; Goode, E.L.; Couch, F.J.; Nevanlinna, H.; Milne, R.L.; Gaudet, M.; Schmidt, M.K.; Broeks, A.; Cox, A.; et al. Associations of breast cancer risk factors with tumor subtypes: A pooled analysis from the breast cancer association consortium studies. J. Natl. Cancer Inst. 2010, 103, 250–263. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Chen, W.Y.; Rosner, B.; Holmes, M.D. Weight, weight gain, and survival after breast cancer diagnosis. J. Clin. Oncol. 2005, 23, 1370–1378. [Google Scholar] [CrossRef]
- Sica, A.; Guarneri, V.; Gennari, A. Myelopoiesis, metabolism and therapy: A crucial crossroads in cancer progression. Cell Stress 2019, 3, 284–294. [Google Scholar] [CrossRef]
- Liu, W.; Lu, X.; Shi, P.; Yang, G.; Zhou, Z.; Li, W.; Mao, X.; Jiang, D.; Chen, C. TNF-α increases breast cancer stem-like cells through up-regulating TAZ expression via the non-canonical NF-κB pathway. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Dethlefsen, C.; Højfeldt, G.; Hojman, P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res. Treat. 2013, 138, 657–664. [Google Scholar] [CrossRef]
- Alan, Ö.; Telli, T.A.; Aktas, B.; Koca, S.; Ökten, I.N.; Hasanov, R.; Basoglu, T.; Arikan, R.; Demircan, N.C.; Ercelep, O.; et al. Is insulin resistance a predictor for complete response in breast cancer patients who underwent neoadjuvant treatment? World J. Surg. Oncol. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Gennari, A.; Amadori, D.; Scarpi, E.; Farolfi, A.; Paradiso, A.; Mangia, A.; Biglia, N.; Gianni, L.; Tienghi, A.; Rocca, A.; et al. Impact of body mass index (BMI) on the prognosis of high-risk early breast cancer (EBC) patients treated with adjuvant chemotherapy. Breast Cancer Res. Treat. 2016, 159, 79–86. [Google Scholar] [CrossRef]
- Strong, A.L.; Ohlstein, J.F.; Biagas, B.A.; Rhodes, L.V.; Pei, D.T.; Tucker, H.A.; Llamas, C.; Bowles, A.C.; Dutreil, M.F.; Zhang, S.; et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015, 17, 1–16. [Google Scholar] [CrossRef]
- Gershuni, V.M.; Ahima, R.S.; Tchou, J. Obesity and breast cancer: A complex relationship. Curr. Surg. Rep. 2016, 4, 14. [Google Scholar] [CrossRef][Green Version]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Rosenblatt, D.N.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Duggan, C.; Irwin, M.L.; Xiao, L.; Henderson, K.D.; Smith, A.W.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Ballard-Barbash, R.; McTiernan, A. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J. Clin. Oncol. 2011, 29, 32–39. [Google Scholar] [CrossRef]
- Van den Brandt, P.A.; Spiegelman, D.; Yaun, S.-S.; Adami, H.-O.; Beeson, L.; Folsom, A.R.; Fraser, G.; Goldbohm, R.A.; Graham, S.; Kushi, L.H.; et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am. J. Epidemiol. 2000, 152, 514–527. [Google Scholar] [CrossRef]
- Whiteman, M.K.; Hillis, S.; Curtis, K.; McDonald, J.A.; Wingo, P.A.; Marchbanks, P.A. Body mass and mortality after breast cancer diagnosis. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2009–2014. [Google Scholar] [CrossRef]
- Nam, S.; Park, S.; Park, H.S.; Kim, S.; Kim, J.Y.; Kim, S.I. Association between insulin resistance and luminal B subtype breast Cancer in postmenopausal women. Medicine 2016, 95, e2825. [Google Scholar] [CrossRef] [PubMed]
- Davison, Z.; De Blacquière, G.E.; Westley, B.R.; May, F.E. Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: Implications for therapy. Neoplasia 2011, 13, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Maiti, B.; Kundranda, M.N.; Spiro, T.P.; Daw, H.A. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res. Treat. 2009, 121, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Yee, L.D.; Mortimer, J.E.; Natarajan, R.; Dietze, E.C.; Seewaldt, V.L. Metabolic health, insulin, and breast cancer: Why oncologists should care about insulin. Front. Endocrinol. 2020, 11, 58. [Google Scholar] [CrossRef]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical management of hyperglycaemia in type 2 diabetes mellitus: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2008, 52, 17–30. [Google Scholar] [CrossRef]
- Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; Nathan, D.M. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.-L. TSC2 Mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Gandini, S.; Puntoni, M.; Heckman-Stoddard, B.M.; Dunn, B.K.; Ford, L.; DeCensi, A.; Szabo, E. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer Prev. Res. 2014, 7, 867–885. [Google Scholar] [CrossRef]
- Heckman-Stoddard, B.M.; Gandini, S.; Puntoni, M.; Dunn, B.K.; DeCensi, A.; Szabo, E. Repurposing old drugs to chemoprevention: The case of metformin. Semin. Oncol. 2016, 43, 123–133. [Google Scholar] [CrossRef]
- Bonanni, B.; Puntoni, M.; Cazzaniga, M.; Pruneri, G.; Serrano, D.; Guerrieri-Gonzaga, A.; Gennari, A.; Trabacca, M.S.; Galimberti, V.; Veronesi, P.; et al. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J. Clin. Oncol. 2012, 30, 2593–2600. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Meric-Bernstam, F. Metformin: A therapeutic opportunity in breast cancer: Fig. 1. Clin. Cancer Res. 2010, 16, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.; Bonanni, B.; Guerrieri-Gonzaga, A.; DeCensi, A. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiol. Biomark. Prev. 2009, 18, 701–705. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cazzaniga, M.; DeCensi, A.; Pruneri, G.; Puntoni, M.; Bottiglieri, L.; Varricchio, C.; Guerrieri-Gonzaga, A.; Gentilini, O.D.; Pagani, G.; Dellorto, P.; et al. The effect of metformin on apoptosis in a breast cancer presurgical trial. Br. J. Cancer 2013, 109, 2792–2797. [Google Scholar] [CrossRef] [PubMed]
- Hadad, S.; Iwamoto, T.; Jordan, L.; Purdie, C.; Bray, S.; Baker, L.; Jellema, G.; Deharo, S.; Hardie, D.G.; Pusztai, L.; et al. Evidence for biological effects of metformin in operable breast cancer: A pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res. Treat. 2011, 128, 783–794. [Google Scholar] [CrossRef]
- Gennari, A.; Foca, F.; Zamarchi, R.; Rocca, A.; Amadori, D.; De Censi, A.; Bologna, A.; Cavanna, L.; Gianni, L.; Scaltriti, L.; et al. Insulin-like growth factor-1 receptor (IGF-1R) expression on circulating tumor cells (CTCs) and metastatic breast cancer outcome: Results from the TransMYME trial. Breast Cancer Res. Treat. 2020, 181, 61–68. [Google Scholar] [CrossRef]
- Bonora, E.; Kiechl, S.; Willeit, J.; Oberhollenzer, F.; Egger, G.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Muggeo, M. Prevalence of insulin resistance in metabolic disorders: The Bruneck Study. Diabetes 1998, 47, 1643–1649. [Google Scholar] [CrossRef]
- De Bono, J.S.; Attard, G.; Adjei, A.; Pollak, M.N.; Fong, P.C.; Haluska, P.; Roberts, L.; Melvin, C.; Repollet, M.; Chianese, D.; et al. Potential applications for circulating tumor cells expressing the insulin-like growth factor-I receptor. Clin. Cancer Res. 2007, 13, 3611–3616. [Google Scholar] [CrossRef]
- Pizon, M.; Zimon, D.S.; Pachmann, U.; Pachmann, K. Insulin-like growth factor receptor I (IGF-IR) and vascular endothelial growth factor receptor 2 (VEGFR-2) are expressed on the circulating epithelial tumor cells of breast cancer patients. PLoS ONE 2013, 8, e56836. [Google Scholar] [CrossRef]
- Spiliotaki, M.; Mavroudis, D.; Kokotsaki, M.; Vetsika, E.; Stoupis, I.; Matikas, A.; Kallergi, G.; Georgoulias, V.; Agelaki, S. Expression of insulin-like growth factor-1 receptor in circulating tumor cells of patients with breast cancer is associated with patient outcomes. Mol. Oncol. 2017, 12, 21–32. [Google Scholar] [CrossRef]
- Strauss, L.; Guarneri, V.; Gennari, A.; Sica, A. Implications of metabolism-driven myeloid dysfunctions in cancer therapy. Cell. Mol. Immunol. 2020, 1–13. [Google Scholar] [CrossRef]
| BC Characteristics | IGF Expression (%) |
|---|---|
| Histological subtype | |
| ER+ | 60 |
| TNBC | 20 |
| Tumor staging | |
| T1-T2 | 35 |
| T3-T4 | 65 |
| Lymph nodes metastasis | |
| Yes | 60 |
| No | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biello, F.; Platini, F.; D’Avanzo, F.; Cattrini, C.; Mennitto, A.; Genestroni, S.; Martini, V.; Marzullo, P.; Aimaretti, G.; Gennari, A. Insulin/IGF Axis in Breast Cancer: Clinical Evidence and Translational Insights. Biomolecules 2021, 11, 125. https://doi.org/10.3390/biom11010125
Biello F, Platini F, D’Avanzo F, Cattrini C, Mennitto A, Genestroni S, Martini V, Marzullo P, Aimaretti G, Gennari A. Insulin/IGF Axis in Breast Cancer: Clinical Evidence and Translational Insights. Biomolecules. 2021; 11(1):125. https://doi.org/10.3390/biom11010125
Chicago/Turabian StyleBiello, Federica, Francesca Platini, Francesca D’Avanzo, Carlo Cattrini, Alessia Mennitto, Silvia Genestroni, Veronica Martini, Paolo Marzullo, Gianluca Aimaretti, and Alessandra Gennari. 2021. "Insulin/IGF Axis in Breast Cancer: Clinical Evidence and Translational Insights" Biomolecules 11, no. 1: 125. https://doi.org/10.3390/biom11010125
APA StyleBiello, F., Platini, F., D’Avanzo, F., Cattrini, C., Mennitto, A., Genestroni, S., Martini, V., Marzullo, P., Aimaretti, G., & Gennari, A. (2021). Insulin/IGF Axis in Breast Cancer: Clinical Evidence and Translational Insights. Biomolecules, 11(1), 125. https://doi.org/10.3390/biom11010125







