Low Concentration of Withaferin a Inhibits Oxidative Stress-Mediated Migration and Invasion in Oral Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
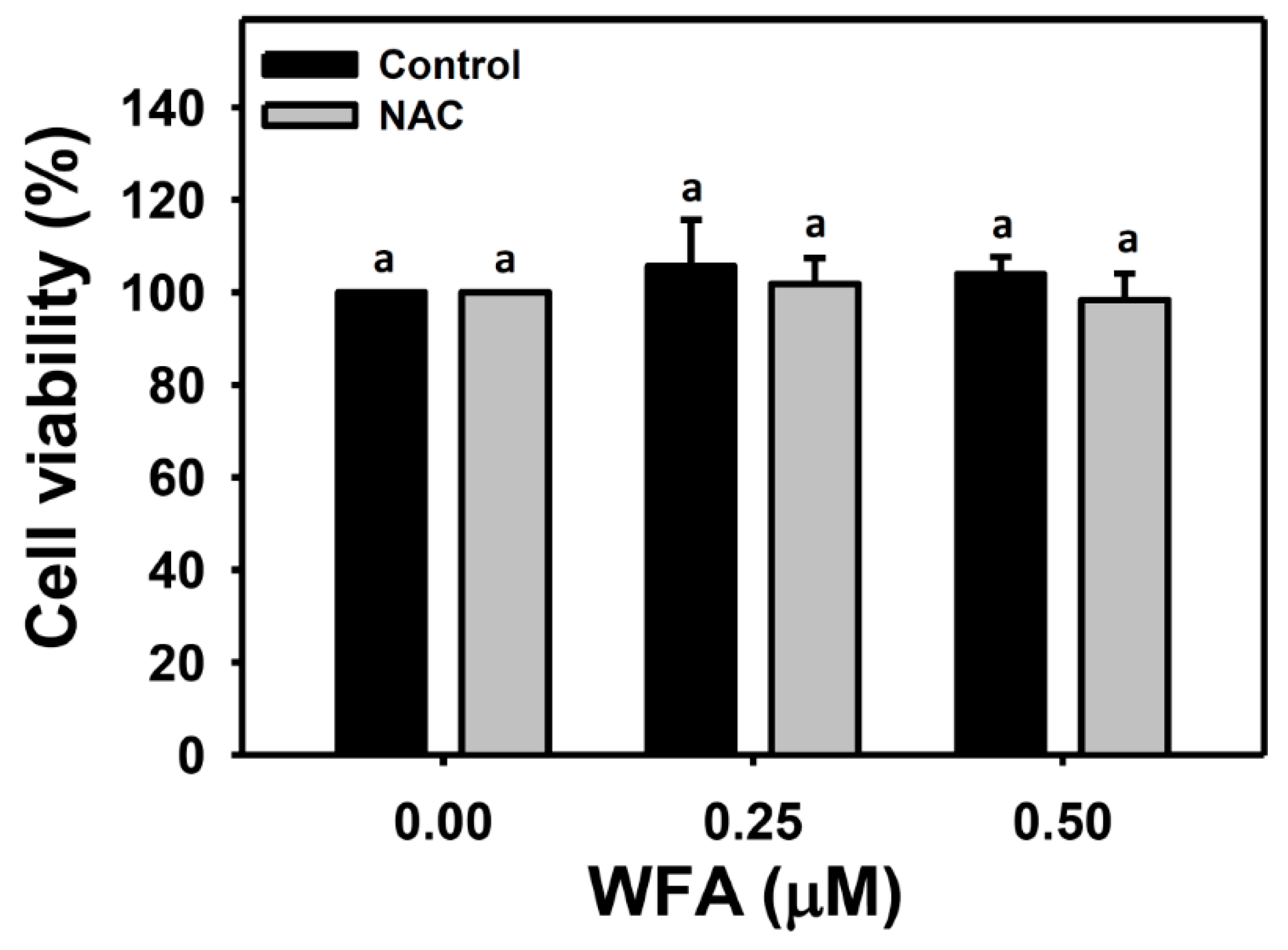
2.2. Cell Viability
2.3. ROS Flow Cytometry
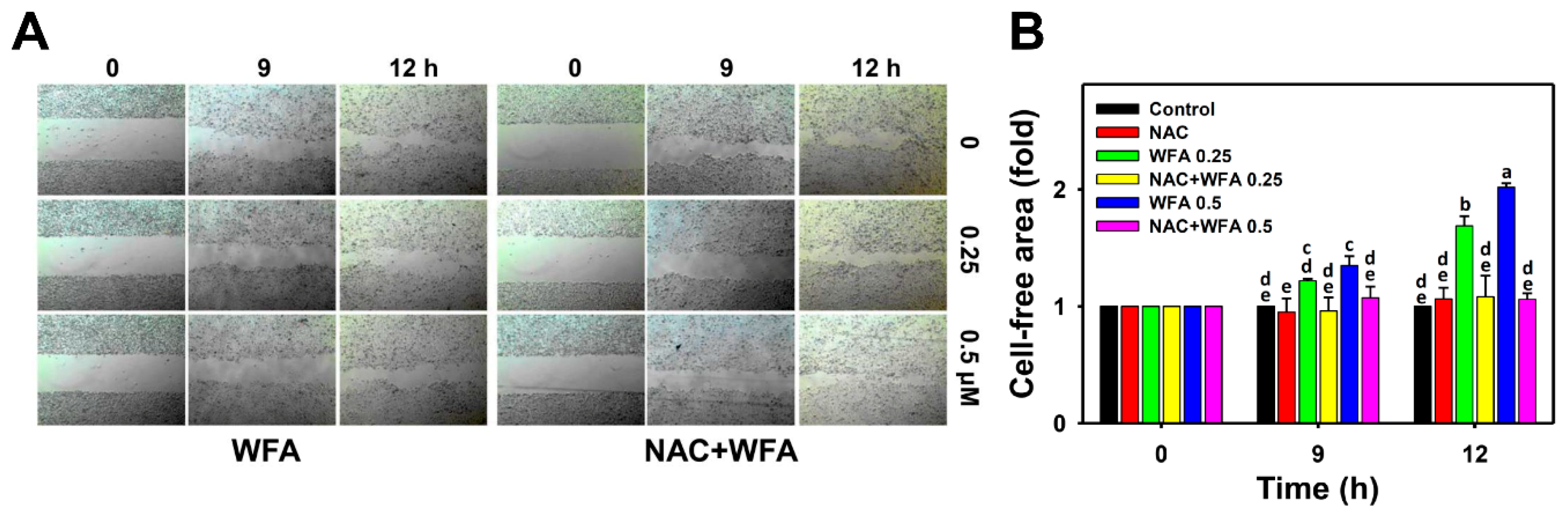
2.4. Wound Healing Assay
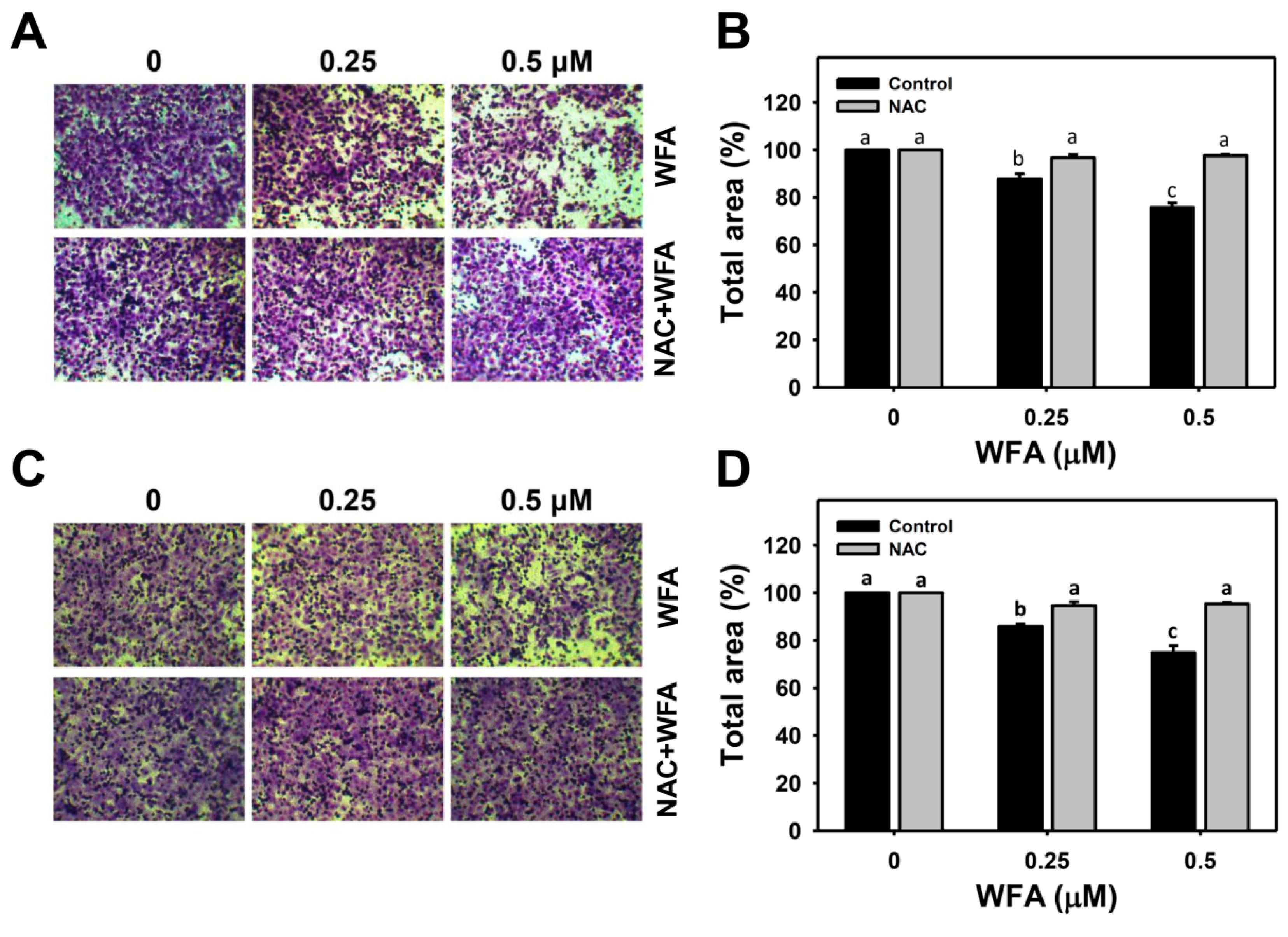
2.5. Cellular 3D Migration and Invasion Assays
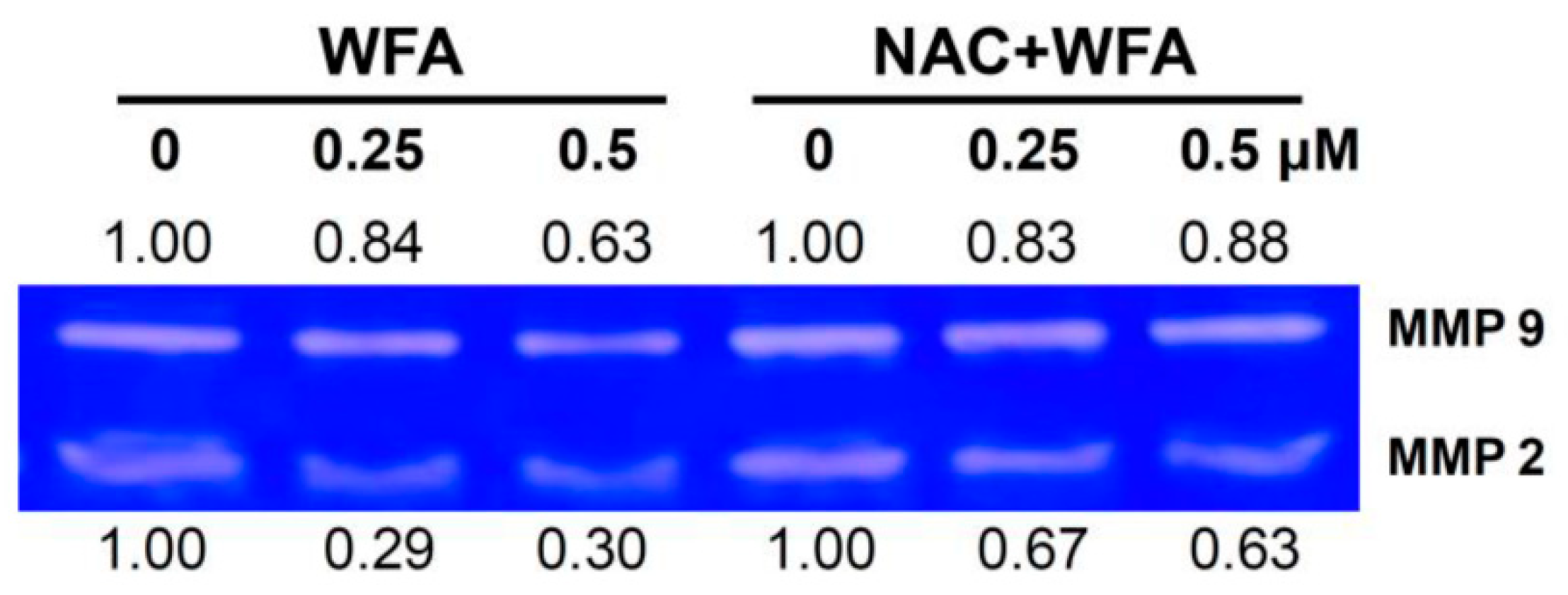
2.6. Zymography for Matrix Metalloproteinase (MMP)-2 and MMP-9 Activities
2.7. Quantitative RT-PCR (qRT-PCR) for Antioxidant-Associated Genes
2.8. Western Blotting for Mitogen-Activated Protein Kinase (MAPK) Expressions
2.9. Statistical Analysis
3. Results
3.1. Identification of the Optimal Concentrations of WFA for Oral Cancer Cell Migration Assay
3.2. ROS Generation of Oral Cancer Ca9-22 Cells at Low Concentrations of WFA
3.3. 2D Migration of Oral Cancer Ca9-22 Cells at Low Concentrations of WFA
3.4. 3D Migration and Invasion Changes in Oral Cancer Ca9-22 Cells at Low Concentrations of WFA
3.5. MMP-2 and MMP-9 Zymography of Oral Cancer Ca9-22 Cells at Low Concentrations of WFA
3.6. Antioxidant Gene Expressions of Oral Cancer Ca9-22 Cells at Low Concentrations of WFA
3.7. Mitogen-Activated Protein Kinase (MAPK) Expressions of Oral Cancer Ca9-22 Cells at Low Concentrations of WFA
4. Discussion
4.1. Low Cytotoxic Concentration of Drugs Is Suitable for Migration Study
4.2. MMP-2 and MMP-9 Activity Changes in WFA-Treated Oral Cancer Cells
4.3. ROS Changes in WFA-Treated Oral Cancer Cells
4.4. Antioxidant Genes Changes in WFA-Treated Oral Cancer Cells
4.5. MAPK Changes in WFA-Treated Oral Cancer Cells
4.6. The Role of ROS in Low Concentration of WFA Induced Migration Changes and Signaling in Oral Cancer Cells
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Bezivin, C.; Tomasi, S.; Lohezic-Le Devehat, F.; Boustie, J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, L.; Jayakar, S.K.; Ow, T.J.; Segall, J.E. Mechanisms of invasion in head and neck cancer. Arch. Pathol. Lab. Med. 2015, 139, 1334–1348. [Google Scholar] [CrossRef]
- Ghate, N.B.; Chaudhuri, D.; Sarkar, R.; Sajem, A.L.; Panja, S.; Rout, J.; Mandal, N. An antioxidant extract of tropical lichen, Parmotrema reticulatum, induces cell cycle arrest and apoptosis in breast carcinoma cell line MCF-7. PLoS ONE 2013, 8, e82293. [Google Scholar] [CrossRef] [PubMed]
- Noguti, J.; De Moura, C.F.; De Jesus, G.P.; Da Silva, V.H.; Hossaka, T.A.; Oshima, C.T.; Ribeiro, D.A. Metastasis from oral cancer: An overview. Cancer Genom. Proteom. 2012, 9, 329–335. [Google Scholar]
- Malik, F.; Kumar, A.; Bhushan, S.; Khan, S.; Bhatia, A.; Suri, K.A.; Qazi, G.N.; Singh, J. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis 2007, 12, 2115–2133. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Kausar, H.; Munjal, C.; Gupta, R.C. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis 2011, 32, 1697–1705. [Google Scholar] [CrossRef]
- Li, X.; Zhu, F.; Jiang, J.; Sun, C.; Wang, X.; Shen, M.; Tian, R.; Shi, C.; Xu, M.; Peng, F.; et al. Synergistic antitumor activity of withaferin A combined with oxaliplatin triggers reactive oxygen species-mediated inactivation of the PI3K/AKT pathway in human pancreatic cancer cells. Cancer Lett. 2015, 357, 219–230. [Google Scholar] [CrossRef]
- Wang, H.C.; Hu, H.H.; Chang, F.R.; Tsai, J.Y.; Kuo, C.Y.; Wu, Y.C.; Wu, C.C. Different effects of 4beta-hydroxywithanolide E and withaferin A, two withanolides from Solanaceae plants, on the Akt signaling pathway in human breast cancer cells. Phytomedicine 2019, 53, 213–222. [Google Scholar] [CrossRef]
- Hsu, J.H.; Chang, P.M.; Cheng, T.S.; Kuo, Y.L.; Wu, A.T.; Tran, T.H.; Yang, Y.H.; Chen, J.M.; Tsai, Y.C.; Chu, Y.S.; et al. Identification of withaferin A as a potential candidate for anti-cancer therapy in non-small cell lung cancer. Cancers 2019, 11, 1003. [Google Scholar] [CrossRef]
- Xia, S.; Miao, Y.; Liu, S. Withaferin A induces apoptosis by ROS-dependent mitochondrial dysfunction in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2018, 503, 2363–2369. [Google Scholar] [CrossRef]
- Yang, I.-H.; Kim, L.-H.; Shin, J.-A.; Cho, S.-D. Chemotherapeutic effect of withaferin A in human oral cancer cells. J. Cancer Ther. 2015, 6, 735–742. [Google Scholar] [CrossRef][Green Version]
- Chang, H.W.; Li, R.N.; Wang, H.R.; Liu, J.R.; Tang, J.Y.; Huang, H.W.; Chan, Y.H.; Yen, C.Y. Withaferin A induces oxidative stress-mediated apoptosis and DNA damage in oral cancer cells. Front. Physiol. 2017, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; De, S.; Mukherjee, S.; Das, S.; Ghosh, A.N.; Sengupta, S.B. Withaferin A induced impaired autophagy and unfolded protein response in human breast cancer cell-lines MCF-7 and MDA-MB-231. Toxicol. In Vitro 2017, 44, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, T.H.; Hwang, E.H.; Chang, K.T.; Hong, J.J.; Park, J.H. Withaferin A inhibits the proliferation of gastric cancer cells by inducing G2/M cell cycle arrest and apoptosis. Oncol. Lett. 2017, 14, 416–422. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Ma, Q.; Wang, Y.; Song, A.L. Withaferin-A inhibits growth of drug-resistant breast carcinoma by inducing apoptosis and autophagy, endogenous reactive oxygen species (ROS) production, and inhibition of cell migration and nuclear factor kappa B (Nf-kappaB)/mammalian target of rapamycin (m-TOR) signalling pathway. Med. Sci. Monit. 2019, 25, 6855–6863. [Google Scholar]
- Hurd, T.R.; DeGennaro, M.; Lehmann, R. Redox regulation of cell migration and adhesion. Trends Cell Biol. 2012, 22, 107–115. [Google Scholar] [CrossRef]
- Chang, Y.T.; Huang, C.Y.; Li, K.T.; Li, R.N.; Liaw, C.C.; Wu, S.H.; Liu, J.R.; Sheu, J.H.; Chang, H.W. Sinuleptolide inhibits proliferation of oral cancer Ca9-22 cells involving apoptosis, oxidative stress, and DNA damage. Arch. Oral Biol. 2016, 66, 147–154. [Google Scholar] [CrossRef]
- Huang, C.H.; Yeh, J.M.; Chan, W.H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ. Toxicol. 2018, 33, 1039–1049. [Google Scholar] [CrossRef]
- Wang, T.S.; Lin, C.P.; Chen, Y.P.; Chao, M.R.; Li, C.C.; Liu, K.L. CYP450-mediated mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ. Toxicol. 2018, 33, 1029–1038. [Google Scholar] [CrossRef]
- Yen, Y.H.; Farooqi, A.A.; Li, K.T.; Butt, G.; Tang, J.Y.; Wu, C.Y.; Cheng, Y.B.; Hou, M.F.; Chang, H.W. Methanolic extracts of Solieria robusta inhibits proliferation of oral cancer Ca9-22 cells via apoptosis and oxidative stress. Molecules 2014, 19, 18721–18732. [Google Scholar] [CrossRef]
- Yen, C.Y.; Chiu, C.C.; Haung, R.W.; Yeh, C.C.; Huang, K.J.; Chang, K.F.; Hseu, Y.C.; Chang, F.R.; Chang, H.W.; Wu, Y.C. Antiproliferative effects of goniothalamin on Ca9-22 oral cancer cells through apoptosis, DNA damage and ROS induction. Mutat. Res. 2012, 747, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Liu, P.L.; Huang, K.J.; Wang, H.M.; Chang, K.F.; Chou, C.K.; Chang, F.R.; Chong, I.W.; Fang, K.; Chen, J.S.; et al. Goniothalamin inhibits growth of human lung cancer cells through DNA damage, apoptosis, and reduced migration ability. J. Agric. Food Chem. 2011, 59, 4288–4293. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Y.; Hsiao, C.C.; Lan, T.H.; Yen, C.Y.; Farooqi, A.A.; Cheng, C.M.; Tang, J.Y.; Yu, T.J.; Yeh, Y.C.; Chuang, Y.T.; et al. Pomegranate extract inhibits migration and invasion of oral cancer cells by downregulating matrix metalloproteinase-2/9 and epithelial-mesenchymal transition. Environ. Toxicol. 2020, 35, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Lin, C.S.; Hua, C.H.; Jou, Y.J.; Liao, C.R.; Chang, Y.S.; Wan, L.; Huang, S.H.; Hour, M.J.; Lin, C.W. Cis-3-O-p-hydroxycinnamoyl ursolic acid induced ROS-dependent p53-mediated mitochondrial apoptosis in oral cancer cells. Biomol. Ther. (Seoul) 2019, 27, 54–62. [Google Scholar] [CrossRef]
- Chang, H.W.; Yen, C.Y.; Chen, C.H.; Tsai, J.H.; Tang, J.Y.; Chang, Y.T.; Kao, Y.H.; Wang, Y.Y.; Yuan, S.F.; Lee, S.Y. Evaluation of the mRNA expression levels of integrins alpha3, alpha5, beta1 and beta6 as tumor biomarkers of oral squamous cell carcinoma. Oncol. Lett. 2018, 16, 4773–4781. [Google Scholar]
- Yen, C.Y.; Huang, C.Y.; Hou, M.F.; Yang, Y.H.; Chang, C.H.; Huang, H.W.; Chen, C.H.; Chang, H.W. Evaluating the performance of fibronectin 1 (FN1), integrin alpha4beta1 (ITGA4), syndecan-2 (SDC2), and glycoprotein CD44 as the potential biomarkers of oral squamous cell carcinoma (OSCC). Biomarkers 2013, 18, 63–72. [Google Scholar] [CrossRef]
- Stagos, D.; Balabanos, D.; Savva, S.; Skaperda, Z.; Priftis, A.; Kerasioti, E.; Mikropoulou, E.V.; Vougogiannopoulou, K.; Mitakou, S.; Halabalaki, M.; et al. Extracts from the mediterranean food plants Carthamus lanatus, Cichorium intybus, and Cichorium spinosum enhanced GSH levels and increased Nrf2 expression in human endothelial cells. Oxid. Med. Cell Longev. 2018, 2018, 6594101. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Brunn, G.J.; Williams, J.; Sabers, C.; Wiederrecht, G.; Lawrence, J.C., Jr.; Abraham, R.T. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996, 15, 5256–5267. [Google Scholar] [CrossRef]
- Fujii, Y.; Yoshihashi, K.; Suzuki, H.; Tsutsumi, S.; Mutoh, H.; Maeda, S.; Yamagata, Y.; Seto, Y.; Aburatani, H.; Hatakeyama, M. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc. Natl. Acad. Sci. USA 2012, 109, 20584–20589. [Google Scholar] [CrossRef]
- Baribeau, S.; Chaudhry, P.; Parent, S.; Asselin, E. Resveratrol inhibits cisplatin-induced epithelial-to-mesenchymal transition in ovarian cancer cell lines. PLoS ONE 2014, 9, e86987. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.C.; Liu, L.C.; Ye, H.Y.; Wu, J.Y.; Yu, Y.L. EZH2 promotes migration and invasion of triple-negative breast cancer cells via regulating TIMP2-MMP-2/-9 pathway. Am. J. Cancer Res. 2018, 8, 422–434. [Google Scholar] [PubMed]
- Chen, J.; Zhang, Z.; Cai, L. Diabetic cardiomyopathy and its prevention by nrf2: Current status. Diabetes Metab. J. 2014, 38, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Do, K.Q. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat. Rev. Neurosci. 2016, 17, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Chu, Y.L.; Hsieh, S.C.; Sheen, L.Y. Diallyl trisulfide inhibits cell migration and invasion of human melanoma a375 cells via inhibiting integrin/facal adhesion kinase pathway. Environ. Toxicol. 2017, 32, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Au, M.K.; Liu, K.L.; Yeh, M.Y.; Lee, C.H.; Lee, M.H.; Lu, H.F.; Yang, J.L.; Wu, R.S.; Chung, J.G. Ouabain impairs cell migration, and invasion and alters gene expression of human osteosarcoma U-2 OS cells. Environ. Toxicol. 2017, 32, 2400–2413. [Google Scholar] [CrossRef]
- Yeh, C.M.; Hsieh, M.J.; Yang, J.S.; Yang, S.F.; Chuang, Y.T.; Su, S.C.; Liang, M.Y.; Chen, M.K.; Lin, C.W. Geraniin inhibits oral cancer cell migration by suppressing matrix metalloproteinase-2 activation through the FAK/Src and ERK pathways. Environ. Toxicol. 2019, 34, 1085–1093. [Google Scholar] [CrossRef]
- Rah, B.; Amin, H.; Yousuf, K.; Khan, S.; Jamwal, G.; Mukherjee, D.; Goswami, A. A novel MMP-2 inhibitor 3-azidowithaferin A (3-azidoWA) abrogates cancer cell invasion and angiogenesis by modulating extracellular Par-4. PLoS ONE 2012, 7, e44039. [Google Scholar] [CrossRef]
- Lee, D.H.; Lim, I.H.; Sung, E.G.; Kim, J.Y.; Song, I.H.; Park, Y.K.; Lee, T.J. Withaferin A inhibits matrix metalloproteinase-9 activity by suppressing the Akt signaling pathway. Oncol. Rep. 2013, 30, 933–938. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hsieh, Y.S.; Yang, S.F.; Chou, M.Y.; Chang, Y.C. Matrix metalloproteinase 2 and matrix metalloproteinase 9 expression in human oral squamous cell carcinoma and the effect of protein kinase C inhibitors: Preliminary observations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 710–716. [Google Scholar] [CrossRef]
- Patel, B.P.; Shah, P.M.; Rawal, U.M.; Desai, A.A.; Shah, S.V.; Rawal, R.M.; Patel, P.S. Activation of MMP-2 and MMP-9 in patients with oral squamous cell carcinoma. J. Surg. Oncol. 2005, 90, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Liao, C.Y.; Lai, L.C.; Tsai, M.H.; Chuang, E.Y. Semaphorin 6A attenuates the migration capability of lung cancer cells via the NRF2/HMOX1 axis. Sci. Rep. 2019, 9, 13302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Li, X.M.; Lian, D.D.; Zhu, M.J.; Yim, S.H.; Lee, J.H.; Jiang, R.H.; Kim, C.D. Tumor suppressive function of NQO1 in cutaneous squamous cell carcinoma (SCC) cells. Biomed. Res. Int. 2019, 2019, 2076579. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lu, M.C.; El-Shazly, M.; Lai, K.H.; Wu, T.Y.; Hsu, Y.M.; Lee, Y.L.; Liu, Y.C. Breaking down leukemia walls: Heteronemin, a sesterterpene derivative, induces apoptosis in leukemia Molt4 cells through oxidative stress, mitochondrial dysfunction and induction of talin Expression. Mar. Drugs 2018, 16, 212. [Google Scholar] [CrossRef]
- Peng, Q.; Deng, Z.; Pan, H.; Gu, L.; Liu, O.; Tang, Z. Mitogen-activated protein kinase signaling pathway in oral cancer. Oncol. Lett. 2018, 15, 1379–1388. [Google Scholar] [CrossRef]
- Mandal, C.; Dutta, A.; Mallick, A.; Chandra, S.; Misra, L.; Sangwan, R.S.; Mandal, C. Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade. Apoptosis 2008, 13, 1450–1464. [Google Scholar] [CrossRef]
- Grogan, P.T.; Sleder, K.D.; Samadi, A.K.; Zhang, H.; Timmermann, B.N.; Cohen, M.S. Cytotoxicity of withaferin A in glioblastomas involves induction of an oxidative stress-mediated heat shock response while altering Akt/mTOR and MAPK signaling pathways. Investig. New Drugs 2013, 31, 545–557. [Google Scholar] [CrossRef]



| Genes | Forward Primers (5′→3′) | Reverse Primers (5′→3′) | Length |
|---|---|---|---|
| TXN | GAAGCAGATCGAGAGCAAGACTG | GCTCCAGAAAATTCACCCACCT | 270 bp |
| GSR | GTTCTCCCAGGTCAAGGAGGTTAA | CCAGCAGCTATTGCAACTGGAGT | 297 bp |
| CAT | ATGCAGGACAATCAGGGTGGT | CCTCAGTGAAGTTCTTGACCGCT | 274 bp |
| SOD1 | AGGGCATCATCAATTTCGAGC [29] | CCCAAGTCTCCAACATGCCTC | 211 bp |
| HMOX1 | CCTTCTTCACCTTCCCCAACAT | GGCAGAATCTTGCACTTTGTTGC | 251 bp |
| NFE2L2 | GATCTGCCAACTACTCCCAGGTT | CTGTAACTCAGGAATGGATAATAGCTCC | 302 bp |
| NQO1 | GAAGGACCCTGCGAACTTTCAGTA | GAAAGCACTGCCTTCTTACTCCG | 258 bp |
| GCLC | ACAAGCACCCTCGCTTCAGTACC | CTGCAGGCTTGGAATGTCACCT | 232 bp |
| GPX1 | AACCAGTTTGGGCATCAGGAG | AGTTCCAGGCAACATCGTTGC | 256 bp |
| GAPDH | CCTCAACTACATGGTTTACATGTTCC [30] | CAAATGAGCCCCAGCCTTCT [31] | 220 bp |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.-J.; Tang, J.-Y.; Ou-Yang, F.; Wang, Y.-Y.; Yuan, S.-S.F.; Tseng, K.; Lin, L.-C.; Chang, H.-W. Low Concentration of Withaferin a Inhibits Oxidative Stress-Mediated Migration and Invasion in Oral Cancer Cells. Biomolecules 2020, 10, 777. https://doi.org/10.3390/biom10050777
Yu T-J, Tang J-Y, Ou-Yang F, Wang Y-Y, Yuan S-SF, Tseng K, Lin L-C, Chang H-W. Low Concentration of Withaferin a Inhibits Oxidative Stress-Mediated Migration and Invasion in Oral Cancer Cells. Biomolecules. 2020; 10(5):777. https://doi.org/10.3390/biom10050777
Chicago/Turabian StyleYu, Tzu-Jung, Jen-Yang Tang, Fu Ou-Yang, Yen-Yun Wang, Shyng-Shiou F. Yuan, Kevin Tseng, Li-Ching Lin, and Hsueh-Wei Chang. 2020. "Low Concentration of Withaferin a Inhibits Oxidative Stress-Mediated Migration and Invasion in Oral Cancer Cells" Biomolecules 10, no. 5: 777. https://doi.org/10.3390/biom10050777
APA StyleYu, T.-J., Tang, J.-Y., Ou-Yang, F., Wang, Y.-Y., Yuan, S.-S. F., Tseng, K., Lin, L.-C., & Chang, H.-W. (2020). Low Concentration of Withaferin a Inhibits Oxidative Stress-Mediated Migration and Invasion in Oral Cancer Cells. Biomolecules, 10(5), 777. https://doi.org/10.3390/biom10050777






