High-Density Lipoprotein Particles and Their Relationship to Posttransplantation Diabetes Mellitus in Renal Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Data Collection
2.3. Laboratory Measurements
2.4. PTDM
2.5. Statistical Analyses
3. Results
3.1. Characteristics of RTRs at Baseline
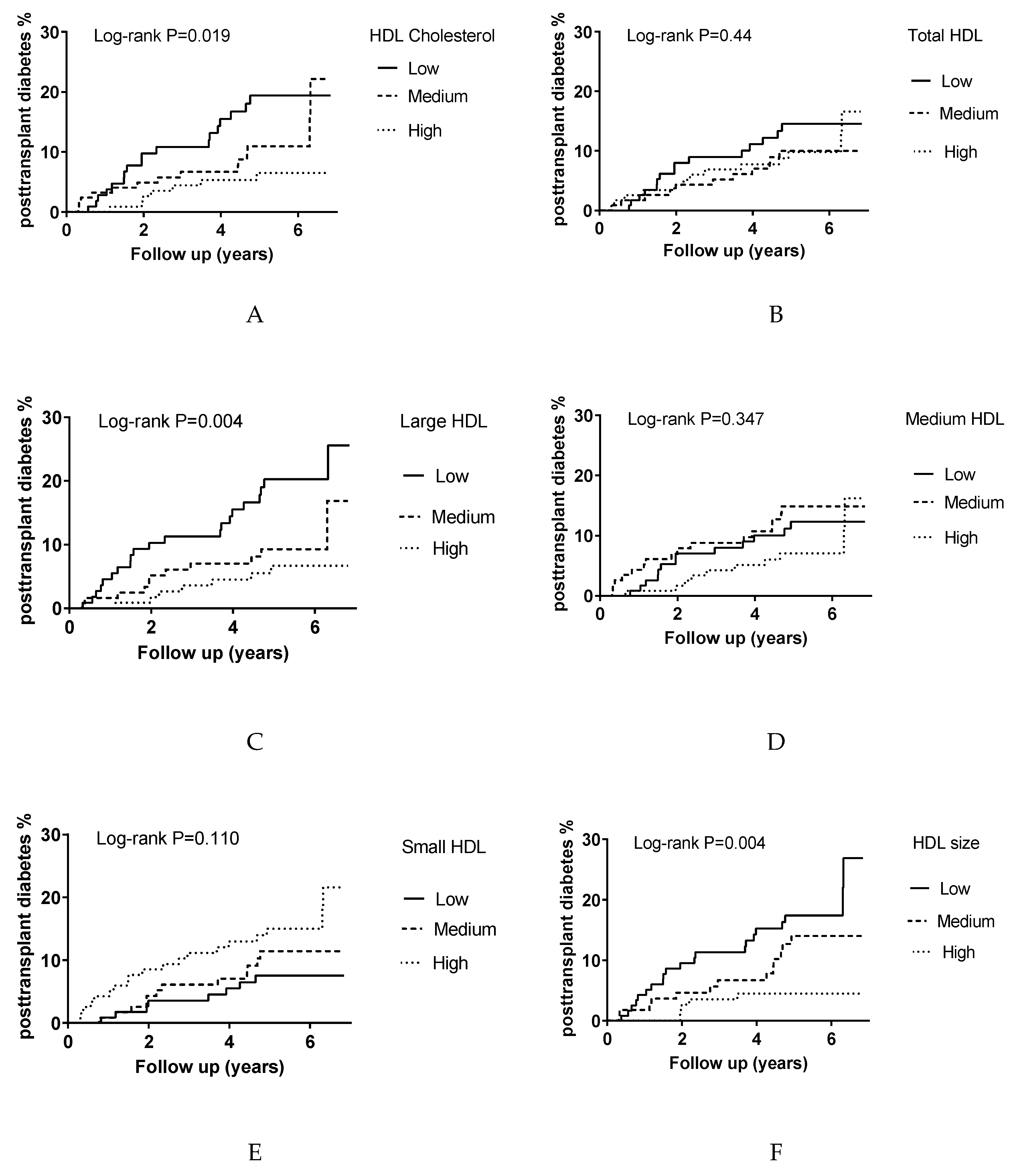
3.2. Association of HDL Cholesterol and HDL Particle Characteristics with Incident PTDM
3.3. Confounding Influence of Other Lipoproteins on the Association of Large HDL Particles with Incident PTDM
3.4. Association of Large HDL Subspecies (H7P and H6P) with Incident PTDM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wauters, R.P.; Cosio, F.G.; Suarez Fernandez, M.L.; Kudva, Y.; Shah, P.; Torres, V.E. Cardiovascular Consequences of New-Onset Hyperglycemia after Kidney Transplantation. Transplantation 2012, 94, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Brennan, D.C.; Schnitzler, M.A. Incidence and Predictors of Myocardial Infarction after Kidney Transplantation. J. Am. Soc. Nephrol. 2005, 16, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Pillado, M.T.; Pita-Fernández, S.; Valdés-Cañedo, F.; Seijo-Bestilleiro, R.; Pértega-Díaz, S.; Fernández-Rivera, C.; Alonso-Hernández, Á.; González-Martín, C.; Balboa-Barreiro, V. Incidence of Cardiovascular Events and Associated Risk Factors in Kidney Transplant Patients: A Competing Risks Survival Analysis. BMC. Cardiovasc. Disord. 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Israni, A.K.; Snyder, J.J.; Skeans, M.A.; Peng, Y.; MacLean, J.R.; Weinhandl, E.D.; Kasiske, B.L. Predicting Coronary Heart Disease after Kidney Transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am. J. Transplant. 2010, 10, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Secchi, A. Post-Transplantation Diabetes in Kidney Transplant Recipients: An Update on Management and Prevention. Acta Diabetol. 2018, 55, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Chakkera, H.A.; Weil, E.J.; Pham, P.T.; Pomeroy, J.; Knowler, W.C. Can New-Onset Diabetes after Kidney Transplant Be Prevented? Diabetes Care 2013, 36, 1406–1412. [Google Scholar] [CrossRef]
- Langsford, D. Dysglycemia after Renal Transplantation: Definition, Pathogenesis, Outcomes and Implications for Management. World J. Diabetes 2015, 6, 1132. [Google Scholar] [CrossRef]
- Li, N.; Fu, J.; Koonen, D.P.; Kuivenhoven, J.A.; Snieder, H.; Hofker, M.H. Are Hypertriglyceridemia and Low HDL Causal Factors in the Development of Insulin Resistance? Atherosclerosis 2014, 233, 130–138. [Google Scholar] [CrossRef]
- Drew, B.G.; Rye, K.-A.; Duffy, S.J.; Barter, P.; Kingwell, B.A. The Emerging Role of HDL in Glucose Metabolism. Nat. Rev. Endocrinol. 2012, 8, 237. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; Meigs, J.B.; Sullivan, L.; Fox, C.S.; Nathan, D.M.; D’Agostino, R.B. Prediction of Incident Diabetes Mellitus in Middle-Aged Adults: The Framingham Offspring Study. Arch. Intern. Med. 2007, 167, 1068–1074. [Google Scholar] [CrossRef]
- Abbasi, A.; Corpeleijn, E.; Gansevoort, R.T.; Gans, R.O.B.; Hillege, H.L.; Stolk, R.P.; Navis, G.; Bakker, S.J.L.; Dullaart, R.P.F. Role of HDL Cholesterol and Estimates of HDL Particle Composition in Future Development of Type 2 Diabetes in the General Population: The PREVEND Study. J. Clin. Endocrinol. Metab. 2013, 98, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.L.; Koh, Y.L.E.; Tan, N.C. The Utility of Diabetes Risk Score Items as Predictors of Incident Type 2 Diabetes in Asian Populations: An Evidence-Based Review. Diabetes Res. Clin. Pract. 2016, 122, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.G.; Duffy, S.J.; Formosa, M.F.; Natoli, A.K.; Henstridge, D.C.; Penfold, S.A.; Thomas, W.G.; Mukhamedova, N.; De Courten, B.; Forbes, J.M.; et al. High-Density Lipoprotein Modulates Glucose Metabolism in Patients with Type 2 Diabetes Mellitus. Circulation 2009, 119, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.C.; Hayashi, T.; Fujimoto, W.Y.; Kahn, S.E.; Leonetti, D.L.; Mcneely, M.J.; Boyko, E.J. Differential Association between HDL Subclasses and the Development of Type 2 Diabetes in a Prospective Study of Japanese Americans. Diabetes Care 2015, 38, 2100–2105. [Google Scholar] [CrossRef]
- Tabara, Y.; Arai, H.; Hirao, Y.; Takahashi, Y.; Setoh, K.; Kawaguchi, T.; Kosugi, S.; Ito, Y.; Nakayama, T.; Matsuda, F. Different Inverse Association of Large High-Density Lipoprotein Subclasses with Exacerbation of Insulin Resistance and Incidence of Type 2 Diabetes: The Nagahama Study. Diabetes Res. Clin. Pract. 2017, 127, 123–131. [Google Scholar] [CrossRef]
- Borggreve, S.E.; De Vries, R.; Dullaart, R.P.F. Alterations in High-Density Lipoprotein Metabolism and Reverse Cholesterol Transport in Insulin Resistance and Type 2 Diabetes Mellitus: Role of Lipolytic Enzymes, Lecithin:Cholesterol Acyltransferase and Lipid Transfer Proteins. Eur. J. Clin. Investig. 2003, 33, 1051–1069. [Google Scholar] [CrossRef]
- Dallinga-Thie, G.M.; Dullaart, R.P.F.; van Tol, A. Derangements of Intravascular Remodeling of Lipoproteins in Type 2 Diabetes Mellitus: Consequences for Atherosclerosis Development. Curr. Diab. Rep. 2008, 8, 65–70. [Google Scholar] [CrossRef]
- Davidson, W.S.; Shah, A.S. High-Density Lipoprotein Subspecies in Health and Human Disease: Focus on Type 2 Diabetes. Methodist Debakey Cardiovasc. J. 2019, 15, 55–61. [Google Scholar] [CrossRef]
- Garvey, W.T.; Kwon, S.; Zheng, D.; Shaughnessy, S.; Wallace, P.; Hutto, A.; Pugh, K.; Jenkins, A.J.; Klein, R.L.; Liao, Y. Effects of Insulin Resistance and Type 2 Diabetes on Lipoprotein Subclass Particle Size and Concentration Determined by Nuclear Magnetic Resonance. Diabetes 2003, 52, 453–462. [Google Scholar] [CrossRef]
- Festa, A.; Williams, K.; Hanley, A.J.G.; Otvos, J.D.; Goff, D.C.; Wagenknecht, L.E.; Haffner, S.M. Nuclear Magnetic Resonance Lipoprotein Abnormalities in Prediabetic Subjects in the Insulin Resistance Atherosclerosis Study. Circulation 2005, 111, 3465–3472. [Google Scholar] [CrossRef]
- Mora, S.; Otvos, J.D.; Rosenson, R.S.; Pradhan, A.; Buring, J.E.; Ridker, P.M. Lipoprotein Particle Size and Concentration by Nuclear Magnetic Resonance and Incident Type 2 Diabetes in Women. Diabetes 2010, 59, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- MacKey, R.H.; Mora, S.; Bertoni, A.G.; Wassel, C.L.; Carnethon, M.R.; Sibley, C.T.; Goff, D.C. Lipoprotein Particles and Incident Type 2 Diabetes in the Multi- Ethnic Study of Atherosclerosis. Diabetes Care 2015, 38, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; Jenkins, A.J.; English, D.R.; O’Dea, K.; Giles, G.G. NMR-Determined Lipoprotein Subclass Profile Predicts Type 2 Diabetes. Diabetes Res. Clin. Pract. 2009, 83, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, E.; Engberink, M.F.; Brink, E.J.; Van Baak, M.A.; Gans, R.O.B.; Navis, G.; Bakker, S.J.L. Dietary Protein, Blood Pressure and Renal Function in Renal Transplant Recipients. Br. J. Nutr. 2013, 109, 1463–1470. [Google Scholar] [CrossRef]
- Eisenga, M.F.; Kieneker, L.M.; Soedamah-muthu, S.S.; Van den Berg, E.; Deetman, P.E.; Navis, G.J.; Gans, R.O.B.; Gaillard, C.A.J.M.; Bakker, S.J.L.; Joosten, M.M. Urinary Potassium Excretion, Renal Ammoniagenesis, and Risk of Graft Failure and Mortality in Renal Transplant Recipients. Am. Soc. Nutr. 2016, 1, 1703–1711. [Google Scholar] [CrossRef]
- Van den Berg, E.; Pasch, A.; Westendorp, W.H.; Navis, G.; Brink, E.J.; Gans, R.O.B.; Van Goor, H.; Bakker, S.J.L. Urinary Sulfur Metabolites Associate with a Favorable Cardiovascular Risk Profile and Survival Bene Fit in Renal Transplant Recipients. Am. Soc. Nephrol. 2014, 25, 1303–1312. [Google Scholar] [CrossRef]
- Wendel-Vos, G.C.W.; Schuit, A.J.; Saris, W.H.M.; Kromhout, D. Reproducibility and Relative Validity of the Short Questionnaire to Assess Health-Enhancing Physical Activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Matyus, S.P.; Braun, P.J.; Wolak-Dinsmore, J.; Jeyarajah, E.J.; Shalaurova, I.; Warner, S.M.; Fischer, T.J.; Connelly, M.A. HDL particle number measured on the Vantera®, the first clinical NMR analyzer. Clin. Biochem. 2015, 48, 148–155. [Google Scholar] [CrossRef]
- Matyus, S.P.; Braun, P.J.; Wolak-Dinsmore, J.; Jeyarajah, E.J.; Shalaurova, I.; Xu, Y.; Warner, S.M.; Clement, T.S.; Connelly, M.A.; Fischer, T.J. NMR Measurement of LDL Particle Number Using the Vantera® Clinical Analyzer. Clin. Biochem. 2014, 47, 203–210. [Google Scholar] [CrossRef]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein Particle Analysis by Nuclear Magnetic Resonance Spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef] [PubMed]
- Berends, A.M.A.; Buitenwerf, E.; Gruppen, E.G.; Sluiter, W.J.; Bakker, S.J.L.; Connelly, M.A.; Kerstens, M.N.; Dullaart, R.P.F. Primary Aldosteronism Is Associated with Decreased Low-Density and High-Density Lipoprotein Particle Concentrations and Increased GlycA, a pro-Inflammatory Glycoprotein Biomarker. Clin. Endocrinol. 2019, 90, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Canivell, S.; Gomis, R. Diagnosis and Classification of Autoimmune Diabetes Mellitus. Autoimmun. Rev. 2014, 13, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Hecking, M.; De Vries, A.P.J.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings from an International Consensus Meeting on Posttransplantation Diabetes Mellitus: Recommendations and Future Directions. Am. J. Transplant. 2014, 14, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Fagot-Campagna, A.; Saaddine, J.; Narayan, K.M.V.; Goldschmid, M.; Howard, B.V. Re: Sex Differences in Risk Factors for Clinical Diabetes Mellitus in a General Population: A 12-Year Follow-up of the Finnmark Study [2]. Am. J. Epidemiol. 1999, 149, 1073–1074. [Google Scholar] [CrossRef]
- Fagot-Campagna, A.; Narayan, K.M.V.; Hanson, R.L.; Imperatore, G.; Howard, B.V.; Nelson, R.G.; Pettitt, D.J.; Knowler, W.C. Plasma Lipoproteins and Incidence of Non-Insulin-Dependent Diabetes Mellitus in Pima Indians: Protective Effect of HDL Cholesterol in Women. Atherosclerosis 1997, 128, 113–119. [Google Scholar] [CrossRef]
- Stern, M.P.; Williams, K.; Haffner, S.M. Identification of Persons at High Risk for Type 2 Diabetes Mellitus: Do We Need the Oral Glucose Tolerance Test? Ann. Intern. Med. 2002, 136, 575–581. [Google Scholar] [CrossRef]
- Rodrigo, E.; Santos, L.; Piñera, C.; San Millán, J.C.R.; Quintela, M.E.; Toyos, C.; Allende, N.; Gómez-Alamillo, C.; Arias, M. Prediction at First Year of Incident New-Onset Diabetes after Kidney Transplantation by Risk Prediction Models. Diabetes Care 2012, 35, 471–473. [Google Scholar] [CrossRef]
- Boloori, A.; Saghafian, S.; Chakkera, H.A.; Cook, C.B. Characterization of Remitting and Relapsing Hyperglycemia in Post-Renal-Transplant Recipients. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef]
- Abderrahmani, A.; Niederhauser, G.; Favre, D.; Abdelli, S.; Ferdaoussi, M.; Yang, J.Y.; Regazzi, R.; Widmann, C.; Waeber, G. Human High-Density Lipoprotein Particles Prevent Activation of the JNK Pathway Induced by Human Oxidised Low-Density Lipoprotein Particles in Pancreatic Beta Cells. Diabetologia 2007, 50, 1304–1314. [Google Scholar] [CrossRef]
- Han, R.; Lai, R.; Ding, Q.; Wang, Z.; Luo, X.; Zhang, Y.; Cui, G.; He, J.; Liu, W.; Chen, Y. Apolipoprotein A-I Stimulates AMP-Activated Protein Kinase and Improves Glucose Metabolism. Diabetologia 2007, 50, 1960–1968. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.; Kontush, A.; James, R.W. Functionality of HDL: Antioxidation and Detoxifying Effects. In High. Density Lipoproteins: From Biological Understanding to Clinical Exploitation Book. Handb. Exp. Pharmacol, 1st ed.; von Eckardstein, A., Kardassis, D., Eds.; Springer International Publishing: New York, NY, USA, 2015; pp. 207–228. ISBN 978-3-319-09665-0. [Google Scholar]
- Camont, L.; Lhomme, M.; Rached, F.; Le Goff, W.; Nègre-Salvayre, A.; Salvayre, R.; Calzada, C.; Lagarde, M.; Chapman, M.J.; Kontush, A. Small, Dense High-Density Lipoprotein-3 Particles Are Enriched in Negatively Charged Phospholipids: Relevance to Cellular Cholesterol Efflux, Antioxidative, Antithrombotic, Anti-Inflammatory, and Antiapoptotic Functionalities. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Parks, J.S. New Roles of HDL in Inflammation and Hematopoiesis. Annu. Rev. Nutr. 2012, 32, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Triolo, M.; Annema, W.; Dullaart, R.P.F.; Tietge, U.J.F. Assessing the Functional Properties of High-Density Lipoproteins: An Emerging Concept in Cardiovascular Research. Biomark. Med. 2013, 7, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.F.; Gruppen, E.G.; Dallinga-Thie, G.M. Paraoxonase-1 Activity Is Positively Related to Phospholipid Transfer Protein Activity in Type 2 Diabetes Mellitus: Role of Large HDL Particles. Clin. Biochem. 2016, 49, 508–510. [Google Scholar] [CrossRef]
- Oterdoom, L.H.; De Vries, A.P.J.; Gansevoort, R.T.; Van Son, W.J.; Van Der Heide, J.J.H.; Ploeg, R.J.; De Jong, P.E.; Gans, R.O.B.; Bakker, S.J.L. Determinants of Insulin Resistance in Renal Transplant Recipients. Transplantation 2007, 83, 29–35. [Google Scholar] [CrossRef][Green Version]
- Otvos, J.D.; Collins, D.; Freedman, D.S.; Shalaurova, I.; Schaefer, E.J.; McNamara, J.R.; Bloomfield, H.E.; Robins, S.J. Low-Density Lipoprotein and High-Density Lipoprotein Particle Subclasses Predict Coronary Events and Are Favorably Changed by Gemfibrozil Therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation 2006, 113, 1556–1563. [Google Scholar] [CrossRef]
- MacKey, R.H.; Greenland, P.; Goff, D.C.; Lloyd-Jones, D.; Sibley, C.T.; Mora, S. High-Density Lipoprotein Cholesterol and Particle Concentrations, Carotid Atherosclerosis, and Coronary Events: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2012, 60, 508–516. [Google Scholar] [CrossRef]
- Sean Davidson, W.; Heink, A.; Sexmith, H.; Dolan, L.M.; Gordon, S.M.; Otvos, J.D.; Melchior, J.T.; Elder, D.A.; Khoury, J.; Geh, E.; et al. Obesity Is Associated with an Altered HDL Subspecies Profle among Adolescents with Metabolic Disease. J. Lipid Res. 2017, 58, 1916–1923. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; De Lemos, J.A.; et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef]
- Rohatgi, A.; Grundy, S.M. Cholesterol Efflux Capacity as a Therapeutic Target Rationale and Clinical Implications. J. Am. Coll. Cardiol. 2015, 66, 2211–2213. [Google Scholar] [CrossRef] [PubMed]
- Ebtehaj, S.; Gruppen, E.G.; Bakker, S.J.L.; Dullaart, R.P.F.; Tietge, U.J.F. HDL (High-Density Lipoprotein) Cholesterol Efflux Capacity Is Associated With Incident Cardiovascular Disease in the General Population. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.F.; Annema, W.; de Boer, J.F.; Tietge, U.J.F. Pancreatic β-Cell Function Relates Positively to HDL Functionality in Well-Controlled Type 2 Diabetes Mellitus. Atherosclerosis 2012, 222, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Szili-Torok, T.; Annema, W.; Anderson, J.L.C.; Bakker, S.J.L.; Tietge, U.J.F. HDL Cholesterol Efflux Predicts Incident New-Onset Diabetes after Transplantation (NODAT) in Renal Transplant Recipients Independent of HDL Cholesterol Levels. Diabetes 2019, 68, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Mutharasan, R.K.; Thaxton, C.S.; Berry, J.; Daviglus, M.L.; Yuan, C.; Sun, J.; Ayers, C.; Lloyd-Jones, D.M.; Wilkins, J.T. HDL Efflux Capacity, HDL Particle Size, & High-Risk Carotid Atherosclerosis in a Cohort of Asymptomatic Older Adults: The Chicago Healthy Aging Study. J. Lipid Res. 2017, 58, 600–606. [Google Scholar] [CrossRef]
- Koekemoer, A.L.; Codd, V.; Masca, N.G.D.; Nelson, C.P.; Musameh, M.D.; Kaess, B.M.; Hengstenberg, C.; Rader, D.J.; Samani, N.J. Large-Scale Analysis of Determinants, Stability, and Heritability of High-Density Lipoprotein Cholesterol Efflux Capacity. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Ebtehaj, S.; Gruppen, E.G.; Parvizi, M.; Tietge, U.J.F.; Dullaart, R.P.F. The Anti-Inflammatory Function of HDL Is Impaired in Type 2 Diabetes: Role of Hyperglycemia, Paraoxonase-1 and Low Grade Inflammation. Cardiovasc. Diabetol. 2017, 16, 1–9. [Google Scholar] [CrossRef]
- Kappelle, P.J.W.H.; de Boer, J.F.; Perton, F.G.; Annema, W.; de Vries, R.; Dullaart, R.P.F.; Tietge, U.J.F. Increased LCAT Activity and Hyperglycaemia Decrease the Antioxidative Functionality of HDL. Eur. J. Clin. Investig. 2012, 42, 487–495. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Kieneker, L.M.; Bakker, S.J.L.; James, R.W.; Dullaart, R.P.F. Incident Type 2 Diabetes Is Associated with HDL, but Not with Its Anti-Oxidant Constituent—Paraoxonase-1: The Prospective Cohort PREVEND Study. Metabolism. 2017, 73, 43–51. [Google Scholar] [CrossRef]
- Dallinga-Thie, G.M.; Dullaart, R.P.F.; Van Tol, A. Concerted Actions of Cholesteryl Ester Transfer Protein and Phospholipid Transfer Protein in Type 2 Diabetes: Effects of Apolipoproteins. Curr. Opin. Lipidol. 2007, 18, 251–257. [Google Scholar] [CrossRef]
- Dullaart, R.P.F.; de Vries, R.; Kwakernaak, A.J.; Perton, F.; Dallinga-Thie, G.M. Increased Large VLDL Particles Confer Elevated Cholesteryl Ester Transfer in Diabetes. Eur. J. Clin. Investig. 2015, 45, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Di Angelantonio, E.; Sarwar, N.; Erqou, S.; Saleheen, D.; Dullaart, R.P.F.; Keavney, B.; Ye, Z.; Danesh, J. Association of Cholesteryl Ester Transfer Protein Genotypes with CETP Mass and Activity, Lipid Levels, and Coronary Risk. JAMA J. Am. Med. Assoc. 2008, 299, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Masson, W.; Lobo, M.; Siniawski, D.; Huerín, M.; Molinero, G.; Valéro, R.; Nogueira, J.P. Therapy with Cholesteryl Ester Transfer Protein (CETP) Inhibitors and Diabetes Risk. Diabetes Metab. 2018, 44, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Vergeer, M.; Boekholdt, S.M.; Sandhu, M.S.; Ricketts, S.L.; Wareham, N.J.; Brown, M.J.; De Faire, U.; Leander, K.; Gigante, B.; Kavousi, M.; et al. Genetic Variation at the Phospholipid Transfer Protein Locus Affects Its Activity and High-Density Lipoprotein Size and Is a Novel Marker of Cardiovascular Disease Susceptibility. Circulation 2010, 122, 470–477. [Google Scholar] [CrossRef]
- Abbasi, A.; Dallinga-Thie, G.M.; Dullaart, R.P.F. Phospholipid Transfer Protein Activity and Incident Type 2 Diabetes Mellitus. Clin. Chim. Acta. 2014, 439, 38–41. [Google Scholar] [CrossRef]
- Von Eckardstein, A.; Widmann, C. High-density lipoprotein, beta cells, and diabeteś. Cardiovasc. Res. 2014, 103, 384–394. [Google Scholar] [CrossRef]
| Variables | Total | Incident PTDM (Posttransplantation Diabetes Mellitus) | p Value | |
|---|---|---|---|---|
| Yes | No | |||
| Participants, n | 351 | 39 | 312 | |
| General characteristics | ||||
| Men, % | 54.1 | 61.5 | 53.2 | 0.325 |
| Age, year (y) | 51.3 ± 13.4 | 52.2 ± 10.4 | 51.2 ± 13.9 | 0.589 |
| Lifestyle parameter | ||||
| Current smoker, % | 14.0 | 17.9 | 13.4 | 0.445 |
| Alcohol use, never, % | 10.1 | 5.4 | 10.6 | 0.320 |
| Physical activity score (time×intensity) | 5640 (3220–8940) | 4710 (2520–10440) | 5760 (3305–8790) | 0.597 |
| Body composition | ||||
| Weight, kg | 78.5 ± 16.1 | 85.5 ± 15.5 | 77.6 ± 15.9 | 0.005 |
| Height, cm | 173.8 ± 9.3 | 175.6 ± 9.4 | 173.6 ± 9.3 | 0.220 |
| BMI, kg/m2 | 25.9 ± 4.5 | 27.7 ± 4.3 | 25.7 ± 4.5 | 0.010 |
| Waist circumference, cm | 96.2 ± 14.4 | 104.3 ± 14.1 | 95.3 ± 14.2 | 0.001 |
| Transplant demographics | ||||
| Time since renal transplantation, y | 4.9 (1.5–11.8) | 3.2 (1.4–11.8) | 5.0 (1.7–11.9) | 0.297 |
| Donor age, y | 44.0 ± 15.2 | 44.4 ± 16.1 | 44.0 ± 15.2 | 0.888 |
| Living donor, % | 37.9 | 43.6 | 37.2 | 0.437 |
| Dialysis duration, months | 26(5–55) | 21 (0–59) | 26 (6–54) | 0.604 |
| Delayed graft function, % | 8.0 | 12.8 | 7.4 | 0.237 |
| Rejection, % | 23.4 | 20.5 | 23.7 | 0.656 |
| CMV infection, % | 25.6 | 26.3 | 20.5 | 0.436 |
| Blood pressure | ||||
| Systolic blood pressure, mmHg | 135.8 ± 17.4 | 142.7 ± 15.9 | 135.0 ± 17.4 | 0.008 |
| Distolic blood pressure, mmHg | 82.8 ± 11.2 | 88.1 ± 11.6 | 82.1 ± 11.0 | 0.003 |
| Hypertension, % | 90.3 | 97.4 | 89.4 | 0.111 |
| Glucose Homeostasis | ||||
| Glucose, mmol/L | 5.1 ± 0.6 | 5.3 ± 0.6 | 5.1 ± 0.06 | 0.064 |
| HbA1c, % | 5.6 ± 0.3 | 6.0 ± 0.3 | 5.6 ± 0.3 | < 0.001 |
| Hs-CRP, mg/L | 1.4 (0.6–3.8) | 1.6 (0.8–2.8) | 1.4 (0.6–4.2) | 0.766 |
| Renal function | ||||
| eGFR, mL/min per 1.73 m2 | 42.9 (30.4-56.8) | 41.1 (25.0-52.3) | 43.0 (31.0–57.8) | 0.172 |
| Urinary Albumin excretion, mg/24 h | 38.9 (9.5–170.5) | 43.2 (6.8–201.6) | 38.5 (10.0–169.7) | 0.615 |
| Medication use | ||||
| Lipid-lowering medication, % | 49.3 | 56.4 | 47.8 | 0.309 |
| Anti-hypertensive medication, % | 88.0 | 94.9 | 87.2 | 0.163 |
| Prednisolone, mg/day | 8.8 ± 1.8 | 9.3 ± 1.3 | 8.8 ± 1.9 | 0.024 |
| Calcineurin inhibitor, % | 58.1 | 76.9 | 55.8 | 0.012 |
| Cyclosporine, % | 41.9 | 53.8 | 40.4 | |
| Tacrolimus, % | 16.5 | 23.1 | 15.7 | |
| Proliferation inhibitor, % | 86.3 | 76.9 | 87.5 | 0.070 |
| Azathioprine,% | 19.9 | 15.4 | 20.5 | |
| Mycophenolic acid, % | 66.4 | 61.5 | 67.0 | |
| Variables | Total | Incident PTDM | p Value | |
|---|---|---|---|---|
| Yes | No | |||
| Participants, n | 351 | 39 | 312 | |
| Triglycerides (total), mg/dL | 149 (103–208) | 173 (126–297) | 146 (102–200) | 0.004 |
| Total cholesterol, mg/dL | 198.9 ± 41.0 | 295.1 ± 45.8 | 198.1 ± 40.3 | 0.365 |
| HDL cholesterol (total), mg/dL | 54.4 ± 14.8 | 48.4 ± 10.6 | 55.2 ± 15.1 | 0.001 |
| TRL particles (total), nmol/L | 189 (138–265) | 219 (148–316) | 184 (136–263) | 0.068 |
| LDL particles (total), nmol/L | 1400 (1171–1632) | 1427 (1335–1673) | 1395 (1158–1622) | 0.212 |
| HDL particles (total), µmol/L | 20.5 ± 3.3 | 20.1 ± 3.2 | 20.5 ± 3.3 | 0.469 |
| Large HDL particles, µmol/L | 2.2 (1.3–3.6) | 1.4 (1.0–2.3) | 2.3 (1.4–3.8) | <0.001 |
| Medium HDL particles, µmol/L | 4.9 ± 2.1 | 4.6 ± 1.9 | 4.9 ± 2.2 | 0.452 |
| Small HDL particles, µmol/L | 13.0 ± 3.3 | 13.8 ± 2.9 | 12.9 ± 3.3 | 0.095 |
| HDL size, nm | 9.1 ± 0.5 | 8.9 ± 0.3 | 9.2 ± 0.5 | 0.001 |
| HDL Subspecies | ||||
| H7P, µmol/L | 0.3 (0.1–0.5) | 0.2 (0.1–0.3) | 0.3 (0.1–0.6) | 0.022 |
| H6P, µmol/L | 0.6 (0.2–1.5) | 0.3 (0.1–0.7) | 0.7 (0.2–1.6) | 0.002 |
| H5P, µmol/L | 0.9 (0.4–1.5) | 0.8 (0.4–1.2) | 0.9 (0.4–1.5) | 0.378 |
| H4P, µmol/L | 1.7 (1.1–2.5) | 1.7 (1.3–2.4) | 1.7 (1.1–2.5) | 0.841 |
| H3P, µmol/L | 2.8 (1.5–4.2) | 2.9 (1.4–4.3) | 2.8 (1.5–4.2) | 0.768 |
| H2P, µmol/L | 9.6 (7.6–11.8) | 11.0 (8.8–12.5) | 9.4 (7.4–11.6) | 0.024 |
| H1P, µmol/L | 3.0 (1.8–4.5) | 3.0 (1.5–4.1) | 3.0 (1.8–4.7) | 0.569 |
| Tertiles | 1 | 2 | 3 | ||
|---|---|---|---|---|---|
| HDL cholesterol, mg/dL | >59 | 47–58 | <47 | Per 1SD | p value |
| Cases | 7 | 14 | 18 | 39 | |
| Crude analysis | 1.00 (ref) | 2.07 (0.84–5.14) | 3.29 (1.37–7.88) | 0.53 (0.36–0.80) | 0.002 |
| Model 1 | 1.00 (ref) | 1.99 (0.79–5.05) | 3.01 (1.22–7.43) | 0.55 (0.36–0.83) | 0.005 |
| Model 2 | 1.00 (ref) | 1.78 (0.69–4.63) | 2.89 (1.16–7.23) | 0.53 (0.34–0.83) | 0.006 |
| Model 3 | 1.00 (ref) | 2.21 (0.85–5.74) | 3.15 (1.26–7.92) | 0.55 (0.36–0.83) | 0.004 |
| Model 4 | 1.00 (ref) | 1.90 (0.74–4.90) | 2.60 (1.02–6.61) | 0.59 (0.39–0.91) | 0.018 |
| Model 5 | 1.00 (ref) | 2.62 (1.01–6.80) | 2.71 (1.05–6.99) | 0.59 (0.38–0.92) | 0.021 |
| Model 6 | 1.00 (ref) | 1.92 (0.76–4.90) | 2.53 (1.00–6.48) | 0.61 (0.40–0.94) | 0.024 |
| Large HDL particles µmol/L | >2.9 | 1.6–2.9 | <1.6 | Per 1SD Log | p value |
| Cases | 7 | 11 | 21 | 39 | |
| Crude analysis | 1.00 (ref) | 1.70 (0.66–4.39) | 3.59 (1.53–8.46) | 0.66 (0.51–0.84) | 0.001 |
| Model 1 | 1.00 (ref) | 1.46 (0.55–3.85) | 3.18 (1.29–7.87) | 0.63 (0.47–0.84) | 0.002 |
| Model 2 | 1.00 (ref) | 1.28 (0.47–3.47) | 3.06 (1.22–7.66) | 0.61 (0.44–0.84) | 0.002 |
| Model 3 | 1.00 (ref) | 1.78 (0.66–4.80) | 3.43 (1.38–8.52) | 0.60 (0.45–0.81) | 0.001 |
| Model 4 | 1.00 (ref) | 1.51 (0.55–4.10) | 3.06 (1.18–7.88) | 0.64 (0.47–0.86) | 0.004 |
| Model 5 | 1.00 (ref) | 1.37 (0.51–3.73) | 2.70 (1.05–6.91) | 0.67 (0.48–0.93) | 0.017 |
| Model 6 | 1.00 (ref) | 1.49 (0.53–3.94) | 2.83 (1.10–7.29) | 0.68 (0.50–0.93) | 0.017 |
| HDL size, nm | >9.2 | 8.9–9.2 | <8.9 | Per 1SD | p value |
| Cases | 5 | 13 | 21 | 39 | |
| Crude analysis | 1.00 (ref) | 3.05 (1.09–8.56) | 4.57 (1.72–12.12) | 0.47 (0.31–0.72) | 0.001 |
| Model 1 | 1.00 (ref) | 2.78 (0.98–7.89) | 4.09 (1.47–11.35) | 0.48 (0.31–0.76) | 0.002 |
| Model 2 | 1.00 (ref) | 2.60 (0.91–7.47) | 3.68 (1.30–10.42) | 0.50 (0.31–0.80) | 0.004 |
| Model 3 | 1.00 (ref) | 3.56 (1.24–10.21) | 4.63 (1.65–13.02) | 0.48 (0.32–0.75) | 0.001 |
| Model 4 | 1.00 (ref) | 2.90 (1.01–8.33) | 3.80 (1.34–10.80) | 0.51 (0.33–0.81) | 0.004 |
| Model 5 | 1.00 (ref) | 2.10 (0.73–6.07) | 3.01 (1.06–8.56) | 0.62 (0.40–0.98) | 0.040 |
| Model 6 | 1.00 (ref) | 2.85 (1.00–8.15) | 3.46 (1.18–10.21) | 0.58 (0.36–0.93) | 0.025 |
| Joint HDL Subclasses | Large HDL Particles | Medium HDL Particles | Small HDL Particles | |||
|---|---|---|---|---|---|---|
| Jointly Models * | HR (95% CI) Per Log1 SD | p Value | HR (95% CI) Per 1 SD | p Value | HR (95% CI) Per 1 SD | p Value |
| Undjusted | 0.68 (0.50–0.93) | 0.014 | 0.97 (0.68–1.38) | 0.85 | 1.05 (0.51–2.15) | 0.75 |
| Adjusted for LDL particles | 0.67 (0.49–0.92) | 0.012 | 0.97 (0.68–1.39) | 0.88 | 1.06 (0.77–1.47) | 0.71 |
| Adjusted for TRL particles | 0.68 (0.50–0.93) | 0.017 | 0.96 (0.67–1.37) | 0.84 | 1.05 (0.75–1.46) | 0.76 |
| Adjusted for LDL and TRL particles | 0.67 (0.49–0.92) | 0.015 | 0.97 (0.68–1.38) | 0.86 | 1.06 (0.76–1.47) | 0.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokooti, S.; Szili-Torok, T.; Flores-Guerrero, J.L.; Osté, M.C.J.; Gomes-Neto, A.W.; Kootstra-Ros, J.E.; Heerspink, H.J.L.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. High-Density Lipoprotein Particles and Their Relationship to Posttransplantation Diabetes Mellitus in Renal Transplant Recipients. Biomolecules 2020, 10, 481. https://doi.org/10.3390/biom10030481
Sokooti S, Szili-Torok T, Flores-Guerrero JL, Osté MCJ, Gomes-Neto AW, Kootstra-Ros JE, Heerspink HJL, Connelly MA, Bakker SJL, Dullaart RPF. High-Density Lipoprotein Particles and Their Relationship to Posttransplantation Diabetes Mellitus in Renal Transplant Recipients. Biomolecules. 2020; 10(3):481. https://doi.org/10.3390/biom10030481
Chicago/Turabian StyleSokooti, Sara, Tamas Szili-Torok, Jose L. Flores-Guerrero, Maryse C. J. Osté, António W. Gomes-Neto, Jenny E. Kootstra-Ros, Hiddo J.L. Heerspink, Margery A. Connelly, Stephan J. L. Bakker, and Robin P. F. Dullaart. 2020. "High-Density Lipoprotein Particles and Their Relationship to Posttransplantation Diabetes Mellitus in Renal Transplant Recipients" Biomolecules 10, no. 3: 481. https://doi.org/10.3390/biom10030481
APA StyleSokooti, S., Szili-Torok, T., Flores-Guerrero, J. L., Osté, M. C. J., Gomes-Neto, A. W., Kootstra-Ros, J. E., Heerspink, H. J. L., Connelly, M. A., Bakker, S. J. L., & Dullaart, R. P. F. (2020). High-Density Lipoprotein Particles and Their Relationship to Posttransplantation Diabetes Mellitus in Renal Transplant Recipients. Biomolecules, 10(3), 481. https://doi.org/10.3390/biom10030481







