Cord Blood Serum (CBS)-Based Eye Drops Modulate Light-Induced Neurodegeneration in Albino Rat Retinas
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of CBS Eye Drops
2.2. Growth Factor and Interleukin Dosage
2.3. Animal Models and Experimental Procedure
2.4. Eye-Drop Treatment
2.5. Light Damage
2.6. Electrophysiological Recordings
2.7. Morphological Analyses and Immunostaining
2.8. Statistical Analysis
3. Results
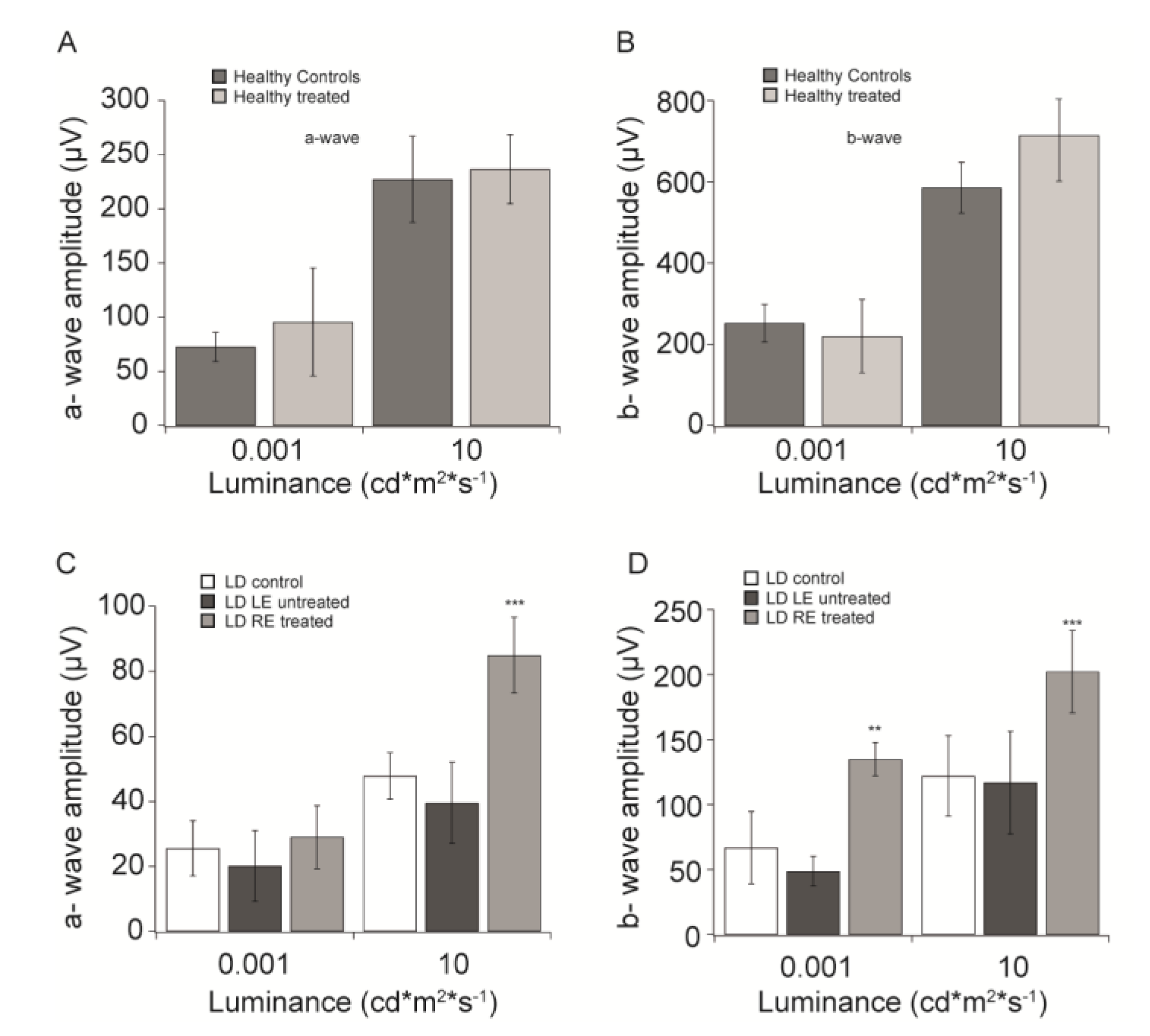
3.1. “In vivo” Functional Analysis
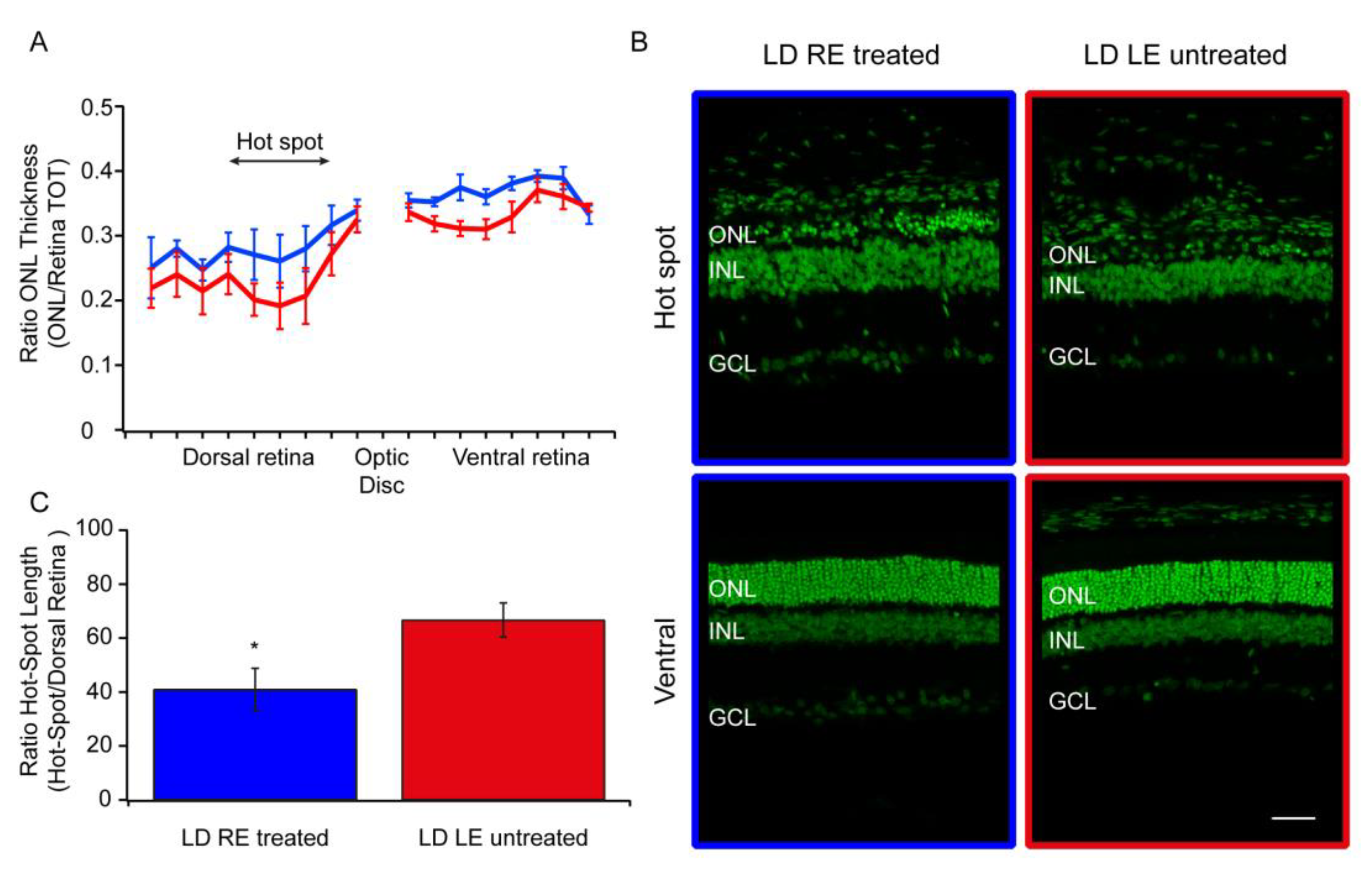
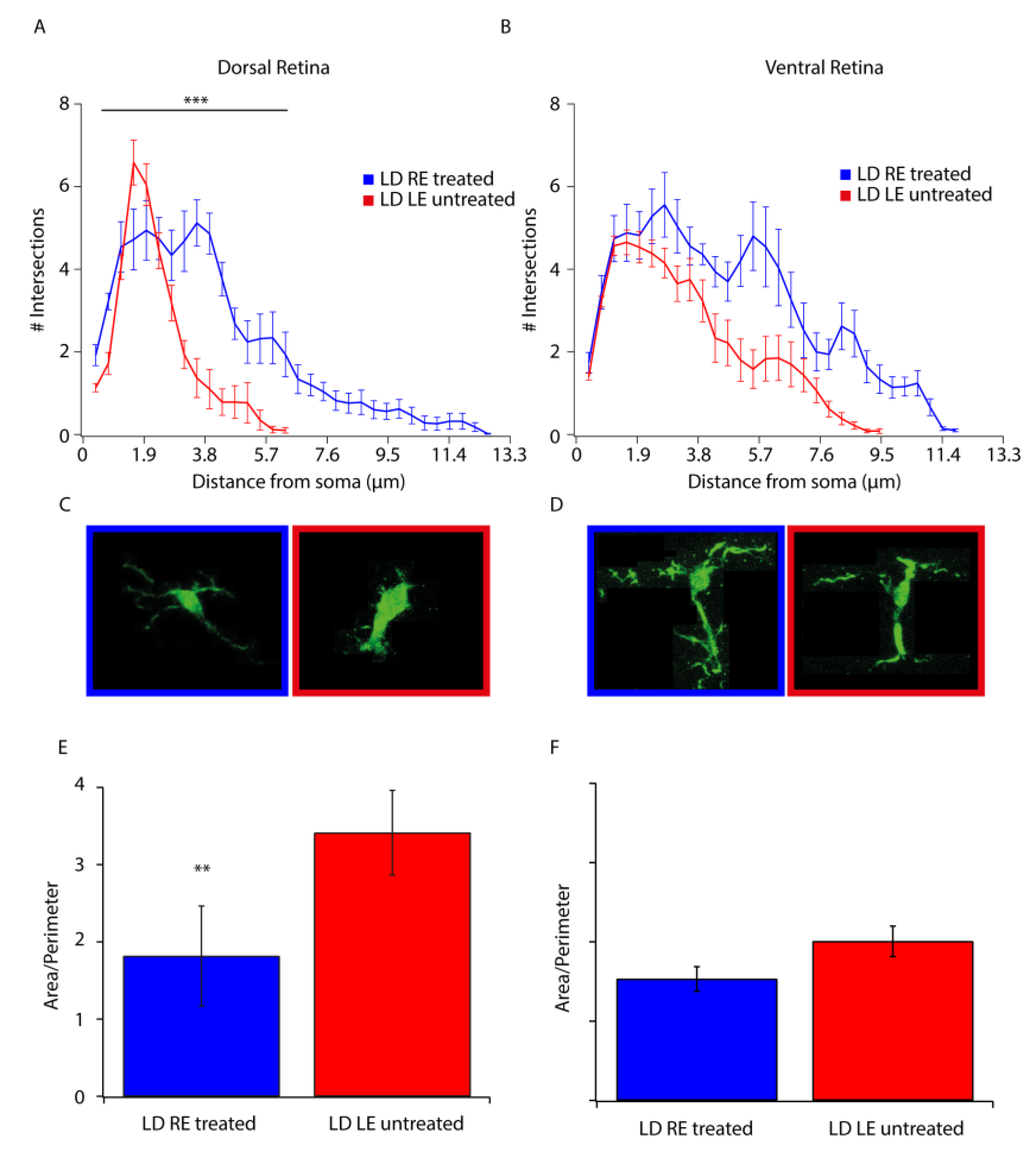
3.2. Morphological Analysis
3.3. Stress and Neuroinflammation Markers
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations:
| AMD | Age-Related Macular degeneration |
| BDNF | Brain-derived neurotrophic factor |
| CB | cord blood |
| CBS | cord blood serum |
| CMV | Cytomegalovirus |
| EGF | Epidermal growth factor |
| fERG | flash Electroretinogram |
| FGF basic | Fibroblast growth factor |
| GCL | ganglion cell layer |
| GF | Growth factor |
| GFAP | Glial fibrillary acidic protein |
| HIV/HBV/HCV-NAT | Human Immunodeficiency Virus/Hepatitis B virus/Hepatitis C Virus -Nucleic Acid Testing |
| HTL | VI/II human T-lymphotropic virus I/II |
| IBA-1 | Ionized calcium-binding adaptor molecule 1 |
| IL-10 and -13 | Interleukin-10 and -13 |
| INL | inner nuclear layer |
| LD | Light damage |
| LE | left eye |
| NGF | Nerve growth factor |
| OCT | optimum cutting temperature |
| ONL | outer nuclear layer |
| Ops | oscillatory potentials |
| PDGF-bb | Platelet-derived growth factor-bb |
| PBS | phosphate-buffered saline |
| RE | right eye |
| RPE | retinal pigmented epithelium |
| TGF-α | Transforming growth factor-α |
| TOXO | Toxoplasmosis |
| TPHA | Treponema pallidum hemagglutination (TPHA) |
References
- Chang, G.-Q.; Hao, Y.; Wong, F. Apoptosis: Final common pathway of photoreceptor death in rd, rds, and mutant mice. Neuron 1993, 11, 595–605. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Rutar, M.; Provis, J.M.; Valter, K. Brief exposure to damaging light causes focal recruitment of macrophages, and long-term destabilization of photoreceptors in the albino rat retina. Curr. Eye Res. 2010, 35, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Demontis, G.C.; Longoni, B.; Marchiafava, P.L. Molecular steps involved in light-induced oxidative damage to retinal rods. Invest. Ophthalmol. Vis. Sci. 2002, 43, 2421–2427. [Google Scholar]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal microglia: Just bystander or target for therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef]
- Maccarone, R.; Di Marco, S.; Bisti, S. Saffron Supplement Maintains Morphology and Function after Exposure to Damaging Light in Mammalian Retina. Investig. Opthalmol. Vis. Sci. 2008, 49, 1254. [Google Scholar] [CrossRef]
- Yamauchi, M.; Tsuruma, K.; Imai, S.; Nakanishi, T.; Umigai, N.; Shimazawa, M.; Hara, H. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur. J. Pharm. 2011, 650, 110–119. [Google Scholar] [CrossRef]
- Fiorani, L.; Passacantando, M.; Santucci, S.; Di Marco, S.; Bisti, S. Cerium Oxide Nanoparticles Reduce Microglial Activation and Neurodegenerative Events in Light Damaged Retina. PLoS ONE 2015, 10, e0140387. [Google Scholar] [CrossRef]
- Rocco, M.L.; Balzamino, B.O.; Aloe, L.; Micera, A. NGF protects corneal, retinal, and cutaneous tissues/cells from phototoxic effect of UV exposure. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 729–738. [Google Scholar] [CrossRef]
- Cerri, E.; Origlia, N.; Falsini, B.; Barloscio, D.; Fabiani, C.; Sansò, M.; Ottino, S.; Giovannini, L.; Domenici, L. Conjunctivally Applied BDNF Protects Photoreceptors from Light-Induced Damage. Transl. Vis. Sci. Technol. 2015, 4, 1. [Google Scholar] [CrossRef]
- Versura, P.; Profazio, V.; Buzzi, M.; Stancari, A.; Arpinati, M.; Malavolta, N.; Campos, E.C. Efficacy of Standardized and Quality-Controlled Cord Blood Serum Eye Drop Therapy in the Healing of Severe Corneal Epithelial Damage in Dry Eye. Cornea 2013, 32, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Buzzi, M.; Giannaccare, G.; Terzi, A.; Fresina, M.; Velati, C.; Campos, E.C. Targeting growth factor supply in keratopathy treatment: Comparison between maternal peripheral blood and cord blood as sources for the preparation of topical eye drops. Blood Transfus. 2016, 14, 145–151. [Google Scholar] [PubMed]
- Campos, E.; Versura, P.; Giannaccare, G.; Terzi, A.; Bisti, S.; Di Marco, S.; Buzzi, M. Topical Treatment with Cord Blood Serum in Glaucoma Patients: A Preliminary Report. Case Rep. Ophthalmol. Med. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, C.; Filardo, G.; Mariani, E.; Kon, E.; Marcacci, M.; Pereira Ruiz, M.T.; Facchini, A.; Grigolo, B. Comparison of platelet-rich plasma formulations for cartilage healing: An in vitro study. J. Bone Jt. Surg. Am. 2014, 96, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, S.; Carnicelli, V.; Franceschini, N.; Di Paolo, M.; Piccardi, M.; Bisti, S.; Falsini, B. Saffron: A multitask neuroprotective agent for retinal degenerative diseases. Antioxidants 2019, 8, 224. [Google Scholar] [CrossRef]
- Stone, J.; Maslim, J.; Valter-Kocsi, K.; Mervin, K.; Bowers, F.; Chu, Y.; Barnett, N.; Provis, J.; Lewis, G.; Fisher, S.K.; et al. Mechanisms of photoreceptor death and survival in mammalian retina. Prog. Retin. Eye Res. 1999, 18, 689–735. [Google Scholar] [CrossRef]
- Stence, N.; Waite, M.; Dailey, M.E. Dynamics of microglial activation: A confocal time-lapse analysis in hippocampal slices. Glia 2001, 33, 256–266. [Google Scholar] [CrossRef]
- Valter, K.; Bisti, S.; Gargini, C.; Di Loreto, S.; Maccarone, R.; Cervetto, L.; Stone, J. Time course of neurotrophic factor upregulation and retinal protection against light-induced damage after optic nerve section. Invest. Ophthalmol Vis. Sci 2005, 46, 1748–1754. [Google Scholar] [CrossRef]
- Gargini, C.; Bisti, S.; Demontis, G.C.; Valter, K.; Stone, J.; Cervetto, L. Electroretinogram changes associated with retinal upregulation of trophic factors: Observations following optic nerve section. Neuroscience 2004, 126, 775–783. [Google Scholar] [CrossRef]
- Falsini, B.; Chiaretti, A.; Rizzo, D.; Piccardi, M.; Ruggiero, A.; Manni, L.; Soligo, M.; Dickmann, A.; Federici, M.; Salerni, A.; et al. Nerve growth factor improves visual loss in childhood optic gliomas: A randomized, double-blind, phase II clinical trial. Brain 2016, 139, 404–414. [Google Scholar] [CrossRef]
- Falsini, B.; Iarossi, G.; Chiaretti, A.; Ruggiero, A.; Luigi, M.; Galli-Resta, L.; Corbo, G.; Abed, E.; Manni, L. NGF eye-drops topical administration in patients with retinitis pigmentosa, a pilot study. J. Transl. Med. 2016, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, A.; Conti, G.; Falsini, B.; Buonsenso, D.; Crasti, M.; Manni, L.; Soligo, M.; Fantacci, C.; Genovese, O.; Calcagni, M.L.; et al. Intranasal Nerve Growth Factor administration improves cerebral functions in a child with severe traumatic brain injury: A case report. Brain Inj. 2017, 31, 1538–1547. [Google Scholar] [CrossRef] [PubMed]

| EGF | TGF-α | PDGFbb | FGF Basic | b-NGF | IL-10 | IL-13 |
|---|---|---|---|---|---|---|
| 890 | 66.6 | 7103 | 77.5 | 0.70 | 15.4 | 262.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Marco, S.; Riccitelli, S.; Di Paolo, M.; Campos, E.; Buzzi, M.; Bisti, S.; Versura, P. Cord Blood Serum (CBS)-Based Eye Drops Modulate Light-Induced Neurodegeneration in Albino Rat Retinas. Biomolecules 2020, 10, 678. https://doi.org/10.3390/biom10050678
Di Marco S, Riccitelli S, Di Paolo M, Campos E, Buzzi M, Bisti S, Versura P. Cord Blood Serum (CBS)-Based Eye Drops Modulate Light-Induced Neurodegeneration in Albino Rat Retinas. Biomolecules. 2020; 10(5):678. https://doi.org/10.3390/biom10050678
Chicago/Turabian StyleDi Marco, Stefano, Serena Riccitelli, Mattia Di Paolo, Emilio Campos, Marina Buzzi, Silvia Bisti, and Piera Versura. 2020. "Cord Blood Serum (CBS)-Based Eye Drops Modulate Light-Induced Neurodegeneration in Albino Rat Retinas" Biomolecules 10, no. 5: 678. https://doi.org/10.3390/biom10050678
APA StyleDi Marco, S., Riccitelli, S., Di Paolo, M., Campos, E., Buzzi, M., Bisti, S., & Versura, P. (2020). Cord Blood Serum (CBS)-Based Eye Drops Modulate Light-Induced Neurodegeneration in Albino Rat Retinas. Biomolecules, 10(5), 678. https://doi.org/10.3390/biom10050678





