In Vitro Anti-Inflammatory, Anti-Oxidant, and Cytotoxic Activities of Four Curcuma Species and the Isolation of Compounds from Curcuma aromatica Rhizome
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Extraction
2.4. Fractionation and Isolation
2.5. Characterization of Curcuma Extracts by UPLC-HRMS
2.6. DPPH Radical-Scavenging Activity Assay
2.7. ABTS Radical Cation Scavenging Assay
2.8. Cell Culture
2.9. MTT Assay
2.10. CytoTox-ONE™ Cytotoxicity Assay
2.11. Luciferase Assay
2.12. Data Analysis
3. Results and Discussion
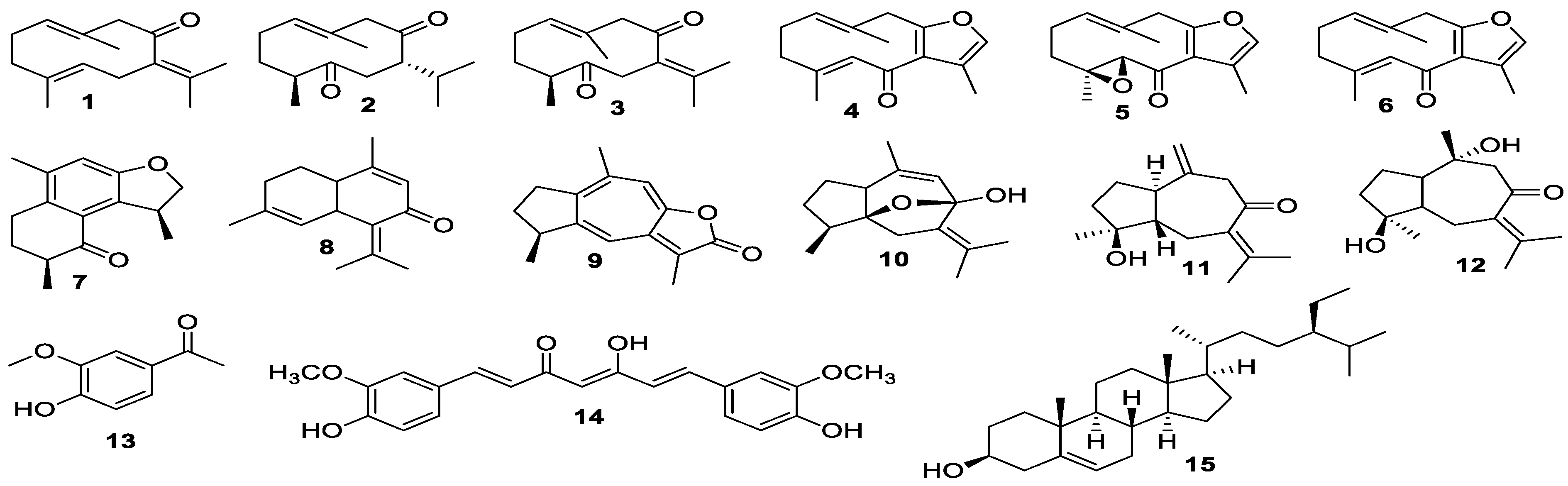
3.1. Isolation of Compounds
3.2. Characterization of Curcuma Extracts by UPLC-HRMS
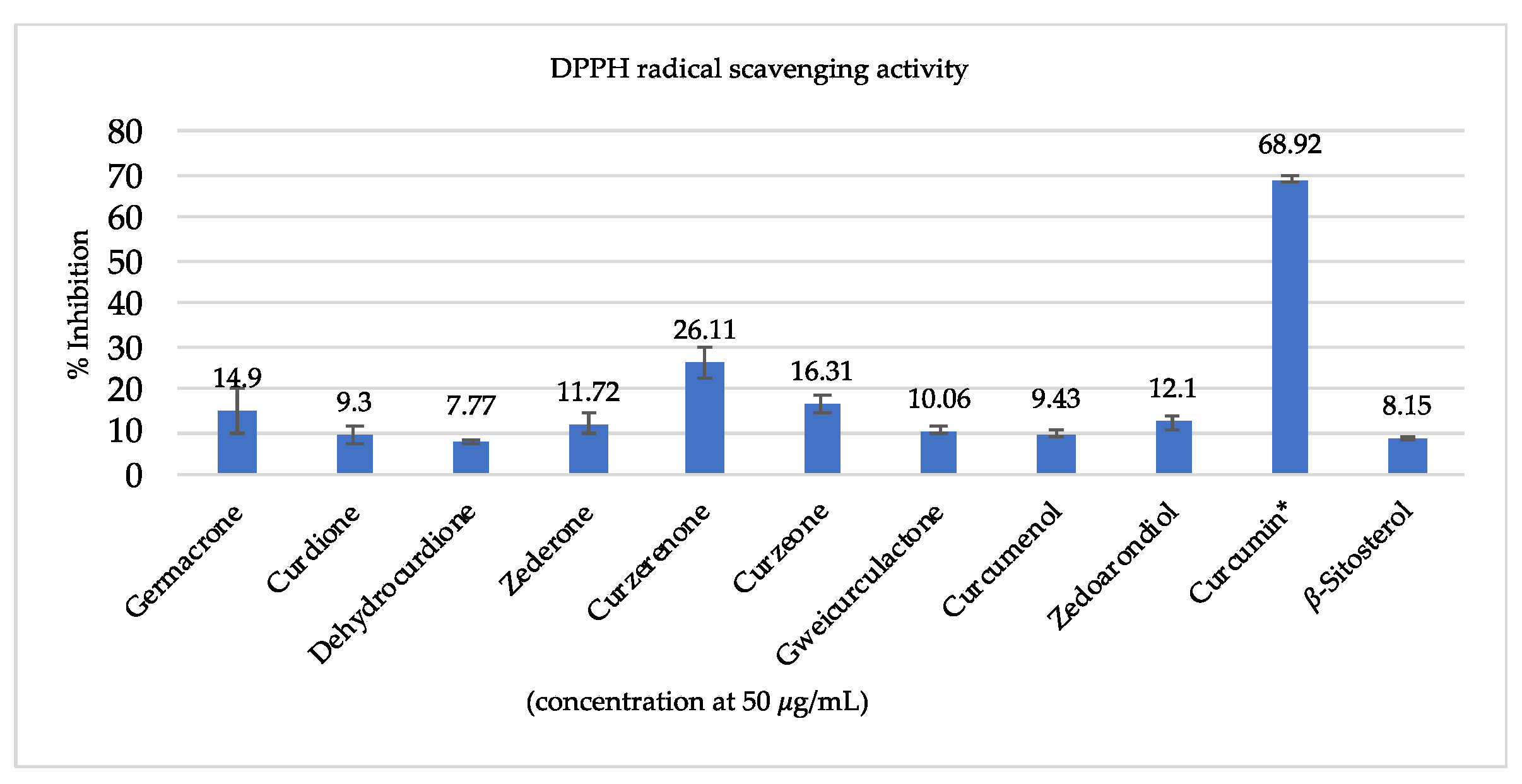
3.3. Antioxidant Activity
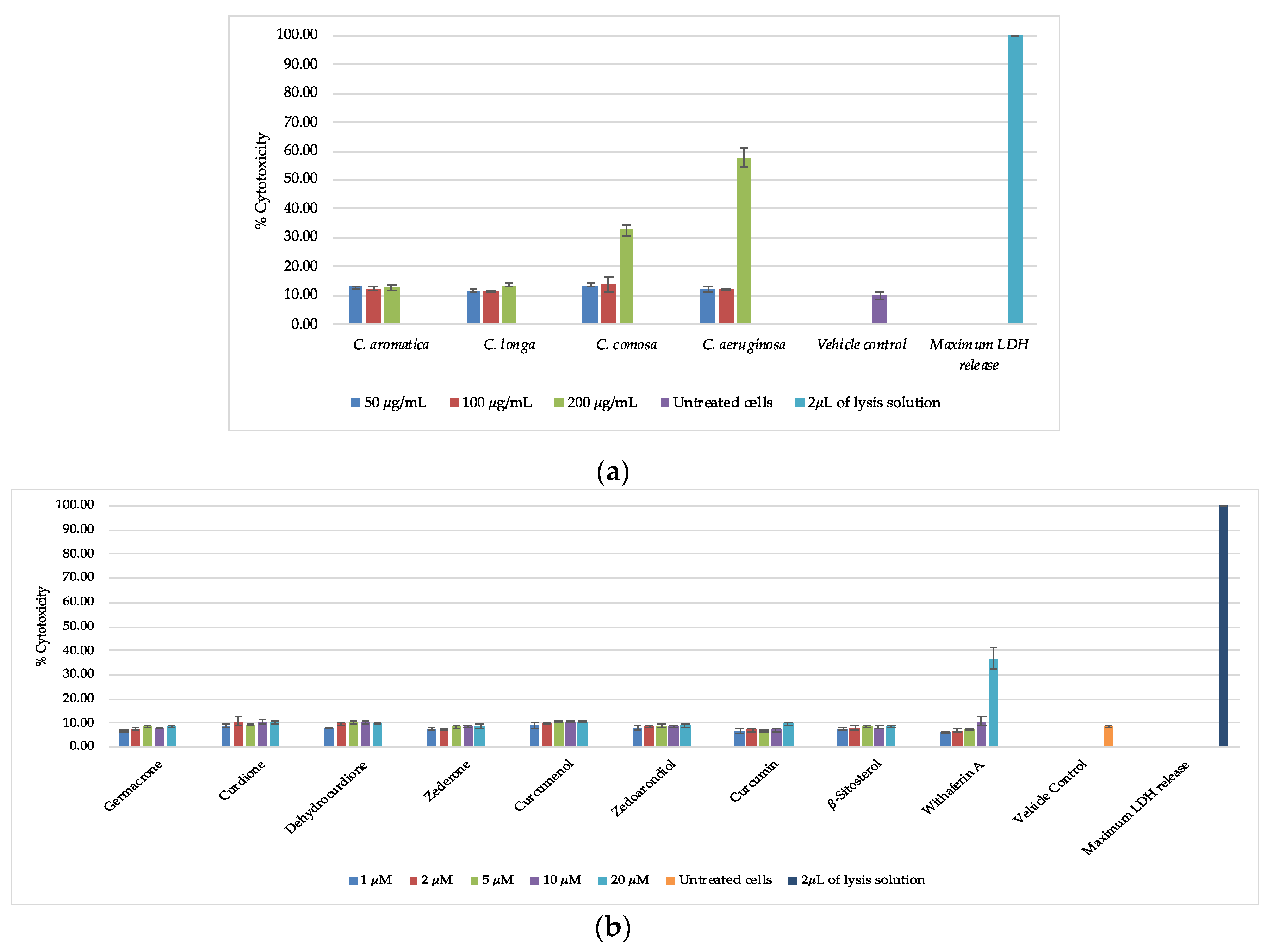
3.4. Cell Viability and Cytotoxicity
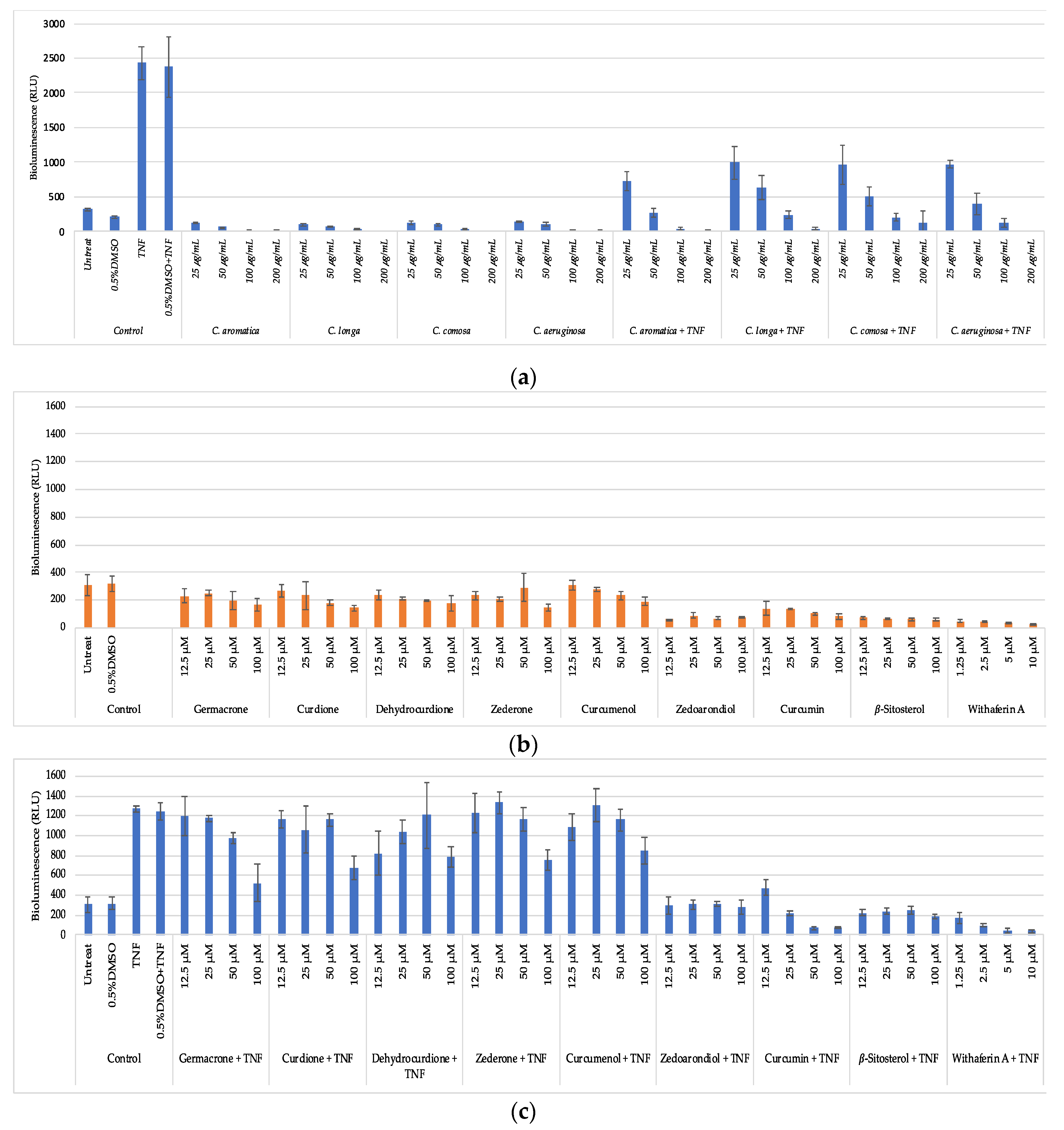
3.5. Anti-Inflammatory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Arya, J.S.; Maiti, K.K.; Gupta, S.S. Curcuma raktakanda induces apoptosis and suppresses migration in cancer cells: Role of reactive oxygen species. Biomolecules 2019, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Srivilai, J.; Rabgay, K.; Knorana, N.; Waranuch, N.; Nuengchamnong, N.; Wisuitiprot, W.; Chuprajob, T.; Changtam, C.; Suksamrarn, A.; Chavasiri, W.; et al. Anti-androgenic curcumin analogues as steroid 5-alpha reductase inhibitors. Med. Chem. Res. 2017, 26, 1550–1556. [Google Scholar] [CrossRef]
- Dong, S.; Luo, X.; Liu, Y.; Zhang, M.; Li, B.; Dai, W. Diarylheptanoids from the root of Curcuma aromatica and their antioxidative effects. Phytochem. Lett. 2018, 27, 148–153. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K.; Lim, K.K.; Joe, C.E.; Lim, T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Bamba, Y.; Yun, Y.S.; Kunugi, A.; Inoue, H. Compounds isolated from Curcuma aromatica Salisb. inhibit human P450 enzymes. J. Nat. Med. 2011, 65, 583–587. [Google Scholar] [CrossRef]
- Liu, B.; Gao, Y.Q.; Wang, X.M.; Wang, Y.C.; Fu, L.Q. Germacrone inhibits the proliferation of glioma cells by promoting apoptosis and inducing cell cycle arrest. Mol. Med. Rep. 2014, 10, 1046–1050. [Google Scholar] [CrossRef]
- Keeratinijakal, V.; Kongkiatpaiboon, S. Distribution of phytoestrogenic diarylheptanoids and sesquiterpenoids components in Curcuma comosa rhizomes and its related species. Rev. Bras. Farm. 2017, 27, 290–296. [Google Scholar] [CrossRef]
- Qu, Y.; Xu, F.; Nakamura, S.; Matsuda, H.; Pongpiriyadacha, Y.; Wu, L.; Yoshikawa, M. Sesquiterpenes from Curcuma comosa. J. Nat. Med. 2009, 63, 102–104. [Google Scholar] [CrossRef]
- Suksamrarn, A.; Ponglikitmongkol, M.; Wongkrajang, K.; Chindaduang, A.; Kittidanairak, S.; Jankam, A.; Yingyongnarongkul, B.; Kittipanumat, N.; Chokchaisiri, R.; Khetkam, P.; et al. Diarylheptanoids, new phytoestrogens from the rhizomes of Curcuma comosa: Isolation, chemical modification and estrogenic evaluation. Bioorg. Med. Chem. 2008, 16, 6891–6902. [Google Scholar] [CrossRef]
- Dong, S.; Li, B.; Dai, W.; Wang, D.; Qin, Y.; Zhang, M. Sesqui- and diterpenoids from the radix of Curcuma aromatica. J. Nat. Prod. 2017, 80, 3093–3102. [Google Scholar] [CrossRef]
- Ramsewak, R.S.; Dewitt, D.L.; Nair, M.G. Cytotoxicity, antioxidant and anti-inflammatory activities of Curcumins I-III from Curcuma longa. J. Phytomed. 2000, 7, 303–308. [Google Scholar] [CrossRef]
- Booker, A.; Frommenwiler, D.; Johnston, D.; Umealajekwu, C.; Reich, E.; Heinrich, M. Chemical variability along the value chains of turmeric (Curcuma longa): A comparison of nuclear magnetic resonance spectroscopy and high performance thin layer chromatography. J. Ethnopharmacol. 2014, 152, 292–301. [Google Scholar] [CrossRef]
- Agnihotri, V.K.; Thakur, S.; Pathania, V.; Chand, G. A new dihomosesquiterpene, termioic acid A, from Curcuma aromatica. Chem. Nat. Compd. 2014, 50, 665–668. [Google Scholar] [CrossRef]
- Keeratinijakal, V.; Kladmook, M.; Laosatit, K. Identification and characterization of Curcuma comosa Roxb., phytoestrogens-producing plant, using AFLP markers and morphological characteristics. J. Med. Plants Res. 2010, 4, 2651–2657. [Google Scholar]
- Jurgens, T.M.; Frazier, E.G.; Schaeffer, J.M.; Jones, T.E.; Zink, D.L.; Borris, R.P. Novel nematocidal agents from Curcuma comosa. J. Nat. Prod. 1994, 57, 230–235. [Google Scholar] [CrossRef]
- Jariyawat, S.; Thammapratip, T.; Suksen, K.; Wanitchakool, P.; Nateewattana, J.; Chairoungdua, A.; Suksamrarn, A.; Piyachaturawat, P. Induction of apoptosis in murine leukemia by diarylheptanoids from Curcuma comosa Roxb. Cell Biol. Toxicol. 2011, 27, 413–423. [Google Scholar] [CrossRef]
- Simoh, S.; Zainal, A. Chemical profiling of Curcuma aeruginosa Roxb. rhizome using different techniques of solvent extraction. Asian Pac. J. Trop. Biomed. 2015, 5, 412–417. [Google Scholar] [CrossRef]
- Waras, N.; Nurul, K.; Muhamad, S.; Maria, B.; Ardyani, I.D.A.A.C. Phytochemical screening, antioxidant and cytotoxic activities in extracts of different rhizome parts from Curcuma aeruginosa Roxb. Int. J. Res. Ayurveda Pharm. 2015, 6, 634–637. [Google Scholar] [CrossRef]
- Jose, S.; Thamas, T.D. Comparative phytochemical and anti-bacterial studies of two indigenous medicinal plants Curcuma caesia Roxb. and Curcuma aeruginosa Roxb. Int. J. Green Pharm. 2014, 8, 65–71. [Google Scholar]
- Takano, I.; Yasuda, I.; Takeya, K.; Itokawa, H. Guaiane sesquiterpene lactones from Curcuma aeruginosa. Phytochemistry 1995, 40, 1197–1200. [Google Scholar] [CrossRef]
- Li, S.; Yuan, W.; Deng, G.; Wang, P.; Yang, P.; Aggarwal, B.B. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharm. Crop. 2011, 2, 28–54. [Google Scholar] [CrossRef]
- Priya, R.; Prathapha, A.; Raghu, K.G.; Menon, A.N. Chemical composition and in vitro antioxidative potential of essential oil isolated from Curcuma longa L. leaves. Asian Pac. J. Trop. Biomed. 2012, S695–S699. [Google Scholar] [CrossRef]
- Gounder, D.K.; Lingamallu, J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, fried and cured turmeric (Curcuma longa) rhizomes. Ind. Crop. Prod. 2012, 38, 124–131. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Sapodilla seed coat as a multifunctional ingredient for cosmetic applications. Process. Biochem. 2011, 46, 2215–2218. [Google Scholar] [CrossRef]
- Firman, K.; Kinoshita, T.; Itai, A.; Sankawa, U. Terpenoids from Curcuma heyneana. Phytochemistry 1988, 27, 3887–3892. [Google Scholar] [CrossRef]
- Harimaya, K.; Gao, J.F.; Ohkura, T.; Kawamata, T.; Iitaka, Y.; Guo, Y.T.; Inayama, S. A series of sesquiterpenes with a 7α-isopropyl side chain and related compounds isolated from Curcuma wenyujin. Chem. Pharm. Bull. 1991, 39, 834–853. [Google Scholar] [CrossRef]
- Hikino, H.; Konno, C.; Agatsuma, K.; Takemoto, T.; Horibe, I.; Tori, K.; Ueyama, M.; Takeda, K. Sesquiterpenoids part XLVII structure configuration conformation and thermal rearrangement of furanodienone, isofuranodienone, curzerenone, epicuraerenone, and pyrocurzerenone, sesquiterpenoids of Curcuma zedoaria. J. Chem. Soc. 1975, 5, 401–524. [Google Scholar]
- Shibuya, H.; Hamamoto, Y.; Cai, Y.; Kitagawa, I. A reinvestigation of the structure of zederone, a furanogermacrane-type sesquiterpene from zedoary. Chem. Pharm. Bull. 1987, 35, 924. [Google Scholar] [CrossRef]
- Shiobara, Y.; Asakawa, Y.; Kodama, M.; Takemoto, T. Zedoarol, 13-hydroxygermacrone and curzeone, three sesquiterpenoids from Curcuma zedoaria. Phytochemistry 1986, 6, 1351–1353. [Google Scholar] [CrossRef]
- Xu, F.; Nakamura, S.; Qu, Y.; Matsuda, H.; Pongpiriyadacha, Y.; Wu, L.; Yoshikawa, M. Structures of new sesquiterpenes form Curcuma comosa. Chem. Pharm. Bull. 2008, 56, 1710–1716. [Google Scholar] [CrossRef]
- Hamdi, O.A.A.; Ye, L.J.; Kamarudin, M.N.A.; Hazni, H.; Paydar, M.; Looi, C.Y.; Shilpi, J.A.; Kadir, H.A.; Awang, K. Neuroprotective and antioxidant constituents from Curcuma zedoaria rhizomes. Rec. Nat. Prod. 2015, 9, 349–355. [Google Scholar]
- Kuroyanagi, M.; Ueno, A.; Koyama, K.; Natori, S. Structures of sesquiterpenes of Curcuma aromatica Salisb. II. Studies on minor sesquiterpenes. Chem. Pharm. Bull. 1990, 38, 55–58. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Ueno, A.; Ujiie, K.; Sato, S. Structures of sesquiterpenes from Curcuma aromatica Salisb. Chem. Pharm. Bull. 1987, 35, 53–59. [Google Scholar] [CrossRef]
- Bandyopadhyay, B.; Banik, B.K. Bismuth nitrate-induced microwave-assisted expeditious synthesis of vanillin from curcumin. Org. Med. Chem. Lett. 2012, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Payton, F.; Sandusky, P.; Alworth, W.L. NMR study of the solution structure of curcumin. J. Nat. Prod. 2007, 70, 143–146. [Google Scholar] [CrossRef]
- Pierre, L.L.; Moses, M.N. Isolation and characterization of stigmasterol and β-sitosterol from Odontonema strictum (Acanthaceae). J. Innov. Pharm. Biol. Sci. 2015, 2, 88–95. [Google Scholar]
- Chirumamilla, C.S.; Palagani, A.; Kamaraj, B.; Declerck, K.; Verbeek, M.W.C.; Oksana, R.; Bosscher, K.D.; Bougarne, N.; Ruttens, B.; Gevaert, K.; et al. Selective glucocorticoid receptor properties of GSK886 analogs with cysteine reactive warheads. Front. Immunol. 2017, 8, 1324. [Google Scholar] [CrossRef]
- Sun, W.; Wang, S.; Zhao, W.; Wu, C.; Guo, S.; Hongwei, G.; Hongxun, T.; Lu, J.J.; Wang, Y.; Chen, X.; et al. Chemical constituents and biological research on plants in the genus Curcuma. Food Sci. Nutr. 2016, 57, 1451–1523. [Google Scholar]
- Kannamangalam, U.; Varakumar, S.; Singhal, R.S. A comparative account of extraction of oleoresin from Curcuma aromatica Salisb by solvent and supercritical carbon dioxide: Characterization and bioactivities. J. Food Sci. Technol. 2019, 116, 108564. [Google Scholar]
- Hassannia, B.; Logie, E.; Vandenabeele, P.; Berghe, T.V. Withaferin A: From ayurvedic folk medicine to preclinical anti-cancer drug. Biochem. Pharmacol. 2020, 173, 113602. [Google Scholar] [CrossRef]
- Kaileh, M.; Berghe, W.V.; Heyerick, A.; Horion, J.; Piette, J.; Libert, C.; Keukeleire, D.D.; Essawi, T.; Haegeman, G. Withaferin A strongly elicits lkB kinase β hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 2006, 282, 4253–4264. [Google Scholar] [CrossRef] [PubMed]

| ESI+ | ESI− | Present in Extract | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Mol. Formula | RT (min) | Measured m/z | Ion | Calculated m/z | Δ (ppm) | MS fragments | Measured m/z | Ion | Calculated m/z | Δ (ppm) | MS fragments | C. aromatica | C. aeruginosa | C. comosa | C. longa |
| Germacrone (1) | C15H22O | 13.8 | 219.1751 | [M + H]+ | 219.1749 | 0.91 | n.d. | x | x | x | x | |||||
| Curdione (2) | C15H24O2 | 11.8 | 237.1858 | [M + H]+ | 237.1855 | 1.26 | n.d. | x | x | x | x | |||||
| Dehydrocurdione (3) | C15H22O2 | 10.9 | 235.1703 | [M + H]+ | 235.1698 | 2.13 | n.d. | x | x | x | x | |||||
| Zederone (5) | C15H18O3 | 11.2 | 247.1339 | [M + H]+ | 247.1334 | 2.02 | 245.1180 | [M − H]− | 245.1178 | 0.82 | x | x | x | x | ||
| Curcumenol (6) | C15H22O2 | 11.1 | 235.1701 | [M + H]+ | 235.1698 | 1.28 | 217.1593; 199.1486; 189.1642; 177.1277 | n.d. | x | x | x | |||||
| Curcumin (13) | C21H20O6 | 11.1 | 369.1345 | [M + H]+ | 369.1338 | 1.90 | 285.1129; 245.1814; 175.0756 | 367.1181 | [M − H]− | 367.1182 | −0.27 | 217.0504, 173.0608 | x | x | ||
| Sample | Antioxidant (IC50, μg/mL) | |
|---|---|---|
| DPPH | ABTS | |
| C. aromatica | 102.4 ± 1.9 | 127.0 ± 1.9 |
| C. longa | 134.9 ± 1.5 | 170.8 ± 1.6 |
| C. comosa | 137.7 ± 5.2 | 171.9 ± 1.9 |
| C. aeruginosa | 187.4 ± 22.1 | 217.9 ± 1.8 |
| Ascorbic acid | 1.80 ± 0.01 | 5.2 ± 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintatum, A.; Maneerat, W.; Logie, E.; Tuenter, E.; Sakavitsi, M.E.; Pieters, L.; Berghe, W.V.; Sripisut, T.; Deachathai, S.; Laphookhieo, S. In Vitro Anti-Inflammatory, Anti-Oxidant, and Cytotoxic Activities of Four Curcuma Species and the Isolation of Compounds from Curcuma aromatica Rhizome. Biomolecules 2020, 10, 799. https://doi.org/10.3390/biom10050799
Pintatum A, Maneerat W, Logie E, Tuenter E, Sakavitsi ME, Pieters L, Berghe WV, Sripisut T, Deachathai S, Laphookhieo S. In Vitro Anti-Inflammatory, Anti-Oxidant, and Cytotoxic Activities of Four Curcuma Species and the Isolation of Compounds from Curcuma aromatica Rhizome. Biomolecules. 2020; 10(5):799. https://doi.org/10.3390/biom10050799
Chicago/Turabian StylePintatum, Aknarin, Wisanu Maneerat, Emilie Logie, Emmy Tuenter, Maria E. Sakavitsi, Luc Pieters, Wim Vanden Berghe, Tawanun Sripisut, Suwanna Deachathai, and Surat Laphookhieo. 2020. "In Vitro Anti-Inflammatory, Anti-Oxidant, and Cytotoxic Activities of Four Curcuma Species and the Isolation of Compounds from Curcuma aromatica Rhizome" Biomolecules 10, no. 5: 799. https://doi.org/10.3390/biom10050799
APA StylePintatum, A., Maneerat, W., Logie, E., Tuenter, E., Sakavitsi, M. E., Pieters, L., Berghe, W. V., Sripisut, T., Deachathai, S., & Laphookhieo, S. (2020). In Vitro Anti-Inflammatory, Anti-Oxidant, and Cytotoxic Activities of Four Curcuma Species and the Isolation of Compounds from Curcuma aromatica Rhizome. Biomolecules, 10(5), 799. https://doi.org/10.3390/biom10050799







